Abstract
The in vivo hollow fiber assay was developed at the National Cancer Institute (NCI) to help bridge the gap between in vitro cell-based assays and human tumor models propagated in immunodeficient mice. The goal was to develop an intermediate assay that could help predict which compounds found active in the 60-cell line panel would be active in a subsequent xenograft system. This was necessary due to the high cost of the traditional xenograft assay in terms of number of animals required, time for assay completion, and financial commitment necessary. To address this problem, investigators of the NCI Developmental Therapeutics Program designed a method of propagating human cancer cells in inert hollow fibers with pores small enough to retain the cancer cells but large enough to permit entry of potential chemotherapeutic drugs, including large proteins and other important substances. Fibers containing proliferating cancer cells are transplanted into the peritoneum or under the skin, the host mice are treated with a test agent and the fibers are subsequently retrieved for analysis of viable cell mass. The assay has been successful in helping investigators from around the world, including our own research group, prioritize compounds active in vitro for further testing in the traditional xenograft system.
Introduction
The fine art of anticancer drug discovery has evolved over time from the serendipitous findings of keenly observant investigators, through empirical animal models of cancer, to today’s rational design of agents that affect exquisitely molecular targets vital to cancer cell survival. Perhaps the most famous example of serendipity in anticancer drug discovery was the effect of the sulfur mustards on white blood cells. During World War I, front line physicians such as Edward B. Krumbhaar observed that, in addition to the known lethal vesicant action caused by mustard gas, exposed soldiers also showed dramatic signs of leukopenia.1,2 Based on these surprising findings, the U.S. Army invested heavily in research on these compounds both for use in chemical warfare and as potential antileukemia drugs.3 This work laid the foundation for the development of modern nitrogen mustards used in the clinic today. It was an ironic twist of fate that one of the first weapons of mass destruction would give rise to one of the first successful agents for the treatment of cancer. Arguably, this is the very antithesis of rational drug design.
Napoleon’s apocryphal assessment of generals notwithstanding, luck is not the most important predictor of future success on the battlefield or in the field of cancer research. Therefore, a rational approach is the cornerstone upon which modern drug discovery programs are built. When the Cancer Chemotherapy National Service Center (CCNSC) was established at the National Cancer Institute (NCI) in 1955, an empirical approach was adopted to screen materials. During the early years, a variety of transplantable models of murine cancer were employed, but by the late 1960s, the majority of natural products screening was conducted with P388 and L1210 murine lymphocytic leukemias. However, some cautioned that relying on rapidly growing rodent leukemias during the screening process might select for compounds that were active only against rapidly growing tumors.4 Detractors pointed to the limited variety of tumor types, rapid growth rate and to the fact that these sorts of models had only identified about 35 new drugs, primarily alkylating agents, from the mid 1950s to the mid 1980s.5–7
During the late 1980s, Boyd and colleagues argued for a fundamental change in the approach of the NCI for anticancer drug discovery.8,9 The idea was to shift away from the previous strategy in which chemical diversity was emphasized while the scope of the biological assays was relatively limited. This strategy was dubbed the “compound-oriented” approach and was successful at discovering agents that affected pathways important to all cancer types (e.g., DNA and protein metabolism or mitosis). However, Boyd et al. hypothesized that many of these leads failed in the clinic because the tumors in patients are far more diverse than the few rodent tumor models then employed as screens.10 He suggested that a “disease-oriented” approach, in which candidate compounds are tested against a wide array of human cancer cell types, might be more successful. Thus was born the 60 human cancer cell line panel for primary drug screening, which currently includes lines representing leukemia, melanoma and cancers of the lung, colon, brain, ovary, breast, prostate, and kidney.8,9 The collective activity pattern of a compound against each of the 60 cell lines constitutes its activity profile or “fingerprint,” which can be queried against the archived profiles of previously tested compounds using the COMPARE (COMputerized, PAttern REcognition) algorithm.11 COMPARE analysis can provide important clues to the mechanism of action of a new agent. For example, a test compound found to have a similar activity fingerprint to a known drug may share a similar mechanism of action or cellular target. Conversely, test substances with a unique activity fingerprint may have a unique mechanism of action.
Before the advent of the hollow fiber assay, compounds found active in the 60-cell panel were then evaluated in the human xenograft assay. Along with establishing in vitro test parameters for the 60 cell lines used in the screen, these same lines were studied for their ability to form tumor xenografts in immunodeficient mice. During the 1970s and ‘80s, assays for grafting human cancer cells into immunodeficient mice were established in the nude mouse, which had recently been discovered, and in the SCID mouse, which was described in 1983.12,13 Monitoring the growth of human cancers propagated in immunodeficient mice has since become an important tool to study the anticancer activities of candidate chemotherapeutic agents.14 Human tumors transplanted to immunodeficient mice grow readily without the need for immunosuppressive treatments and develop into tumors that reflect the histologic appearance, karyotype and molecular pathology of the donor patient’s tumor.14,15 In addition, cells isolated from human xenografts and donor patient’s tumors show similar treatment sensitivities in vitro. Human xenografts also show organ-specific metastatic patterns similar to those of the donor patient’s tumor.16 Therefore, compared to the rodent models, human xenografts offered superior biological diversity necessary for a disease-oriented screen.
Compounds found active in the 60-cell line panel were then tested for activity in the xenograft models using tumors that had been derived from the lines showing the most activity in the in vitro screen. Since the in vitro screen is so much more rapid than the associated xenograft assay, a backlog of in vitro active compounds accrued. This problem underscored the need for a means by which these active compounds could be prioritized for the in vivo assay. Hollingshead and colleagues solved this dilemma by developing the hollow fiber assay, which serves as a bridge between the in vitro screen and the xenograft assay. The same cell lines are used in all three assays. The hollow fiber assay is similar to the in vitro screen in that the assay is rapid and relatively inexpensive. The hollow fiber assay is similar to the xenograft assay in that the cells are propagated and treated in a mouse with all of the associated pharmacokinetic, pharmacodynamic and toxicologic dimensions of an in vivo assay. Furthermore, the hollow fiber assay allows drug metabolism to have a role in the activity determined. Thus, the hollow fiber assay serves as a filter to help investigators rapidly and economically select the best leads for further analysis in the xenograft assay, which is costly in terms of time, money, animals and compound quantity required. Also, the assay allows investigators to abide by the 3Rs - the ‘replacement’ ‘refinement’ and ‘reduction’ of animal use, by decreasing the assay time (refinement) and the number of animals (reduction) needed for drug discovery.17–19 Below is a brief description of our experience with the assay, other uses for this versatile assay outside cancer drug discovery and some thoughts on future applications.
