Abstract
Single erythropoietin (EPO) glycoforms with defined mature oligosaccharide structures and amino acid sequences are essential to elucidate the molecular mechanisms by which carbohydrates exert various physiological and metabolic functions and to explore the possible links between carbohydrates and the prevention or management of diseases. To demonstrate that it is possible to generate EPO even without recourse to cysteine-based NCL, a concise synthesis of the partially-protected EPO fragment (78-166) bearing fully mature N- and O-glycans is described.
Introduction
Traditionally in the mindset of the pharmaceutical sciences, there has prevailed an unstated but still sharp division between the worlds of “biologics” and “small molecules”. Chemists have played relatively minor roles in the space of “biologics”. Rather, chemistry has typically been confined to the realm of “small molecules” where its capacities for synthesis are widely recognized.
In our judgment, there is considerable opportunity for the discovery process to benefit substantially from focused chemistry even in the field of “biologics”.1 The thought was that revolutionary developments in the field of chemical synthesis, if properly appreciated and optimized, could change the prevailing assumptions concerning biologics. We have been pursuing this vision, particularly in the context of complex carbohydrates, paving the way for instance, for the design of fully synthetic vaccines.2 Of course, the central element of our attempt at constructive intervention of chemistry in the realm of “biologics” is our proposed synthesis of the glycoprotein, erythropoietin (EPO).
In launching the EPO project, we took note that the functions of many proteins are modulated by post-translational glycosylations.3 Indeed, protein glycosylation has been implicated in mediating protein folding, in protecting against proteolysis, in cellular differentiation, and in cell-cell communication.4-7 Mammalian cell-expressed glycoproteins are often heterogeneous mixtures of glycoforms with potentially different biological properties. Access to homogeneous glycoproteins might facilitate the exploration of specific functions associated with specific structures. Of course, the natural accessibility of homogeneous entities in this area is severely limited by difficulties in the isolation and purification of specific glycoforms from highly complex mixtures. Therefore, the development of technologies which allow access to homogeneous and structurally defined glycoproteins, would be of significant value for systematic study of the effects of protein glycosylation on stability and function.
Despite the daunting challenges associated with the preparation of glycopeptides in the laboratory, we are pursuing the possibility that chemical synthesis could yet prove to be the most viable way to provide the field of glycobiology with homogeneous ‘precision tools’ for study.1,8,9 In this connection, our laboratory is engaged in an ongoing program devoted to the de novo synthesis of a homogeneous erythropoietic agent. Erythropoietin (EPO) is a 166-residue conserved protein possessing four sites of glycosidation.10-19 Three oligosaccharide chains are N-linked to specific asparagine residues and one is O-linked to a serine residue. Although studies of human erythropoietin and recombinant erythropoiesis stimulating proteins have provided a wide array of information on the biological properties of EPO, a rigorous comparison of the therapeutic value of defined glycoforms of EPO has not been possible owing to the huge difficulties in obtaining homogeneous EPO from natural sources.20,21 Chemical synthesis of a homogeneous functional EPO should greatly advance our understanding of the biological roles of EPO glycans.
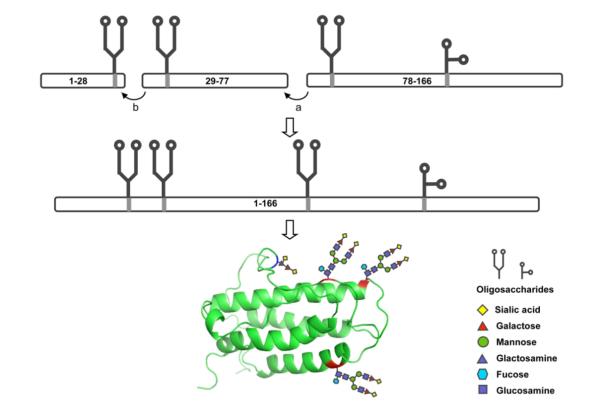
Our governing plan anticipates that EPO will be assembled by sequential fragment condensations22,23 (including native chemical ligation (NCL) reactions24,25) from three discrete glycopeptide fragments, EPO(78-166), EPO(29-77) and EPO(1-28) (Figure 1). Each fragment terminates in a glycine residue. In this way, we hope to facilitate fragment coupling, and to prevent epimerization during the coupling reactions. For such a plan to be realized, accessibility by synthesis to defined homogeneous glycopeptide fragments bearing N- and O-glycans is crucial.
Figure 1.
Synthetic strategy toward homogeneous erythropoietin.
(a) Fragment condensation between G77 and Q78; (b) Native chemical ligation between G28 and C29. The ribbon diagram of the tertiary structure of human EPO protein is shown in green. N-linked glycosylation sites, N24, N38 and N83 are shown in red and O-linked glycosylation site, S126, in blue.
