Abstract
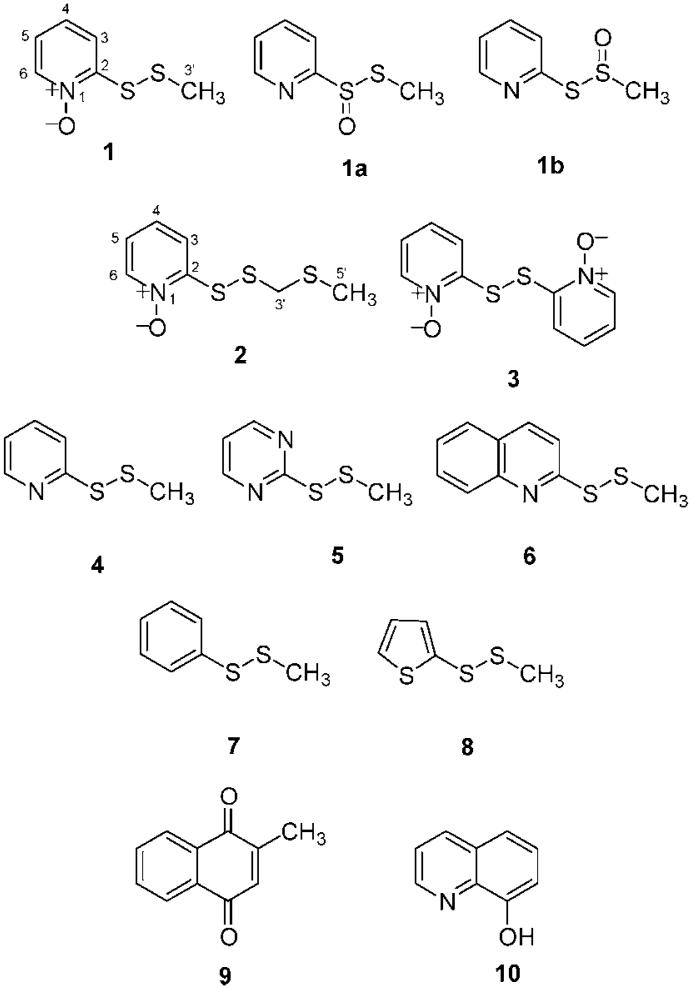
From Allium stipitatum, three pyridine-N-oxide alkaloids (1-3) possessing disulfide functional groups were isolated. The structures of these natural products were elucidated by spectroscopic means as 2-(methyldithio)pyridine-N-oxide (1), 2-[(methylthiomethyl)dithio]pyridine-N-oxide (2), and 2,2′-dithio-bis-pyridine-N-oxide (3). The proposed structure of 1 was confirmed by synthetic S-methylthiolation of commercial 2-thiopyridine-N-oxide. Compounds 1 and 2 are new natural products, and 3 is reported for the first time from an Allium species. All compounds were evaluated for activity against fast-growing species of Mycobacterium, methicillin-resistant Staphylococcus aureus, and a multidrug-resistant (MDR) variants of S. aureus. Compounds 1 and 2 exhibited minimum inhibitory concentrations (MICs) of 0.5-8 μg/mL against these strains. A small series of analogues of 1 were synthesized in an attempt to optimize antibacterial activity, although the natural product had the most potent in vitro activity. In a whole-cell assay at 30 μg/mL, 1 was shown to give complete inhibition of the incorporation of 14C-labeled acetate into soluble fatty acids, indicating that it is potentially an inhibitor of fatty acid biosynthesis. In a human cancer cell line antiproliferative assay, 1 and 2 displayed IC50 values ranging from 0.3 to 1.8 μM with a selectivity index of 2.3 when compared to a human somatic cell line. Compound 1 was evaluated in a microarray analysis that indicated a similar mode of action to menadione and 8-quinolinol by interfering with the thioredoxin system and up-regulating the production of various heat shock proteins. This compound was also assessed in a mouse model for in vivo toxicity.
The genus Allium is a rich source of bioactive natural products and a prolific producer of sulfur-containing metabolites. Common garlic (Allium sativum L.) has a long history of use as an antibacterial material, and the major active principle, allicin, was isolated and characterized in the 1940s.1-3 Garlic was even used clinically in the United States and known as Russian penicillin.4 We have been investigating extracts of bulbs from the Liliaceae and Alliaceae families for their ability to produce antibacterial compounds.5,6 The rationale is that it is highly likely that plants produce defensive antimicrobial compounds in their subterranean organs, given the richness of soil in terms of its microbial content. Soil is rich in filamentous bacteria such as Streptomyces and Mycobacterium, and it is conceivable that plants have evolved defensive secondary metabolites with activity against these genera. A number of species of the genus Allium have also been used medicinally in Tajikistan and Uzbekistan for various uses including the promotion of wound healing.7
There is a pressing need for new classes of antibacterials to deal with emerging threats such as extensively drug-resistant (XDR) tuberculosis and multidrug resistance (MDR) increasingly associated with clinically relevant bacteria such as Staphylococcus aureus. Plants are a relatively untapped source of antibacterial compounds, and some of the examples in the literature are striking, in terms of their preliminary in vitro potency.8,9 However, many of these studies lack depth in terms of their biological evaluation, and in the majority of cases there is little evaluation of mammalian cell cytotoxicity or determination of potential mode of action. Unfortunately there have been only a small number of new antibacterial substances based on new carbon skeletons developed over the last 10 years, and these include daptomycin, a lipopeptide, arguably from a well-known class of natural product, and linezolid, a totally new oxazolidinone class derived synthetically. Limited reports of resistance to these new agents have been reported,10,11 and therefore new classes of antibacterials would be valuable. There are several compelling reasons to evaluate plants as a source of new antibacterial agents. First, given the structural dissimilarity that exists between many plant antibacterials and conventional antibiotics such as tetracyclines, fluoroquinolones, and macrolides, it is possible that these phytochemical antibacterials exert their effects through new modes of action. Second, there are countless examples of ethno-medical usage of plant extracts to treat topical and systemic bacterial infections in many systems of traditional medicine.12
Given the need for new antibacterial substances and the pressing problems of bacterial MDR, we have evaluated Allium stipitatum Regel, which is grown horticulturally in Europe for its beautiful spherical flowers with tightly packed umbels. Herein, we describe the isolation, structure elucidation, and in-depth in vitro and in vivo biological evaluation of new antibacterial pyridine-N-oxide disulfides isolated from the bulbs of this species.