In Vivo Hollow Fiber Assay as a Tool for Anticancer Drug Discovery at UIC
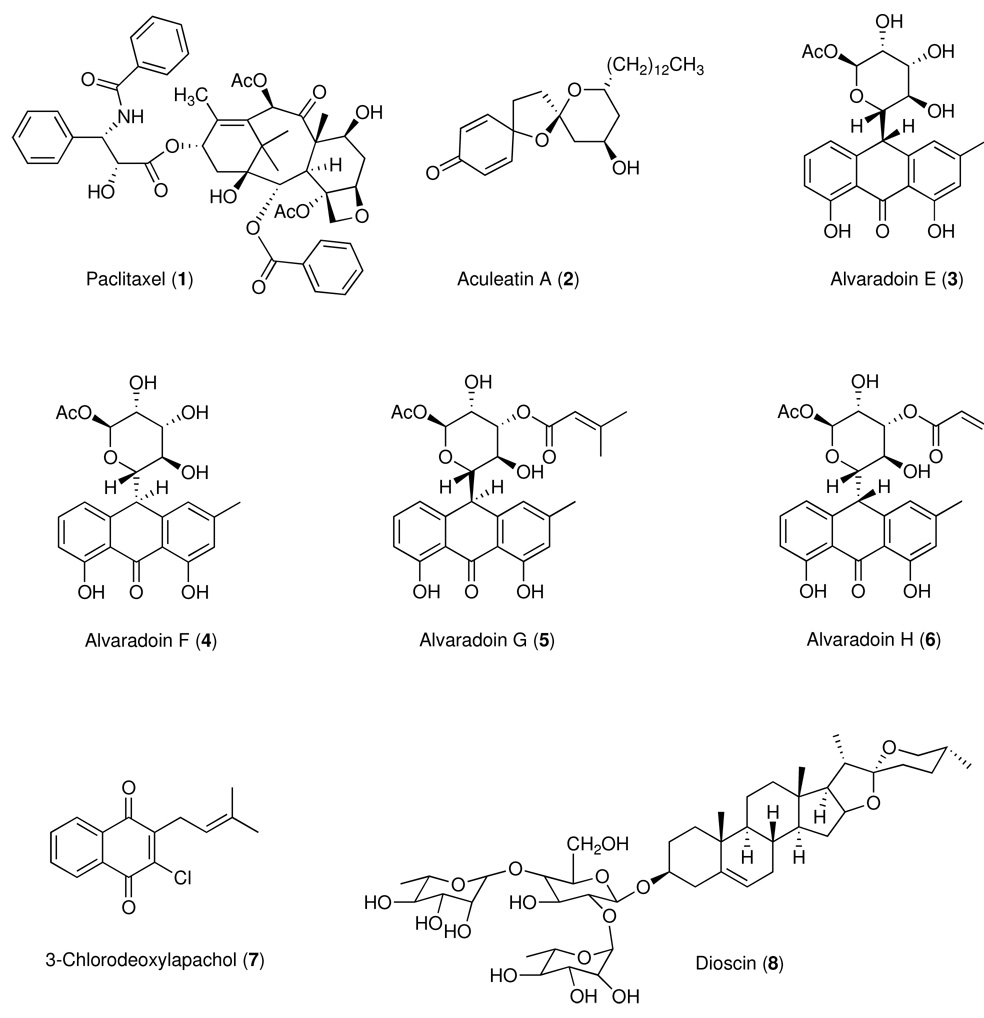
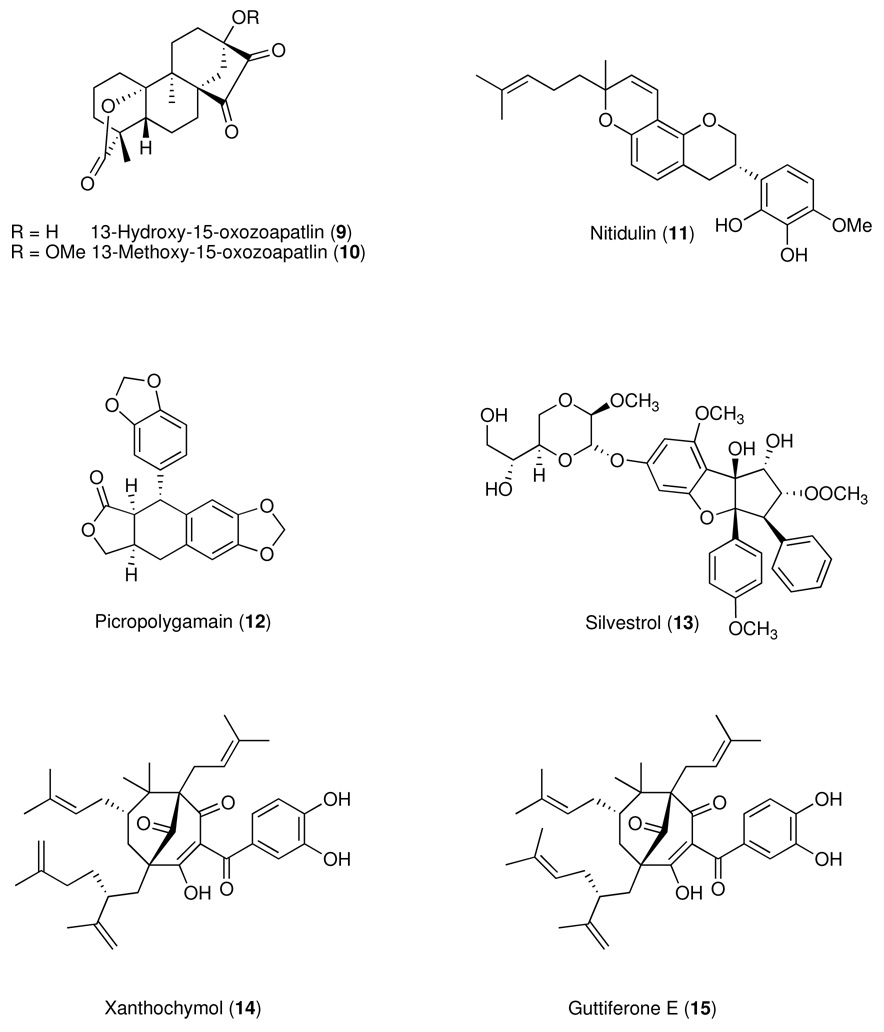
Between the period 1990–2005, our anticancer drug discovery effort was supported by the National Cooperative Drug Discovery Group (NCDDG) program of the U.S. National Cancer Institute (NCI), National Institutes of Health, which fosters broad, multi-disciplinary approaches to the discovery of new, synthetic or natural-source derived anticancer drugs.20 Our research group has consisted of teams from The Ohio State University (OSU), the University of Illinois at Chicago (UIC), Research Triangle Institute (RTI), Research Triangle Park, North Carolina, and Glaxo Medicines Research Centre, Stevenage, U.K. (for the period 1990–1995) followed by Bristol-Myers Squibb (B-MS), Pharmaceutical Research Institute, Wallingford, Connecticut and Princeton, New Jersey (1995–2005). While our original effort was focused exclusively on plant sources, we have recently substantially revised our strategy and now include cyanobacteria and filamentous fungi as source materials for our discovery project, which is currently supported by the program project (P01) grant award mechanism of the NCI (2007–2012). Mycosynthetix, Inc. of Hillsborough, NC has recently joined our consortium and will provide the filamentous fungi for analysis and biological evaluation. The overall goal of the integrated studies is to discover novel chemicals for development as cancer chemotherapeutic agents, particularly for tumors that cannot be cured by present treatment methods. Some of the compounds with activity in the hollow fiber assay that we have reported over the years are summarized in Table 1 and Chart 1.
Table 1.