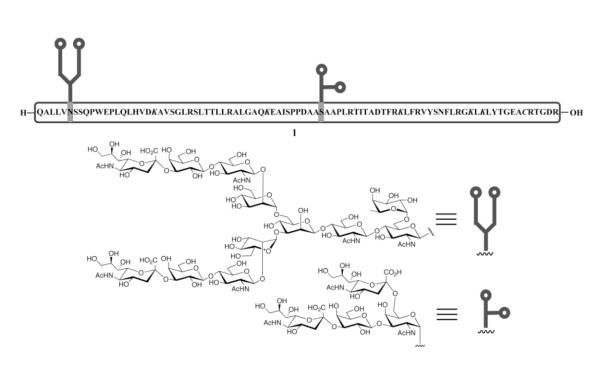
The results reported herein describe the synthesis of a major subunit of erythropoietin, i.e. the partially-protected EPO fragment 1 (see Figure 2). It will be noted that 1, which comprises more than half of the EPO molecule (78-166) bearing fully mature N- and O-glycans, will be arranged for incorporation into several possible assembly permutations to enable the total synthesis of a homogeneous EPO. Aside from its potential ramifications for probing chemobiology of complex glycopeptides, we viewed our EPO project as providing an opportunity to learn more about the chemical synthesis of complex glycopeptides, especially those with relatively few cysteine residues. We hoped that a successful program to reach a homogeneous fragment of the scope of this target would be likely to enable a much broader menu of technical options than had been available for gaining access to other complex glycopeptides.
Figure 2.
Illustration of partially-protected EPO(78-166) glycopeptides with the mature N- and O-linked glycans. The protected amino acid residues, K97, K116, K140, K152, K154 and C161, are shown in italic.
Results and Discussion
Several strategies have been entertained for the synthesis of target 1. At the outset, we favored a convergent approach, which would involve the merger of two long glycopeptide fragments, EPO(78-113) and EPO(114-166), through auxiliary-based cysteine-free NCL.14,26 For such a synthesis to be realized, the first stage would entail the preparation of homogeneous glycopeptidic fragments bearing defined N- and O-glycans. Toward this end, we recently disclosed the construction of the O-linked glycopeptide EPO(114-166) through cysteine-free NCL.12 Depending on the situation, the N-linked glycopeptides could be prepared as a single block, or in the form of segments, which could be subsequently ligated to form EPO(78-113). In either case, the need for the glycan to be efficiently coupled to a peptide chain and to be maintained through the synthesis requires careful selection of glycosylation conditions and protecting groups.16,27,28
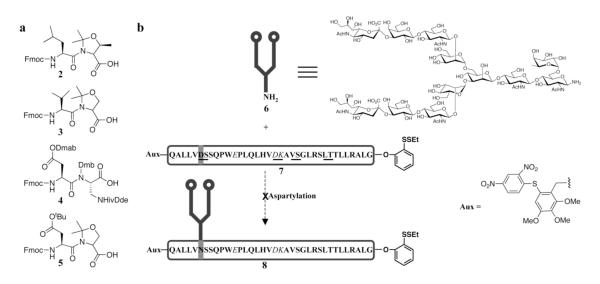
We first attempted to implement the seemingly more straightforward single-block plan toward 1. By this strategy, the N-linked glycopeptide EPO(78-113) would be generated by direct coupling of a carbohydrate moiety (cf. 6) with a complete peptide construct, possessing a C-terminal 2-(ethyldithio)-phenol ester and/or an N-terminal auxiliary (7, Figure 3b). However, an issue presented itself in this otherwise attractive coupling strategy. The EPO(78-113) peptide fragment, which is long and hydrophobic, was extremely difficult to prepare under the standard Fmoc solid-phase peptide synthesis (SPPS) conditions.29
Figure 3.
The single block strategy toward EPO(78-113) glycopeptide fragment.a
a(a) Pseudoproline and Dmb dipeptides used to enhance the synthetic efficiency of the peptidic fragment of EPO(78-113). (b) Overview of the direct aspartylation strategy for the synthesis of EPO(78-113) glycopeptide. The dipeptides used in SPPS are underlined. The protected amino acid residues are shown in italic. Reagents and conditions, HATU, DIEA, DMSO.
Fortunately, as demonstrated by Mutter and co-workers, fully protected long hydrophobic peptide fragments can be efficiently prepared through the use of secondary amino acid surrogates, which take advantage of the natural propensity of proline and N-alkyl amino acids to disrupt the formation of secondary structures during peptide assembly.30 Furthermore, the use of secondary amino acid surrogates significantly improves the solubility of fully protected peptides, which is essential for subsequent esterification of the C-terminal carboxylic group in CH2Cl2.