Results and Discussion
Compound 1 was isolated as a colorless waxy solid from the chloroform extract of A. stipitatum. The high-resolution ESIMS indicated a [M + H]+ ion at m/z 174 and a [M + Na]+ ion at m/z 196, suggesting a molecular formula of C6H8NOS2, and the spectrum also gave the isotope pattern for two sulfur atoms. The compound displayed a positive reaction with Dragendorff's reagent when analyzed by normal-phase TLC, indicating that it may be an alkaloidal natural product.
The 1H spectrum (Table 1) displayed a deshielded methyl singlet at δ 2.50 and four hydrogens indicative of an ABCD aromatic system. The 13C NMR and DEPT-135 spectra (Table 1) showed four methine aromatic carbons at δ 140.5-123.4, a deshielded quaternary carbon at δ 153.4, and a methyl carbon at δ 22.1. Given the deshielded nature of one of the hydrogens (δ 8.35, H-6) and that the coupling constant for this resonance was typical for a hydrogen α to the nitrogen of a pyridine,13 compound 1 could be shown to be a pyridine ring-containing natural product. The aromatic hydrogens of this system were identified by correlations observed in the COSY spectrum. Specifically, a double-doublet of H-3 (δ 8.07; J = 8.0, 1.5 Hz) coupled to an H-4 triplet (δ 7.67; J = 7.5 Hz), which coupled to a second triplet of H-5 (δ 7.38; J = 7.0 Hz), and finally coupled to the α H-6 doublet (δ 8.35; J = 6.5 Hz).
Table 1.
1H, 13C, and HMBC NMR Data for 1 and 2
| 1 |
2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| no. | 1H | 13C | 2J | 3J | 1H | 13C | 2J | 3J |
| 2 | 153.4 | 153.5 | ||||||
| 3 | 8.07 dd (1.5, 8.0) | 123.4 | C-5 | 8.09 dd (1.5, 8.5) | 140.3 | C-5 | ||
| 4 | 7.67 t (1.0, 8.5) | 130.6 | C-3 | C-2, C-6 | 7.64 t (1.5, 8.5) | 130.4 | C-3, C-5 | C-2, C-6 |
| 5 | 7.38 t (1.5, 7.0) | 123.9 | C-6 | C-3 | 7.37 t (2.0, 8.0) | 123.8 | C-6 | C-3 |
| 6 | 8.35 d (6.5) | 140.5 | C-3, C-5 | C-2, C-4 | 8.34 d (7.0) | 124.0 | C-5 | C-2, C-4 |
| 3′ | 2.50 | 22.1 | C-2 | 4.02 s | 44.9 | C-2 | ||
| 5′ | 2.29 s | 15.7 | C-7 | |||||
A methyl group appeared as a singlet at δ 2.50 displaying no couplings to the remaining hydrogens and suggesting that it was isolated from the aromatic nucleus. Its downfield appearance in the 1H spectrum at 2.50 ppm indicated that it was slightly deshielded and was attached to a heteroatom such as one of the sulfur atoms. A weak correlation (4J) in the HMBC spectrum from the methyl hydrogens to the quaternary carbon C-2 suggested it was in the terminal position on a disulfide side-chain α to the nitrogen. Furthermore, a NOE was also evident in the NOESY spectrum from the methyl hydrogens to H-3. The aromatic hydrogen H-3 was deshielded due to its positioning β to the nitrogen of the pyridine ring and α to the disulfide side-chain. From the molecular formula of 1, this left the placement of one oxygen atom in the structure of 1. This posed a dilemma, as there were three structural possibilities that could exist without appreciably great differences in terms of their NMR spectroscopic data. For example, the placement of the oxygen atom on the nitrogen would give a pyridine-N-oxide natural product (structure 1). However, placement of the oxygen could also occur at the sulfur directly attached to the aromatic ring (1a) or to the sulfur to which the methyl group was attached (1b), resulting in thiosulfinate natural products that occur widely in the genus Allium and include the well-known example allicin.14 This was resolved by the synthesis of compound 1 by the simple method of Kitson and Loomes.15 Briefly, commercially available 2-thiopyridine-N-oxide was treated with base and reacted with S-methyl methanethiosulfonate, resulting in S-methylthiolation of the thiol to generate 2-(methyldithio)pyridine-N-oxide, which was identical in terms of its spectroscopic and biological data with compound 1. Pyridine-N-oxides have not been described from the genus Allium previously, and this is the first report of this compound as a natural product. The compound is, however, the subject of two Japanese patents.16,17 These describe the use of 1 for the disinfection of seeds against Pyrenophora graminea16 and the use of this compound as an antibacterial against a number of bacteria including Staphylococcus aureus, Pyricularia oryzae, and Aspergillus niger.17
Compound 2 was isolated as a yellow oil from the chloroform extract. The high-resolution ESIMS indicated a [M + H]+ ion at m/z 220 and a [M + Na]+ ion at m/z 242, suggesting a molecular formula of C7H10NOS3, differing from compound 1 by CH2S. The ESIMS gave an isotope pattern for three sulfur atoms. The compound also displayed a positive reaction with Dragendorff's reagent when analyzed by normal-phase TLC, and the NMR data were very similar to those of 1. The 1H spectrum (Table 1) also revealed the presence of four aromatic hydrogens with chemical shifts and coupling patterns similar to those for 1, again indicative of a substituted pyridine-N-oxide. A deshielded methyl singlet (δ 2.29) and a methylene singlet (δ 4.02) were evident. The 13C NMR and DEPT-135 spectra indicated the presence of four aromatic methine carbons at δ 140.3-123.8, a quaternary aromatic carbon at δ 153.5, a methylene at δ 44.9, and a methyl at δ 15.7 (Table 1). The methyl singlet was again slightly deshielded (2.29 ppm), indicating that it was attached to a sulfur atom. The deshielded nature of the methylene resonance at 4.02 ppm was also suggestive of attachment to at least one heteroatom, although oxygen could be ruled out due to the upfield nature of the carbon resonance (44.0 ppm). The downfield appearance of this resonance could be explained by attachment to two sulfur atoms. A correlation was evident in the HMBC spectrum from the methyl hydrogens to this methylene carbon. A 4J correlation from the methylene hydrogens to the quaternary carbon of C-2 was also evident, and this was analogous to the 4J correlation seen in compound 1. These data suggested that a 2-(methylthio)methyldithio side-chain (R-S-S-CH2-S-CH3) was present in compound 2 and would account for the additional CH2S seen in the molecular formula of 2, when compared to 1. Both the methyl and methylene hydrogens displayed NOE correlations to the H-2 aromatic hydrogen in the NOESY spectrum. This is the first time the isolation and NMR data have been described for 2-[(methylthiomethyl)dithio]pyridine-N-oxide and the first reported isolation of the compound as a natural product.