Growth Inhibitory Effects of Test Substances with Activity in the Hollow Fiber Modela
| Compound | Cell Lines | In vitro ED50 µg/ml (µM or as indicated)b | Selected In Vivo Dosesc (mg/kg) | Growth Inhibition (%) | References | |||
|---|---|---|---|---|---|---|---|---|
| ip | sc | |||||||
| 1 | Paclitaxel | KB | 0.020 | (0.023) | 3.75, 7, 15, 30 | 70 – 85d | 20 – 27e | Mi et al.24 |
| SW626 | 10 pg/ml | (12 pM) | 98 – 100d | 0 – 22e | ||||
| 2 | Aculeatin A | MCF-7 | 6.25, 12.5, 25,50 | 10 – 58d | 0e | Chin et al.34 | ||
| 3 | Alvaradoin E | KB | 0.10 | (0.23) | 0.195, 0.39,0.78, 1.56 | 40 – 58d | 5 – 11e | Mi et al.29 |
| LNCaP | 0.06 | (0.14) | 60 – 80d | 10 – 19e | ||||
| Col2 | 0.10 | (0.23) | 31 – 74d | 6 – 16e | ||||
| 4 | Alvaradoin F | KB | 0.10 | (0.23) | 0.195, 0.39, 0.78, 1.56 | 32 – 58d | 2 – 18e | Mi et al.29 |
| LNCaP | 0.05 | (0.12) | 59 – 72d | 3 – 15e | ||||
| Col2 | 0.10 | (0.23) | 21 – 72d | 1 – 10e | ||||
| 5 | Alvaradoin G | KB | 0.20 | (0.39) | 6.25, 12.5, 25 | 79 – 85d | 13 – 60d | Phifer et al.90 |
| LNCaP | 0.15 | (0.29) | 52 – 78d | 24 – 58d | ||||
| Col2 | 0.96 | (1.3) | 84 – 86d | 0 – 24e | ||||
| 6 | Alvaradoin H | KB | 0.15 | (0.29) | 0.78, 1.56, 3.125 | 43 – 58d | 1 – 28e | Phifer et al.91 |
| LNCaP | 0.15 | (0.29) | 47 – 67d | 10 – 16e | ||||
| 7 | 3-Chlorodeoxylapachol | KB | 3.2 | (12) | 6.25, 12.5, 25,50,100 | 0 – 65d | 45 – 48d | Jones et al.92 |
| 8 | Dioscin | Lu1 | 1.0 | (1.2) | 6.2, 12.5, 25 | 25 – 95d | 0 – 2e | Mi et al.24 |
| LNCaP | 1.5 | (1.7) | 37 – 72d | 36 – 45e | ||||
| KB | 18 | (21) | 79 – 100d | 4 – 48e | ||||
| 9 | 13-Hydroxy-15-oxozoapatlin | KB | 1.2 | (3.6) | 25, 50, 75, 100 | 0 – 69d | 0e | Braca et al.93 |
| LNCaP | 1.5 | (4.5) | 0 – 88d | 0 – 2e | ||||
| 10 | 13-Methoxy-15-oxozoapatlin | SW626 | 0.20 | (0.58) | 25, 50, 100 | 0 – 92d | 16 – 60d | Mi et al.24 |
| LNCaP | 0.40 | (1.2) | 50 – 58d | 0e | ||||
| Mel2 | 0.40 | (1.2) | 15 – 85d | 11 – 60d | ||||
| KB | 2.2 | (6.4) | 21 – 100d | 6 – 45d | ||||
| MCF-7 | 0.30 | (0.87) | 34 – 89d | 0 – 35e | ||||
| 11 | Nitidulin | LNCaP | 4.1 | (9.7) | 10, 20, 40 | 54 – 59d | 0 – 18e | Chin et al.31 |
| 12 | Picropolygamain | LNCaP | 1.1 | (3.1) | 5, 10, 20 | 21 – 53d | 3 – 34e | Rivero-Cruz et al.94 |
| 13 | Silvestrol | LU1 | 0.79 ng/ml | (1.2 nM) | 0.625, 1.25, 2.5, 5 | 12 – 63d | 0 – 27e | Hwang et al.28 |
| LNCaP | 0.98ng/ml | (1.5 nM) | 15 – 83d | 12 – 16e | ||||
| MCF-7 | 0.98 ng/ml | (1.5 nM) | 20 – 77d | 5 – 23e | ||||
| 14 | Xanthochymolf | LNCaP | 4.8 | (8.0) | 12.5, 25 | 44 – 60d | 65 – 66d | Kim et al.95 |
| 15 | Guttiferone Ef | |||||||
Animals were treated with PBS (control) or the indicated doses of test substance, once daily by intraperitoneal injection from day 3–6 after implantation. On day 7, mice were sacrificed, and fibers were retrieved and analyzed by MTT assay. Results are shown as the average percentage cell growth inhibition relative to control.
Doses are expressed as µg/ml or µM unless specified otherwise. All incubations with compound were 72 h in duration.
Doses were selected as described previously.24
Statistical significance achieved with one or more dose levels.
Statistical significance not achieved with one or more dose levels.
These compounds occurred in their plant of origin as an inseparable mixture, as reported by others.96
Chart 1.
Descriptions of our collaborative work with the former NCDDG project have been published.21,22 Our original approach to screening of natural product extracts has mirrored the strategy used at the NCI, which relied heavily on cell proliferation/cytotoxicity assays and human tumor xenograft approaches. When the hollow fiber assay was developed at the NCI by Hollingshead et al.23 we adopted it for use in our drug discovery program to help us prioritize leads for subsequent analysis in the traditional xenograft models.24–34 We have established growth conditions for cells implanted at the intraperitoneal (ip) and subcutaneous (sc) compartments of athymic mice. These lines include the human cancer cells designated HL-60 (leukemia), HUVEC (umbilical endothelium), Ishikawa (endometrium), KB, KB-V1 (both epidermal), LNCaP (prostate), Lu1 (lung), MCF-7 (breast), Mel2 (melanoma), SW626 (ovary) and the murine leukemia line designated P-388. Several laboratories have published excellent reviews on the technology and methodology of the assay23,35–40 and the NCI Developmental Therapeutics Program Web site has a detailed experimental protocol.41
Many different types of cell lines are amenable for use in the hollow fiber assay, and this versatility is a major strength of the procedure. We have used adherent lines, but cell lines propagated in suspension also work well. One major criterion is that the cell line is tumorigenic in immunodeficient mice, which will permit follow up studies using the traditional xenograft assay. Also, the line must exhibit a minimum proliferation rate within the fibers over the course of culture in the mouse such that significant differences in cell mass can be observed over the course of the assay. A range of cell sizes and proliferation rates among lines can be accommodated by simply altering the number of cells seeded into the fibers. Hollingshead and colleagues have established the optimal seeding conditions for many of the cell lines of the 60-cell line panel used for screening at the NCI.39 We have also optimized the seeding density for the cells used on our projects.24 The range for seeding the fibers is usually between 2 × 106 and 10 × 106 cells per ml, which translates into about 3 × 104 to 60 × 104 cells per fiber that is 1mm in inner diameter and 2 cm long.
As is the practice at the NCI, we propagate all our cells in RPMI-1640 medium supplemented with fetal bovine serum (5% vol/vol) and 2 mM glutamine at 37 °C in a 5% CO2atmosphere. Cells in late log-phase growth are released from the plastic dish by brief digestion with trypsin, washed and suspended in medium supplemented with fetal bovine serum (to 5% vol/vol) at the seeding density predetermined as optimal for the line. The cells are then gently infused into sterile conditioned23 polyvinylidene fluoride hollow fibers that have a molecular weight exclusion of about 500 kDa. The fibers are then heat sealed at two-cm intervals and cut in the middle of the seals to generate the fibers for study. Prior to implantation, the fibers are cultured overnight at 37 °C in a 5% CO2 atmosphere. On the following day (designated day zero of the assay) a set of fibers representative of each cell line under test is evaluated for viable cell mass by a modified MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay.42 Another set of fibers remains in culture to confirm sterility. The largest group of fibers is transplanted into immunodeficient hosts (we use male and female NCr nu/nu mice). For intraperitoneal implants, a small incision is made through the skin and musculature of the dorsal abdominal wall of the mouse, the fiber samples are inserted into the peritoneal cavity in a craniocaudal direction, and the incision is closed with skin staples. For subcutaneous implants, a small skin incision is made at the nape of the neck to allow insertion of an 11-gauge tumor implant trocar. The trocar, containing the hollow fiber samples, is inserted caudally through the subcutaneous tissues and fibers were deposited during withdrawal of the trocar. The incision is closed with skin staples.