The synthesis of the EPO(78-113) peptide commenced with the NovaSyn® TGT resin, preloaded with Fmoc-Gly. As shown in Figure 3a, three pseudoproline dipeptides (2, 3 and 5) were incorporated into the peptide during SPPS to improve the purity and solubility of the peptidic product. A Dmb (2,4-dimethoxy benzyl) dipeptide (4) was incorporated to block aspartimide formation.31 The glutamic acid residue was also equipped with the Dmab protecting group and the lysine residue was introduced with ivDde [1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl]. The protective use of Dmab and ivDde enabled selective appendage of the oligosaccharide domain to the D83 residue of this fragment. Unfortunately, when partially protected peptide 7 and glycopeptide 8 were exposed to Lansbury aspartylation conditions,1,28 the desired coupling did not proceed to a useful level, owing to many side reactions, including aspartimide formation.
Given the difficulties in achieving direct aspartylation between the complex oligosaccharide units and the complex peptide fragments of EPO, we wondered about reaching EPO(78-113) glycopeptide through fragment coupling between a relatively small glycopeptide fragment and a longer peptidic fragment. To avoid epimerization during esterification of the C-terminus of the peptide, we planned to ligate this fragment at either the Pro87-Trp88 or Pro90-Leu91 site. Based on our previous work,1,16 we reasoned that the use of such a short peptide with fewer side-chain functional groups could well enable more efficient aspartylation of the dodecamer saccharide, by exploiting the logic of sequential Kochetkov-Lansbury amination and aspartylation protocols.1,16
Initially, our thinking was strongly influenced by the success14 of our auxiliary-based cysteine-free NCL protocol in glycopeptide couplings. These considerations prompted the synthesis and screening of various truncated possibilities for the synthesis of the EPO(78-113) glycopeptide.14 Unfortunately, but not surprisingly, due to several significant application limitations, auxiliary-based NCL at non-glycine, non-alanine sites did not yield promising results.
We next redirected our efforts to reach EPO(78-113) through thiocarboxyl segment condensations. This approach relied on a proposal that a peptide/glycopeptide fragment condensation between Pro and Trp/Leu can be accomplished under Blake-Aimoto conditions, although study of the literature revealed surprisingly few encouraging examples without a Gly at the joining site.22,23,32,33 The validity of this hypothesis was first field-tested in the context of the synthesis of EPO(78-113) peptide. Our venture commenced with the preparation of four peptide fragments EPO(78-87), EPO(78-90), EPO(88-113) and EPO(91-113). For reasons discussed above, the pseudoproline dipeptides were used for preparing these peptide fragments. Happily, under carefully optimized reaction conditions, AgCl-mediated fragment condensation worked well at both Pro87-Trp88 and Pro90-Leu91 sites. It should be noted that the condensation between Pro87 and Trp88 is perhaps more attractive due to the absence of a glutamate δ-allyl ester, which would require introduction of an additional allyl protecting group.
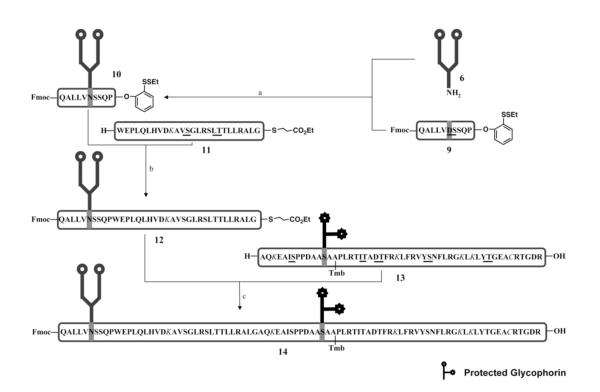
Thus encouraged, we undertook the challenge of reaching the EPO(78-87) glycopeptide, bearing the dodecamer oligosaccharide, through condensation at the Pro87-Trp88 site (Figure 4). The approach of EPO(78-87) glycopeptide synthesis used here had already been charted,1,16 but the implementation was now modified and systematically optimized to avoid undesired aspartimide formation. The other issue is that of appropriate differentiation of the C-terminus of the EPO(78-87) glycopeptide and the EPO(88-113) peptide. Toward this end, a productive solution to the problem has been charted recently.18 Synthesis of EPO(78-113) glycopeptide with a C-terminal S-alkyl thioester could be accomplished through selective condensation at Pro87-Trp88 site between C-terminal 2-(ethyldithio)-phenolic ester and N-terminal amino group under TCEP•HCl conditions (Figure 4). Another key issue to be faced now was whether the methodology used for relatively short glycopeptide models could be transferred to building a long glycopeptide. Potentially severe hindrance of the sugar moieties and issues arising from the difficultly predictable conformation of the peptide could not be evaluated in advance. Great efforts were invested in optimizing the direct coupling of EPO(78-87) glycopeptide 10 and EPO(88-113) peptide 11. Once again, condensation at the Pro87-Trp88 site using modified Blake-Aimoto conditions furnished the EPO(78-113) glycopeptide 12 in 33% isolated yield.