Compound 3 was isolated as an orange oil from the chloroform extract. The ESIMS indicated a [M + H]+ ion at m/z 253 and an [M + Na]+ ion at m/z 275, suggesting a molecular formula of C10H8N2O2S2. The only signals in the 1H spectrum were two doublet and two triplet aromatic hydrogens, again supportive of a 1,2,3,4 splitting pattern. The 13C, DEPT-135, and HMQC spectra revealed four methine aromatic carbons and a quaternary aromatic carbon, indicating that 3 is a dimeric analogue of 1, but lacking the methyl group. The presence of a monosubstituted pyridine ring system was characterized through HMBC correlations. The H-4 triplet (δ 7.41; J = 7.0 Hz) and the H-6 doublet (δ 8.46; J = 6.0 Hz) displayed 3J correlations to the quaternary carbon C-2 (δ 147.3). Furthermore, the coupling constant of H-6 was again indicative of a hydrogen α to the nitrogen of a pyridine ring. H-3 displayed a 3J correlation to C-5 (δ 123.5), and H-5, in turn, displayed a 3J correlation to C-3 (δ 121.8) and a 2J correlation to C-6 (δ 138.5). This completed the assignment of the pyridine-N-oxide ring. Analysis of the ESIMS data suggested that compound 3 is a dimer with a disulfide chain linking two pyridine-N-oxide rings in a symmetrical C-2-C-2′ linkage. This compound has been previously isolated from a basidiomycete mushroom of the Cortinarius genus,18 and the observed NMR data are in accordance with this report.
A series of methyl sulfide analogues were prepared and with the natural products they were assayed against a panel of bacteria (Table 2). Compounds 1-6 were highly active (MIC < 4 μg/mL) against the three Staphylococcus aureus strains. These included a multidrug-resistant strain that overexpresses the NorA efflux transporter (SA1199B); a tetracycline-effluxing strain that is also a MRSA (XU212); and EMRSA-15, one of the major epidemic strains of MRSA responsible for bacteraemias in U.K. hospitals.19 The levels of activity of the natural products against this EMRSA strain are particularly noteworthy at 0.5 μg/mL. The natural products were also marginally more active than the synthetic compounds against the fast-growing mycobacteria (FGM) (Table 2) with MIC values in the range 2-16 mg/mL. Compounds 7 and 8 were either inactive or only marginally active, indicating that the presence of the disulfide moiety is not the only factor responsible for activity. It is possible that this disulfide is strongly “activated” by the presence of electron-withdrawing functional groups such as pyridine, pyridine-N-oxide, pyrimidine, and quinoline, whereas phenyl and thiophene are poorly electron-withdrawing and therefore have little effect on the “reactivity” of the disulfide bond.
Table 2.
Minimum Inhibitory Concentration (MIC) Values of Compounds (μ/mL)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Nora | Oxb | Ethc | Isod | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. fortuitum | 8 | 8 | 16 | 8 | 8 | 4 | > 128 | > 128 | -e | -e | 2 | 0.25 |
| M. smegmatis | 8 | 4 | 16 | 8 | 8 | 8 | 64 | 64 | -e | -e | 32 | 0.25 |
| M. smegmatis (mc2 2700) | 8 | 4 | -e | -e | -e | -e | -e | -e | -e | -e | -e | -e |
| M. phlei | 2 | 2 | 16 | 16 | 16 | 8 | > 128 | > 128 | -e | -e | 2 | 128 |
| S. aureus (SA-1199B) | 2 | 1 | 1 | 2 | 2 | 4 | 128 | 128 | 32 | 0.25 | -e | -e |
| S. aureus (XU212) | 1 | 1 | 1 | 4 | 4 | 4 | > 128 | > 128 | 16 | 128 | -e | -e |
| S. aureus (EMRSA-15) | 0.5 | 0.5 | 0.5 | 2 | 2 | 4 | > 128 | > 128 | 0.5 | 32 | -e | -e |
Norfloxacin.
Oxacillin.
Ethambutol.
Isoniazid.
Not tested (-).