Shnyder and colleagues have recently reported that immunocompetent mice such as NMRI also can be used, which can significantly lower operating costs.43 The fibers are available in several different colors, which facilitates the culture of up to three different cell lines per mouse. Routinely, we place three fibers ip and three in the sc space of the animal’s back. On day three, the mice are ready to be treated with test agent. Our standard regimen is to administer the test compound at two dose levels in four daily ip injections on days 3, 4, 5 and 6 followed by fiber retrieval on day 7. During agent administration, each mouse is weighed daily and carefully monitored for toxicity, which is objectively determined as a 20% or greater loss of body weight or subjectively judged by lethargic behavior, scruffy coat or hunched posture.
Many of the compounds we study in our natural products drug discovery program have limited solubility in water. This issue can complicate cell free studies but may limit bioavailability to the point where studies in animals are impossible. Thus, the issue of solubility is very serious indeed. To enhance the solubility of a wide range of chemical skeletons, we have employed the technique of co-precipitation with polyvinylpyrrolidone (PVP). This polymer has excellent wetting properties, is used as a stabilizer in some food products and is employed in the pharmaceutical industry as an excipient.44 Our approach is to separately dissolve a known mass of the test compound and the PVP in miscible, volatile solvents, mix the solutions thoroughly, and dry the solvent mixture under vacuum or a gentle stream of nitrogen gas. The resulting precipitate is dissolved in an aqueous solution appropriate for the subsequent biological assay. This method has been used by other laboratories studying the biology of natural products such as reserpine45 and digitoxin.46
The dose levels chosen for each test compound are determined by performing acute toxicity tests for each agent as described by the Food and Drug Administration47 and the NCI Developmental Therapeutics Program.48 One mouse is given a single ip injection at 400 mg/kg body weight; another mouse is administered 300 mg/kg and a third mouse is given 100 mg/kg. The mice are observed for two weeks and sacrificed if they lose 20% or more of their body weight or exhibit outward signs of toxicity as indicated above. If all three mice die or must be sacrificed, three lower doses (e.g., 50, 25 and 10 mg/kg) are tested. The process is repeated until the maximum tolerated dose (MTD) is identified. We routinely use 40% of the single-dose MTD as the highest dose in our 4-daily-dose treatment schedules. The initial level of exposure that we choose for the acute toxicity study is based on the activity of the compound in cell cytotoxicity tests. For example, one of the compounds we have studied recently, silvestrol (13), exhibited an ED50 of about 3 nM in our cell line screens at the University of Illinois at Chicago and in the 60-cell line panel at NIH. This concentration translates into about 2 ng/mL or 2 µg/kg (1 ml weighs 1 g) for cells continuously exposed to silvestrol for two days. Based on these data, we conducted acute toxicity testing with a high dose of 10 mg/kg. Ultimately, silvestrol demonstrated an MTD of 2.5 mg/kg ip in the mouse.28
On day 7 of the experiment, all mice are sacrificed, and the fibers are retrieved. Necropsies are performed on each mouse to assess and record gross toxicity to major organs. The fibers are then placed into 6-well plates, with each well containing culture medium and allowed to equilibrate for 30 min at 37 °C. The viable cell mass contained within each hollow fiber is determined with a MTT [3-(4,5-demethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] dye conversion method.23,24,42 After incubating suitable aliquots of the culture medium and the MTT solution for 4 hours, the culture medium is removed, and 2.5% protamine sulfate solution added, with the plates stored at 4 °C for 2–4 h. To assess the optical density of the samples, fibers are transferred to 24-well plates, cut in half, and dried overnight. Formazan is then extracted from each sample with DMSO for 4 h on a rotation platform. Aliquots of the extracted formazan are then transferred to individual wells of 96-well plates and assessed for optical density at 540 nm. The percent net growth for each cell line in each treatment group is calculated by subtracting the day-zero absorbance from the day 7 absorbance and dividing this difference by the difference between the net growth in the day 7 vehicle-treated controls minus the day-zero values. A 50% or greater reduction in net cell growth in the treated samples compared to the vehicle control samples is considered a positive result.
Additional Applications of the Hollow Fiber Assay
The focus in our laboratories has been the discovery of natural product inhibitors of cancer.22,24–28,30–34,49 However, the simplicity and versatility of the hollow fiber assay lends itself to other applications. For example, we have explored the potential of natural products to modulate the multidrug-resistant phenotype of cancer cells.49 Further, many types of mechanistic experiments that can be conducted in vitro on a given cell line can be extended to the in vivo setting using this assay. Along these lines, Hall and colleagues have used the hollow fiber assay to study the effects of cell cycle inhibitors on Rb expression and phosphorylation and PCNA expression.37 Temmink and colleagues used the model to study the in vivo role of thymidine phosphorylase/platelet-derived endothelial cell growth factor in the cytotoxicity and pharmacodynamics in colon cancer cells of a formulation of trifluorothymidine and a thymidine phosphorylase inhibitor.50 Sader et al. have adapted the assay to study the molecular events involved as a human prostate cancer cell line (LNCaP) progresses to hormone independence.51 Below are summarized some of the most commonly used applications of the hollow fiber assay.
Screening for Anti-HIV Activity
One of the first applications of the hollow fiber assay was as a tool for anti-human immunodeficiency virus (HIV) drug discovery.52 Overall, the procedure is very similar to the method used for the anticancer assay with several key differences. One difference is that the fibers are filled with human lymphoid CD4 positive cells (designated CEM-SS). Hollingshead and colleagues have demonstrated that CEM-SS cells can proliferate in the PVDF fibers cultivated either sc or ip in severe combined immunodeficient (SCID) mice while supporting HIV replication as judged by reverse transcriptase activity.52 Another difference is the treatment rotocol, which begins immediately prior to fiber implantation and continues every 8 hours (usually by ip injections) until day 6. The mice are sacrificed on day 7 and blood, peritoneal wash and fibers are harvested for analysis. When SCID mice implanted with fibers harboring HIV-infected CEM-SS cells were treated with the AIDS drugs 3’-azido-3’-deoxythymidine (AZT) or dideoxycytidine (ddC), cell proliferation was inhibited and HIV production was suppressed.52 Finally, more endpoint analyses are conducted for the HIV hollow fiber SCID mouse assay than for the anticancer version of the procedure. In addition to the stable endpoint MTT assay to measure CEM-SS cell viability, p24 antigen and reverse transcriptase are measured to assess HIV protein and activity, respectively.
The purpose of the HIV hollow fiber SCID mouse assay is the same as the purpose of its anticancer counterpart: to provide a relatively low cost, high throughput in vivo screen for preliminary evaluation that can help investigators better prioritize compounds for subsequent, well established assays that are costly assays in terms of time, compound required, animals needed and financial commitment. In the case of HIV drug discovery, the HIV hollow fiber SCID mouse assay serves as a filter for compounds to be tested in the SCID/hu (Thy/Liv) model. Since HIV does not infect rodent cells, human hematolymphoid organs that can support HIV replication are implanted in immunodeficient hosts such as SCID mice.53–55 In this model, about 1 mm3 of human fetal thymus and liver tissue (or other tissue sources of hematopoietic progenitor cells) that can support HIV replication, are implanted under the renal capsules of SCID mice. Three to five months later, exploratory surgery is performed to determine if the tissues grew to a minimum of 30 mm3 and, if so, treatments are initiated and the transplanted tissues are injected with virus.56 The HIV hollow fiber SCID mouse assay is much faster, requires fewer surgeries and is simpler than the SCID/hu assay. Therefore, this model can be used as a pharmacologic gatekeeper to help separate active and inactive agents and select the best lead compounds for further animal model testing such as the SCID/hu assay.