Figure 4.
The fragment condensation strategy for the synthesis of EPO(78-113) glycopeptide.a
aThe protected glycans are shown in star shape. Tmb is an abbreviation for 4,5,6-trimethoxy-2-methylthiobenzyl. The dipeptides used in SPPS are underlined. The protected amino acid residues are shown in italic. Reagents and conditions: (a) HATU, DIEA, DMSO, 29%; (b) TCEP•HCl, HOOBt, DIEA, DMSO, 33%; (c) AgCl, HOOBt, DIEA, DMSO, 30%.
In planning the synthesis of EPO(78-166) glycopeptide, we anticipated the possibility that the synthetic target might be prepared by joining the two fragments, EPO(78-113) and EPO(114-166), either through auxiliary-based Cys-free NCL or through Cys-free fragment condensation. Inspection of these two glycopeptide coupling methods revealed that, although both methods might be able to generate the desired product, Cys-free NCL requires an extra step for the attachment of the auxiliary. On the basis of these and other considerations, we preferred to implement a fragment condensation approach.
As a model for what had to be accomplished, we began our study with a simple condensation between the peptide fragments. Following implementation of the standard fragment condensation procedure, EPO(78-113) peptide, which was equipped with C-terminal S-ethyl propionate thioester,32 was successfully condensed with partially protected EPO(114-166) to afford uncapped EPO(78-166) peptide in 53% isolation yield.
The next task was that of preparing the EPO(78-166) glycopeptide. With the N-linked EPO(78-113) glycopeptide (12) in hand, we were prepared to take on the challenge of synthesizing our target compound: EPO(78-166) glycopeptide with both N- and O-linked glycans. Happily, under AgCl-mediated conditions, coupling of EPO(78-113) glycopeptide (12) with EPO(114-166) glycopeptide (13) produced the desired partially protected EPO(78-166) glycopeptide (14) in 30% isolated yield (Figure 4). Unfortunately, all attempts to cleave the acid-labile auxiliary without destroying the N-linked oligosaccharide failed.
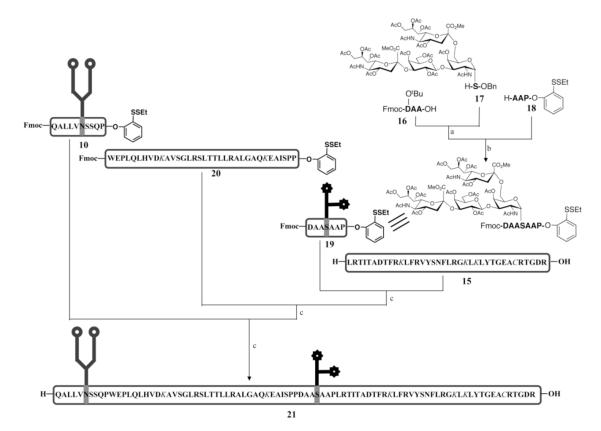
Since auxiliary-based native chemical ligation had been shown to be unsuitable for the synthesis of EPO(78-166) glycopeptide, we developed a method for the synthesis of acid-sensitive sialic acid-containing glycopeptides which takes advantage of Cys-free fragment condensation. While a variety of options for eventually reaching auxiliary-free EPO(78-166) glycopeptide 21 merited consideration, based on our early experiences we were particularly disposed toward the route shown in Figure 5. According to this strategy, EPO(78-166) glycopeptide (21) would be assembled from two short glycopeptides, EPO(78-87) (10) and EPO(123-129) (19), and two long peptides, EPO(88-122) (20) and EPO(130-166) (15), containing all the side chain protecting groups. In formulating a prospective plan, we desired that the proposed synthesis be flexible, allowing for optimization by varying the protecting groups of the peptide fragments, without modifying the complicated glycopeptide synthesis. This synthetic plan also fulfilled the requirement that the precious N-linked glycopeptide be the limiting reagent in the fragment condensation process.
Figure 5.
Synthesis of the EPO(78-166) glycopeptide with both N-linked glycan and O-linked protected glycans.a
a The protected amino acid residues are shown in italic. Reagents and conditions: (a) DCC, DMAP, CH2Cl2, 70%; (b) 1. Pd/C, H2, MeOH, AcOH, 2. DCC, DMAP, CH2Cl2, 3. TFA, PhOH, TIPS, H2O, 25%; (c) 1. TCEP•HCl, HOOBt, DIEA, DMSO, 2. Piperidine, DMSO, 43% for the first condensation; 53% for the second condensation; 39% for the third condensation. The side chain of aspartic acid residue in peptide 16 is protected with tert-butyl group. The side chains of lysine and cysteine residues in 15, 20 and 21 are protected with ivDde and Acm, respectively.
