Abstract
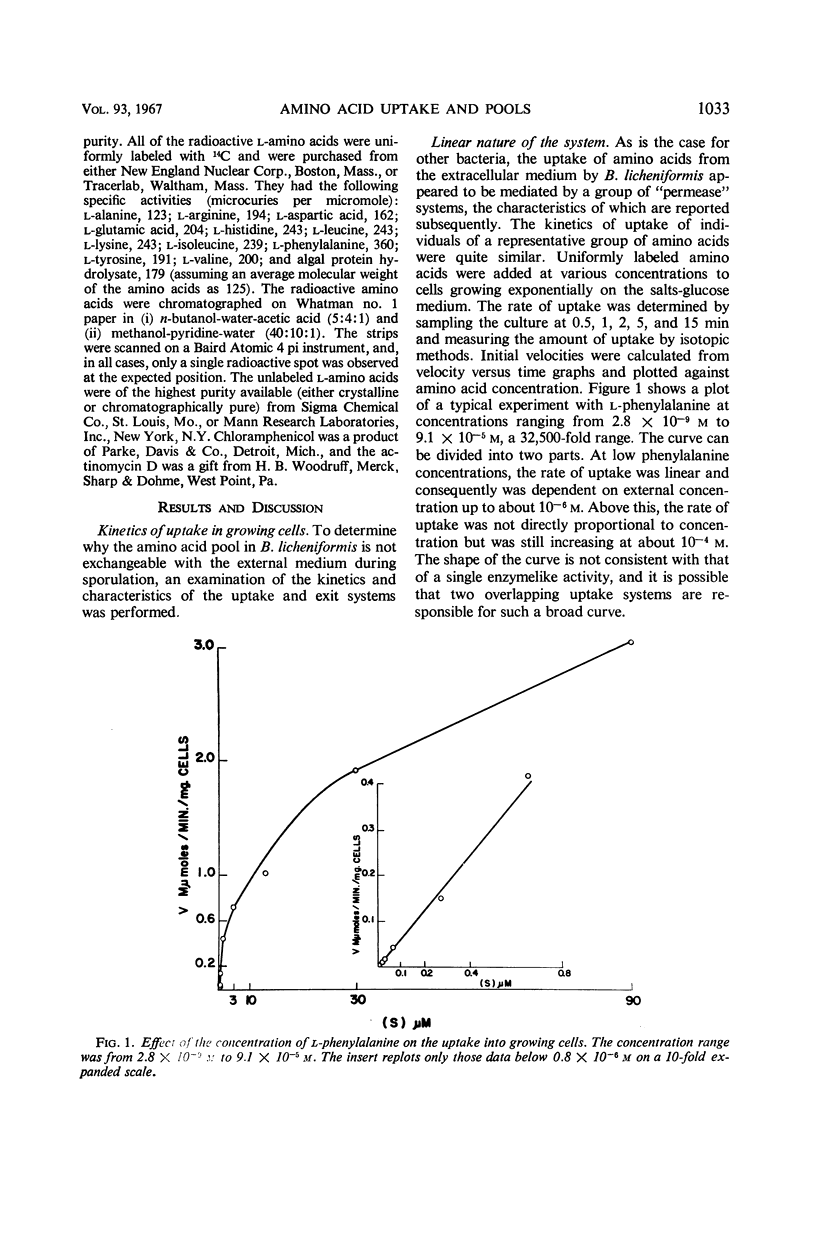
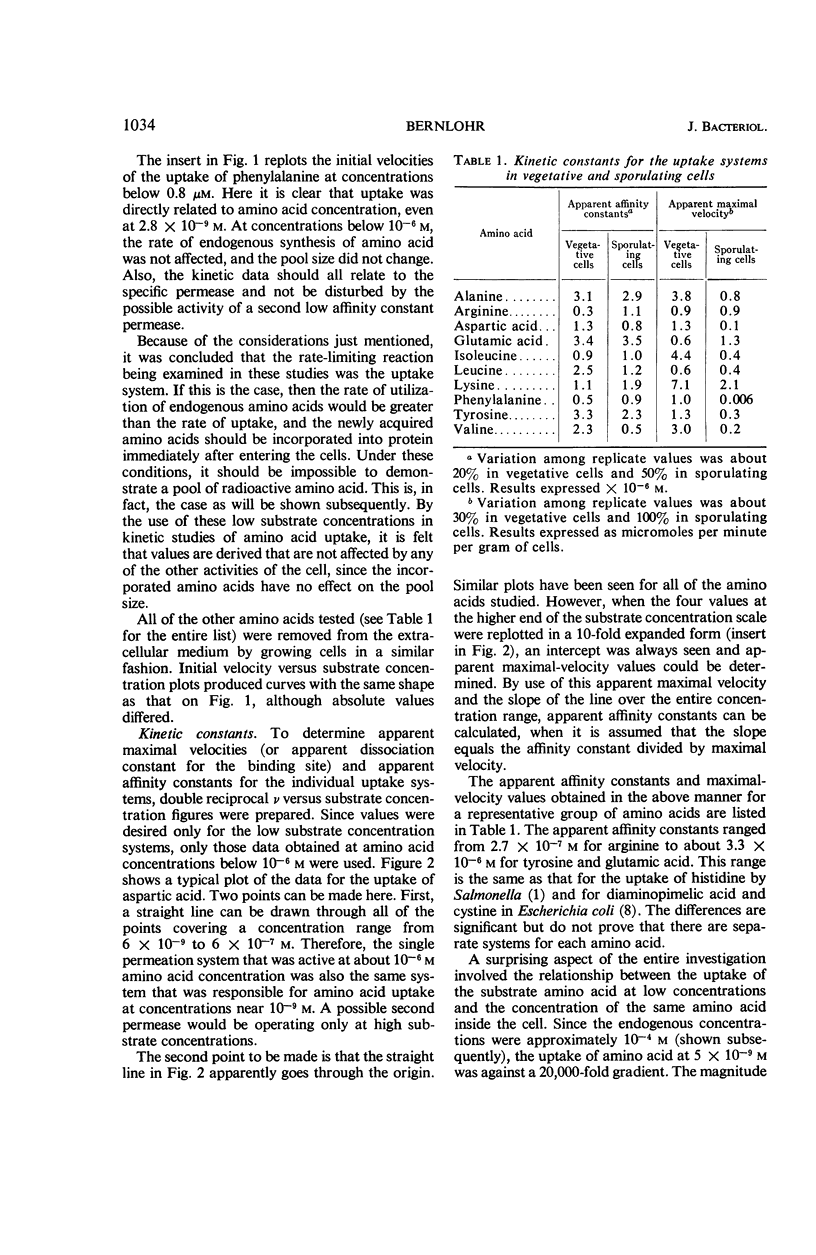
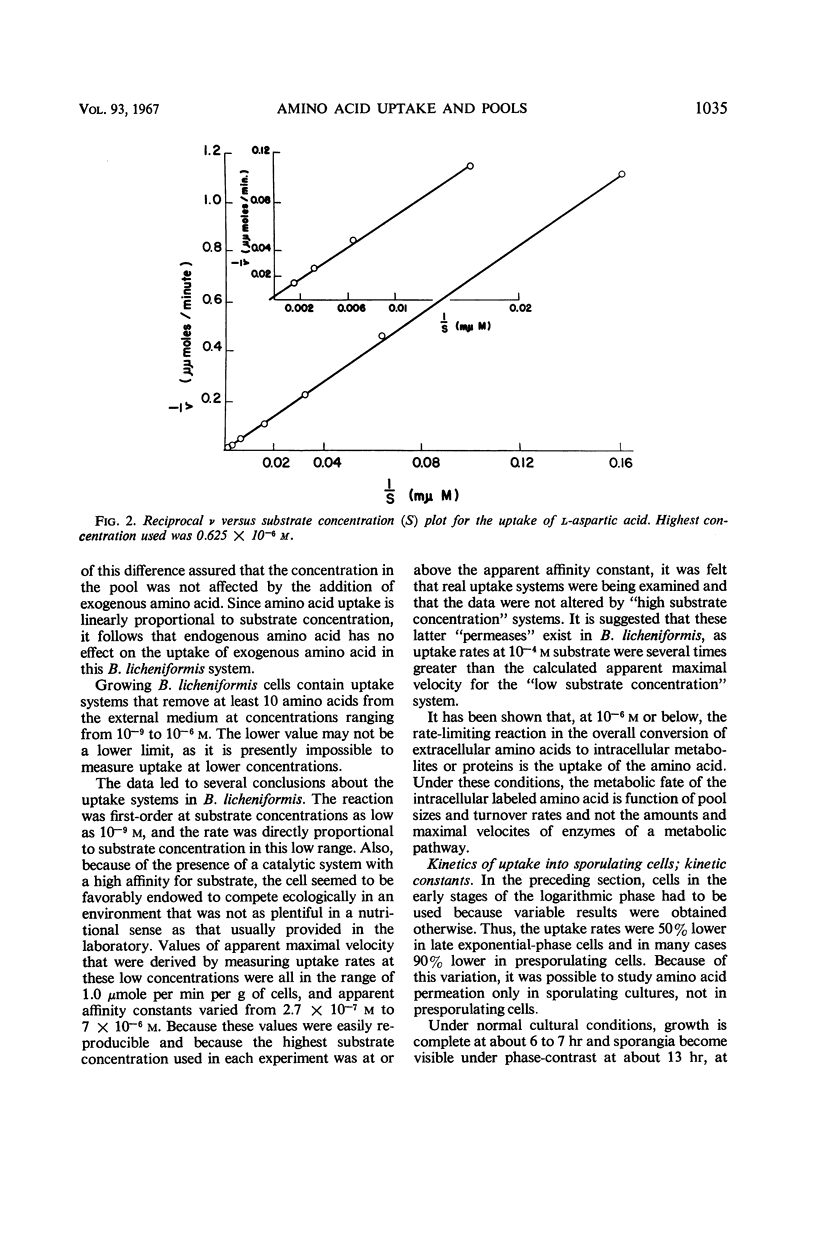
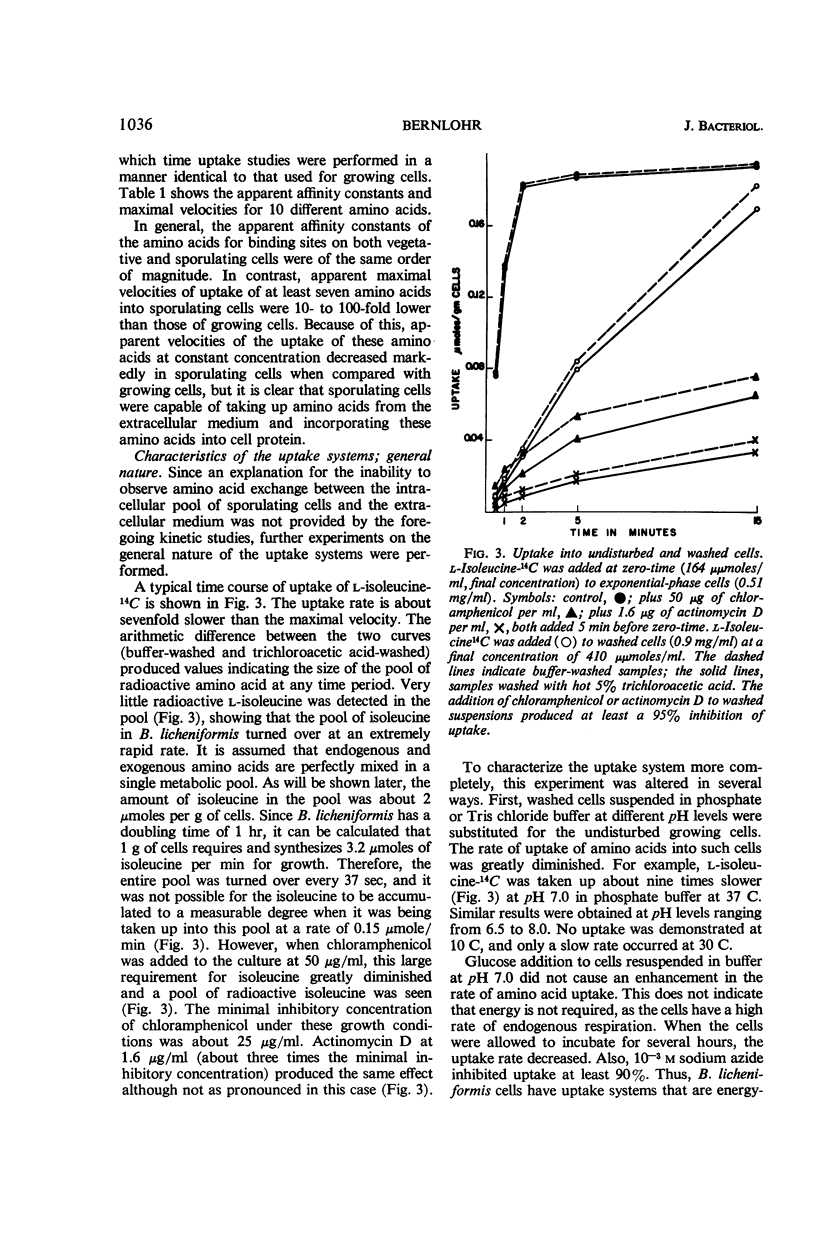
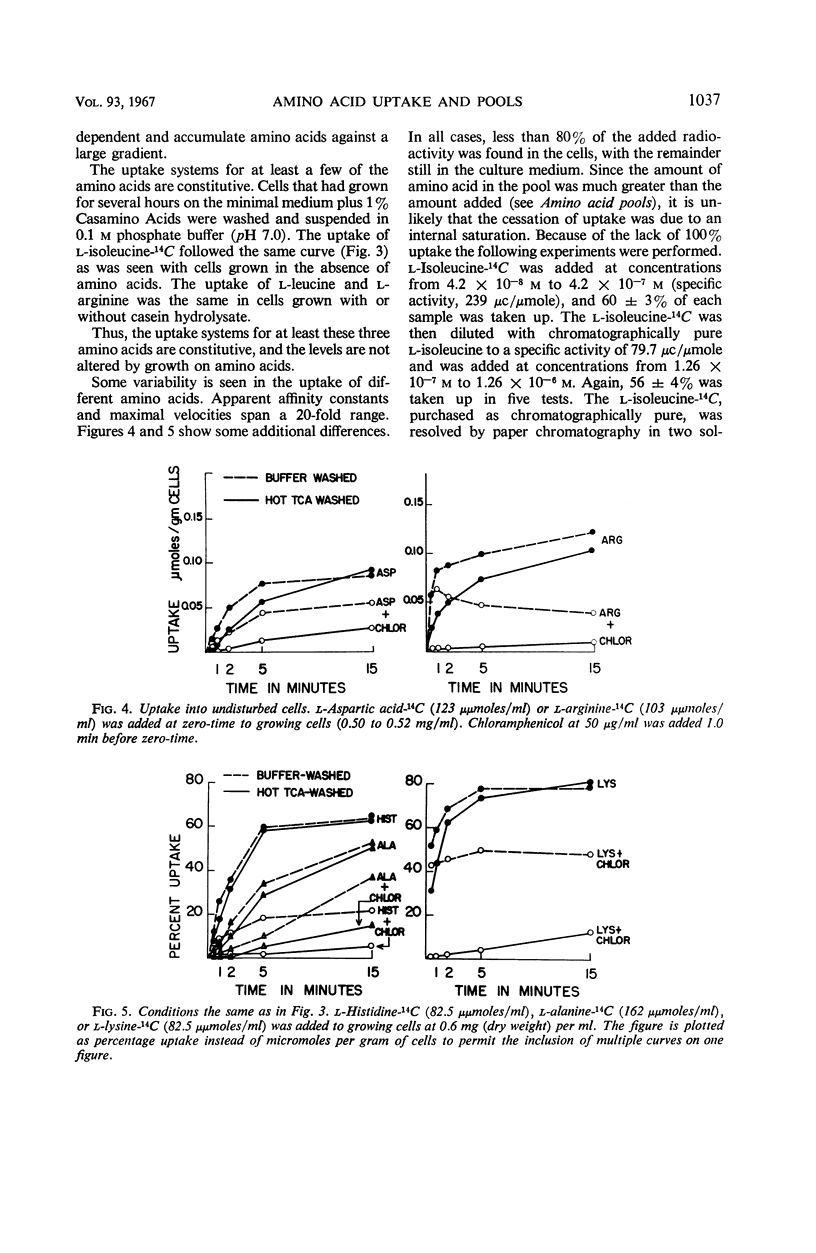
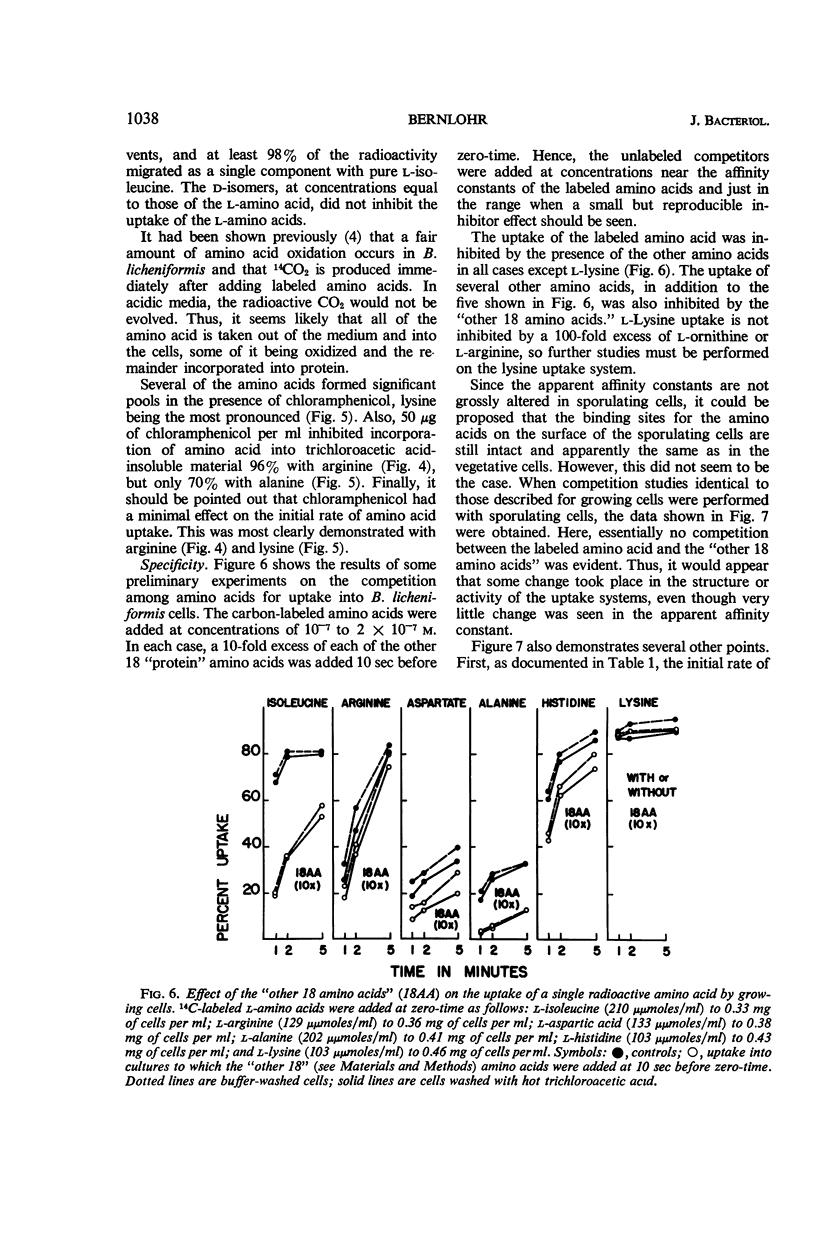
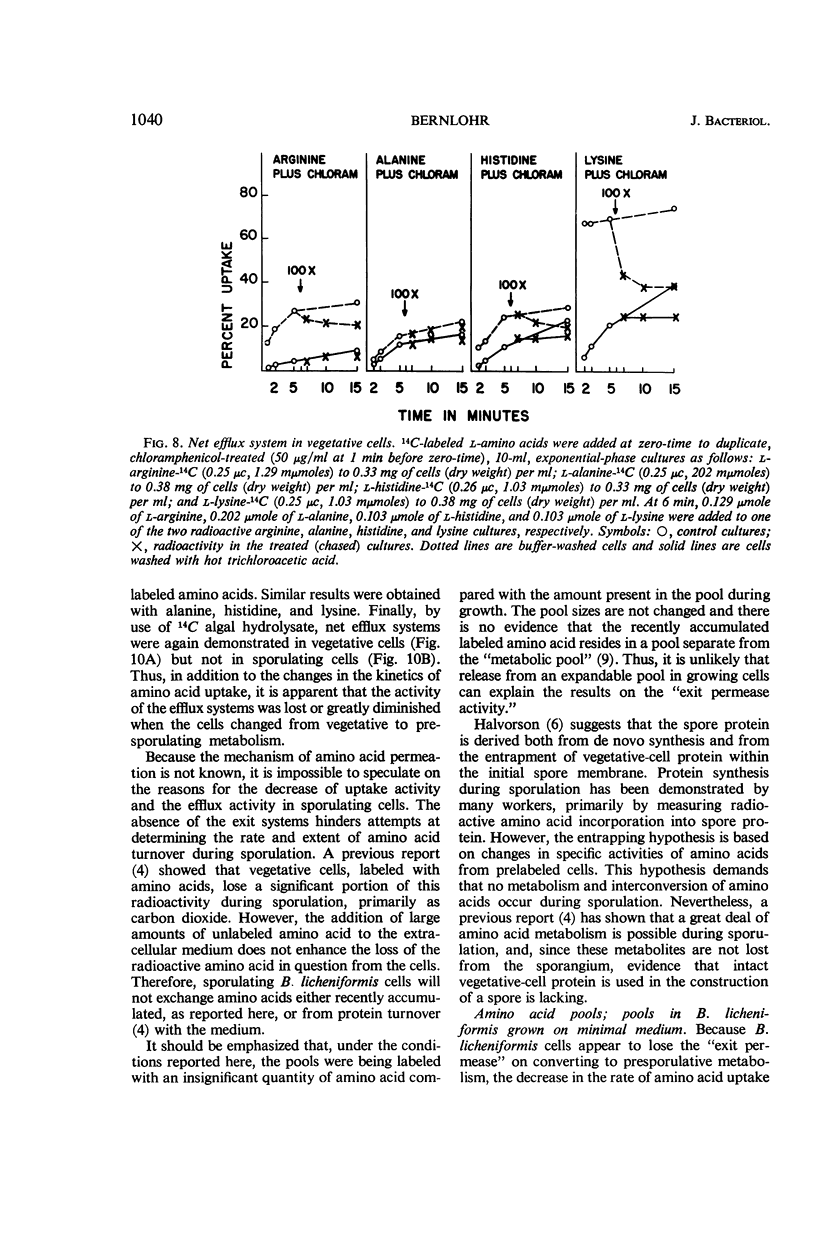
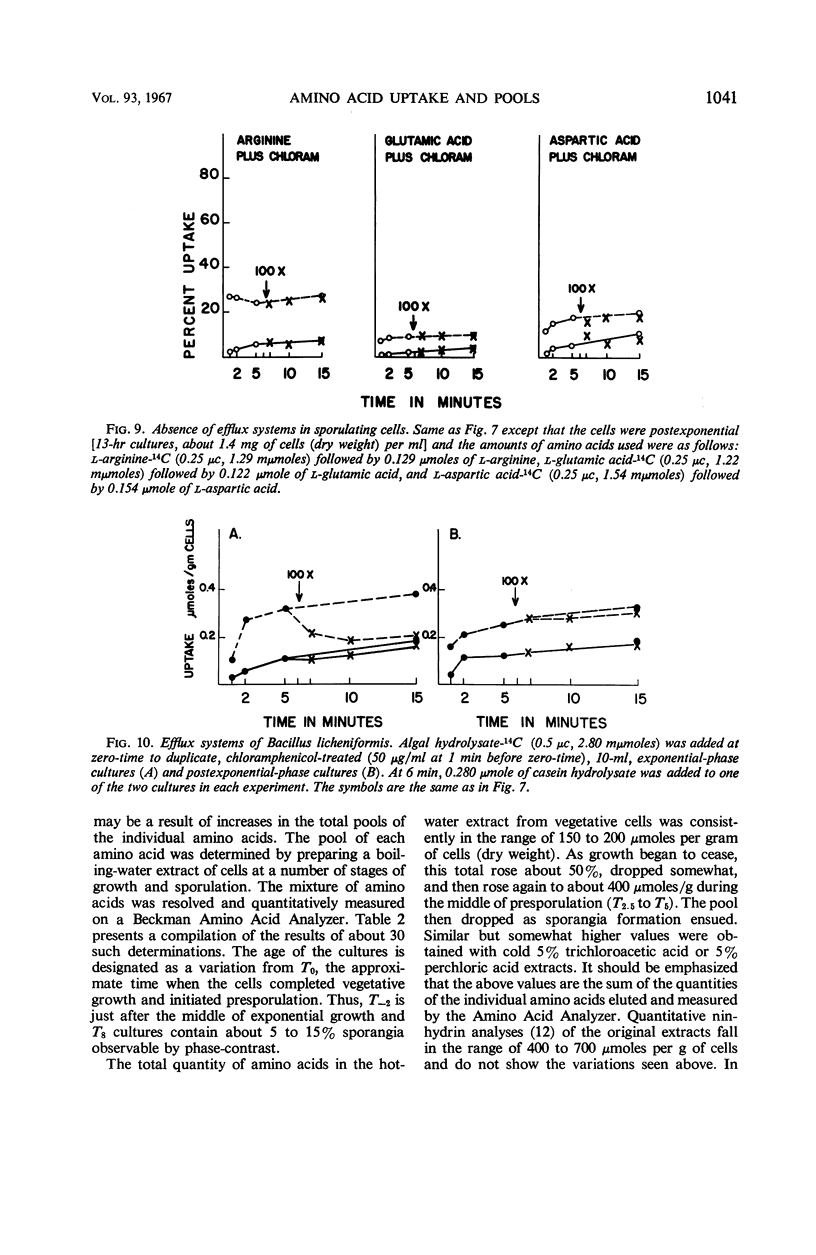
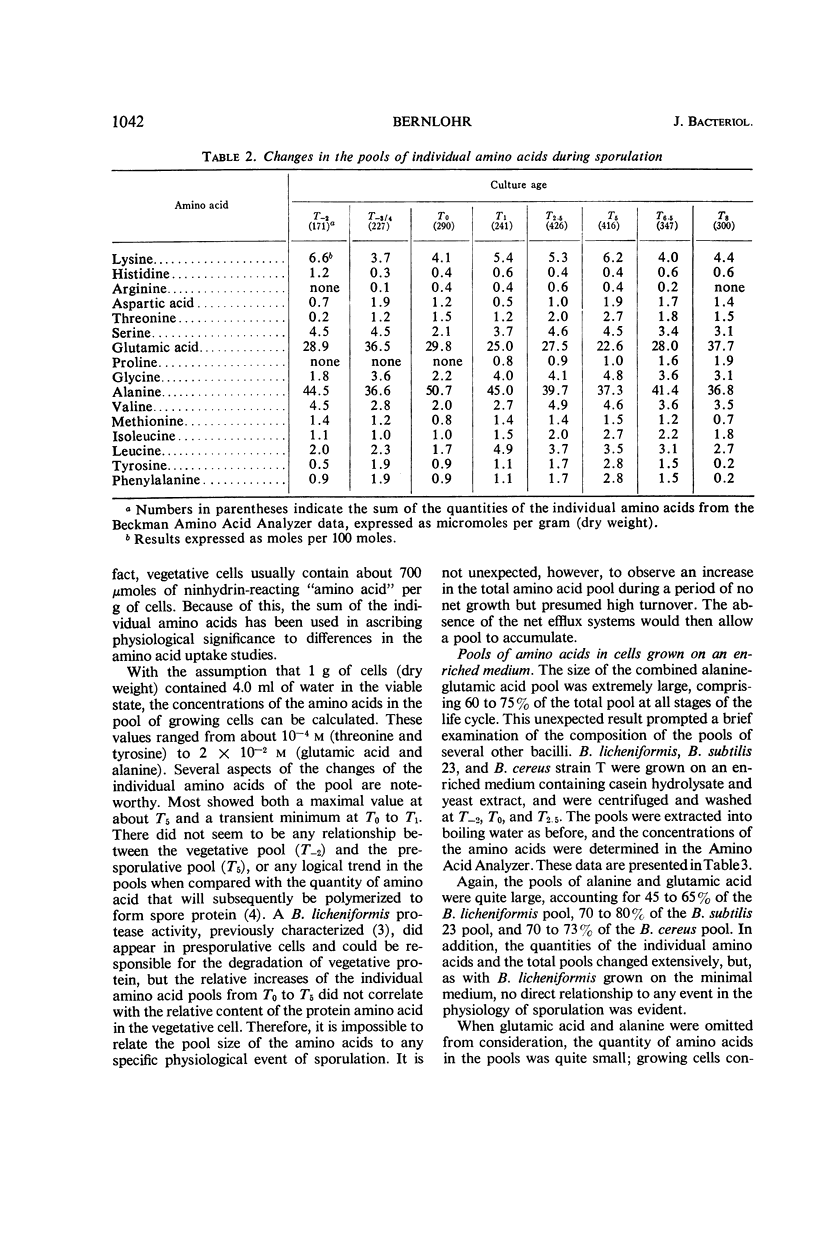