Monitoring Molecular Pathways by Bioluminescence
Bioluminescence has emerged as a highly sensitive and quantitative technique to measure biological processes within cells and, more recently, within whole animals.57 Research in imaging technologies such as bioluminescence has been intense in recent years and has yielded significant improvements. Bioluminescence imaging is highly sensitive, quantitative, non-invasive, and allows for longitudinal studies before, during and after treatment. At the core of the technique is the oxidation of a luciferin by a luciferase enzyme, a reaction that releases energy in the form of light at around 562 nm. There are many luciferins and luciferases that occur in microbes, marine organisms, and insects. The most commonly used luciferin in biomedical research is a benzothiazole isolated from the male firefly (Photinus pyralis). Bioluminescence is used to track cells within an animal or monitoring gene expression within cells. For example, a subline can be cloned from a tumorigenic cancer cell line, stably transfected to express firefly luciferase and subsequently inoculated into an immunodeficient mouse using the same procedures as the xenograft model or the hollow fiber assay. HollFingshead58,59 and others60,61 have shown that the progress of tumor growth can be monitored by bioluminescence detection well before the tumors are even palpable, let alone measurable by calipers. To image the tumor cells that constitutively express luciferase, the mice are given a single ip injection of luciferin and imaged about an hour later. Thus, the procedure is simple, rapid and multiple mice can be imaged simultaneously. The resulting bioluminescence is capable of penetrating the hollow fibers, thereby permitting investigators to monitor the growth of cells in the fibers over the course of a hollow fiber assay.58–61 Companies such as Caliper Life Sciences (Hopkinton, MA) manufacture sensitive imagers as well as biologic reagents, such as cancer cells stably transfected with luciferase. In addition to following the fate of cancer cells inoculated into immunocompromised mice, bioluminescence can be used to track the activity of specific biochemical pathways in cancer cells propagated in hollow fibers. Zhang60,61 and olleagues at Merck Research Laboratories used bioluminescence imaging of cells propagated in vivo in hollow fibers to monitor the nuclear factor KB (NF-κB) pathway in vivo. Activation of NF-κB by lipopolysaccharide and tumor necrosis factor-α was stimulated in tumor cell lines genetically engineered to express luciferase controlled by an NF-κB -responsive element. These results demonstrate that optical imaging of hollow fibers containing reporter tumor cells can be used to evaluate antitumor activities of anticancer drugs and for measurement of specific molecular pathways.
Modeling Solid Tumors in Vitro
Another interesting application for the hollow fiber assay is to allow cells propagated within the fibers to proliferate beyond a monolayer of cells adherent to the polyvinylidene fluoride surface of the fiber. Casciari et al.35 and Hassan et al.62have shown that if the fibers are seeded with a high number of cells, or if the fibers are propagated in vitro for an extended period of time, the fibers can become completely filled with cancer cells, thereby modeling, to some degree, a solid tumor mass. Once the fibers’ inner volume is filled with cells, the cell proliferation rate drops significantly, but the cell mass is maintained.62 This model has several important characteristics. First, it addresses a concern that screening substances through a panel of rapidly proliferating cells in suspension or adherent culture may select for agents that can treat rapidly growing tumors, but not the many relatively slow growing solid tumors for which there are currently few treatment options. Recall that the rapid growth rate of the rodent tumor models used by the CCNSC was considered a weakness of those systems.5–7 Another advantageous characteristic is that the three-dimensional architecture of the cells in the fiber mimics the special orientation of cancer cells in a natural tumor and the types of barriers that anticancer drugs must penetrate to access cells at the core of a small neoplasm. Also, this system is amenable to the use of primary cells derived from a patient’s tumor. Selection for cells that can propagate on plastic or glass is minimized affording investigators additional experimental options for evaluating clinical specimens.
Angiogenesis
A concern about the hollow fiber assay is that, compared to fibers implanted ip, the fibers implanted at the sc site provide a less accurate prediction of the subsequent success or failure of a compound in the xenograft assay.63 One reason for this discrepancy is that the 7-day period during which the fibers are implanted in the host is not enough time for the fibers to become vascularized. Phillips et al. hypothesized that, given sufficient time, angiogenesis would be stimulated by the cancer cells within the fibers.64 To test this idea, these investigators designed experiments in which hollow fibers containing murine colon adenocarcinoma cells (MAC 15A) or medium only were implanted subcutaneously. At various time points between 4 and 32 days, the mice were sacrificed, the skin was peeled back to reveal the fibers, and gross vascularization around the fibers was documented by photography. The results indicated that between 7 and 32 days post implantation, substantial vascularization was stimulated toward hollow fibers that contained the cancer cells, but not the cell-free control fibers. The degree of vacularization can be quantified if the blood vessel number is scored in paraffin embedded tissue sections.65 Hasan and colleagues used this approach to demonstrate that heparin oligosaccharides inhibit the angiogenesis induced by large cell lung cancer cells (NCI-H460) that express high levels of vascular endothelial growth factor, a potent inducer of angiogenesis.66 In addition, Fu et al showed that the retinoid X receptor ligand LGD1069 can inhibit angiogenesis stimulated by a combination of A549 (human lung carcinoma), MCF-7 (human breast adenocarcinoma) and HT-29 (colorectal adenocarcinoma) human cancer cells propagated in hollow fibers over a period of 21 days.67
These experiments demonstrate that it takes at least a week for cancer cell-containing hollow fibers to stimulate new blood vessel growth when implanted in the sc space. Phillips et al. proposed that this lack of vascularization during the brief period of the standard hollow fiber assay may account for the relative lack of activity of most compounds against cells in fibers implanted sc compared to cells in fibers implanted ip64 To test this hypothesis, these investigators compared the doxorubicin susceptibility of MAC 15A cells that had been propagated in hollow fibers implanted in mice for either 4 days (no observable vascularization) or 28 days (well vascularized). Doxorubicin was significantly more active against cells in fibers that were vascularized compared to cells in fibers that were not vascularized.64 These results suggest that a lack of activity in fibers implanted sc may yield a false negative in some instances due to impaired drug delivery. Nonetheless, the system has utility and, as with all models, strengths and limitations must be taken into account.
Summary and Conclusions
The hollow fiber assay was originally designed by Hollingshead and colleagues to provide a means of efficiently prioritizing compounds found active in the 60-cell line panel for subsequent analysis in the human tumor xenograft assay.23 The assay has successfully met this goal. In 2001, the NCI published a study designed to test how predictive the hollow fiber and the xenograft assays were for the discovery of effective clinical agents.63 Thirty-nine agents that had progressed through phase II trials and that had been tested in the tumor xenograft assay were evaluated.63 Compounds that showed in vivo activity in at least one-third of xenograft models tested also demonstrated activity in some Phase II trials, which underscores the utility of the xenograft assay for predicting clinical activity. These investigators also compared the activity of 564 compounds in the hollow fiber assay and tumor xenograft models. The result indicated that the likelihood of finding xenograft activity in at least one-third of the models rose with increasing ip hollow fiber activity, from 8% for all compounds tested to 20% for agents active in more than 6 fibers implanted ip. Intraperitoneal hollow fiber activity was also found to be a better predictor of xenograft activity than sc hollow fiber activity. These findings were confirmed and extended in a subsequent analysis of 690 compounds tested in both models.36 The authors concluded that activity in hollow fibers implanted ip is a useful predictor of subsequent activity in the xenograft assay. A similar conclusion was drawn by Voskoglou-Nomikos et al in the Canadian NCI review of the utility of the xenograft model in predicting clinical efficacy.68Furthermore, as discussed above, the hollow fiber assay is reasonably simple, rapid and affords investigators the ability to advance compounds through the drug discovery process in a manner that minimizes the use of animals, which is a significant advancement from an animal welfare standpoint.17–19 Therefore, the assay has successfully fulfilled the mission for which it was originally designed.
