Abstract
New stable cationic organogold(III) complexes containing the ‘pincer’ iminophosphorane ligand (2-C6H4-PPh2=NPh) have been prepared by reaction of the previously described [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}Cl2] 1 and a combination of sodium or silver salts and appropriate ligands. The presence of the P atom in the PR3 fragment has been used as a “spectroscopic marker” to study the in vitro stability (and oxidation state) of the new organogold complexes in solvents like DMSO and water. Compounds with dithiocarbamato ligands and water-soluble phosphines of the general type [Au{κ2-C,NC6H4(PPh2=N(C6H5)-2}(S2CN-R2)]PF6 (R = Me 2; Bz 3) and [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(PR3)nCl]PF6 (PR3 = P{Cp(m-C6H4-SO3Na)2} n = 1 4, n = 2 TPA {1,3,5-triaza-7-phosphaadamantane} 5) have been synthesized and characterized in solution and in the solid state (the crystal structure of 2 has been determined by X-ray diffraction studies). Complexes 1–5 have been tested as potential anticancer agents and their cytotoxicity properties were evaluated in vitro against HeLa human cervical carcinoma and Jurkat-T acute lymphoblastic leukemia cells. Compounds 2 and 3 are quite cytotoxic for these two cell lines. There is a preferential induction of apoptosis in HeLa cells after treatment with 1–5. However in the case of the more cytotoxic complex (2), cell death is activated due to both apoptosis and necrosis. The interactions of 1–5 with Calf Thymus DNA have been evaluated by Thermal Denaturation methods. All these complexes show no or little (electrostatic) interaction with DNA. The interaction of 2 with two model proteins (cytochrome c and thioredoxin reductase) has been analyzed by spectroscopic methods (vis-UV and fluorescence). Compound 2 manifests a high reactivity toward both proteins. The mechanistic implications of these results are discussed here.
Introduction
The discovery of the antiproliferative properties of platinum(II) complexes was serendipitous.1 The subsequent successful development of antitumor platinum drugs has paved the way for studying other metal-based chemotherapeutic compounds.2 The high effectiveness of cisplatin in the treatment of several types of tumors is severely hindered by some clinical problems related to its use in curative therapy, such as normal tissue toxicity and the frequent occurrence of initial and acquired resistance to the treatment.1–8 One promising family of non-platinum anticancer agents may be found in gold complexes. Attention was directed towards gold compounds9 for two reasons: (1) gold(III) centers are isoelectronic to Pt(II) compounds and adopt square-planar configurations similar to that of cisplatin, and (2) gold(I) compounds are well known pharmaceuticals,9,10 some of which are currently being used to treat rheumatoid arthritis.11 During the last few years there has been a renewed interest in the application of gold(I) and gold(III) compounds in cancer chemotherapy driven by a few novel compounds, endowed with improved stability and with encouraging pharmacological properties.12–20 Moreover, gold(I) compounds are nowadays being designed specifically as mitochondria-targeted chemotherapeutics.21
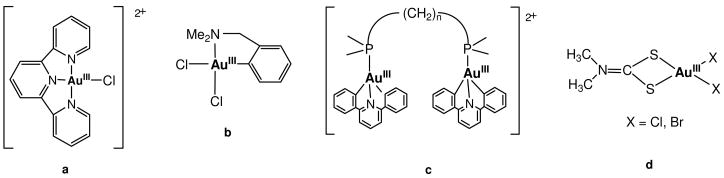
Three main types of gold(III) complexes have shown activity and these are the following: a) coordination compounds (with N-polydentatee.g. 22–24 (Chart 1a) or macrocyclic ligands25,26 or dithiocarbamato ligands with S-donor atoms (Chart 1d)27–29); b) organometallic complexes with an N-C30 (e.g. DAMP = o-C6H4CH2NMe2, Chart 1b)31–36 or CNC-pincer backbone37 or c) gold(III) complexes containing bioligands (e.g. naturally occurring amino acids).38–41 Furthermore, dinuclear gold(III) complexes with bipyridyl ligands42,43 and multinuclear gold (III) compounds with CNC-backbones and chelating phosphines (fig. 1c)37 have been reported recently.
Chart 1.
Relevant examples of gold (III) compounds that display anti-tumor properties: a) gold (III) with a chelating N,N,N ligand;22–24 b) gold(III) with a pincer C,N ligand;31–36 c) dinuclear gold(III) compound with a combination of a C,N,C pincer and diphosphine ligands;37 d) gold(III) with dithiocarbamato ligands.27–29
Figure 1.
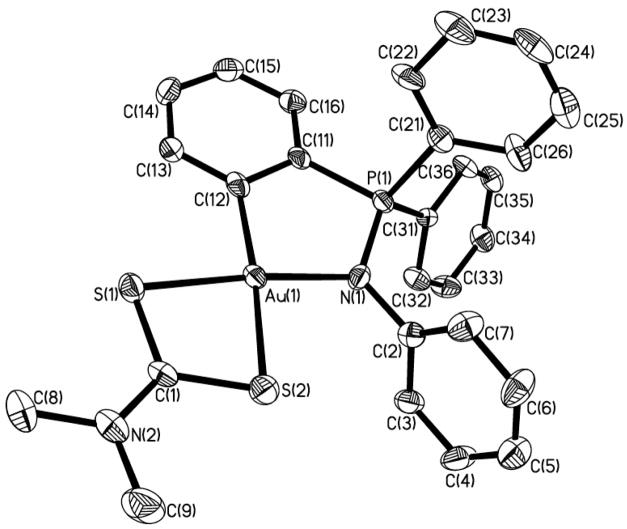
Molecular drawing of the cation in compound [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(S2CN-Me2)]PF6 2 with the atomic numbering scheme.
While the mechanism of action for platinum relies on covalent binding to DNA purine bases, it seems that gold(III) compounds behave in a different way. There is a large body of evidence to believe that DNA does not represent the primary target for many gold(III) complexes. There is none or very weak interaction between most gold(III) complexes and calf thymus DNA. Recently it has been reported that some gold(III) compounds promote apoptosis via mitochondrial pathways44–46 and it has been assessed that selective modification of surface proteins residues by gold(III) compounds could be the molecular basis for their biological effects on the basis of the reaction of gold(III) compounds with a few model proteins.14,42,47
We report here on the synthesis of new cationic cycloaurated organogold(III) complexes containing the iminophosphorane group (2-C6H4-PPh2=NPh) with different ligands that have allowed us to tune the lipophilicity/hydrophilicity of the resulting compounds. The main advantage of the iminophosphorane ligand is that provides a C,N-backbone that stabilizes the resulting square-planar cycloaurated complexes. An extra advantage is that the P atom in the PR3 fragment can be used as a “spectroscopic marker” to study the in vitro stability (and oxidation state) by 31P NMR. We and others48,49 reported on the synthesis, characterization and catalytic activity (C-C and C-O bond formations) of neutral [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}Cl2] 1. This compound displayed moderated cytotoxicity on a P388 murine leukaemia cell line49 due mainly to lack of solubility in biologically relevant solvents. We have prepared cationic compounds soluble in dimethyl sulfoxide (DMSO), mixtures of DMSO/water or water that have also displayed cytotoxicity against HeLa human cervical carcinoma and Jurkat-T acute lymphoblastic leukemia cells with preferential induction of apoptosis. We present here the results of the interactions of these compounds with DNA and two model proteins and the mechanistic implications derived from these data.
Experimental Section
1. Synthesis and Characterization of the Gold(III) complexes
Solvents were purified by use of a PureSolv purification unit from Innovative Technology, Inc.; all other chemicals were used as received. Elemental analyses were carried out by Atlantic Microlab, Inc. (US). Infrared spectra (4000–400 cm−1) were recorded on a Nicolet 380 FT-IR infrared spectrophotometer on KBr pellets. The 1H, 13C{1H} and 31P{1H} NMR spectra were recorded in d6-acetone, d6-DMSO or D2O solutions at 25 °C on a Bruker 400 and spectrometer (δ, ppm; J, Hz); 1H and 13C{1H} were referenced using the solvent signal as internal standard while 31P{1H} was externally referenced to H3PO4 (85%). The mass spectra (ESI) were recorded from acetone or water solutions by the mass spectrometry facility of the University of California Riverside (US). Compound 1 was prepared as previously reported.48 The preparation of phosphine P{Cp(m-C6H4-SO3Na)2} will be reported elsewhere. Calf Thymus DNA, cytochrome c for heart horse, thioredoxin reductase from Escherichia Coli, DL-dithiothreitol (DTT), buffers and solvents were purchased from Sigma-Aldrich. Spectrophotometric studies and thermal denaturation experiments were performed on an Agilent 8453 diode-array spectrophotometer equipped with a HP 89090 Peltier temperature control accessory.
[Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(S2CN-R2)]PF6 (R = Me 2; Bz 3)
To a solution of 1 (0.261g, 0.34 mmol) in 40 mL of MeOH, Na[Me2NCS2] (0.657 g, 0.34 mmol) for the preparation of 2 or Na[Bz2NCS2] (0.118 g, 0.34 mmol) for the preparation of 3 was added. The resulting mixture was stirred at RT during 2 h and a solution of NaPF6 (0.08 g, 0.5 mmol) in 7 mL of MeOH was added. The resulting suspension was stirred at RT during 30 min and then filtered through celite (to remove the NaCl formed). The resulting yellow solution was concentrated under vacuum to dryness. The yellow residue was extracted with 20 mL of acetone and the solvent was reduced under reduced pressure to ca. 2 mL. Addition of 20 mL of Et2O afforded 2 and 3 as yellow solids that were filtered and dried under vacuum and used without further purification. 2: Yield: 0.224 g, 81%. Anal. Calc. for C27H25AuF6N2P2S2 (814.55): C, 39.81; H, 3.09; N, 3.44; S, 7.87 found: C, 39.90; H, 2.73; N, 3.77; S, 8.45. MS(ESI+) [M/Z, (%)]: 669 [M]+. IR: ν(N-CSS) = 1562 cm−1; ν(C-S) 986 cm−1. 31P{1H} NMR (d6-acetone) δ = 65.7 (s); −144.1 (sept). 1H NMR (d6-acetone) δ = 3.45 (s, 3H, Me), 3.62 (s, 3H, Me); 7.06 (dt, 2H, H2+H6, NAr, 3JH-H = 7.9, 4JH-H = 4), 7.15 (td, 1H, H4, NAr, 3JH-H = 7.8, 4JH-H = 1.2), 7.26 (br t, 2H, H3+H5, NAr, 3JH-H = 7.9), 7.49 (dd, 1H, H3′, C6H4, 3JH-H = 7.9, 3JP-H = 3), 7.60–7.78 (m, 10H, Hp+Ho+Hm, PPh2), 7.89 (td, 1H, H4′, C6H4, 3JH-H = 8 Hz, 4JP-H = 2), 7.95 (dd, 1H, H5′, C6H4, 3JH-H = 12, 5JP-H = 1.5), 7.98 (br dd, 1H, H6′, C6H4, 3JH-H = 12). 13C{1H} NMR (d6-acetone) δ = 40.03 (Me), 41.40 (Me), 124.90 (d, Ci, PPh2, 1JPC = 92.5), 126.49 (br, C4, NAr), 128.71 (d, C6, NAr, 3JPC = 5.5), 129.18 (s, C5, NAr), 129.19 (d, C4′, C6H4, 3JPC = 13.5), 129.86 (d, Cm, PPh2, 3JPC = 12.3), 130.22 (d, C3′, C6H4, 2JPC = 12.8), 132.34 (d, C6′, C6H4, 3JPC = 19.16); 134.02 (d, Co, PPh2, 2JPC = 10.50); 134.62 (d, C5′, C6H4, 4JPC = 2.54); 134.83 (d, Cp, PPh2, 4JPC = 2.4); 137.48 (d, C2′, C6H4, 1JPC = 128.8); 143.22 (br d, C1, NAr); 148.59 (d, C1, C6H4, 2JPC = 17.2), 194.97 (NCS2). C3, NAr (not seen). 31P{1H} NMR (d6-DMSO) δ = 64.9 (s); −144.1 (sept). 1H NMR (d6-DMSO) δ = 3.20 (s, 3H, Me), 3.49 (s, 3H, Me); 6.97 (br d, 2H, H2+H6, NAr, 3JH-H = 8), 7.05 (br t, 1H, H4, NAr, 3JH-H = 12), 7.19 (br t, 2H, H3+H5, NAr, 3JH-H = 8), 7.43 (br dd, 1H, H3′, C6H4, 3JH-H = 8, 3JP-H = 4), 7.58 (m, 1H, H5′, C6H4), 7.65–7.78 (m, 8H, Hp+Ho, PPh2), 7.85 (m, 5H, Hm,, PPh2; H4′, C6H4; H6′, C6H4).
3
Yield: 0.231 g, 60%. Anal. Calc. for C39H33AuF6N2P2S2 (966.73): C, 48.45; H, 3.44; N, 2.90; S, 6.63 found: C, 47.96; H, 3,31; N, 2.68; S, 6.44. MS(MALDI+) [M/Z, (%)]:821.78 [M]+. IR: ν(N-CSS) = 1533 cm−1; ν(C-S) 983 cm−1. 31P{1H} NMR (d6-acetone) δ = 66.2 (s); −144.1 (sept). 1H NMR (d6-acetone) δ = 5.08 (d, 4H, CH2, 2JH-H = 36), 7.1 (td, br, 2H, H2+H6, NAr, 3JH-H = 8), 7.15 (br, 1H, H4, NAr, 3JH-H = 8), 7.30–7.80 (m, 15H, Hp+Ho+Hm, PPh2, C6H5 Bz), 7.9 (td, br, 1H, H4′, C6H4, 3JH-H = 8), 8.0 (q, 2H, H5′+H6′, C6H4). 13C{1H} NMR (d6-acetone) δ = 54.37 (Ph-CH2), 55.97 (Ph-CH2), 124.84 (d, Ci, PPh2, 1JPC = 93.6), 126.64 (d, br, C6, NAr), 128.31 (d, C6, NAr, 3JPC = 3.59), 128.86 (br, C5, NAr), 128.88 (d, C4′, C6H4, 3JPC = 10.65); 129. 14 (d, Cm, PPh2, 3JPC = 7.29), 129. 24 (C4, Bz); 129.88 (d, C3′, C6H4, 2JPC =13.01); 130.28 (d, Co, PPh2, 2JPC = 11.98); 132.47 (C6′, C6H4, 3JPC = 13.53); 133.54 (C3, C5 Bz), 133.64, (C2, C6, Bz) 134.71 (d, Cp, PPh2, 4JPC = 2.9), 134.90 (d, C3, NAr, 2JPC = 2.64); 137.33 (d, C2′, C6H4, 1JPC = 130.3), 143.17 (br d, C1, NAr), 146.96 (Ci, Bz), 148.26 (d, C1, C6H4, 2JPC = 20.5), 198.96 (NCS2).31P{1H} NMR (d6-DMSO) δ = 65.6 (s); −144.1 (sept). 1H NMR (d6-DMSO) δ = 5.04 (d, 4H, CH2, 2JH-H = 36), 6.97 (d, br, 2H, H2+H6, NAr, 3JH-H = 8), 7.05 (t, br, 1H, H4, NAr, 3JH-H = 8), 7.18 (t, br, 2H, H3+H5, NAr, 3JH-H = 12); 7.25 (d, br, 2H, 3JH-H = 8), 7.30–7.48 (m, 10H, C6H5 Bz), 7.58 (m, 1H, H5′, C6H4), 7.64–7.75 (m, 8H, Ho+Hm, PPh2), 7.82–7.91 (m, 6H, Hm,, PPh2; H4′, C6H4; H4′, C6H4; H6′, C6H4).
[Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(PR3)nCl]PF6 (PR3 = P{Cp(m-C6H4-SO3Na)2} 4 n = 1, TPA 5 n=2)
4
To a solution of 1 (0.217 g, 0.35 mmol) in 20 mL of dry acetonitrile, AgPF6 (0.097 g, 0.385 mmol) in 5 mL of acetonitrile was added and the flask protected from light exposure. The reaction mixture was stirred at RT during 30 min and it was subsequently filtered through celite (to remove AgCl). To the resulting yellow filtrate P{Cp(m-C6H4-SO3Na)2} (0.136 g, 0.31 mmol) in a mixture of 2 mL of acetonitrile and 0.5 mL of H2O was added at 0 °C. The reaction mixture was stirred at 0 °C for 15 min and at RT for another 15 min. All solvents were subsequently removed under reduced pressure. The oily yellow residue was dissolved in 2 mL of acetonitrile and by addition of Et2O a yellow solid precipitated which was filtered and washed with 2 portions of Et2O (10 mL). The solid was dried under vacuum to afford pure 4. Yield: 0.217 g, 59%. Anal. Calc. for 4.8H2O: C41H52AuClF6NNa2O14P3S2 (1332.31.): C, 36.96; H, 3.93; N, 1.05; found: C 36.47; H, 3.82; N, 0.61; MS(ESI+) [M/Z, (%)]: 590.07 [M −PR3]+. 31P{1H} NMR (d6-DMSO) δ = 57.47 (s), 45.84 (s), −144.1 (sept). 1H NMR (d6-DMSO) δ = 1.3–1.8 (br m, 9H, Cp), 6.97 (m, 4H, H2+H6, NAr; H4, m-C6H4-SO3Na), 7.13 (m, 5H, H4 + H3 + H5 NAr; H3, m-C6H4-SO3Na), 7.35 (m, 1H, H3′, C6H4),7.43 (m, 1H, H5′, C6H4), 7.50 (d, 4H, H2, m-C6H4-SO3Na, 3JP-H = 12), 7.54–7.77 (m, 3H, H5′, C6H4; Ho, PPh2), 7.72–8.08 (br m, 8H, Hp + Hm,, PPh2; H4′, C6H4; H6′, C6H4; H1, m-C6H4-SO3Na). 13C{1H} NMR (d6-dmso) δ = 26.48 (d, 2JP-C = 19.10 Hz, Cb Cp), 30.2 (br, Cc Cp), 34.2 (vbr, Ca Cp), 121.9 (br, C4, NAr), 124.45 (d, Ci, PPh2, 1JPC = 92.1), assignation of the peaks due to the other arylic carbons becomes very difficult due to the overlapping of broad signals of all the aryl groups and couplings in this molecule, 126.00 (s), 128,39 (d), 128.49 (s), 128.63, 128.99 (br), 129.41 (vbr, m). 129.92 (m), 130.54 (d, JPC = 12.37); 130.93 (m) 131.2 (br), 134.14 (d, JPC = 10.24); 134.30 (br), 135.29 (br), 143.6 (br), 149.65 (s, C1, C6H4), 151.1 (brd), 152.9 (brd).