Both compounds 1 and 2 were evaluated against Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv (Figure 1) and were shown to be highly active. Compound 1 is bactericidal against nonreplicating cells of M. tuberculosis, with a concentration of 1.25 μg/mL, resulting in a 99% decrease in viable counts of anaerobically adapted cells after one week of exposure.20 To investigate the macromolecular processes that were affected by treatment with this disulfide, we analyzed the effects on translation and fatty acid synthesis. The disulfide structure stimulated us first to test the effect of 1 on protein biosynthesis; however, application of 1 at 10-20-fold of the MIC followed by incubation with 35S methionine yielded no effect of 1 on 35S methionine incorporation into protein. Therefore, protein biosynthesis is not affected by 1. We next investigated the ability of 1 to inhibit the incorporation of labeled acetate into soluble fatty acids. Replicating M. smegmatis bacilli were treated with 1 at a concentration of 30 μg/mL for 2 h before adding [1-14C] acetate for another 2 h followed by soluble lipid extraction (which yields mostly fatty acids) and comparison of the counts per minute to the untreated control as previously described.21 Incubation with 1 resulted in complete inhibition of acetate incorporation into soluble lipids, indicating that this natural product may inhibit fatty acid synthesis. The primary possibility for this finding is that 1 inhibits FAS-I, which should be next tested by an in vitro assay. The antibacterial activity against S. aureus (which synthesizes fatty acids through a FAS-II system) then may be due to inhibition of a FAS-II enzyme. Other possibilities that could explain the observed results are inhibition of the enzyme acetyl-CoA carboxylase AccD1, which mediates the formation of malonyl CoA,22 or inhibition of the acyl-CoA condensation reaction with malonyl-AcpM in a reaction catalyzed by mtFabH.23,24 However, the possibility that the observed arrest in fatty acid synthesis is a nonspecific result of a generalized toxic effect can not be excluded.
Figure 1.
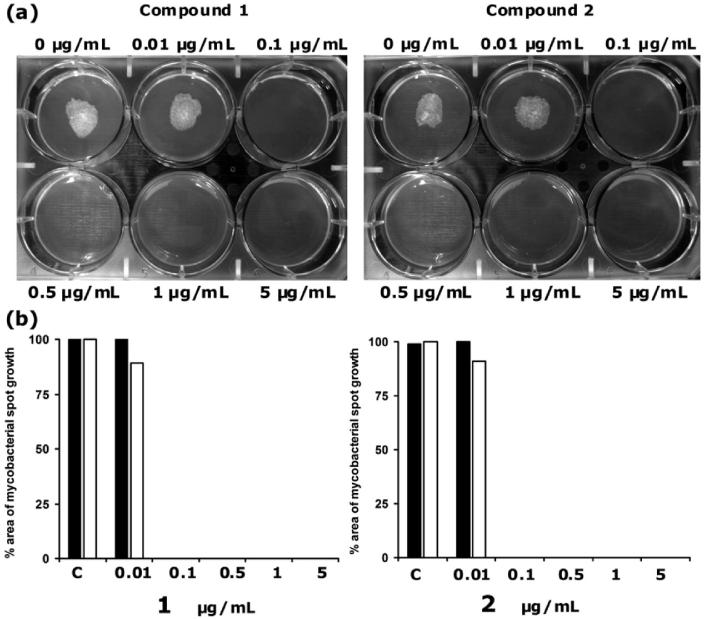
Effect of 1 and 2 on the growth of mycobacteria. (a) Growth of M. bovis BCG on solid agar at 37 °C in the presence of different concentrations of compounds 1 and 2. The MIC values of compounds 1 and 2 are both 0.1 μg/mL (a) Approximately 103 M. bovis BCG cells were spotted on solidified Middlebrook 7H10 agar containing different concentrations of 1 and 2 (0.01, 0.1, 0.5, 1, and 5 μg/mL) in a six-well plate. Pictures of cultures that grew as spots were taken on the 14th day of inoculation using a BioDoc-ItTM imaging system. (b) M. tuberculosis H37Rv was grown in the presence of similar concentrations of 1 and 2 as in (a) above. A comparison of inhibitory effects of 1 and 2 on M. bovis BCG (black bars) versus M. tuberculosis H37Rv (open bars) is illustrated on the bar graphs.
Microarray analysis of RNA extracted from cells treated with 1 revealed that transcriptional profiles elicited were similar to the profiles generated during treatment of cells with compounds such as menadione (9) and 8-quinolinol (10) that result in oxidative stress. Genes that characterized the response to compound 1, menadione, and 8-quinolinol included the thioredoxin system components encoded by trxB2 and trxC as well as several genes associated with the heat shock response such as clpB, sigH, dnaJ, dnaK, and hsp. Other genes that have been found to be up-regulated during treatment of M. tuberculosis at high temperatures25 were also up-regulated, including Rv0331, Rv3463, Rv3054c, and Rv1334 Rv1335. These results suggest that compound 1 possibly generates damaged proteins and other oxidative stress signals as part of its mechanism of action.
Compound 1 was also evaluated in vivo, including efficacy in a mouse model of TB infection. The maximum tolerated dose (MTD) was determined using an escalating single dose of drug given to mice by oral gavage. Acute adverse effects were observed at doses of 100 and 300 mg/kg. No adverse effects, reactions, or toxicity was observed at oral doses of 30 mg/kg (Table 3). Subsequently, the efficacy of 1 was evaluated in mice at a dose of 30 mg/kg administered via oral gavage in infected GKO C57BL/6 mice.26 Compound 1 was found to be inactive in that it did not effectively reduce the bacterial numbers in the lungs and spleens with respect to the untreated controls at the dose tested (Table 4).
Table 3.
In Vivo Toxicity Data of 1 after 3-day Dosing in Mice
| dose (mg/kg) | days Rx | % survival + comments |
|---|---|---|
| 300 | 3 | 1/5 lethality after 3 days of dosing, 1/5 additional lethality after 3 days of dosing |
| 100 | 3 | transient lethargy after dosing |
| 30 | 3 | no adverse effects |
Table 4.