To efficiently merge mature glycopeptide and peptide fragments to afford 21, it would be necessary to first develop an enabling non-Cys-based fragment condensation protocol. For practical reasons, we began with four peptide fragments, having the same amino acid sequences as the glycopeptides in Figure 5. The viability of both the AgCl and the TCEP-actuated fragment—fragment coupling approaches were evaluated in the context of these peptides.17 Although both approaches could be harmonized to allow the coupling process to proceed from N- to C-terminus, one-pot Fmoc deprotection reaction following each condensation reaction is clearly not feasible for the AgCl-mediated fragment coupling due to silver-mediated acetamidomethyl (Acm) removal. Although the addition of excess dithiothreitol (DTT) served to eliminate such side reactions, trace amounts of DTT remaining in the substantially “purified” product apparently complicated further fragment condensation reactions.
Thus, the EPO(78-166) peptide was synthesized using TCEP-mediated fragment condensation.18 The condensation conditions were further optimized to enhance the reaction rates and to reduce the formation of 3-phosphinyl-propionamide by lowering the concentration of TCEP•HCl. At this stage, we were, in fact, well aware of the relatively low isolation yield associated with obtaining peptides protected with hydrophobic protecting groups, including side chain ivDde, N-terminal Fmoc and C-terminal phenolic ester. Despite potential difficulties associated with the TCEP-mediated fragment condensation,18 it is our judgment that this approach still represents one of the most promising means by which to generate Cys-deficient peptides and glycopeptides.
Having devised what seemed to be a feasible protocol, we prepared permutations of the fragments required for the assembly of EPO(78-166) glycopeptide. For instance, two peptide fragments, 15 and 20, and the N-linked glycopeptide 10 could be easily prepared through the techniques mentioned above. However, we still needed to develop a practical synthetic route to reach the O-linked glycopeptide, 19. While previous work had shown that the protected the O-linked glycoamino acids could be directly used as building blocks for SPPS, the general record from glycophorin-serine glycoamino acids as SPPS building blocks suggested that excess amounts of protected glycoamino acids are required to obtain satisfactory yields.34,35 We hoped to exploit an alternative approach, outlined in Figure 5, as a means of meeting our primary requirement: that access to a fully synthetic O-glycoamino acid be the limiting reagent in the synthetic sequence. Our route to 19 first involved the merging of the glycoamino acid 17 with a small peptide 16, to generate a glycopeptide intermediate. Following benzyl deprotection, this intermediate was annealed with another trimeric peptide, 18, through DCC-mediated peptide coupling. Treatment of 18 with TFA, as described, afforded the desired O-glycosylated peptide 19. We were well aware that some of the C-terminal residues might be quite susceptible to racemization under the coupling conditions used here. Special attention was directed to minimization of epimerization.
With the four required subfragments in hand, we were able to employ the Cys-free fragment coupling strategy to gain access to the EPO(78-166) glycopeptide fragment. Not surprisingly, upon exposure to TCEP•HCl, glycopeptide 19 underwent reductive disulfide cleavage to generate the active thioester moiety. Amide bond formation under modified Blake-Aimoto conditions17 followed by Fmoc removal provided EPO(123-166) glycopeptide with the protected glycophorin in place. This resultant intermediate was then advanced to partially protected glycopeptide product 21 through a two-step sequence, as shown in Figure 5, using the same reaction conditions.
The next goal en route to 1 was the deprotection of the O-linked glycophorin. We proceeded with circumspection. In our initial experiments, it was pleasing to observe nearly quantitative deprotection of small glycopeptide 19 under basic conditions (NaOH in H2O and MeOH), as determined by LCMS.36 We also found that the NaOH method can slowly remove the ivDde functionality. Thus encouraged, we sought to determine whether such NaOH-based protocols would be compatible with the extensive functionality present in our large, complex glycopeptide substrate systems. Unfortunately, we found a significant drawback in the method: it is unable to accommodate the Acm functionality, which is commonly employed as a protecting group for cysteine. In addition to small amounts of the desired deprotected product, we typically observed extensive side product formation, probably resulting from β-elimination of the Acm-protected Cys residues and subsequent addition reaction between dehydroalanine and methanol.
