Controlling polymer topology presents challenging synthetic obstacles as well as exciting opportunities for tuning macromolecular properties.1 In particular, nanoscale molecular architectures with well-defined shapes and dimensions may provide significant advancements in areas such as drug delivery and nanotechnology. Over the last decade, breakthroughs in polymer syntheses have greatly increased the variety of macromolecular architectures that may be obtained, including dendronized, cylindrical, star, hyperbranched, and cyclic polymers as well as various block copolymers.2,3 However, the synthesis of circular nanostructures remains challenging due in large part to the difficulty in preparing functionalized cyclic polymers.4 Furthermore, reliance on macrocyclization routes to cyclic polymers restricts attachment of large side chains or dendrons to post-polymerization, and an efficient route to cyclic hybrid architectures of high purity is yet to be realized. Following recent developments in ring-expansion metathesis polymerization (REMP)5 and linear dendronized polymers,6 we aimed to interface these two areas to achieve a direct, efficient route to cyclic organic nanostructures.7 Herein, we report the REMP of a dendronized macromonomer (MM) as well as confirmation of cyclic polymer topology via atomic force microscopy (AFM).
REMP utilizes Ru-based metathesis catalysts (Figure 1) capable of producing cyclic polymers directly from cyclic olefin monomers, thus avoiding linear polymeric synthons. Earlier studies revealed that N-heterocyclic carbene backbone saturation greatly increased overall catalyst activity, while tether length influenced the relative rates of propagation vs catalyst release. With access to a range of catalyst activities, we envisioned that REMP of dendronized MMs may achieve dendronized cyclic polymers in a single operation. In addition, REMP can produce high molecular weight (MW) cyclic polymers, a goal not easily accomplished using macrocyclization processes.
Figure 1.
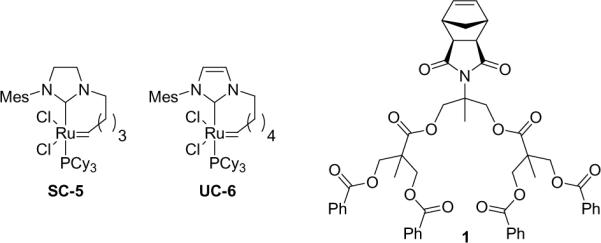
Structures of the cyclic REMP catalysts and dendronized macromonomer used in this study.
While the polymerization of sterically hindered MMs presents an inherent challenge, it was shown that 1 (Figure 1) could be efficiently polymerized via ROMP using a highly active Ru-based metathesis catalyst. This approach to dendritic polymers is particularly attractive, in that post-polymerization modifications are unnecessary, ensuring that complete dendron functionality is present along the polymer backbone.2c Thus, we examined the efficiency of REMP using 1 in combination with cyclic catalysts SC-5 and UC-6.
Saturated catalyst SC-5 was found to mediate REMP of 1 and key data are summarized in Table 1. In general, polymerizations required higher temperatures (55 °C) and higher loadings of [1]0/[SC-5]0 than used in previous studies involving cyclooctene monomers, which is ascribed to the steric bulk of MM 1. Interestingly, an inverse relationship between reaction concentration and degree of polymerization (DP) of polymer P1 was observed. Specifically, REMP of 1 ([1]0/[SC-5]0 = 50:1) at [1]0 = 0.05, 0.10, 0.20, and 0.33 M resulted in polymers having DPs of 5840, 4510, 4330, and 2190, respectively (entries 1, 2, 5, and 6). Since each polymerization reached full monomer conversion, this trend suggests that the initiation rate increases more with increased reaction concentration than does the propagation rate. Additionally, because incomplete initiation is observed with catalyst SC-5, the increased reaction concentration essentially led to a greater number of growing polymer rings and therefore a lower average MW.
Table 1.