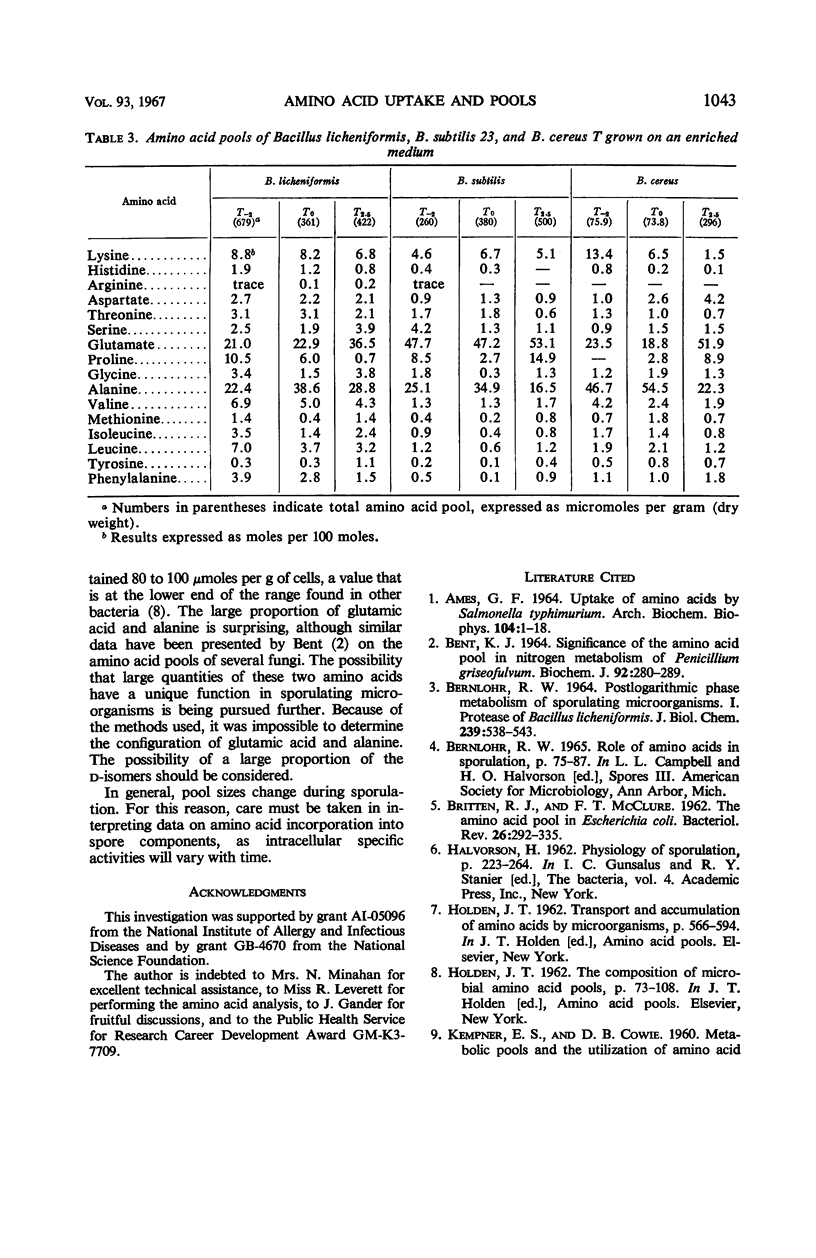
Changes in amino acid uptake in Bacillus licheniformis and in the amino acid pools of three Bacillus species were investigated, by use of cells from different stages of the life cycle. B. licheniformis contains catalytic uptake systems for all of the 10 amino acids studied. The apparent maximal velocities of uptake decreased during sporulation but