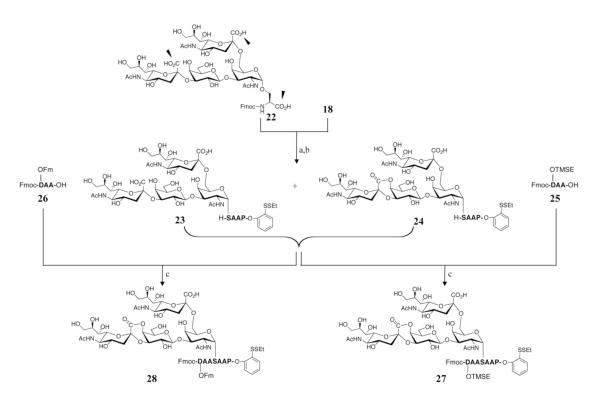
To overcome the problems caused by the requirement of global deprotection at the level of large glycopeptide constructs, we sought to develop a general synthetic method for O-linked glycopeptides. It was necessary that the method be tolerant of glycoamino acids with unmasked carbohydrate moieties. We began our study by taking note of the work of Meldal et. al. in 1993, which described a solid phase glycopeptide synthesis from glycoamino acid containing unprotected O-glycans.37 This approach has since been employed in the synthesis of a few simple glycopeptides with some success.38-40 On the basis of this work, we considered the possibility of utilizing a similar strategy to prepare our O-glycopeptide. We noted that, apparently, there was a significant difference between the unprotected glycophorin-Ser and the previously used O-glycoamino acids: the glycophorin amino acid contains two extra carboxylic acid groups, which may react with the amine containing starting materials (Fig. 6, black arrows). Happily, in practice, the HATU-mediated peptide coupling reaction between 18 and 22 proceeded very selectively to afford the desired products, 23 and 24, in more than 30% isolation yield, in spite of our initial concerns. This reaction also occurred quite rapidly, with complete consumption of the limiting reagent 18, within 1 min. We took this result to suggest that, under HATU-mediated conditions, the carboxylic groups could be clearly differentiated on the basis of their β-carbon substitution patterns. The proximity-accelerated irreversible lactone formation reaction could be another reason for the selectivity. The structures of the products were assigned by analyzing their fragmentation patterns in electrospray ionization-mass spectrometry (ESI-MS). The two compounds, 23 and 24, were then submitted concurrently to the next step, which involved another HATU-mediated peptide coupling with tripeptide 25. Again, the reaction proceeded rapidly with complete consumption of 23 and 24 in 1 min, leading to the formation of the unprotected glycopeptide 27. The selection of 2-(trimethylsilyl)ethyl (TMSE) as the side chain protecting group for Asp was influenced, to some extent, by a perception that the alkyl ester would be less “sticky” to the HPLC column, thus improving the O-glycopepide synthesis yield.
Figure 6.
Demonstration of a novel strategy for the synthesis of unprotected O-glycopeptides.a
aReagents and conditions: (a) HATU, DIEA, DMSO, 35%; (b) Piperidine, DMSO, 88%; (c) HATU, DIEA, DMSO, 51% for 27; 35% for 28.
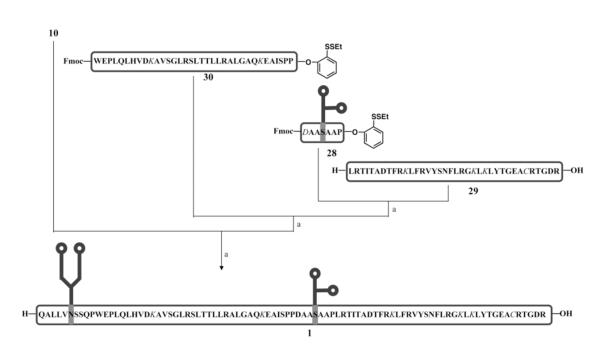
In addition to the O-linked carbohydrate deprotection problem, at this stage we also were concerned about the removal of ivDde. Based on previous work in this area, ivDde had been chosen as the side chain protecting group for Lys.12,14 Because NaOH could not be utilized for ivDde deprotection, these groups would have to be removed by hydrazine. In our initial study, we were pleased to find that the deprotection of ivDde protected EPO(78-166) peptide could be accomplished with 5% hydrazine in DMSO, without affecting the Acm functionality. However, further study of the literature revealed that hydrazine is often capable of removing carbohydrate chains from glycopeptides and glycoproteins. We therefore had to choose another amino protecting group. Based on our previous experiments, we envisioned that the allyloxycarbonyl (Alloc) protecting group could well meet the goal of orthogonality in the presence of Cys(Acm), N- and O-glycans.41,42 It was also anticipated that, having a much simpler structure, the Alloc group would have the ability to decrease the hydrophobicity of the final product.