REMP of 1 to give cyclic dendeonized polymer P1a.![]()
| Entry | Catalyst | [1]0(M) | [1]0/[C]0 | Mw (MDa)b | DP/1000 | PDIb |
|---|---|---|---|---|---|---|
| 1 | SC-5 | 0.05 | 50 | 5.26 | 5.84 | 1.29 |
| 2 | SC-5 | 0.10 | 50 | 4.06 | 4.51 | 1.25 |
| 3c | SC-5 | 0.10 | 100 | 4.47 | 4.97 | 1.51 |
| 4d | SC-5 | 0.10 | 200 | 5.33 | 5.92 | 1.49 |
| 5 | SC-5 | 0.20 | 50 | 3.90 | 4.33 | 1.33 |
| 6 | SC-5 | 0.33 | 50 | 1.97 | 2.19 | 1.17 |
| 7 | UC-6 | 0.10 | 250 | 3.79 | 4.21 | 1.18 |
Unless noted otherwise, reactions were conducted under dry N2 and monitored via 1H NMR spectroscopy until conversions were >90%. [1]0 = initial concentration of 1; [C]0 = initial catalyst concentration
Molecular weight data obtained via GPC with multiangle laser light scattering.
Maximum conversion = 60%.
Maximum conversion = 39%
Increasing the [1]0/[SC-5]0 resulted in incomplete monomer conversion due to catalyst death over the extended reaction periods. However, as expected, increased [1]0/[SC-5]0 ratios resulted in higher Mw values. Specifically, using [1]0/[SC-5]0 = 100:1 polymer P1 with Mw = 4.47 MDa (entry 3) was obtained, whereas Mw = 5.33 MDa was obtained with [1]0/[SC-5]0 (entry 4). Notably, catalyst UC-6 efficiently polymerized 1 to >95% conversion at [11]0/[UC-6]0 = 250:1. These conditions provided P1 with a Mw = 3.79 MDa (entry 7).
Catalyst release and reincorporation have been shown to guide the thermodynamically driven MW of REMP polymers. In the present case, catalyst reincorporation and chain transfer are unlikely considering the steric bulk of MM 1. Notably, the MW of P1 did not change upon prolonged standing after 100% monomer conversion, or upon injection of fresh catalyst into the reaction mixtures. This indicates that thermodynamic equilibration of ring sizes is not taking place. Furthermore, the MW of isolated P1 did not increase upon treatment with additional 1, suggesting that no significant amount of active Ru species remain in the cyclic polymer. Collectively the data suggests efficient, irreversible catalyst release, and an absence of chain transfer events. These experiments provide an initial investigation into the kinetically controlled MW profile of REMP.
We next turned our attention toward visualization of the polymers via AFM.8 Samples of P1 were prepared by spin-coating a 9 ng/mL solution of the polymer in CHCl3 onto freshly cleaved mica. As shown in Figure 2, toroidal features were observed with diameters of ca 35 - 40 nm, heights ranging from 5 - 9 Å, and internal diameters ranging from ca 5 - 7 nm. Multiple rings were observed (Figure 2A) without any detectable linear polymers, and analysis of the line scans of the toroids revealed highly uniform profile features (Figure 2D). Considering the high DP values obtained for P1, the dimensions observed via AFM suggested that the polymer backbone is not fully extended and may be adopting a zigzag orientation.
Figure 2.
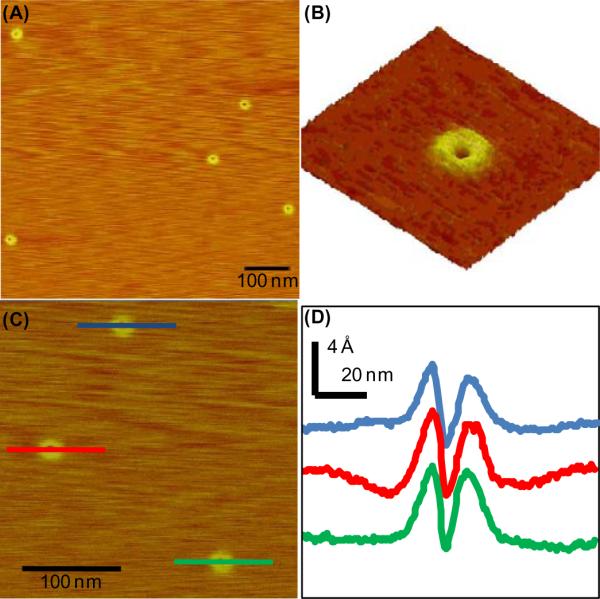
(A) AFM images of P1 (Table 1, entry 7) on mica. (B) 3-D plot of toroidal feature. (C) Three toroids from image (A). (D) Line scans of toroids in image (C).