5
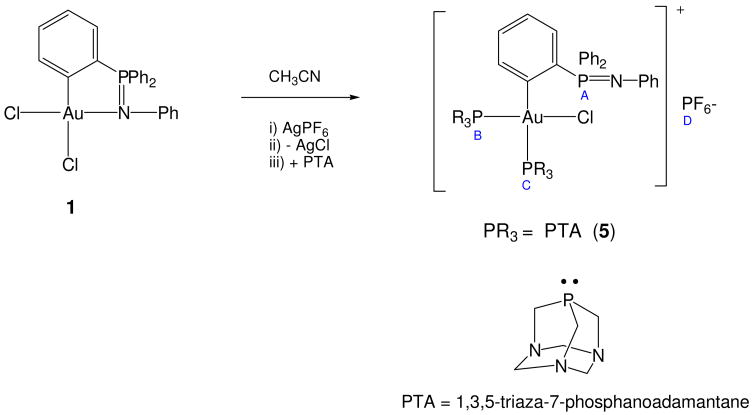
To a solution of 1 (0.155 g, 0.25 mmol) in 10 mL of dry acetonitrile, AgPF6 (0.069 g, 0.29 mmol) in 4 mL of acetonitrile was added and the flask protected from light exposure. The reaction mixture was stirred at RT during 30 min and it was subsequently filtered through celite (to remove AgCl). To the resulting yellow filtrate PTA (0.035 g, 0.22 mmol) in a mixture of 1 mL of acetonitrile and 1 mL of H2O was added at 0 °C. The reaction mixture was stirred at 0 °C for 15 min and at RT for another 15 min. All solvents were subsequently removed under reduced pressure. The white off residue was dissolved in 2 mL of acetonitrile and by addition of Et2O (10 mL) a pale yellow solid precipitated which was filtered and dried under vacuum to afford pure 5. Yield: 0.062 g, 54%. Anal. Calc. for C36H37AuClF6N7P4 (1038.03): C, 41.65; H, 3.59; N, 9.45; found: C, 41.15; H, 4.17; N, 9.14; MS(ESI+) [M/Z, (%)]: 509 [Au(TPA)2]+. 31P{1H} NMR (d6-dmso) δ = 7.44 (s, PA); −56.6 (s + vbr, PB + Pc), −144.1 (sept, PD). 1H NMR (d6-dmso) δ = 4.07 and 4.58 (AB system, 6H, N-CH2-N), 4.32 (s, 6H, NCH2P), 4.41 (t, 6H, 2JH-H = 12), 5.07 (d, 6H, 2JP-H = 8, NCH2P), 6.77 (br d, 2H, H2+H6, NAr, 2JH-H = 8), 6.83 (br t, 1H, H4, NAr, 2JH-H = 8), 7.16 (br t, 2H, H3+H5, NAr, 2JH-H = 8), 7.58–7.85 (m, 10H, H3′+H5′, C6H4, Hp+Ho, PPh2), 7.85–8.09 (m, 5H, Hm,, PPh2; H4′, C6H4; H6′, C6H4). 13C{1H} NMR (d6-dmso) δ = 50.88 (d, 1JP-C = 16.0 Hz, P-CH2-N), 53.02 (d, 1JPC = 31.2, P-CH2-N), 71.25 (d, 3JP-C = 7.4, N-CH2-N), 72.13 (d, 3JPC = 10.2, N-CH2-N), 120.91 (br, C4, NAr), 125.51 (d, C6, NAr, 3JPC = 11.39), 129.61 (s, C5, NAr), 129.84 (d, Cm, PPh2, 3JPC = 11.7 Hz), 130.06 (s, Cp, PPh2), 130.45 (s, C5′, C6H4), 133.07 (br d, C1, NAr), 133.36 (d, Co, PPh2, 2JPC = 9.68 Hz), 133.61 (s, C4′, C6H4), 133.44 (br dd, C3′, C6H4), 136.31 (dm, C2′, C6H4, 1JPC = 122.7), 148.23 (s, C1, C6H4). C3, NAr (not seen).
2. Cytotoxicity Assay
Adherent HeLa-GFP human cervical carcinoma cells (Montoya et al)57 and Jurkat-GFP acute lymphoblastic leukemia cells (to be described in a subsequent publication) were seeded, in 24 well plate format, 100,000 cells/well using 1 ml DMEM media (HyClone, Logan, UT) supplemented with antibiotics and 10% heat-inactivated newborn calf serum (HyClone, Logan, UT). After overnight incubation, to allow cell attachment, they were exposed for 22 h to several concentrations of chemical compounds. Floating cells were collected in a ice-cold tube and placed on ice, while attached cells were treated for 15 min with 0.25% of trypsin solution (Invitrogen, Carlsbad, CA), diluted in serum free DMEM, and incubated at 37°C. Cells from each individual well, included both those harvested by trypsinization and those floating, were centrifuged at 1,400 rpm for 5 min at 4°C. The media was then removed and cells resuspended in 500 μl of staining solution, containing 2 μg/ml Propidium iodide dissolved in FACS buffer (PBS, 0.5 mM EDTA, 2% heat inactivated fetal bovine serum, and 0.1% sodium azide), incubated in the dark at room temperature for 15 min and analyzed by flow cytometry, using Cytomic FC 500 (Beckman-Coulter, Miami, FL). The data were analyzed using CXP software (Beckman-Coulter).
3. Apoptosis Assay
Adherent HeLa cells (American Type Culture Collection, Manassas, VA) were seeded in 24 well plate format at 100,000 cells/well using 1ml DMEM media (HyClone, Logan, UT) supplemented with antibiotics and 10% heat-inactivated newborn calf serum (also referred as complete media). Cells were exposed for 8 or 16 h to IC50 concentrations of the chemical compounds as determined by the cytotoxicity assays. Floating and attached cells were collected from tissue culture plates as described above. Cells from each individual well were centrifuged and washed, first with cold complete media, and second with cold PBS. Treated cells were then resuspended in staining solution and immediately analyzed by flow cytometry. Prior to data acquisition, the flow cytometer was set up and calibrated utilizing unstained, single-(PI or Annexin V-FITC) and double-(Annexin V-FITC plus PI) stained cells.
Staining protocol for apoptosis assay
After incubation with chemical compound, cells were transferred to ice-cold 5 ml flow-cytometry tubes and washed twice; first with ice-cold complete media and second with ice-cold PBS. The staining procedure was performed with cells resuspended in 100 μl binding buffer (0.1 M HEPES, pH 7.4; 1.4 M NaCl; 25 mM CaCl2) containing 1 μl of 25 μg/ml Annexin V-FITC (Beckman Coulter, Miami, FL) and 5 μl of 250 μg/ml PI. After incubation for 15 min on ice in the dark, cell suspensions were added to 400 μl of ice-cold binding buffer, gently homogenized and analyzed by flow cytometry. For each sample, 10,000 individual events were collected and analyzed using CXP software (Beckman Coulter, Miami, FL). After the exposure of cells to chemical compounds and trypsinization, all subsequent procedures were carried out on ice or at 4°C to arrest or slow down progression of cell damage, from viability to stages of apoptosis and necrosis.
4. Interactions with DNA
Melting curves were recorded in media containing 50 mM NaClO4 and 5 mM Tris/HCl buffer (pH=7.29). The absorbance at 260 nm was monitored for solutions of Calf Thymus DNA (35 μM) before and after incubation with a solution of the drug under study (17.5 μM in Tris/HCl buffer) for 1 h at room temperature. The temperature was increased by 0.5 °C/min between 65 and 82°C and by 3°C/min between 25 and 65°C and between 82 and 97°C.
5. Interactions with Proteins. UV-Visible Absorption Spectra and Fluorescence Studies
Electronic spectra of each selected protein (horse heart cytochrome c and thioredoxin reductase) at 5 μM were recorded in buffer (0.1 mol % DMSO) consisting of 50 mM NaClO4 and 5 μM Tris/HCl (pH=7.29). Subsequently solutions of cytochrome c (5 mM) and gold compound 2 in different concentrations (5, 15 and 25 μM) were prepared in 3000 μL of buffer (0.1 % DMSO) and recorded at 25.9 °C and after incubation during 24 hours at 37 °C (see figure 6 in section 3.2). Solutions of cytochrome c (5 μM) and DTT in different concentrations (5, 15 and 25 μM) were prepared in 3000 μL of buffer (0.1 % DMSO) and recorded at 25.9 °C.62
Fluorescence spectra were recorded on a XFLUORSAFIREII spectrofluorimeter at 25.9 °C and excitation wavelength 285 nm (emission wavelength start: 310 nm; emission wavelength end: 700 nm). Fluorescence spectra of thioredoxin reductase (from Escherichia coli) at 20 μM in 100 μL of buffer (10 mM phosphate, 20 mM NaCl, pH = 7.4) 0.1% in DMSO was recorded. We recorded spectra of gold compound 2 in higher concentrations (200 μM and 600 μM) in the same conditions (buffer, 0.1% of DMSO). Subsequently, solutions of thioredoxin reductase (20 μM) and gold compound 2 in different concentrations (10, 30, 60, 100, 200 and 600 μM) were prepared in 100 μL of buffer (0.1% DMSO) and recorded at 25.9 °C (see figure 7 in section 3.2).
Results
1. Chemistry
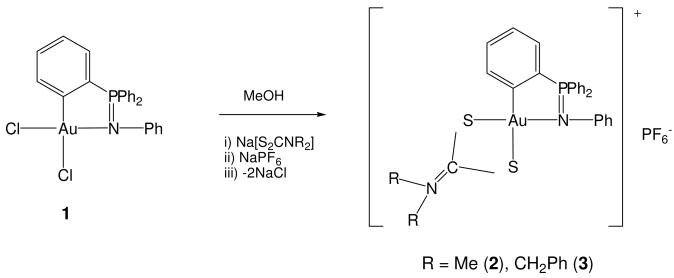
The cycloaurated compound [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}Cl2] 1 was prepared as described previously by the greener transmetallation process with [Au{C6H4(PPh2=N(C6H5))-2}(PPh3)] and K[AuCl4] that avoids the use of more toxic organomercurial reagents.48 By reaction of 1 and sodium dithiocarbamato salts and addition of 1 equivalent of NaPF6 (Scheme 1) cationic compounds with dithiocarbamato ligands of the general type [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(S2CN-R2)]PF6 (R = Me 2; Bz 3) were synthesized. These bright yellow cationic compounds are soluble in DMSO and in mixtures of DMSO/H2O.