Bacterial Numbers in Lungs and Spleens of Mycobacterium tuberculosis-Infected Mice at the Start of Treatment (15 days after aerosol infection) and after 9 Days of Drug Treatment (24 days after aerosol infection)
| log10 CFU ± SEM |
|||
|---|---|---|---|
| treatment regimen | dose | lung | spleen |
| day 15, start of treatment (pretreated controls) |
- | 6.08 ± 0.06 | 4.00 ± 0.19 |
| day 24, untreated controls | - | 8.13 ± 0.11 | 6.35 ± 0.13 |
| day 24, isoniazid | 25 | 5.33 ± 0.10 | 2.16 ± 0.26 |
| day 24, 1 | 30 | 8.17 ± 0.11 | 6.17 ± 0.29 |
Both compounds 1 and 2 showed significant activity in antiproliferative assays using MCF7 breast carcinoma and A549 non-small-cell lung carcinoma cancer cells, as well as human colon adenocarcinoma HT29 cells (Table 5). They showed greater selectivity to a human normal fibroblast cell line than the established clinical anticancer agent cisplatin, indicating their potential for anticancer activity, although at this stage no information is available on their mechanism of action.
Table 5.
Short-Term Cytotoxicity Data for Compounds 1, 2, and Cisplatin (IC50) in Human Breast, Lung, and Colorectal Cancer Cell Lines and Normal Human Fibroblast Cell Linea,b
| human breast cancer (MCF7) | human lung cancer (A549) | human colorectal cancer (HT29) | lung fibroblast (WI38) | selectivity index (WI88IC50/MCF7 IC50) |
|
|---|---|---|---|---|---|
| 1 | 0.35 | 0.22 | 1.84 | 0.8 | 2.3 |
| 2 | 0.39 | 0.78 | c | 0.89 | 2.3 |
| cisplatin | 0.76 | 2.47 | 3.27 | 1 | 1.4 |
Data expressed in μM.
Cells were exposed to compounds for 96 h and stained with sulforhodamine B.
Not determined.
It is possible that compound 1, in being an N-oxide, is rapidly excreted, and this would presumably be the main route of metabolism of the parent pyridine methyldisulfide (4). Compound 4 displays a similar level of antibacterial activity to 1 and 2 and is also likely to possess toxicity to mammalian cell lines. An investigation of the activity of 4 in a mouse model of infection and in a xenograft antitumor assay would be worthwhile to gauge the in vivo utility of this class of compound.
Experimental Section
General Experimental Procedures
UV spectra were recorded on a Thermo Electron Corporation Helios spectrophotometer, and IR spectra were recorded on a Nicolet 360 FT-IR spectrophotometer. NMR spectra were recorded on a Bruker AVANCE 500 MHz spectrometer. Chemical shift values (δ) are reported in parts per million (ppm) relative to appropriate internal solvent standard, and coupling constants (J values) are given in Hz. Mass spectra were recorded on a Finnigan MAT 95 high-resolution, double-focusing, magnetic sector mass spectrometer. Accurate mass measurement was achieved using voltage scanning of the accelerating voltage. This was nominally 5 kV, and an internal reference of heptacosa was used. Resolution was set between 5000 and 10 000.
Plant Material
The fresh bulbs of Allium stipitatum were purchased from Gee Tee Garden Products, Spalding, Lincolnshire, U.K., and a voucher specimen (GO'D/017B) has been deposited in their herbarium.
Extraction and Isolation
A 4.43 kg quantity of macerated bulbs was extracted exhaustively with hexane, chloroform, and methanol to form three extracts with MIC values of 16, 8, and >512 μg/mL against Mycobacterium fortuitum. The chloroform extract (4.9 g) was separated on silica gel by VLC, eluting 11 fractions (200 mL) with hexane and 10% increments of ethyl acetate, followed by one 100% chloroform fraction (300 mL) and a final 100% methanol wash (500 mL). The final fraction (13) exhibited an MIC of 8 μg/mL against M. fortuitum. Then, 1000 mg of VLC fraction 13, eluted in the 100% methanol wash, was subjected to reversed-phase preparative HPLC (Waters XTerra Prep MS C18 column, 10 μM, 19 × 300 mm, 100% water to 100% acetonitrile from 2 to 15 min, 50 mL/min) to yield three peaks, eluting at 10.4 (3, 361.1 mg), 12.8 (1, 250.5 mg), and 14.8 min (2, 72.1 mg).
2-(Methyldithio)pyridine-N-oxide (1)
colorless, waxy solid; UV (MeOH) λmax (log ε) 239.0 (4.37) nm; IR (film) νmax 3410 (br), 3090, 1463, 1419, 1260, 1220, 1138, 1079, 836, 760 cm-1; 1H and 13C NMR (CDCl3, 500 and 125 MHz), see Table 1; HRESIMS m/z 174.0043 [M + H]+ (calcd for C6H8NOS2 174.0042).
2-[(Methylthiomethyl)dithio]pyridine-N-oxide (2)
yellow oil; UV (MeOH) λmax (log ε) 239.0 (4.18), 204.0 (4.00) nm; IR (film) νmax 3386 (br), 3096, 2916, 1418, 1259, 1221, 836, 760 cm-1; 1H and 13C NMR (CDCl3, 500 and 125 MHz), see Table 1; HRESIMS m/z 219.9931 [M + H]+, 241.9751 [M + Na]+ (calcd for C7H10NOS3 219.9919).
2,2′-Dithio-bis-pyridine-N-oxide (dipyrithione, 3)
yellow oil; UV (MeOH) λmax (log ε) 266.0 (4.17), 240.5 (3.73) nm; IR (film) νmax 3420, 1647, 1558, 1379, 1022, 1000, 822, 760 cm-1; 1H and 13C NMR (methanol-d4) in agreement with those published;18 HRESIMS m/z 253.0102 [M + H]+ (calcd for C10H9N2S2O2 253.0105).