did not fall below the range observed for other microorganisms. In sporulating cells, the apparent affinity constants of the uptake systems for individual amino acids remained about the same as in growing cells, i.e., from 2 × 10−7m to 7 × 10−6m, whereas, in some cases, the apparent maximal velocities decreased significantly. Because the velocity of uptake showed an atypical dependence on substrate concentration, it was postulated that these cells contain two or more uptake systems for each amino acid. Only one of these systems appeared to be operative at a substrate concentration below 10−6m. Working at these low substrate concentrations, catalytic activities producing a net efflux of amino acids were demonstrable in vegetative cells in the presence of chloramphenicol, but these exit systems were lost during sporulation. A pool formed by the addition of radioactive algal hydrolysate will exchange with the external medium in vegetative cells but not in sporulating cells. Glutamic acid and alanine comprise at least 60% of the amino acid pool of B. licheniformis A-5, B. subtilis 23, and B. cereus T during all stages of growth and sporulation. The concentrations of the other amino acids in the pool varied extensively, but reflected, in general, the amino acid turnover known to occur during sporulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent K. J. Significance of the amino acid pool in nitrogen metabolism of Penicillium griseofulvum. Biochem J. 1964 Aug;92(2):280–289. doi: 10.1042/bj0920280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMPNER E. S., COWIE D. B. Metabolic pools and the utilization of amino acid analogs for protein synthesis. Biochim Biophys Acta. 1960 Aug 26;42:401–408. doi: 10.1016/0006-3002(60)90817-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Davis B. D. The transport of diaminopimelate and cystine in Escherichia coli. J Biol Chem. 1965 Nov;240(11):4362–4369. [PubMed] [Google Scholar]
- TROLL W., CANNAN R. K. A modified photometric ninhydrin method for the analysis of amino and imino acids. J Biol Chem. 1953 Feb;200(2):803–811. [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. II. Spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:483–498. doi: 10.1083/jcb.6.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]