Scheme 1.
Preparation of organogold(III) compounds with dithiocarbamato ligands.
The 31P NMR spectra of the complexes allow us to asses their stability in solution, the oxidation state of the metal and the binding mode of the iminophosphorane ligand.48 Cyclometalallated gold(III) complexes 2 and 3 have distinct 31P NMR chemical shifts in d6-acetone δ 65.7 (2), 66.2 (3) ppm consistent with C,N-coordination of the iminophosphorane ligand to the gold (III) center in a square-planar arrangement (δ in CDCl3 65.4 ppm (1)) completed by the two S donor atoms of the dithiocarbamato ligands. The chemical shift due to the PF6− as an anion are visible for the spectra of both compounds at −144.1 ppm (sept). The cationic character of these species can be also inferred from the slight downfield shift of the 31P signal with respect to compound 1. Chemical shifts of the 31P signals in d6-DMSO solutions are very similar: δ 64.9 (2), 65.6 (3) ppm and −144.0 (sept) ppm for the PF6− anion for both compounds. More importantly the spectra remained the same over time. Solutions of 2 and 3 in d6-DMSO at RT do not show any changes in the chemical shifts even after 3 months (see 31P NMR spectra in supplementary material). 2 and 3 are thus quite stable in solution and do not decompose to Au(I) complexes or metallic gold.
For these complexes, in which both ligands are chelated, signals from the R groups of the dithiocarbamato ligands are non-equivalent (two signals for the two methyl groups in the 1H and 13C NMR spectra of 2). In the case of the Bz derivative (3) this non-equivalence is only apparent in the 13C NMR spectrum. This reflects the absence of a plane of symmetry between the two different ligands (iminophosphorane and dithiocarbamato).
The crystal structure of 2 has been elucidated by X-ray diffraction studies. The structure of the gold(III) cation is shown in Fig. 1 while selected bond and angles are presented in Table 1.
Table 1.
Selected Bond lengths [A] and angles [°] for 2.
| Au(1)-C(12) | 2.031(9) | C(12)-Au(1)-S(2) | 174.0(2) |
| Au(1)-N(1) | 2.053(7) | N(1)-Au(1)-S(2) | 100.0(2) |
| Au(1)-S(1) | 2.305(2) | S(1)-Au(1)-S(2) | 75.31(9) |
| Au(1)-S(2) | 2.376(2) | C(1)-S(1)-Au(1) | 87.2(3) |
| S(1)-C(1) | 1.721(9) | N(1)-P(1)-C(11) | 102.6(4) |
| P(1)-N(1) | 1.613(8) | N(1)-P(1)-C(31) | 111.7(4) |
| C(12)-Au(1)-N(1) | 85.9(3) | C(11)-P(1)-C(31) | 112.0(4) |
| C(12)-Au(1)-S(1) | 98.8(2) | C(2)-N(1)-P(1) | 123.6(6) |
| N(1)-Au(1)-S(1) | 174.8(2) |
The AuIII ion is four-coordinated in a square-planar geometry as expected. The Au-C and Au-N bond lengths are 2.053(7) and 2.031(9) Å, similar to those found for neutral compound 1 (both distances are 2.035(4) Å).49 The AuIII-S bond lengths for the S atoms of the dithiocarbamato ligands are 2.305(2) and 2.376(2) Å, which reflects the higher trans-influence of C versus N (longer Au-S(2) distance). A similar trans influence was reported for the dithiocarbamate complexes of the C,N-pincer damp (o-C6H4CH2NMe2) ligand.50 For instance for the compound [Au(dmtc)(damp)]BPh4 (dmtc = S2CN-Me2) the distance Au-S(1) was 2.179(9) and Au-S(2) 2.340(9) Å.50 In our case Au-S(1) bond length is similar to those found recently for [Au(ESDT)Br2] (ESDT = CH3CH2O(CO)CH2N(CH3)CS2−) of 2.302(2) and 2.319(2) Å.43
The coordination geometry around the gold atom is slightly distorted from square-planarity, with the C(12)-Au(1)-N(1) angle of 85.9(3)° suggesting a rigid ‘bite’ angle. The C(12)-Au(1)-S(1) also deviates (98.8(2)°). The five-membered metallocyclic ring is puckered, with deviations of −0.1566 [N(1)] and 0.14 [P(1)] from the least-squares plane. The coordination plane for gold and the metallocyclic plane are slightly twisted to give an angle of 5.66 ° between them. The metallocyclic ring is essentially more planar than that for related compound 1.49 The N-bonded phenyl ring is twisted 80.7° from the metallocycle plane.
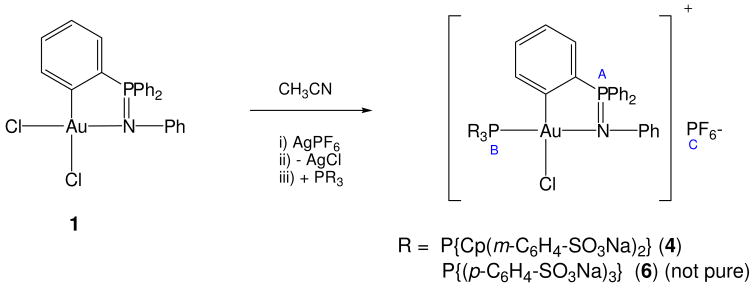
In order to improve the solubility of the compounds in water new complexes the type [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(PR3)nCl]PF6 (PR3 = P{Cp(m-C6H4-SO3Na)2} n = 1 4, n = 2 PTA 5) were prepared by reaction of 1 with 1 eq. of AgPF6 and subsequent addition of 1 eq. of water soluble phosphine (Schemes 2 and 3). Compound 4 (yellow solid) resulted totally soluble in water while compound 5 (off white solid) resulted only soluble in DMSO and in mixtures DMSO-water. The 31P NMR spectra in d6-DMSO of compound 4 shows clearly the cyclometallation for the gold(III) center (δPA = 57.5 (4)), the incorporation of the phosphine (δPB = 45.8 (4)) and its cationic nature as the signal for the anion PF6− can be observed at δPC = −144.1 ppm.
Scheme 2.
Preparation of cycloaurated compounds with water-soluble phosphines containing sulfonated groups.
Scheme 3.
Reaction of compound 1 with the PTA ligand.
Our attempts to prepare [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(PR3)nCl]PF6 6 with the less basic and commercially available phosphine TPPTS P{(4-C6H4-SO3Na)3} were not successful and we got a mixture of two compounds. The major one was compound 6 (δPA = 60.9, δPB = 40.2, δPC = −144.1 ppm) and a minor compound with a δP = 25.8 ppm was always obtained. 4 and 6 are hydrolized in water as it can be seen in their 31P NMR spectra (D2O). New major broad signals appear at δPA = 56.2 and δPB = 47.7 ppm (4) and δPB = 45.5 and δPA = 10 ppm (6). In the case of compound 6 the chemical shift of PA indicates that the iminophosphorane ligand decoordinates from the metallic center. In DMSO a new signal appears for 4 at δP = 33.5 ppm over time. After 12 days the signals that can be assigned to 4 still remained (ca. 60%). The signals at δP = 33.5 ppm (4) and δP = 25.8 ppm (6) can be assigned to Au(III)-PR3 derivatives (with loss of the iminophosphorane ligand) and that for 6 constitute the only impurity in their preparation. The more basic, less hydrophobic phosphine P{Cp(m-C6H4-SO3Na)2} seems to stabilize more the resulting gold(III) complexes. Reduction to Au(I)-PR3 species is not proposed since we did not find signals that could be assigned to compounds of the type AuCl(PR3).51–52
The reaction in the same conditions of 1 with PTA (1,3,5-triaza-7-phosphaadamantane) afforded a totally different compound regardless of the mol ratio of phosphine employed (scheme 3). The iminophosphorane ligand is only coordinated through the C-atom and the incorporation of two PTA phosphines is evident from microanalysis and spectroscopic data. The structure described in scheme 3 is the most plausible one. The coordination of the iminophosphorane ligand through the carbon atom only is evident from the chemical shift in the 31P NMR (d6-DMSO) for the phosphorous atom from the N-PPh2 fragment (δPA = 7.4 ppm) and from the high-field displacement of the signals due to the protons of the N-phenyl ring (see experimental). The 31P NMR (CDCl3) chemical shift for N-PPh2 in [Au{C6H4(PPh2=N(C6H5))-2}(PPh3)] and [Hg{C6H4(PPh2=N(C6H5))-2}Cl] are 8.8 and 8.6 ppm respectively.48 The 1H NMR and 13C{1H} NMR spectra (in d6-DMSO) shows clearly two PTA phosphines that are coordinated to the gold(III) center differently. The protons that can be assigned to one coordinated PTA ligand appear as a singlet at 4.32 (6H, NCH2P) and an AB system at 4.07 and 4.58 ppm (6H, JA-B = 16 Hz, NCH2N). A similar pattern is found for some Pt(II) with phosphines in trans and some gold compounds containing PTA ligands.52 The lack of P-H coupling for the NCH2P protons is not uncommon in some PTA metal-transition complexes.53 Signals that can be assigned to the protons of another coordinated PTA ligand appear at 4.41 ppm (br t, JH-H = 12 Hz, N-CH2-N) and 5.07 (d, JH-P = 8 Hz, NCH2P). The ratio of PTA ligands to iminophosphorane ligand is thus 2:1. The proton spectrum is in accordance with the structure shown in scheme 3. The 13C{1H} NMR spectrum (see experimental) is also in accordance with two phosphines coordinated to the gold(III) center (one in cis and one in trans) as two doublets can be observed for the methylene groups of each phosphine (higher JP-C coupling constants should correspond to the methylene groups of the phosphine in trans Pc). However, the 31P NMR spectrum results more complex. The signal corresponding to PA appears, as mentioned before at 7.4 ppm while the PF6− signal appears at −144.1 ppm (sept). There is a signal at −56.7 ppm that appears as a singlet and that overlaps a very broad signal in the same region. The ratio of these signals PB and PC with respect to PA is 2:1. We believe that there is not an exchange between a cis and trans configuration for these system since these phosphines are quite rigid. However there may be an equilibrium and coordination-decoordination of one of the phosphines and coordination-decoordination of a molecule of DMSO. This would explain the broad signal that coalesces at RT in the 31P NMR. The signals do not coalesce at RT in the 1H NMR spectrum. Unfortunately compound 5 is only soluble in DMSO and partly soluble in H2O (like [AuCl(PTA)]54) and is totally insoluble in acetone or MeOH, solvents in which we could have performed a low temperature 31P to observe the two distinct signals that correspond to PB and PC. Similar 31P chemical shifts due to the PTA ligand in square-planar Pt(II) complexes with thionate ligands of the type [Pt(SR2)(P)2] have been reported recently.53
Compound 5 gives rise to a new compound in DMSO solution after 4 days (40%) with signals at δPA = 31.8 (s), δPB = −11.5 (s) and δPC = −53.2 (s) ppm that might correspond to a situation of partial coordination of the N of the N-Ph fragment and displacement of the chloride. The ratio of 5: new compound remains the same over time. Again, signals that could be assign to Au(I) complexes were not observed.54 Overall we saw that these complexes are not as stable as the dithiocarbamato derivatives in solution.