For comparison, we also examined linear analogues via AFM. Because the high DPs obtained via REMP were difficult to achieve using acyclic ROMP catalysts, we investigated ring-opening of P1 via sonication. Given that polymer chain scission can be induced via ultrasound, 9 and that the steric congestion may weaken the backbone of P1, we subjected a solution of cyclic polymer (Mw = 2.99 MDa, 1 mg/mL in THF) to ultrasound irradiation for 30 min to give P1son. GPC analysis of P1son revealed an Mw = 959 kDa, which suggests that chain scission accompanied ring-opening. In addition, P1son displayed shorter elution times at the same MW in comparison with P1, consistent with linear and cyclic topologies, respectively (Figure 3).10 AFM imaging of P1son revealed features consistent with a linear topology (see the Supporting Information). Notably, some features resembled those of the cyclic polymers in diameter and shape; however these did not appear toroidal as no central void was observed. This may be ascribed to aggregation, which is more commonly observed upon AFM imaging of the linear polymers in comparison with cyclic P1.
Figure 3.
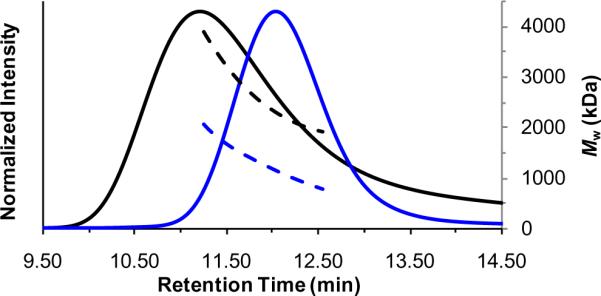
GPC data for P1 (black) and P1son (blue): normalized refractive index detector intensities (solid lines) and Mw values (dashed lines) vs retention times.
In conclusion, we have demonstrated the first direct synthesis of cyclic dendronized polymers via REMP of a dendronized macromonomer. AFM imaging has confirmed the cyclic topology, revealing uniform cyclic features with no detectable linear polymer contaminants. The kinetically controlled molecular weight profiles and relative initiation versus propagation rates of REMP resulted in polymer Mw values that increased with decreasing initial concentrations. Despite the steric challenges inherent to the polymerization of these dendronized monomers, very high molecular weights were achieved for this novel class of hybrid macromolecules.
Supplementary Material
Acknowledgment
Financial support of this research by the National Science Foundation (CHE-0410425 and DMR-0906638) and the Department of Energy (DE-FG02-05ER46218). AJB thanks the National Institutes of Health for a postdoctoral fellowship. TWH thanks the National Science Foundation for a Graduate Research Fellowship.
