Abstract
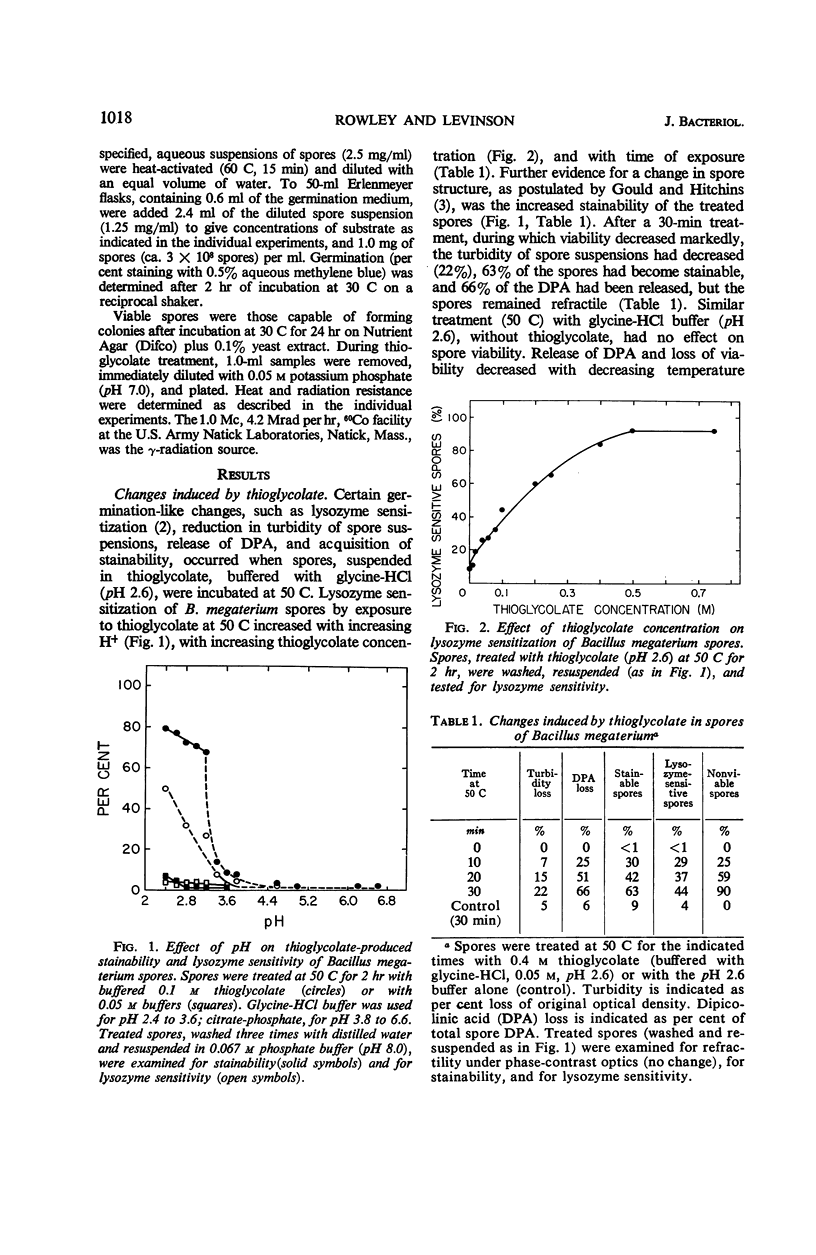
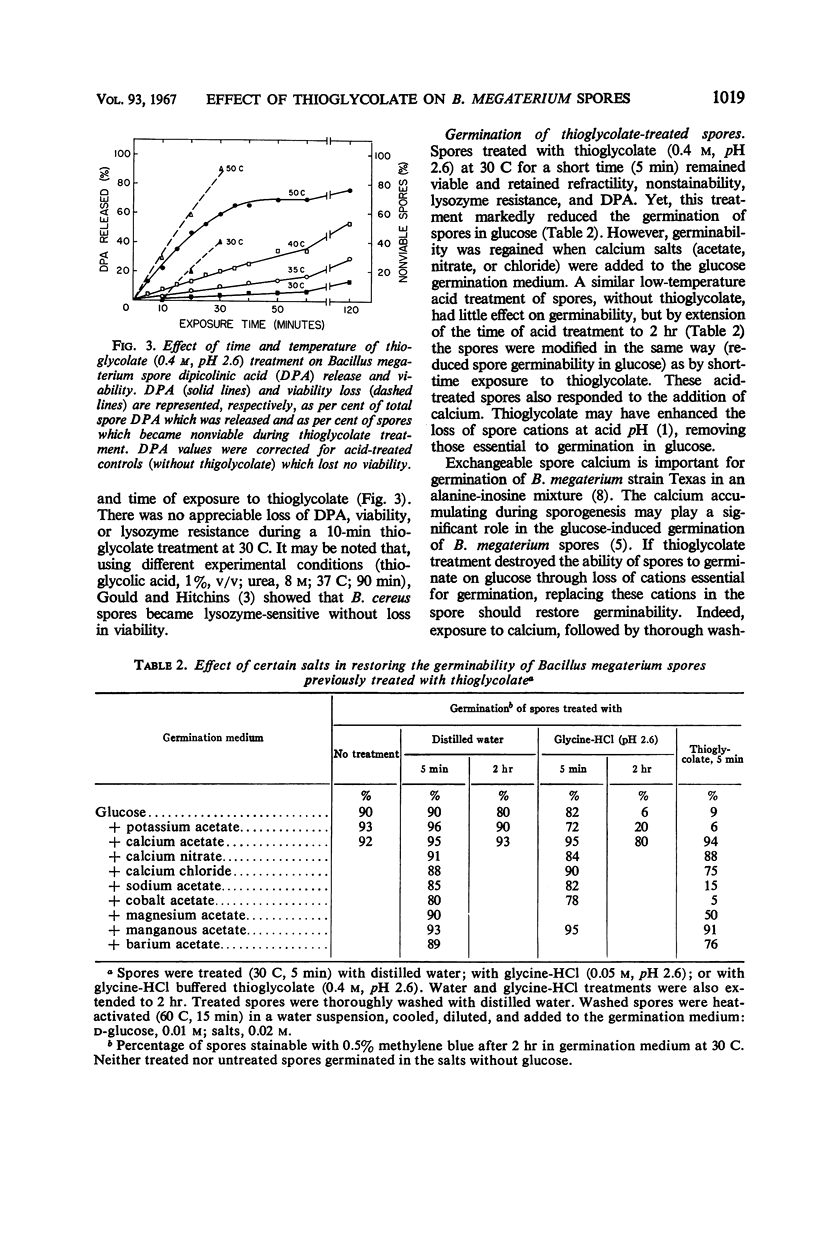
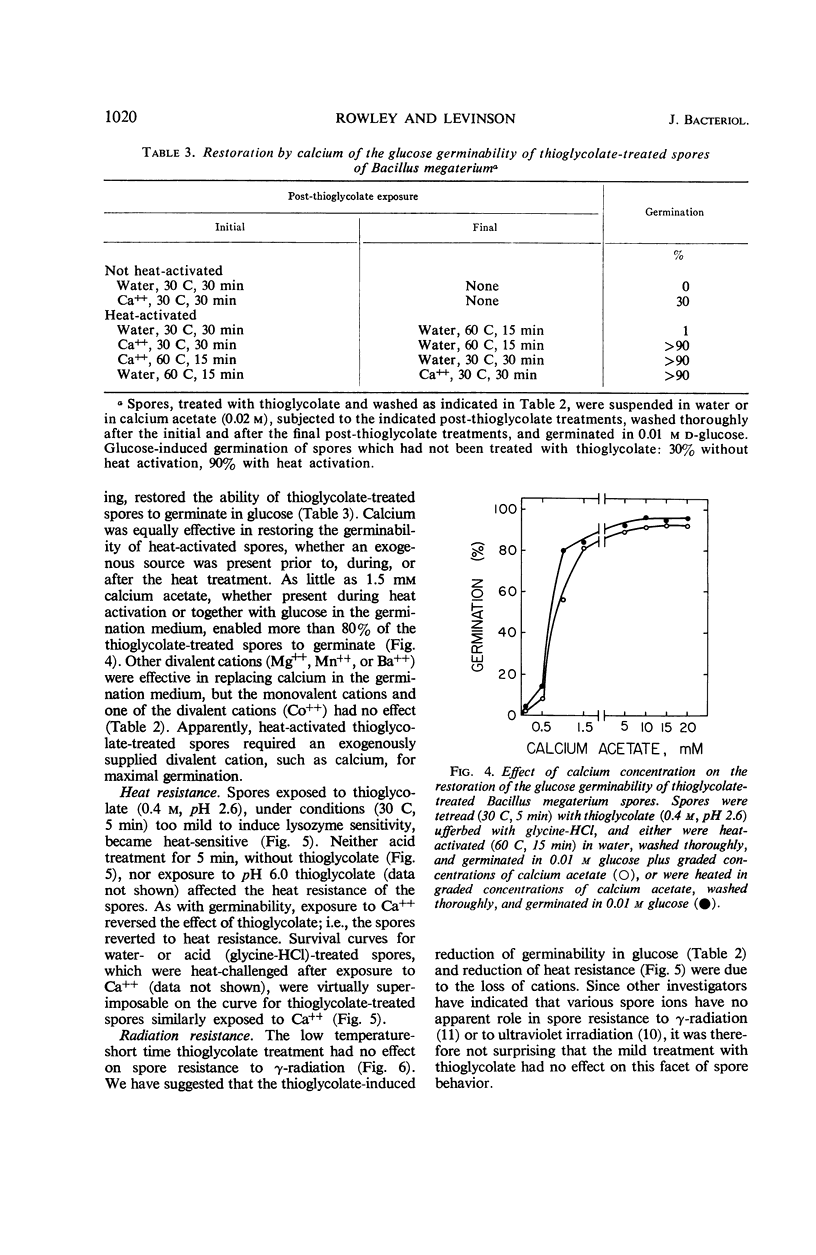
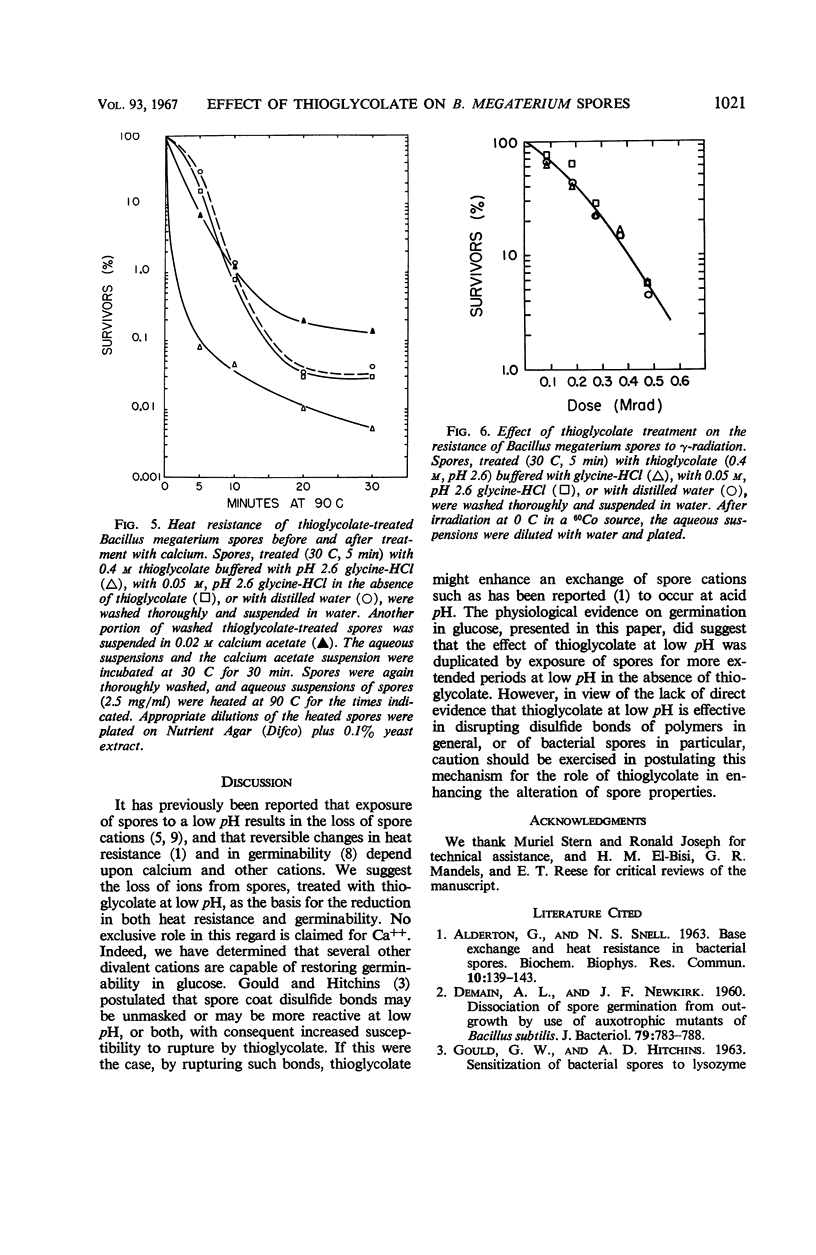
Spores of Bacillus megaterium QM B1551 treated with thioglycolate (0.4 m, pH 2.6) at 50 C for 30 min remained refractile, but they became stainable, lysozymesensitive, and nonviable, and they lost dipicolinic acid (DPA). The loss of DPA and of viability were functions of the time and temperature of exposure to thioglycolate. Spores treated with thioglycolate at a lower temperature and for a shorter time (30 C, 5 min) retained DPA, viability, and nonstainability. Although these spores also retained