2. Cytotoxic Properties
2.1. Cytotoxicity
The cytotoxic properties of gold(III) 1–5 compounds were analyzed in vitro according to a procedure described by Montoya et al 55 utilizing two human cell lines, HeLa cervical carcinoma and Jurkat T-cell acute lymphoblastic leukemia cells, that express the Green Fluorescence Protein (GFP) primarily in the nucleus. Before use, all tested compounds were dissolved in DMSO, except for 4 which was dissolved in water, and dilutions of each compound were then added to the cells in normal growth medium. The final solvent concentration (0.1%) had no discernible effect on cell killing. All the tested complexes have proven, by 31P NMR studies, to be stable in DMSO over 24 hours or more (see above). Cisplatin was used as a positive control as the cytotoxity assays were performed under different conditions as those previously reported (eg. Shekan et al,56 and Alley et al 57). The procedure reported here measured the cytotoxicity of the tested compounds after 22 hours instead of after 2457 or, most commonly, 72 hours56 of exposure to the chemicals and the results are shown in Table 2. As cell death was strongly induced after 8 hours (see Figures 2 and 3), incubation for prolonged periods (>24 hrs) of time were not deemed necessary and would make dead cell detection impractical as cell lysis would likely eliminate many cells from the viability counts.
Table 2.
IC50 values of iminophosphorane gold(III) compounds, compared to cisplatin.
| IC50(μM)a | ||||||
|---|---|---|---|---|---|---|
| Cell lines | cisplatin | 1 | 2 | 3 | 4 | 5 |
| HeLa-GPF | 14.9 | 33.7 | 6.2 | 7.75 | 109 | 35 |
| Jurkat-GFP | 31 | 35 | 1 | 2.5 | 52.83 | 40 |
IC50 is defined as the concentration of drug required to disrupt the plasma membrane of 50% of cell population, compared to untreated cells, after 22 hours of incubation. Cells with compromised plasma membrane were monitored using Propidium iodide (PI) and flow cytometry. Cisplatin was used as reference compound.
Figure 2.
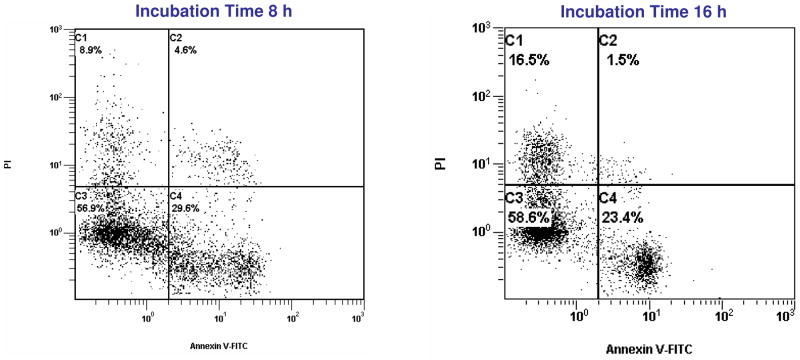
Apoptosis of HeLa (no GFP-expressing) cells induced by 1 (33.75 μM) measured by using two-colour flow cytometric analysis, at two different times of incubation.
Figure 3.
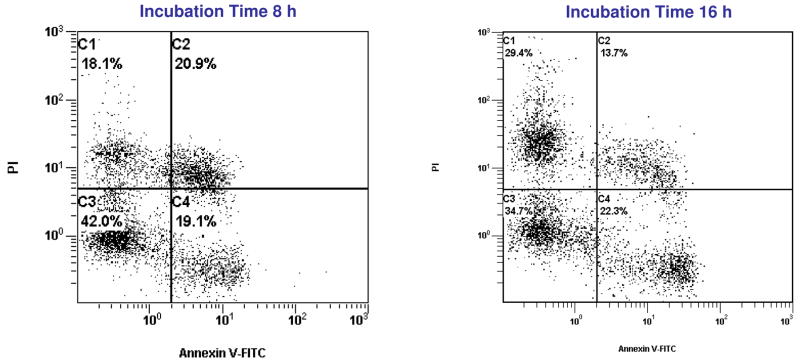
Apoptosis of HeLa (no GFP-expressing) cells induced by 2 (6.25 μM) measured by using two-colour flow cytometry, at two different incubation times.
It is apparent that some of the gold(III) compounds exhibit strong cytotoxicity toward the HeLa cell line. Remarkably, the dithiocarbamate derivatives 2 and 3 appear to be much more potent than cisplatin or 1, with IC50 values 4-low lower than that for 1 or half of the value of the reference drug. Similar values were obtained for this cell line with different gold(III) coordination compounds containing a variety of dithiocarbamate ligands [(DMDT)AuX2], [(ESDT)AuX2] (DMDT = N,N-dimethyldithiocarbamate; ESDT = ethylsarcosinedithiocarbamate; X= Cl, Br).28 The IC50 values for these complexes were between 2.1–8.2 μM (measured after 24 hours by the method described by Alley et al 57). The cytotoxicity of this type of gold(III) complexes was also studied with other cell lines like HL60.27 In this case, drug sensitivity profiles of HL60 cells toward the gold compounds (studied after 3h, 6h, 8 h and 24 h incubation) showed complexes were more cytotoxic after longer incubation periods (IC50 values decreased over time).27 The cytotoxicity of water soluble 4 was much lower for the HeLa-GPF cell lines and the cytotoxicity of 5 was comparable to that of 1 and, therefore, lower than that for cisplatin. Remarkably while Jurkat-GPF cells were more resistant to the effects of cisplatin (IC50 = 31 μM), compounds 2 and 3 were quite cytotoxic for this cell line (IC50 = 1 (2) and 2.5 (3) μM). This is in accordance to the cytotoxicity of some gold(III)12–20 and, more recently copper(II) compounds,58 against cisplatin resistant cancer cell lines. Compounds 1 and 5 had a cytotoxicity comparable to cisplatin. 4 again displayed the lowest cytotoxicity but still was more cytotoxic for Jurkat-GFP cells than for HeLa-GPF cells.
2.2. Apoptosis Studies
In order to gain some insight of the type of cell death that the more cytotoxic gold complexes induce in the cancer cell lines, we performed some apoptosis assays with the HeLa cells (without GFP) with complexes 1–5 (see experimental for details). As cells may undergo programmed cell death (apoptosis) or necrosis, the mode of death mediated by our compounds was investigated. In early stages of apoptosis, one of the significant biochemical features is loss of plasma membrane phospholipid asymmetry, due to translocation of phosphatidylserine (PS) from cytoplasmic to extracellular side. This characteristic allows detection of externalized PS by the specific binding of Annexin V (FITC-conjugatated). Initiation of cell death will eventually result in the permeabilization of the cell membrane, allowing PI to stain DNA within the nucleus. As shown in Fig. 3, each histogram is divided into four quadrants with the left top quadrant detecting necrotic cells without Annexin-V FITC signal. The right top quadrant shows cells with compromised membranes that are permeable to PI and stained with Annexin V-FITC, which is indicative of late apoptosis. The left bottom quadrant shows live cells that have intact membranes (not stained) while the right bottom quadrant represents cells that were stained (bound) with Annexin V-FITC, which is indicative of early apoptosis.
Using fluorescein-labeled Annexin V, we have quantified the percentage of apoptotic cells by flow cytometry. As shown in Figure 2, upon treatment with 1 (33.75 μM) for 8 or 16 hours, 34.2% of the cells (8 hours) or 24.9% (16 hours) were found to undergo apoptosis (either early or late) as visualized by the Annexin V-FITC fluorescence. As mentioned earlier, necrosis almost doubled from 8.9% at 8 hours to 16.5% by 16 hours.