Footnotes
Supporting Information Available Detailed experimental procedures and AFM images of P1son. This materials is available free of charge via the Internet at http://pubs.acs.org
References
- 1).(a) Nasongkla N, Chen B, Macaraeg N, Fox ME, Fréchet JMJ, Szoka FC. J. Am. Chem. Soc. doi: 10.1021/ja900062u. in press (DOI: 10.1021/ja900062u) [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Eugene DM, Grayson SM. Macromolecules. 2008;41:5082–5084. [Google Scholar]; (c) Guan Z. J. Polym. Sci., Part A: Polym. Chem. 2003;41:3680–3692. [Google Scholar]; (d) Guan Z. Chem. Eur. J. 2002;8:3086–3092. doi: 10.1002/1521-3765(20020715)8:14<3086::AID-CHEM3086>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]; (e) Harth EM, Hecht S, Helms B, Malmstrom EE, Fréchet JMJ, Hawker CJ. J. Am. Chem. Soc. 2002;124:3926–3938. doi: 10.1021/ja025536u. [DOI] [PubMed] [Google Scholar]; (f) Edgecombe BD, Stein JA, Fréchet JMJ, Xu Z, Kramer EJ. Macromolecules. 1998;31:1292–1304. [Google Scholar]
- 2).For reviews, see: Matyjaszewski K, Xia J. Chem. Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g..Kamigaito M, Ando T, Sawamoto M. Chem. Rev. 2001;101:3689–3746. doi: 10.1021/cr9901182..Schlüter AD, Rabe JP. Angew. Chem. Int. Ed. 2000;39:864–883. doi: 10.1002/(sici)1521-3773(20000303)39:5<864::aid-anie864>3.0.co;2-e..
- 3).(a) Tezuka Y, Fujiyama K. J. Am. Chem. Soc. 2008;127:6266–6270. doi: 10.1021/ja042198j. [DOI] [PubMed] [Google Scholar]; (b) Jeong W, Hedrick JL, Waymouth RM. J. Am. Chem. Soc. 2007;129:8414–8415. doi: 10.1021/ja072037q. [DOI] [PubMed] [Google Scholar]; (c) Culkin DA, Jeong W, Csihony S, Gomez ED, Balsara NP, Hedrick JL, Waymouth RM. Angew. Chem. Int. Ed. 2007;46:2627–2630. doi: 10.1002/anie.200604740. [DOI] [PubMed] [Google Scholar]; (d) Pyun J, Kowalewski T, Matyjaszewski K. Macromol. Rapid Commun. 2003;24:1043–1059. [Google Scholar]; (e) Bosman AW, Vestberg R, Heumann A, Fréchet JMJ, Hawker CJ. J. Am. Chem. Soc. 2003;125:715–728. doi: 10.1021/ja028392s. [DOI] [PubMed] [Google Scholar]; (f) Shu L, Schlüter AD, Ecker C, Severin N, Rabe JP. Angew. Chem. Int. Ed. 2001;40:4666–4669. doi: 10.1002/1521-3773(20011217)40:24<4666::aid-anie4666>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4).Schappacher M, Deffieux A. J. Am. Chem. Soc. 2008;130:14684–14689. doi: 10.1021/ja804780s. [DOI] [PubMed] [Google Scholar]
- 5).(a) Boydston AJ, Xia Y, Kornfield JA, Gorodetskaya IA, Grubbs RH. J. Am. Chem. Soc. 2008;130:12775–12782. doi: 10.1021/ja8037849. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xia Y, Boydston AJ, Gorodetskaya IA, Kornfield JA, Grubbs RH. J. Am. Chem. Soc. 2009;131:2670–2677. doi: 10.1021/ja808296a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Rajaram S, Choi T-L, Rolandi M, Fréchet JMJ. J. Am. Chem. Soc. 2007;129:9619–9621. doi: 10.1021/ja0741980. [DOI] [PubMed] [Google Scholar]
- 7).Laurent BA, Grayson SM. Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 2008;49:287–288. [Google Scholar]
- 8).Sheiko SS, Möller M. Chem. Rev. 2001;101:4099–4124. doi: 10.1021/cr990129v. [DOI] [PubMed] [Google Scholar]
- 9).Mason TJ, Lorimer JP, editors. Applied Sonochemistry. Wiley-VCH Verlag GmbH; Weinheim: 2002. [Google Scholar]
- 10).Zimm BH, Stockmayer WH. J. Chem. Phys. 1949;17:1301–1314. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