their resistance to γ radiation and to lysozyme, they lost thermo-resistance. Their percentage of germination over a 2-hr period in glucose was markedly reduced. Germinability and heat resistance were restored by exogenous cations, suggesting that the thioglycolate treatment (30 C, 5 min) resulted in the loss of spore ions essential for normal germination in glucose and for heat resistance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDERTON G., SNELL N. Base exchange and heat resistance in bacterial spores. Biochem Biophys Res Commun. 1963 Jan 31;10:139–143. doi: 10.1016/0006-291x(63)90039-1. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Newkirk J. F. DISSOCIATION OF SPORE GERMINATION FROM OUTGROWTH BY USE OF AUXOTROPHIC MUTANTS OF BACILLUS SUBTILIS. J Bacteriol. 1960 Jun;79(6):783–788. doi: 10.1128/jb.79.6.783-788.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD G. W., HITCHINS A. D. SENSITIZATION OF BACTERIAL SPORES TO LYSOZYME AND TO HYDROGEN PEROXIDE WITH AGENTS WHICH RUPTURE DISULPHIDE BONDS. J Gen Microbiol. 1963 Dec;33:413–423. doi: 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. EFFECT OF SPORULATION MEDIUM ON HEAT RESISTANCE, CHEMICAL COMPOSITION, AND GERMINATION OF BACILLUS MEGATERIUM SPORES. J Bacteriol. 1964 Apr;87:876–886. doi: 10.1128/jb.87.4.876-886.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., SEVAG M. G. Stimulation of germination and respiration of the spores of Bacillus megatherium by manganese and monovalent anions. J Gen Physiol. 1953 May;36(5):617–629. doi: 10.1085/jgp.36.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Influence of exchangeable ions on germinability of bacterial spores. J Bacteriol. 1966 Apr;91(4):1582–1588. doi: 10.1128/jb.91.4.1582-1588.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J., Foster J. W. Quantitative aspects of exchangeable calcium in spores of Bacillus megaterium. J Bacteriol. 1966 Apr;91(4):1589–1593. doi: 10.1128/jb.91.4.1589-1593.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]