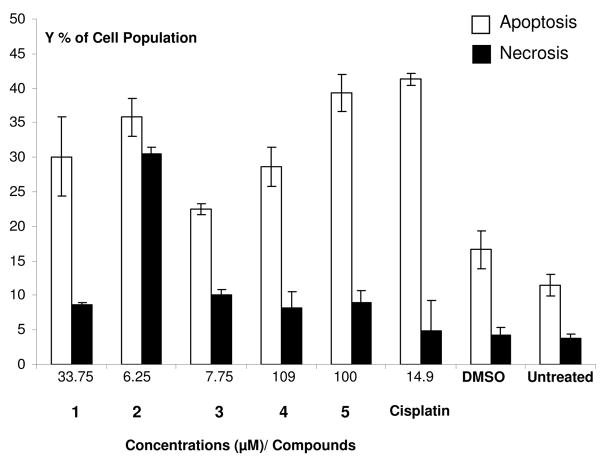
In Figure 3, we can see that upon treatment with 6.25 μM of 2, 41% and 35% of the cells were found to undergo apoptosis by 8 and 16 hours, respectively. As in the previous experiments with 1, necrosis was found to increase from 18.1% at 8 h to 29.4% by 16 h. These studies were also performed with compounds 3–5 and Figure 4 shows the preferential induction of apoptosis in HeLa cells after treatment with 1–5. Remarkably for compound 2, necrosis is an important cell death pathway. These results are similar to those found by Fregona and Bindoli with other gold(III)-dithiocarbamato complexes.26,44,45 They found (by studying PARP cleavage) that these complexes were able to induce cell death in a dose-dependent way and they hypothesized that their compounds triggered cell death by activating not only apoptotic pathways but also other death mechanisms such as necrosis. This is in contrast to what Che and Chiu found with a gold(III) porphyrin complex.59 In this case the gold compound induced cytotoxicity through an apoptotic way only as demonstrated by laser confocal and flow cytometry studies.26,59
Figure 4.
Preferential induction of apoptosis in HeLa cells after treatment with the various organogold (III) complexes. IC50 was the concentration used for each organogold compound and cisplatin. The total percent of cells reacting with Annexin V-FITC is expressed as the sum of both percentage of early and late stage of apoptosis. Each bar represents average and standard deviation results from triplicate samples. The concentration of DMSO was 0.1%. White and black bars represent percentages of apoptotic and necrotic cells, respectively.
3. Mechanistic Studies
3.1. Reactions with Calf Thymus DNA
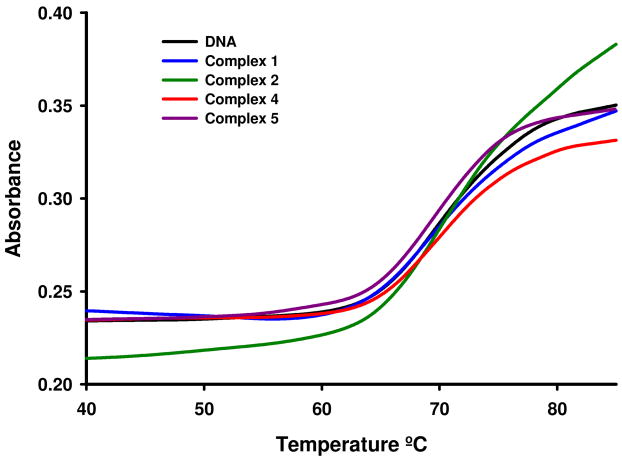
To either prove or rule out the possibility of a DNA-drug interaction, some Thermal Denaturation experiments were carried out. The melting technique is a sensitive and easy tool to detect even slight DNA conformational changes. It is known that a destabilizing interaction with the double helix (typically, covalent) is observed as a decrease in the Tm, while a stabilizing interaction (usually by intercalation or by electrostatic attraction) induces an increase of the Tm. Bearing that in mind, Calf Thymus DNA was incubated for 1 hour with each drug at a ratio DNA:drug 2:1. The results are summarized in table 3 and figure 5. Under solution conditions, compound 3 was not completely soluble and its interaction with DNA could not be studied by this technique.
Table 3.
Changes in the Tm of CT DNA after incubation with complexes 1, 2, 4 and 5 for 1h in 5mM tris/NaClO4 buffer at pH 7.39 and r = 0.5.
| Complex | ΔT(Tm DNA/Complex-Tm DNA) °C |
|---|---|
| 1 | − 0.30 |
| 2 | 0.00 |
| 3 | 0.30 |
| 4 | 0.05 |
Figure 5.
Melting curves for free CT DNA and CT DNA after incubation with complexes 1, 2, 4 and 5 for 1h in 5mM tris/NaClO4 buffer at pH 7.39 and r = 0.5.
None of the complexes were able to modify the melting temperature of a solution of calf thymus DNA beyond the experimental error. This fact suggests that either they do not interact with DNA or that interaction is so weak that can not be detected by this technique, indicating that the mechanism for which the complexes are cytotoxic are not likely related to DNA damage.
3.2. Reactions with representative proteins
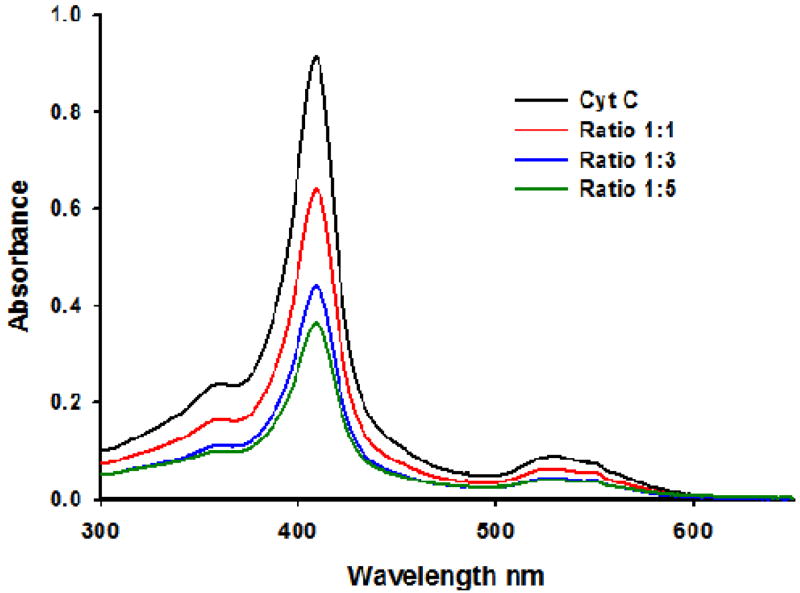
As mentioned earlier, gold(III) complexes have been suggested to exert cytotoxicity by inducing apoptosis.26,44–46,58 Since the binding of these complexes to DNA is generally weak, several groups have investigated their interaction with model proteins such as ubiquitin, cytochrome c and thioredoxin reductase. Indeed, Messori et al. reported significant spectral changes in gold(III) binuclear oxo compounds as characterized by UV-vis spectroscopy that were indicative of their interaction with these proteins.14,42 In our case, incubation for 24 hours at 37 °C of compound 2 (the most cytotoxic and solution stable of the compounds) with the protein cytochrome c did reveal changes in the representative protein spectral bands (figure 6). There was a significant decrease in the intensity of the Soret band (409 nm) of the hemo group in the protein when treated with increasing amounts of the gold complex. This effect suggests protein-drug60 interaction that is not related to a redox-process (via initial reduction of 2 to gold(I) and subsequent reduction of the Fe(III) of the heme group by reoxidation to gold(III)).61 On the other hand, the bands of the gold compound (200–300 nm) could not be studied as a consequence of an overlapping with the DMSO used to dissolve it and some other bands of the cytochrome c.
Figure 6.
UV-vis absorption spectra of the titration of 5 μM of cytochrome c with Gold-Derivative 2 after incubation at 37 °C during 24 h. The mol ratios studied were 1:1, 1:3 and 1:5.
We also studied the interaction of 2 with thioredoxin reductase. In this case the characteristic bands for this protein are overlapped by the bands of the gold compound in the range of 200–300 nm when absorption experiments were carried out. As an alternative approach we monitored the interaction of thioredoxin reductase using fluorescence spectroscopy.
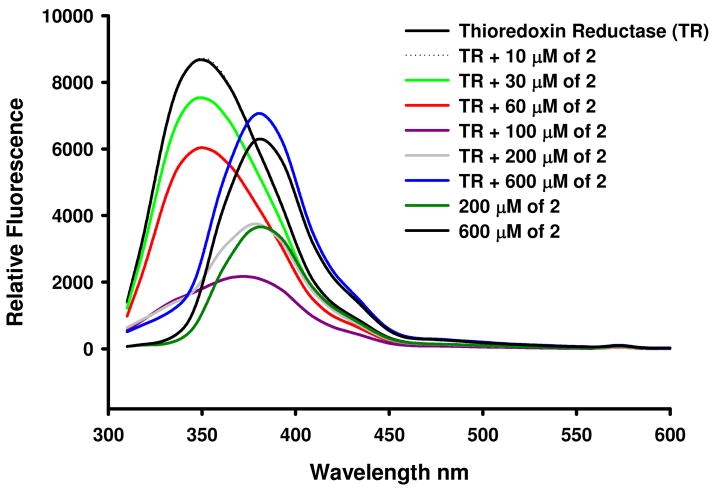
As shown in figure 7, thioredoxin reductase displays typical fluorescence emission spectra with a maximum at 349 nm that is indicative of tryptophan fluorescence. Titration of the protein with compound 2 resulted in an immediate and significant reduction in tryptophan (trp 53) fluorescence emission intensity. Incubation at 37 °C was not necessary. The spectra revealed changes immediately after addition of 2 at room temperature. Above mol:mol ratios of 1:5, the fluorescence emission maxima shifted towards higher wavelengths to reach correspondence with the spectra of compound 2. This spectral behavior resembles that of compound imidazolium[trans-tetrachlorobis(imidazol)ruthenate(III)] when incubated at 37 °C with human serum albumin (HAS).62 In this case there is also a quenching of the tryptophan fluorescence from HSA. The authors claimed this quenching was due to the fact that tryptophan residues in the Ru-im-HAS system are considered to be brought to a more hydrophilic environment as a result of the ruthenium bindings in the proximity of the tryptophan residue. Based on our results, we hypothesize that compound 2 interacts with thioredoxin reductase.