Synthesis of Methyl Disulfides
Aromatic thiols (2-thiopyridine, 2-thiopyrimidine, 2-thioquinoline, thiobenzene, and 2-thiothiophene) and S-methyl methanethiosulfonate were purchased from Sigma-Aldrich, Gillingham, U.K. The method of Kitson and Loomes15 was adapted as follows. The appropriate thiol (2.5 mmol) was dissolved in water (5 mL) containing NaOH (0.10 g, 2.5 mmol, 1 equiv). S-Methyl methanethiosulfonate (0.315 g, 2.5 mmol, 1 equiv) was then added, and the solution stirred for 1 h at room temperature. The cloudy suspension formed was extracted with dichloromethane (20 mL). The resulting dichloromethane solution was then dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to afford the pure disulfide.
2-(Methyldithio)pyridine (4)
pale yellow oil; UV (MeOH) λmax (log ε) 283.0 (3.66), 237.5 (3.93), 201.0 (3.82) nm; IR (film) νmax 1571, 1559, 1444, 1415, 1307, 1274, 1143, 1112, 1082, 1042, 985, 953, 755, 716 cm-1; 1H NMR (500 MHz, methanol-d4) δ 8.39 (1H, dt, J = 5.2, 4.8 Hz), 7.77 (2H, m), 7.17 (1H, dt, J = 9.6, 8.4 Hz), 2.47 (3H, s); 13C NMR (125 MHz, methanol-d4) 161.2 (s), 150.6 (d), 139.2 (d), 122.3 (d), 120.8 (d), 23.4 (q); HRESIMS m/z 158.0103 [M + H]+ (calcd for C6H7NS2 158.0098).
2-(Methyldithio)pyrimidine (5)
yellow oil; UV (MeOH) λmax (log ε) 238.5 (3.94) nm; IR (film) νmax 1558, 1546, 1372, 1189, 1166, 955, 800, 770, 741 cm-1; 1H (500 MHz, methanol-d4) δ 8.66 (2H, d, J = 4.8 Hz), 7.26 (t, J = 5.2, 4.8 Hz), 2.54 s (3H, s); 13C NMR (125 MHz, methanol-d4) δ 172.4 (s), 159.4 (d), 119.6 (d), 23.0 (q); HRESIMS m/z 159.0051 [M + H]+ (calc for C5H6N2S2 159.0051).
2-(Methyldithio)quinoline (6)
yellow oil; UV (MeOH) λmax (log ε) 334.0 (3.84), 325.0 (3.79), 250.5 (4.39), 212.0 (4.62) nm; IR (film) νmax 1615, 1582, 1553, 1492, 1447, 1291, 1137, 1103, 1087, 952, 939, 851, 815, 778, 744 cm-1; 1H NMR (500 MHz, CDCl3) δ 8.12 (1H, d, J = 8.8 Hz), 8.02 (1H, d, J = 8.4 Hz), 7.87 (1H, d, J = 8.8 Hz), 7.79 (1H, d, J = 8.4 Hz), 7.71 (1H, t, J = 8.4 Hz), 7.50 (1H, t, J = 8.0 Hz), 2.56 (3H, s); 13C NMR (125 MHz, CDCl3) δ 160.4 (s), 148.3 (s), 137.1 (d), 130.3 (d), 128.5 (d), 127.9 (d), 126.4 (s), 126.2 (d), 117.3 (d), 23.5 (q); HRESIMS m/z 208.0256 [M + H]+ (calcd for C10H10NS2 208.0255).
2-(Methyldithio)benzene (7)
pale yellow oil; UV (MeOH) λmax (log ε) 239.0 (3.56), 205.0 (3.56) nm; IR (film) νmax 1576, 1475, 1437, 1305, 1136, 1067, 1022, 951, 735, 686 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.43 (2H, dd, J = 5.2, 1.2 Hz), 7.25 (1H, dd, J = 3.6, 1.2 Hz), 7.00 (2H, dd, J = 5.2, 3.6 Hz), 2.55 (3H, s); 13C NMR (125 MHz, CDCl3) δ 137.1 (s), 129.2 (d), 127.7 (d), 127.0 (d), 23.1 (q); ESIMS m/z 156 [M + H]+ (calcd for C7H8S2 156).
2-(Methyldithio)thiophene (8)
orange oil; UV (MeOH) λmax (log ε) 311.5 (3.80), 240.0 (3.40), 203.5 (3.94) nm; IR (film) νmax 1397, 1327, 1303, 1250, 1214, 1133, 1083, 1023, 987, 950, 846, 742, 699 cm-1; 1H NMR (500 MHz, CDCl3) δ 7.55 (1H, dd, J) 8.4, 1.2 Hz), 7.35 (1H, t, J) 8.0 Hz), 7.26 (1H, bd, J) 7.2 Hz), 2.46 (3H, s); 13C NMR (125 MHz, CDCl3) δ 136.6 (s), 134.2 (d), 131.0 (d), 127.8 (d), 23.3 (q); ESIMS m/z 142 [M + H]+ (calcd for C6H6S2 142).
Antibacterial Assay with Fast-Growing Mycobacterium species and Staphylococcus aureus
Mycobacterium species were acquired from the NCTC, Salisbury, U.K. Strains were grown on Columbia blood agar (Oxoid) supplemented with 7% defibrinated horse blood (Oxoid) and incubated for 72 h at 37 °C prior to minimum inhibitory concentration (MIC) determination. S. aureus strains were the generous gift of Dr. Edet Udo (XU212), Prof. Glenn W. Kaatz (SA1199B), and Dr. Paul Stapleton (EMRSA-15). Antibacterial assays were performed as previously described.5,27 Ethambutol and isoniazid were used as positive controls for the mycobacteria, and norfloxacin and oxacillin were used as positive controls for the staphylococci.