With the synthesis of the unprotected O-glycopeptide 27 and Alloc protected peptide fragments 29 and 30 accomplished, we were now prepared to investigate the viability of our strategy for the synthesis of EPO(78-166) glycopeptide 1, which has both unprotected N- and O-carbohydrate domains. Condensation of 27 with 29 proceeded very well. However, one-pot deprotection of TMSE-ester and Fmoc under known conditions did not give rise to the desired product.43 From these lessons, a more straightforward idea for reaching 1 presented itself. The side chain of Asp would be protected by fluorenylmethyl (Fm) ester, which could also be removed by treatment with piperidine. As expected, joining the four fragments under the same conditions that we have previously developed in the synthesis of 21, gave 1 in 15% isolated yield (Figure 7). Although the Alloc and Acm protecting groups are readily removable by treatment with Pd(0)44 and with Hg(II)/Ag(I)45, they were intentionally maintained on the glycopeptide 1 to facilitate the subsequent fragment condensation reactions with Epo(29-77) and EPO(1-28). ESI mass spectrometry analysis confirmed the identity of the glycopeptide 1 (Supporting Information, Figure 29).46 The mass data of 1 also indicated the presence of lactones involving the sialic acid and the neighboring galactose units,47 which will be hydrolyzed under mild conditions after the completion of the full-length EPO synthesis.48
Figure 7.
Synthesis of EPO(78-166) glycopeptide 1.a
aKey: The protected amino acid residues are shown in italic. Reagents and conditions: (a) 1. TCEP·HCl, HOOBt, DIEA, DMSO, 2. Piperidine, DMSO, 59% for the first condensation; 52% for the second condensation; 50% for the third condensation.
Conclusion
In summary, we have described herein the synthesis of the complex partially-protected glycopeptide EPO(78-166). We are confident of the broad applicability of the methods described above for the preparation of complex glycopeptides. Perhaps even more telling is the demonstration that it is possible to generate glycopeptides even without recourse to cysteine-based NCL. The synthetic routes reported above will hopefully prove to be valuable in furthering progress in the synthesis of homogeneous glycopeptides and glycoproteins.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health (Grant CA28824).
Footnotes
Supporting Information Available. Experimental procedures and spectroscopic and analytical data for all new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- (1).Wilson RM, Danishefsky SJ. Pure Appl. Chem. 2007;79:2189–2216. [Google Scholar]
- (2).Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Expert Rev. of Vaccines. 2005;4:677–685. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- (3).Wold F. Annual Review Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]
- (4).Dwek RA. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- (5).Roth J. Chem. Rev. 2002;102:285–304. doi: 10.1021/cr000423j. [DOI] [PubMed] [Google Scholar]
- (6).Ohtsubo K, Marth JD. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- (7).Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. Annual Review Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- (8).Yamamoto N, Tanabe Y, Okamoto R, Dawson PE, Kajihara Y. J. Am. Chem. Soc. 2008;130:501–10. doi: 10.1021/ja072543f. [DOI] [PubMed] [Google Scholar]
- (9).Denkewalter RG, Veber DF, Holly FW, Hirschmann R. J. Am. Chem. Soc. 1969;91:503–4. doi: 10.1021/ja01030a051. [DOI] [PubMed] [Google Scholar]
- (10).Warren JD, Miller JS, Keding SJ, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:6576–6578. doi: 10.1021/ja0491836. [DOI] [PubMed] [Google Scholar]
- (11).Chen G, Warren JD, Chen J, Wu B, Wan Q, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:7460–7462. doi: 10.1021/ja061588y. [DOI] [PubMed] [Google Scholar]
- (12).Chen J, Chen G, Wu B, Wan Q, Tan Z, Hua Z, Danishefsky SJ. Tetrahedron Lett. 2006;47:8013–8016. doi: 10.1016/j.tetlet.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Chen J, Warren JD, Wu B, Chen G, Wan Q, Danishefsky SJ. Tetrahedron Lett. 2006;47:1969–1972. doi: 10.1016/j.tetlet.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wu B, Chen J, Warren JD, Chen G, Hua Z, Danishefsky SJ. Angew. Chem. Int. Ed. 2006;45:4116–4125. doi: 10.1002/anie.200600538. [DOI] [PubMed] [Google Scholar]