Figure 7.
Fluorescence spectra of the titration of 20 μM of thioredoxin reductase with 2. Excitation wavelength 285 nm. The mol ratios studied were 1:0.5; 1:1.5, 1:3; 1:5, 1:10, 1:30. In the figure the colored lines correspond to different concentrations of gold compound with respect to 20 μM of protein (10, 30, 60, 100, 200 and 600 μM). We have also included the spectra of the TR alone (0 μM in gold compound) as well as 2 alone (200, 600 μM alone).
Discussion
The last five years have been very important in the field of gold compounds as potential anti-tumor agents.12–21 Several gold(III) and organogold(III) derivatives have displayed encouraging in vitro pharmacological properties. The search for new biomolecular targets has attracted great interest lately since it seems that DNA is not a primary target for most gold(III) complexes.14–19 Recent reports favor the idea that the cytotoxic effects of gold (III) compounds are primarily a consequence of protein interactions and mitochondrial damage.13,27–29,42–45 Thus, the search of new gold compounds and, more importantly, studies to understand the mechanism of action of gold compounds, becomes extremely relevant. For instance, direct inhibition of thioredoxin reductase that may activate apoptosis after triggering mitochondrial cyt c release has been recently reported.44 We report here a series of cationic organogold(III) compounds with a C,N backbone that stabilizes the resulting square-planar cycloaurated complexes. Besides, the P atom in the PR3 fragment can be used as a “spectroscopic marker” to study the in vitro stability (and oxidation state) by 31P NMR. All cationic compounds exhibit a high solubility in DMSO and a reasonable solubility within a 99:1 water/DMSO or buffer/DMSO environment. 4 is completely soluble in water. We have used 31P NMR spectroscopy to establish their stability and oxidation state in solutions of DMSO or water. While compounds with dithiocarbamato ligands [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(S2CN-R2)]PF6 (R = Me 2; Bz 3) result extremely stable in DMSO or DMSO/water solutions new complexes with water-soluble phosphines [Au{κ2-C,N-C6H4(PPh2=N(C6H5)-2}(PR3)nCl]PF6 (PR3 = P{Cp(m-C6H4-SO3Na)2} n = 1 4, n = 2 PTA 5) give rise to solvation products with total or partial decoordination of the N atom of the iminophosphorane ligand and/or decomposition to Au(III)-PR3 species over time. The cytotoxic properties of these compounds were evaluated in vitro against HeLa and Jurkat cells. Compounds 2 and 3 resulted more cytotoxic than parent neutral compound 1 and their IC50 values (HeLa) where comparable to those reported for other gold(III) dithiocarbamato derivatives of the type [(DMDT)AuX2], [(ESDT)AuX2] (DMDT = N,N-dimethyldithiocarbamate; ESDT = ethylsarcosinedithiocarbamate; X= Cl, Br).29 The cytotoxicity of 4 and 5 was significantly lower (4) or comparable (5) to that of parent compound 1 supporting the idea that the stability afforded by the C,N-backbone is an important consideration. The fact that the organogold (III) derivatives with dithiocarbamato ligands have very similar cytotoxicity to dithiocarbamato compounds with halides is of interest since it will allow a possibility to further functionalize and tune electronic and steric factors and lipohilicity/hydrophilicity of dithiocarbamato compounds by inclusion of some other ligands similar to the one described here (iminophosphorane). The apoptosis studies that we have carried out have shown that our compounds trigger cell death by activating not only apoptotic pathways (major) but also other death mechanisms such as necrosis like in the case of the dithiocarbamato halide complexes. This effect is more marked with complex 2. To gain more information on the mechanistic aspects of the cytotoxic compounds 1–5 we have carried out spectrophotometric studies (UV-vis) on their interaction with DNA. None of the complexes were able to modify the melting temperature of a solution of calf thymus DNA. This fact suggests that either they do not interact with DNA or that interaction is very weak and electrostatic in nature. This is in accordance to what has been reported for most gold(III) compounds.14 The reactivity of the more cytotoxic compound 2 towards model biomolecules was studied spectroscopically. By UV-vis, we could assess a slow interaction with cytochrome c. By fluorescence spectroscopy, we have been able to confirm that compound 2 interacts with protein thioredoxin reductase and that at high concentrations, it likely induces an irreversible denaturation. This in accordance to the behavior exhibited by recently reported gold(III) compounds with bipyridil42,43 and dithiocarbamato ligands44,45 and supports the idea that mitochondrial proteins may be the target of gold(III) compounds that subsequently activate apoptosis. Fregona et al. found that the gold(III) dithiocarbamato complexes of the type [Au(SS)X2] (SS = DMDT: N,N-dimethyldithiocarbamate; ESDT = ethylsarcosinedithiocarbamate; X = Cl, Br) inhibited thioredoxin reductase activity and hypothesized a model suggesting that deregulation of the thioredoxin reductase/thioredoxin redox system is a major mechanism involved in the anticancer activity of those gold(III) compounds.44
Conclusions
The present study gives support to previous reports that the cytotoxicity displayed by gold(III) compounds is not related to DNA-damage. We have demonstrated that our compounds do not interact with DNA. We have also demonstrated that compound 2 interacts with proteins like cytochrome c and thioredoxin reductase. The use of 31P NMR spectroscopy as a simple characterization tool to asses stability and oxidation state of the gold compounds in solution has been demonstrated. Further modification of the iminophosphorane ligand is currently underway to prepare new gold(III) compounds with higher hydrophilicity and improved pharmacological properties. More detailed studies on mitochondrial damage and interaction of these compounds with the proteins (by different techniques) are also underway.
Supplementary Material
Tables of thermal parameters and observed and calculated structure factors (crystal structure of compound 2) have been deposited at the Cambridge Crystallographic Data Center. Any request for this material should quote a full literature citation and the reference number CCDC 704381, and may be obtained from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +441233336033; email: deposit@ccdc.cam.ac.uk or www:http://ccdc.cam.ac.uk). Selected 31P{1H} NMR spectra of compounds 2–6 in different solvents is available online at……….
Acknowledgments
We thank Brooklyn College (start-up budget M.C.), NSF (#DBI 0353887) and NIH RCMI Grant (2G112RR08124) at University of Texas at El Paso for financial support and the Arnold and Ruth T. Kaufman Chemistry Fund at Brooklyn College for a summer research undergraduate award (N.S.). We thank Prof. Iban Ubarretxena-Belandia (Mt. Sinai Hospital, New York) for useful discussions and assistance with the fluorescence measurements and Prof. István T. Horváth (Eötvös University, Budapest, Hungary) for the generous donation of the water-soluble phosphine P{Cp(m-C6H4-SO3Na)2}. This paper is dedicated to Maria Antonia Casas Gurpegui (grandmother of M.C. and supporter of her scientific career) who died of cancer in 2006.