Determination of MIC Value in M. bovis BCG and in M. tuberculosis Using Spot-Culture Growth Inhibition Assays
M. bovis BCG was cultured at 37 °C in an incubator with rotation at two revolutions per minute in Middlebrook 7H9 broth supplemented with 10% (v/v) albumin-dextrose-catalase (ADC; Difco) and 0.05% Tween 80 until the midexponential phase (OD600 of 1.0). M. tuberculosis H37Rv was grown at 37 °C in an incubator as standing culture in 30 mL unbreakable universals containing Middlebrook 7H9 broth supplemented with 10% (v/v) oleic acid-albumin-dextrose-catalase (OADC; Difco) and 0.05% Tween 80 with occasional stirring until the midexponential phase (OD600 of 0.6) was attained. For the quality control of the mycobacterial cultures, they were stained each time with a modified Ziehl-Neelsen staining protocol using a Tb-color kit, Bund Deutscher Hebammen Laboratory (Karlsruhe, Germany), followed by bright field microscopy.
To measure anaerobic cidal activity of the compounds, M. tuberculosis H37Rv (ATCC27294) was cultured in a self-generated oxygen-depletion model as described by Wayne28 using 19.5 × 145 mm tubes with a magnetic stirrer. Tubes were sealed with Teflon-lined caps and subsequently with paraplast and incubated for 3 weeks at 37 °C on a magnetic stirrer. The tubes were opened in an anaerobic chamber and diluted 10-fold into anaerobic Dubos medium, and 1 mL volumes were treated with various concentrations of the compound in 24-well plates. Control cultures were treated with DMSO. Positive and negative drug controls were metronidazole and isoniazid, respectively. The plates were sealed in anaerobic bags and incubated for 7 days at 37 °C. Serial dilutions were subsequently plated on 7H11 Middlebrook agar to monitor bacterial survival.
For the spot-culture growth inhibition assay,20 bacilli in the culture were serially diluted in order to give a final concentration of 103 cells/mL. Then, 5 μL of the diluted culture was spotted onto 5 mL of Middlebrook 7H10 agar medium, supplemented with 10% (v/v) OADC in a six-well plate containing various concentrations of compounds 1 and 2, and incubated at 37 °C for 14 days. A well with no compound as a positive control was also used. MIC was determined as the lowest concentration at which there is no growth.
Sulforhodamine B Short-Term Cytotoxicity Assay
Short-term growth inhibition was measured using the SRB assay as described previously.29 Briefly, cells were seeded (4000 cells/wells) into the wells of 96-well plates in DMEM and incubated overnight at 37 °C and 5% CO2 to allow the cells to attach. Subsequently, cells were exposed to freshly made solutions of compounds at increasing concentrations of 0.1 to 25 μM in quadruplicate and incubated for a further 96 h. Following this, the cells were fixed with ice-cold trichloroacetic acid (TCA) (10% w/v) for 30 min and stained with 0.4% SRB dissolved in 1% acetic acid for 15 min. All incubations were carried out at room temperature. The IC50 value, the concentration required to inhibit cell growth by 50%, was determined from the mean absorbance at 540 nm for each drug concentration expressed as a percentage of the control for untreated well absorbance.
Microarray Analysis
M. tuberculosis H37Rv (ATCC 27294) was grown in 7H9 Middlebrook medium supplemented with 0.2% glycerol, 0.5% bovine serum albumin fraction V, 0.05% Tween 80, 0.08% NaCl, and 0.2% glucose to an OD650nm of 0.3 and was treated with either 2, 5, or 10 μg/mL of compound 1 or an equivalent amount of DMSO for 6 h before harvesting cells for RNA isolation. RNA was isolated and labeled cDNA synthesized, and microarray hybridizations and microarray analysis were performed as described by Boshoff et al.30 Genes that were predictive for various treatment groups were determined by a class prediction software developed by Tibshirani et al.31
Maximum Tolerated Dose (MTD)
C57BL/6 female mice were orally administered (by gavage) a single dose of drug at 30, 100, or 300 mg/kg, using three mice per dose. Mice were observed postad-ministration at 4 and 6 h and then twice daily for the duration of the study (1 week).
Evaluation of in Vivo Efficacy against M. tuberculosis
Eight- to 10-week-old female specific-pathogen-free C57BL/6-Ifngtm1ts mice (gamma interferon gene-disrupted [GKO] mice) were purchased from Jackson Laboratories, Bar Harbor, ME. Mice were infected via low-dose aerosol exposure to M. tuberculosis Erdman using a Middlebrook aerosol generation device (Glas-Col Inc., Terre Haute, IN), and the short-course mouse model was performed as described previously. One day postinfection, three mice were sacrificed to verify the uptake of 50 to 100 CFU of bacteria per mouse. Negative control mice remained untreated. An isoniazid (INH) control group (isoniazid administered via oral gavage at 25 mg/kg/day) was included in each study. Each treatment group consisted of five mice. Treatment was started 14 days postinfection and continued for nine consecutive days. Five infected mice were killed at the start of treatment as pretreatment controls. Drugs were administered daily by oral gavage using 0.5% methyl cellulose (200 μL volume).
Statistical Analysis
The viable counts were converted to logarithms, which were then evaluated by a one-way ANOVA test, followed by a multiple comparison analysis of variance by a one-way Tukey test (SigmaStat software program). Differences were considered significant at the 95% level of confidence.
Acknowledgment
We thank the Engineering and Physical Sciences Research Council for the award of a doctoral scholarship to G.O.
Footnotes
Dedicated to Dr. David G. I. Kingston of Virginia Polytechnic Institute and State University for his pioneering work on bioactive natural products.