The hollow fiber assay has been incorporated into the drug discovery programs of many laboratories around the world including Argentina,69 Austria,70 France,71 Germany,72 India,73Italy,74–78 New Zealand,79 Poland,80,81 Spain,82,83 Sweden,84 and the United Kingdom.85–89Laboratories that focus on chemical synthesis or on natural roduct isolation and structure elucidation usually choose to have their compounds tested by the NCI rather than have the assay set up in their own laboratories. This underscores the multidisciplinary and collaborative nature of drug discovery and emphasizes the important role that the NCI plays in the drug discovery efforts of academic laboratories.
It has been nearly 20 years since the disease-oriented 60-cell line panel was launched by the NCI for anticancer drug discovery. Over that period of time, great strides have been made to understand the molecular basis of cancer. The success of drugs such as imatinib mesylate (Gleevec®), which interact with specific molecular targets within the cancer cell, demonstrates the need to continue to develop targeted therapies for the treatment of the many forms of cancer. The current trend is to screen libraries of compounds against validated molecular targets critical for neoplastic transformation or vital to the survival of the cancer cell. The initial screen is typically conducted using a cell-free, high-throughput system. Active leads are pursued using cell-based systems in which the target has been shown to be vital to cell survival. Ultimately, lead compounds are tested in animals, often in the xenograft assay using tumors derived from the same cell line used for the in vitro studies. As key molecular targets are discovered and validated, mice are genetically engineered such that dysregulation of the target contributes to tumor formation that mimics the pathogenesis observed in man. What role might the hollow fiber assay play in this “target-oriented” approach to cancer drug discovery? It is clear that, for the foreseeable future, all drugs will need to be carefully tested for efficacy and safety in animals prior to clinical evaluation. The flexibility, efficiency and economy of the hollow fiber assay make this technique well suited to bridge the gap between a wide range of in vitro studies and many different types of animal studies.
Acknowledgments
The authors recognize with deep admiration the immeasurable contributions of the late Monroe Wall, a dear friend and colleague, who fully participated in these projects during the period of 1990–2000. We are also grateful to Joseph G. Mayo and Melinda G. Hollingshead for instrumental help in establishing the hollow fiber assay at the UIC, and the superb technical support of Daniel D. Lantvit. We are grateful to our botanist collaborators in several countries for their cooperation in regard to the plant collections made. We also thank the many staff and faculty colleagues, postdoctoral associates, and graduate students who have participated in this collaborative research project, and whose names are indicated in the reference section below. The authors acknowledge financial support through Public Health Service grants U19-CA52956, U19 CA52956–09S3, and P01-CA125066 from the NCI, National Institutes of Health, Bethesda, MD as well as NCI contract support for compound development through the RAID program.
Footnotes
Dedicated to Dr. David G. I. Kingston of Virginia Polytechnic Institute and State University for his pioneering work on bioactive natural products.
References and Notes
- 1.Krumbhaar EB. J. Amer. Med. Assoc. 1919;72:39–41. [Google Scholar]
- 2.Krumbhaar EB, Krumbhaar HD. J. Med. Res. 1919;40:497–508. [PMC free article] [PubMed] [Google Scholar]
- 3.Gilman A, Philips FS. Science. 1946;103:409–415. [PubMed] [Google Scholar]
- 4.Venditti JM, Wesley RA, Plowman J. Adv. Pharmacol. Chemother. 1984;20:1–20. doi: 10.1016/s1054-3589(08)60263-x. [DOI] [PubMed] [Google Scholar]
- 5.Alley MC, Hollingshead MG, Dykes DJ, Waud WR. In: Anticancer Drug Development Guide. Teicher BA, Andrews PA, editors. Totowa NJ: Humana Press; 2004. pp. 125–152. [Google Scholar]
- 6.Corbett TH, Valeriote FA, Dykes DJ, Baker LH. Invest. New Drugs. 1987;5:3–20. doi: 10.1007/BF00217664. [DOI] [PubMed] [Google Scholar]
- 7.Sausville EA, Burger AM. Cancer Res. 2006;66:3351–3354. doi: 10.1158/0008-5472.CAN-05-3627. [DOI] [PubMed] [Google Scholar]
- 8.Boyd MR, Paull KD. Drug Dev. Res. 1995;34:91–109. [Google Scholar]
- 9.Boyd MR. In: Anticancer Drug Development Guide. Teicher BA, Andrews PA, editors. Totowa, NJ: Humana Press; 2004. pp. 41–61. [Google Scholar]
- 10.Shoemaker RH, Monks A, Alley MC, Scudiero DA, Fine DL, McLemore TL, Abbott BJ, Paull KD, Mayo JG, Boyd MR. Prog. Clin. Biol. Res. 1988;276:265–286. [PubMed] [Google Scholar]
- 11.Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero DA, Rubinstein L, Plowman J, Boyd MR. J. Natl. Cancer. Inst. 1989;81:1088–1092. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan SP. Genet. Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 13.Bosma GC, Custer RP, Bosma MJ. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 14.Teicher BA. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval. Totowa, NJ: Humana Press; 1997. p. 311. [Google Scholar]
- 15.Boven E, Winograd B. The Nude Mouse in Oncology Research. Boca Raton, FL: CRC Press; 1991. p. 340. [Google Scholar]
- 16.Rofstad EK. Int. J. Cancer. 1995;63:744–749. doi: 10.1002/ijc.2910630523. [DOI] [PubMed] [Google Scholar]