- (15).Wu B, Hua Z, Warren JD, Ranganathan K, Wan Q, Chen G, Tan Z, Chen J, Endo A, Danishefsky SJ. Tetrahedron Lett. 2006;47:5577–5579. doi: 10.1016/j.tetlet.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wu B, Tan Z, Chen G, Chen J, Hua Z, Wan Q, Ranganathan K, Danishefsky SJ. Tetrahedron Lett. 2006;47:8009–8011. doi: 10.1016/j.tetlet.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wu B, Warren JD, Chen J, Chen G, Hua Z, Danishefsky SJ. Tetrahedron Lett. 2006;47:5219–5223. doi: 10.1016/j.tetlet.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chen G, Wan Q, Tan Z, Kan C, Hua Z, Ranganathan K, Danishefsky SJ. Angew. Chem., Int. Ed. 2007;46:7383–7387. doi: 10.1002/anie.200702865. [DOI] [PubMed] [Google Scholar]
- (19).Wan Q, Danishefsky SJ. Angew. Chem., Int. Ed. 2007;46:9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- (20).Jelkmann W. Intern. Med. 2004;43:649–659. doi: 10.2169/internalmedicine.43.649. [DOI] [PubMed] [Google Scholar]
- (21).Sytkowski AJ. Erythropoietin. WILEY-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004. [Google Scholar]
- (22).Blake J, Li CH. Proc. Natl. Acad. Sci. USA. 1981;78:4055–8. doi: 10.1073/pnas.78.7.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hojo H, Aimoto S. Bull. Chem. Soc. Japan. 1991;64:111–117. [Google Scholar]
- (24).Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- (25).Tam JP, Lu YA, Liu CF, Shao J. Proc. Natl. Acad. Sci. USA. 1995;92:12485–12489. doi: 10.1073/pnas.92.26.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Offer J, Boddy CN, Dawson PE. J. Am. Chem. Soc. 2002;124:4642–4646. doi: 10.1021/ja016731w. [DOI] [PubMed] [Google Scholar]
- (27).Likhosherstov LM, Novikova OS, Derevitskaja VA, Kochetkov NK. Carbo. Res. 1986;146:C1–C5. [Google Scholar]
- (28).Cohen-Anisfeld ST, Lansbury PT. J. Am. Chem. Soc. 1993;115:10531–10537. [Google Scholar]
- (29).Robert S. J. Pep. Sci. 2003;9:545–552. [Google Scholar]
- (30).Haack T, Mutter M. Tetrahedron Lett. 1992;33:1589–1592. [Google Scholar]
- (31).Zahariev S, Guarnaccia C, Zanuttin F, Pintar A, G. E, Maravic G, Krust B, Hovanessian AG, Pongor S. J. Pept. Sci. 2005;11:17–28. doi: 10.1002/psc.577. [DOI] [PubMed] [Google Scholar]
- (32).Saburo A. Pept. Sci. 1999;51:247–265. [Google Scholar]
- (33).Atherton E, Holder JL, Meldal M, Sheppard RC, Valerio RM. J. Chem. Soc., Perkin Trans. 1. 1988:2887–2894. [Google Scholar]
- (34).Kunz H. Angew. Chem. Int. Ed. 1987;26:294–308. [Google Scholar]
- (35).Takano Y, Hojo H, Kojima N, Nakahara Y. Org. Lett. 2004;6:3135–3138. doi: 10.1021/ol048827b. [DOI] [PubMed] [Google Scholar]
- (36).Ragupathi G, Koide F, Livingston PO, Cho YS, Endo A, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O, Wilson RM, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:2715–2725. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- (37).Reimer KB, Meldal M, Kusumoto S, Fukase K, Bock K. J. Chem. Soc., Perkin Trans. 1. 1993:925–932. [Google Scholar]
- (38).Takemura T, Hojo H, Nakahara Y, Ishimizu T, Hase S. Org. Biomol. Chem. 2004;2:133–136. doi: 10.1039/b312413d. [DOI] [PubMed] [Google Scholar]
- (39).Seitz O, Wong CH. J. Am. Chem. Soc. 1997;119:8766–8776. [Google Scholar]
- (40).Ichiyanagi T, Takatani M, Sakamoto K, Nakahara Y, Ito Y, Hojo H, Nakahara Y. Tetrahedron Lett. 2002;43:3297–3300. [Google Scholar]
- (41).Kunz H, Dombo B. Angew. Chem. Int. Ed. 1988;27:711–713. [Google Scholar]
- (42).Nakahara Y, Ando S, Itakura M, Kumabe N, Hojo H, Ito Y, Nakahara Y. Tetrahedron Lett. 2000;41:6489–6493. [Google Scholar]
- (43).Marlowe CK. Bioorg. Med. Chem. Lett. 1993;3:437–440. [Google Scholar]
- (44).López PE, Isidro-Llobeta A, Graciaa C, Cruza LJ, García-Granadosb A, Parrab A, Álvareza M, Albericioa F. Tetrahedron Lett. 2005;46:7737–7741. [Google Scholar]
- (45).Durek T, Torbeev VY, Kent SB. Proc. Natl. Acad. Sci. USA. 2007;104:4846–4851. doi: 10.1073/pnas.0610630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).See the Supporting Information for a discussion of impurities, which are often observed in glycopeptides displaying the N-linked dodecasaccharide.
- (47).Pudelko M, Lindgren A, Tengel T, Reis CA, Elofsson M, Kihlberg J. Org. Biomol. Chem. 2006;4:713–720. doi: 10.1039/b514918e. [DOI] [PubMed] [Google Scholar]
- (48).Ando S, Nakahara Y, Ito Y, Ogawa T, Nakahara Y. Carbo. Res. 2000;329:773–780. doi: 10.1016/s0008-6215(00)00235-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.