References and Notes
- (1).Cavallito CJ, Buck JS, Suter CM. J. Am. Chem. Soc. 1944;66:1950–1951. [Google Scholar]
- (2).Cavallito CJ, Bailey JH. J. Am. Chem. Soc. 1944;66:1952–1954. [Google Scholar]
- (3).Cavallito CJ, Bailey JH, Buck JS. J. Am. Chem. Soc. 1945;67:1032–1033. [Google Scholar]
- (4).Bolton SG, Null G, Troetel WM. J. Am. Pharm. Assoc. 1982;22:40–43. doi: 10.1016/s0160-3450(16)31735-4. [DOI] [PubMed] [Google Scholar]
- (5).O'Donnell G, Bucar F, Gibbons S. Phytochemistry. 2006;67:178–182. doi: 10.1016/j.phytochem.2005.10.023. [DOI] [PubMed] [Google Scholar]
- (6).O'Donnell G, Gibbons S. Phytother. Res. 2007;21:653–657. doi: 10.1002/ptr.2136. [DOI] [PubMed] [Google Scholar]
- (7).Keusgen M, Fritsch RM, Hisoriev H, Kurbonova PA, Khassanov FO. J. Ethnobiol. Ethnomed. 2006;2:18. doi: 10.1186/1746-4269-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gibbons S. Planta Med. 2008;74:594–602. doi: 10.1055/s-2008-1074518. [DOI] [PubMed] [Google Scholar]
- (9).Gibbons S. Nat. Prod. Rep. 2004;21:263–277. doi: 10.1039/b212695h. [DOI] [PubMed] [Google Scholar]
- (10).Bennett JW, Murray CK, Holmes RL, Patterson JE, Jorgensen JH. Diagn. Microbiol. Infect. Dis. 2008;60:437–40. doi: 10.1016/j.diagmicrobio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- (11).Schulte B, Heininger A, Autenrieth IB, Wolz C. Epidemiol. Infect. 2007;25:1–3. doi: 10.1017/S0950268807009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Smith JE, Tucker D, Watson K, Lloyd Jones G. J. Ethnopharmacol. 2007;112:386–393. doi: 10.1016/j.jep.2007.03.031. [DOI] [PubMed] [Google Scholar]
- (13).Zhu J, Wang M, Wu W, Ji Z, Hu Z. Phytochemistry. 2002;61:699–704. [Google Scholar]
- (14).Naznin MT, Akagawa M, Okukawa K, Maeda T, Morita N. Food Chem. 2008;106:1113–1119. [Google Scholar]
- (15).Kitson TM, Loomes KM. Anal. Biochem. 1985;146:429–430. doi: 10.1016/0003-2697(85)90563-9. [DOI] [PubMed] [Google Scholar]
- (16).Nakayama K, Hisada Y, Yamashita N. Jap. patent, JP 61056104. 1986 [Google Scholar]
- (17).Sumitomo Chemical Co., Ltd. Jap. patent, JP 59163368. 1984 [Google Scholar]
- (18).Nicholas GM, Blunt JW, Munro MHG. J. Nat. Prod. 2001;64:341–344. doi: 10.1021/np000408+. [DOI] [PubMed] [Google Scholar]
- (19).Johnson AP, Aucken HM, Cavendish S, Ganner M, Wale MCJ, Warner M, Livermore DM, Cookson BD. J. Antimicrob. Chemother. 2001;48:143–144. doi: 10.1093/jac/48.1.143. [DOI] [PubMed] [Google Scholar]
- (20).Madikane VE, Bhakta S, Russell AJ, Campbell WE, Claridge TD, Elisha BG, Davies SG, Smith P, Sim E. Bioorg. Med. Chem. 2007;15:3579–3586. doi: 10.1016/j.bmc.2007.02.011. [DOI] [PubMed] [Google Scholar]
- (21).Zimhony O, Cox JS, Welch JT, Vilchèze C, Jacobs WR., Jr. Nat. Med. 2000;6:1043–1047. doi: 10.1038/79558. [DOI] [PubMed] [Google Scholar]
- (22).Gande R, Dover LG, Krumbach K, Besra GS, Sahm H, Oikawa T, Eggeling L. J. Bacteriol. 2007;189:5257–5264. doi: 10.1128/JB.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Choi KH, Kremer L, Besra GS, Rock CO. J. Biol. Chem. 2000;275:28201–28207. doi: 10.1074/jbc.M003241200. [DOI] [PubMed] [Google Scholar]
- (24).He X, Reynolds KA. Antimicrob. Agents Chemother. 2002;46:1310–1318. doi: 10.1128/AAC.46.5.1310-1318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Stewart GR, Wernisch L, Stabler R, Mangan JA, Hinds J, Laing KG, Young DB, Butcher PD. Microbiology. 2002;148:3129–3138. doi: 10.1099/00221287-148-10-3129. [DOI] [PubMed] [Google Scholar]
- (26).Lenaerts AJM, Gruppo V, Brooks JV, Orme IM. Antimicrob. Agents Chemother. 2003;47:783–785. doi: 10.1128/AAC.47.2.783-785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gibbons S, Udo EE. Phytother. Res. 2000;14:139–140. doi: 10.1002/(sici)1099-1573(200003)14:2<139::aid-ptr608>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- (28).Wayne LG. In: Mycobacterium Tuberculosis Protocols. Parish T, Stoker NG, editors. Humana Press; Totowa, NJ: 2001. pp. 247–270. [Google Scholar]
- (29).Moore MJ, Schultes CM, Cuesta J, Cuenca F, Gunaratnam M, Tanious FA, Wilson WD, Neidle S. J. Med. Chem. 2006;49:582–599. doi: 10.1021/jm050555a. [DOI] [PubMed] [Google Scholar]
- (30).Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE., III J. Biol. Chem. 2004;279:40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- (31).Tibshirani R, Hastie T, Narashiman B, Chu G. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]