- 17.Russell WM. Altern. Lab. Anim. 1995;23:298–304. [PubMed] [Google Scholar]
- 18.Russell WM, Burch RL. The Principles of Humane Experimental Technique. London: Methuen; 1959. [Google Scholar]
- 19.Suggitt M, Cooper PA, Shnyder SD, Bibby MC. Int. J. Oncol. 2006;29:1493–1499. [PubMed] [Google Scholar]
- 20.Hallock Y, Cragg GM. Pharm. Biol. 2003;41 (Suppl.):78–91. [Google Scholar]
- 21.Kinghorn AD, Farnsworth NR, Soejarto DD, Cordell GA, Pezzuto JM, Udeani GO, Wani MC, Wall ME, Navarro HA, Kramer RA, Menendez AT, Fairchild CR, Lane KE, Vyas DM, Lam KS, Shu Y-Z. Pure Appl. Chem. 1999;71:1611–1618. [Google Scholar]
- 22.Kinghorn AD, Farnsworth NR, Soejarto DD, Cordell GA, Swanson SM, Pezzuto JM, Wani MC, Wall ME, Oberlies NH, Kroll DJ, Kramer RA, Rose WC, Vite GD, Fairchild CR, Peterson RW, Wild R. Pharm. Biol. 2003;41:53–67. [Google Scholar]
- 23.Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- 24.Mi Q, Lantvit D, Reyes-Lim E, Chai H, Zhao W, Lee IS, Peraza-Sanchez S, Ngassapa O, Kardono LB, Riswan S, Hollingshead MG, Mayo JG, Farnsworth NR, Cordell GA, Kinghorn AD, Pezzuto JM. J. Nat. Prod. 2002;65:842–850. doi: 10.1021/np010322w. [DOI] [PubMed] [Google Scholar]
- 25.Diaz F, Chavez D, Lee D, Mi Q, Chai HB, Tan GT, Kardono LB, Riswan S, Fairchild CR, Wild R, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. J. Nat. Prod. 2003;66:865–867. doi: 10.1021/np0300784. [DOI] [PubMed] [Google Scholar]
- 26.Diaz F, Chai HB, Mi Q, Su BN, Vigo JS, Graham JG, Cabieses F, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. J. Nat. Prod. 2004;67:352–356. doi: 10.1021/np030479j. [DOI] [PubMed] [Google Scholar]
- 27.Gu JQ, Graf TN, Lee D, Chai HB, Mi Q, Kardono LB, Setyowati FM, Ismail R, Riswan S, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kroll DJ, Falkinham JO, 3rd, Wall ME, Wani MC, Kinghorn AD, Oberlies NH. J. Nat. Prod. 2004;67:1156–1161. doi: 10.1021/np040027m. [DOI] [PubMed] [Google Scholar]
- 28.Hwang BY, Su BN, Chai H, Mi Q, Kardono LB, Afriastini JJ, Riswan S, Santarsiero BD, Mesecar AD, Wild R, Fairchild CR, Vite GD, Rose WC, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. J. Org. Chem. 2004;69:3350–3358. doi: 10.1021/jo040120f. [DOI] [PubMed] [Google Scholar]
- 29.Mi Q, Lantvit D, Reyes-Lim E, Chai H, Phifer SS, Wani MC, Wall ME, Tan GT, Cordell GA, Farnsworth NR, Kinghorn AD, Pezzuto JM. Anticancer Res. 2005;25:779–787. [PubMed] [Google Scholar]
- 30.Chin YW, Jones WP, Mi Q, Rachman I, Riswan S, Kardono LB, Chai HB, Farnsworth NR, Cordell GA, Swanson SM, Cassady JM, Kinghorn AD. Phytochemistry. 2006;67:1243–1248. doi: 10.1016/j.phytochem.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Chin YW, Mdee LK, Mbwambo ZH, Mi Q, Chai HB, Cragg GM, Swanson SM, Kinghorn AD. J. Nat. Prod. 2006;69:1649–1652. doi: 10.1021/np060418w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang L, Ito A, Chai HB, Mi Q, Jones WP, Madulid DR, Oliveros MB, Gao Q, Orjala J, Farnsworth NR, Soejarto DD, Cordell GA, Swanson SM, Pezzuto JM, Kinghorn AD. J. Nat. Prod. 2006;69:332–337. doi: 10.1021/np058083q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones WP, Lobo-Echeverri T, Mi Q, Chai HB, Soejarto DD, Cordell GA, Swanson SM, Kinghorn AD. J. Nat. Prod. 2007;70:372–377. doi: 10.1021/np060534z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin YW, Salim AA, Su BN, Mi Q, Chai HB, Riswan S, Kardono LB, Ruskandi A, Farnsworth NR, Swanson SM, Kinghorn AD. J. Nat. Prod. 2008;71:390–395. doi: 10.1021/np070584j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casciari JJ, Hollingshead MG, Alley MC, Mayo JG, Malspeis L, Miyauchi S, Grever MR, Weinstein JN. J. Natl. Cancer. Inst. 1994;86:1846–1852. doi: 10.1093/jnci/86.24.1846. [DOI] [PubMed] [Google Scholar]
- 36.Decker S, Hollingshead M, Bonomi CA, Carter JP, Sausville EA. Eur. J. Cancer. 2004;40:821–826. doi: 10.1016/j.ejca.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 37.Hall LA, Krauthauser CM, Wexler RS, Hollingshead MG, Slee AM, Kerr JS. Anticancer Res. 2000;20:903–911. [PubMed] [Google Scholar]
- 38.Hall LA, Krauthauser CM, Wexler RS, Slee AM, Kerr JS. Methods Mol. Med. 2003;74:545–566. doi: 10.1385/1-59259-323-2:545. [DOI] [PubMed] [Google Scholar]
- 39.Hollingshead MG, Plowman J, Alley MC, Mayo JG, Sausville EA. In: Relevance of Tumor Models for Anticancer Drug Development. Fiebig HH, Burger AM, editors. Basel: Karger AG; 1999. pp. 109–120. [Google Scholar]
- 40.Phillips RM, Bibby MC. In: Angiogenesis Protocols. Murray JC, editor. Totowa, NJ: Humana Press; 2001. pp. 87–93. [Google Scholar]
- 41.Biological Testing Branch. [Accessed October, 2008];NCI. Hollow Fiber Assay Protocols for Tumor Cell Lines. 1991 http://dtp.nci.nih.gov/branches/btb/pdf/cancer_protocol_hollow_fiber.pdf.
- 42.Alley MC, Pacula-Cox CM, Hursey ML, Rubinstein LR, Boyd MR. Cancer Res. 1991;51:1247–56. [PubMed] [Google Scholar]
- 43.Shnyder SD, Cooper PA, Scally AJ, Bibby MC. Anticancer Res. 2006;26:2049–2052. [PubMed] [Google Scholar]
- 44.BÜhler V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone, and Copovidone. Berlin: Springer; 2005. p. 254. [Google Scholar]
- 45.Stupak EI, Rosenberg HA, Bates TR. J. Pharmacokinet. Biopharm. 1974;2:511–524. doi: 10.1007/BF01070945. [DOI] [PubMed] [Google Scholar]
- 46.Stupak EI, Bates TR. J. Pharm. Sci. 1973;62:1806–1809. doi: 10.1002/jps.2600621114. [DOI] [PubMed] [Google Scholar]
- 47.Food and Drug Administration. Fed. Regist. 2006;61:43934. [Google Scholar]
- 48.Developmental Therapeutics Program. [Accessed October, 2008];NCI. http://dtp.nci.nih.gov/branches/btb/acute_tox.html.