References
- 1.Kelland L. Nature Rev. 2007;7:573. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Bruijnincx PCA, Sadler PJ. Current Opinion in Chem Biol. 2008;12:197. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reedijk J. Platinum Metals Rev. 2008;52(1):2. [Google Scholar]
- 4.Lippert B. Cisplatin: chemistry and biochemistry of a leading anticancer drug. Willey-VCH; Zurich: 1999. [Google Scholar]
- 5.Lippert B. Coord Chem Rev. 1999;182:263. [Google Scholar]
- 6.Jamieson ER, Lippard SJ. Chem Rev. 1999;99:2467. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Berners-Price SJ, Thorburn DR, Antalis T, Dickinson J, Hurst T, Qiu L, Khoo SK, Parsons PC. Biochem Pharmacol. 1997;53:1673. doi: 10.1016/s0006-2952(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 8.Guo Z, Sadler PJ. Angew Chem Int Ed. 1999;38:1512. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Parish RV, Cottrill SM. Gold Bull. 1987;20:3. and references therein. [Google Scholar]
- 10.Tiekink ERT. Bioinorg Chem Appl. 2003;1:53. doi: 10.1155/S1565363303000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw CF., III Chem Rev. 1999;99:2589. [Google Scholar]
- 12.Tiekink ERT. Inflammopharmacology. 2008;16:138. doi: 10.1007/s10787-007-0018-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Guo Z. Dalton Trans. 2008:1521. doi: 10.1039/b715903j. [DOI] [PubMed] [Google Scholar]
- 14.Casini A, Hartinger C, Gabbiani C, Mini E, Dyson PJ, Keppler BK, Messori L. J Inorg Biochem. 2008;102:564. doi: 10.1016/j.jinorgbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Milacic V, Fregona D, Dou QP. Histol Histopathol. 2008;23:101. doi: 10.14670/HH-23.101. [DOI] [PubMed] [Google Scholar]
- 16.Sun WY, Ma DL, Wong ELH, Che CM. Dalton Trans. 2007:4884. doi: 10.1039/b705079h. [DOI] [PubMed] [Google Scholar]
- 17.Gabbiani C, Casini A, Messori L. Gold Bull. 2007;40:73. [Google Scholar]
- 18.Barnard PJ, Berners-Price SJ. Coord Chem Rev. 2007;251:1889. [Google Scholar]
- 19.Messori L, Chiara G. Metal Compounds in Cancer. Chemotherapy. 2005:355. [Google Scholar]
- 20.Tiekink ER. Gold Bull. 2003;36:117. [Google Scholar]
- 21.Hickey JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price S, Filipovska A. J Am Chem Soc. 2008;138:12570. doi: 10.1021/ja804027j. [DOI] [PubMed] [Google Scholar]
- 22.Shi P, Jiang Q, Lin J, Zhao Y, Lin L, Guo Z. J Inorg Biochem. 2006;100:939. doi: 10.1016/j.jinorgbio.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Palanichamy K, Ontko AC. Inorg Chim Acta. 2006;359:44. [Google Scholar]
- 24.Marcon G, Carotti S, Coronnello M, Messori L, Mini E, Orioli P, Mazzei T, Cinellu MA, Minghetti G. J Med Chem. 2002;45:1672. doi: 10.1021/jm010997w. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, He QY, Sun RWY, Che CM, Chiu JF. Eur J Pharmacol. 2007;554:113. doi: 10.1016/j.ejphar.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Che CM, Sun RWY, Ko CB, Zhu N, Sun H. Chem Commun. 2003:1718. doi: 10.1039/b303294a. [DOI] [PubMed] [Google Scholar]
- 27.Aldinucci D, Lorenzon D, Stefani L, Giovagnini L, Colombatti A, Fregona D. Anti-Cancer Drugs. 2007;18:323. doi: 10.1097/CAD.0b013e328011ae98. [DOI] [PubMed] [Google Scholar]
- 28.Ronconi L, Marzano C, Zanello P, Corsini M, Macca C, Trevisan A, Fregona D. J Med Chem. 2006;49:1648. doi: 10.1021/jm0509288. [DOI] [PubMed] [Google Scholar]
- 29.Ronconi L, Giovagnini L, Marzano C, Bettio F, Grziani R, Pilloni G, Fregona D. Inorg Chem. 2005;44:1867. doi: 10.1021/ic048260v. [DOI] [PubMed] [Google Scholar]
- 30.Kilpin KJ, Henderson W, Nicholson BK. Polyhedron. 2007;26:434. [Google Scholar]
- 31.Kilpin KJ, Henderson W, Nicholson BK. Polyhedron. 2007;26:204. [Google Scholar]
- 32.Casas JS, Castaño MV, Cifuentes MC, García-Monteagudo JC, Sánchez A, Sordo J, Abram U. J Inorg Biochem. 2004;98:1009. doi: 10.1016/j.jinorgbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Fang D, Yang CT, Ranford JD, Lee PF, Vittal JJ. Dalton Trans. 2003:2680. [Google Scholar]
- 34.Goss CHA, Henderson W, Wilkins AL, Evans C. J Organomet Chem. 2003;679:194. [Google Scholar]
- 35.Messori L, Abbate F, Marcon G, Orioli P, Fontani M, Mini E, Mazzei T, Carotti S, O’Connell T, Zanello P. J Med Chem. 2000;43:3541. doi: 10.1021/jm990492u. [DOI] [PubMed] [Google Scholar]
- 36.Buckley R, Elsome A, Fricker S, Henderson G, Theobald B, Parish R, Howe B, Kelland L. J Med Chem. 1996;39:5208. doi: 10.1021/jm9601563. [DOI] [PubMed] [Google Scholar]
- 37.Li CKL, Sun RWY, Kui SCF, Zhu N, Che CM. Chem Eur J. 2006;12:5253. [Google Scholar]
- 38.Criado JJ, Manzano JL, Rodriguez-Fernández E. J Inorg Biochem. 2003;96:311. doi: 10.1016/s0162-0134(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 39.Carrasco J, Criado JJ, Macias RI, Manzano JL, Marin JJ, Medarde M, Rodriguez E. J Inorg Biochem. 2001;84:287. doi: 10.1016/s0162-0134(01)00172-6. [DOI] [PubMed] [Google Scholar]
- 40.Cuadrado JA, Zhang W, Hang W, Majidi V. J Environ Monit. 2000;2:355. doi: 10.1039/b000085j. [DOI] [PubMed] [Google Scholar]
- 41.Carotti S, Marcon G, Marussich M, Mazzei T, Messori L, Mini E, Orioli P. Chem Biol Interact. 2000;125:29. doi: 10.1016/s0009-2797(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 42.Casini A, Cinellu MA, Minghetti G, Gabbiani C, Coronnello M, Mini E, Messori L. J Med Chem. 2006;49:5524. doi: 10.1021/jm060436a. [DOI] [PubMed] [Google Scholar]
- 43.Gabbiani C, Casini A, Messori L, Guerri A, Cinellu MA, Minghetti G, Corsini M, Rosani C, Zanello P, Arca M. Inorg Chem. 2008;47:2368. doi: 10.1021/ic701254s. [DOI] [PubMed] [Google Scholar]
- 44.Saggioro D, Rigobello MP, Paloschi L, Folda L, Moggach SA, Parsons S, Ronconi L, Fregona D, Bindoli A. Chem Biol. 2007;14:1128. doi: 10.1016/j.chembiol.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Milacic V, Chen D, Ronconi L, Landis-Piwowar KR, Fregona D, Dou Dou QP. Cancer Res. 2006;66:21. doi: 10.1158/0008-5472.CAN-06-3017. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, He QY, Che CH, Tsa SW, Sun RWY, Chiu JF. Biochem Pharmacol. 2008;75:1282. doi: 10.1016/j.bcp.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 47.Casini A, Guerri A, Gabbiani C, Messori L. J Inorg Biochem. 2008;102:995. doi: 10.1016/j.jinorgbio.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Aguilar D, Contel M, Navarro R, Urriolabeitia EP. Organometallics. 2007;26:4604. [Google Scholar]
- 49.Brown SDJ, Henderson W, Kilpin KJ, Nicholson BK. Inorg Chim Acta. 2007;360:1310. [Google Scholar]
- 50.Parish RV, Howe BP, Wright JP, Mack J, Pritchard RG. Inorg Chem. 1996;35:1659. doi: 10.1021/ic950343b. [DOI] [PubMed] [Google Scholar]
- 51.31P{1H} NMR (D2O): δ[AuClP{Cp(m-C6H4-SO3Na)2] : 45.1 (s) ppm (to be reported elsewhere).
- 52.31P{1H} NMR (D2O): δ [AuCl(TPPS)]: 33.4 (s) ppm. Sanz S, Jones LA, Mohr F, Laguna M. Organometallics. 2007;26:952.
- 53.Miranda S, Vergara E, Mohr F, de Vos D, Cerrada E, Media A, Laguna M. Inorg Chem. 2008;47:5641. doi: 10.1021/ic7021903. [DOI] [PubMed] [Google Scholar]
- 54.31P{1H} NMR (d6-DMSO): δ [AuCl(PTA)]: −51.4 (s) ppm. Assefa Z, McBurnett G, Staples RJ, Fickler JP, Jr, Assman B, Angermaler K, Schmidbaur H. Inorg Chem. 1995;34:75.
- 55.Montoya J, Varela-Ramirez A, Estrada A, Martinez LE, Garza K, Aguilera RJ. Biochem Biophys Res Commun. 2004;325:1517. doi: 10.1016/j.bbrc.2004.10.196. [DOI] [PubMed] [Google Scholar]
- 56.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. J Natl Cancer Inst. 1990;82(13):1107. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 57.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Cancer Res. 1988;48:589. [PubMed] [Google Scholar]
- 58.Giovagnini L, Sitran S, Montpoli M, Caparrotta L, Corsini M, Rosani C, Zanello P, Dou QP, Fregona D. Inorg Chem. 2008;47:6336. doi: 10.1021/ic800404e. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, He QY, Sun RWY, Che CM, Chiu JF. Eur J Pharmacol. 2007;554:113. doi: 10.1016/j.ejphar.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 60.Similar spectroscopic changes for interactions of metals with serum proteins have been reported. Timerbaev AR, Hartinger CG, Aleksenko SS, Keppler BK. Chem Rev. 2006;106:2224. doi: 10.1021/cr040704h. and refs. therein.
- 61.We performed a titration of cytochrome c with dithiothreitol DTT (in mol ratios protein:DTT 1:1, 1:3 and 1:5, concentration of protein 5 μm) in buffer containing 0.1 mol% DMSO and the vis-UV spectra shows a shift of the Soret band of the hemo group at 409 nm to 415 nm but also two characteristic bands at 521 and 550 nm consistent with the reduction of Fe(III) to Fe(II) that we do not see in the experiment of titration of cytochrome c with 2.
- 62.Trynda-Lemiesz L, Keppler BK, Kozlowski H. J Inorg Biochem. 1999;73:123. doi: 10.1016/s0162-0134(99)00004-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables of thermal parameters and observed and calculated structure factors (crystal structure of compound 2) have been deposited at the Cambridge Crystallographic Data Center. Any request for this material should quote a full literature citation and the reference number CCDC 704381, and may be obtained from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +441233336033; email: deposit@ccdc.cam.ac.uk or www:http://ccdc.cam.ac.uk). Selected 31P{1H} NMR spectra of compounds 2–6 in different solvents is available online at……….