- 49.Mi Q, Cui B, Silva GL, Lantvit D, Lim E, Chai H, Hollingshead MG, Mayo JG, Kinghorn AD, Pezzuto JM. Cancer Lett. 2002;184:13–20. doi: 10.1016/s0304-3835(02)00202-1. [DOI] [PubMed] [Google Scholar]
- 50.Temmink OH, Prins HJ, van Gelderop E, Peters GJ. Br. J. Cancer. 2007;96:61–66. doi: 10.1038/sj.bjc.6603507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadar MD, Akopian VA, Beraldi E. Mol. Cancer Ther. 2002;1:629–637. [PubMed] [Google Scholar]
- 52.Hollingshead M, Roberson J, Decker W, Buckheit R, Jr, Elder C, Malspeis L, Mayo J, Grever M. Antiviral Res. 1995;28:265–279. doi: 10.1016/0166-3542(95)00055-q. [DOI] [PubMed] [Google Scholar]
- 53.McCune JM. Science. 1997;278:2141–2142. doi: 10.1126/science.278.5346.2141. [DOI] [PubMed] [Google Scholar]
- 54.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 55.Rabin L, Hincenbergs M, Moreno MB, Warren S, Linquist V, Datema R, Charpiot B, Seifert J, Kaneshima H, McCune JM. Antimicrob. Agents Chemother. 1996;40:755–762. doi: 10.1128/aac.40.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taggart BR, Harrington P, Hollingshead M. Antiviral Res. 2004;63:1–6. doi: 10.1016/j.antiviral.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Weissleder R, Ntziachristos V. Nat. Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 58.Hollingshead MG, Alley MC, Kaur G, Stinson SF. In: Anticancer Drug Development Guide. Teicher BA, Andrews PA, editors. Totowa, NJ: Humana Press; 2004. pp. 153–182. [Google Scholar]
- 59.Hollingshead MG, Bonomi CA, Borgel SD, Carter JP, Shoemaker R, Melillo G, Sausville EA. Eur. J. Cancer. 2004;40:890–898. doi: 10.1016/j.ejca.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Zhang GJ, Chen TB, Bednar B, Connolly BM, Hargreaves R, Sur C, Williams DL. Neoplasia. 2007;9:652–656. doi: 10.1593/neo.07421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang GJ, Chen TB, Hargreaves R, Sur C, Williams DL., Jr Nat. Protoc. 2008;3:891–899. doi: 10.1038/nprot.2008.52. [DOI] [PubMed] [Google Scholar]
- 62.Hassan SB, de la Torre M, Nygren P, Karlsson MO, Larsson R, Jonsson E. Anticancer Drugs. 2001;12:33–42. doi: 10.1097/00001813-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Br. J. Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips RM, Pearce J, Loadman PM, Bibby MC, Cooper PA, Swaine DJ, Double JA. Cancer Res. 1998;58:5263–5266. [PubMed] [Google Scholar]
- 65.Hasan J, Shnyder SD, Bibby M, Double JA, Bicknel R, Jayson GC. Angiogenesis. 2004;7:1–16. doi: 10.1023/B:AGEN.0000037338.51851.d1. [DOI] [PubMed] [Google Scholar]
- 66.Hasan J, Shnyder SD, Clamp AR, McGown AT, Bicknell R, Presta M, Bibby M, Double J, Craig S, Leeming D, Stevenson K, Gallagher JT, Jayson GC. Clin. Cancer Res. 2005;11:8172–8179. doi: 10.1158/1078-0432.CCR-05-0452. [DOI] [PubMed] [Google Scholar]
- 67.Fu J, Ding Y, Huang D, Li H, Chen X. Cancer Lett. 2007;248:153–163. doi: 10.1016/j.canlet.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 68.Voskoglou-Nomikos T, Pater JL, Seymour L. Clin. Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 69.Di Chenna PH, Benedetti-Doctorovich V, Baggio RF, Garland MT, Burton G. J. Med. Chem. 2001;44:2486–2489. doi: 10.1021/jm010050u. [DOI] [PubMed] [Google Scholar]
- 70.Easmon J, Purstinger G, Thies KS, Heinisch G, Hofmann J. J. Med. Chem. 2006;49:6343–6350. doi: 10.1021/jm060232u. [DOI] [PubMed] [Google Scholar]
- 71.Dallemagne P, Khanh LP, Alsaidi A, Varlet I, Collot V, Paillet M, Bureau R, Rault S. Bioorg. Med. Chem. 2003;11:1161–1167. doi: 10.1016/s0968-0896(02)00654-5. [DOI] [PubMed] [Google Scholar]
- 72.Kock I, Heber D, Weide M, Wolschendorf U, Clement B. J. Med. Chem. 2005;48:2772–2777. doi: 10.1021/jm0490888. [DOI] [PubMed] [Google Scholar]
- 73.Sharma VM, Prasanna P, Seshu KV, Renuka B, Rao CV, Kumar GS, Narasimhulu CP, Babu PA, Puranik RC, Subramanyam D, Venkateswarlu A, Rajagopal S, Kumar KB, Rao CS, Mamidi NV, Deevi DS, Ajaykumar R, Rajagopalan R. Bioorg. Med. Chem. Lett. 2002;12:2303–2307. doi: 10.1016/s0960-894x(02)00431-6. [DOI] [PubMed] [Google Scholar]
- 74.Antonini I, Polucci P, Magnano A, Sparapani S, Martelli S. J. Med. Chem. 2004;47:5244–5250. doi: 10.1021/jm049706k. [DOI] [PubMed] [Google Scholar]
- 75.Bigioni M, Benzo A, Irrissuto C, Maggi CA, Goso C. Anticancer Drugs. 2005;16:1083–1089. doi: 10.1097/00001813-200511000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Congiu C, Cocco MT, Lilliu V, Onnis V. J. Med. Chem. 2005;48:8245–8252. doi: 10.1021/jm050711d. [DOI] [PubMed] [Google Scholar]
- 77.Pinna GA, Pirisi MA, Grella GE, Gherardini L, Mussinu JM, Paglietti G, Ferrari AM, Rastelli G. Arch Pharm. 2001;334:337–344. doi: 10.1002/1521-4184(200112)334:11<337::aid-ardp337>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 78.Pinna GA, Pirisi MA, Mussinu JM, Murineddu G, Loriga G, Pau A, Grella GE. Farmaco. 2003;58:749–763. doi: 10.1016/S0014-827X(03)00131-9. [DOI] [PubMed] [Google Scholar]
- 79.Yao B, Prinsep MR, Nicholson BK, Gordon DP. J. Nat. Prod. 2003;66:1074–1077. doi: 10.1021/np030104y. [DOI] [PubMed] [Google Scholar]
- 80.Dzierzbicka K, Kolodziejczyk AM. J. Med. Chem. 2003;46:183–189. doi: 10.1021/jm020991m. [DOI] [PubMed] [Google Scholar]
- 81.Dzierzbicka K, Kolodziejczyk AM, Wysocka-Skrzela B, Mysliwski A, Sosnowska D. J. Med. Chem. 2001;44:3606–3615. doi: 10.1021/jm001115g. [DOI] [PubMed] [Google Scholar]
- 82.Solano B, Junnotula V, Marin A, Villar R, Burguete A, Vicente E, Perez-Silanes S, Aldana I, Monge A, Dutta S, Sarkar U, Gates KS. J. Med. Chem. 2007;50:5485–5492. doi: 10.1021/jm0703993. [DOI] [PubMed] [Google Scholar]
- 83.Zarranz B, Jaso A, Aldana I, Monge A. Bioorg. Med. Chem. 2004;12:3711–3721. doi: 10.1016/j.bmc.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 84.Gullbo J, Lindhagen E, Bashir-Hassan S, Tullberg M, Ehrsson H, Lewensohn R, Nygren P, De La Torre M, Luthman K, Larsson R. Invest. New Drugs. 2004;22:411–420. doi: 10.1023/B:DRUG.0000036683.10945.bb. [DOI] [PubMed] [Google Scholar]
- 85.Bridges EM, Bibby MC, Burchill SA. J. Pediatr. 2006;149:103–111. doi: 10.1016/j.jpeds.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 86.Hartley JA, Spanswick VJ, Brooks N, Clingen PH, McHugh PJ, Hochhauser D, Pedley RB, Kelland LR, Alley MC, Schultz R, Hollingshead MG, Schweikart KM, Tomaszewski JE, Sausville EA, Gregson SJ, Howard PW, Thurston DE. Cancer Res. 2004;64:6693–6699. doi: 10.1158/0008-5472.CAN-03-2941. [DOI] [PubMed] [Google Scholar]
- 87.Leese MP, Hejaz HA, Mahon MF, Newman SP, Purohit A, Reed MJ, Potter BV. J. Med. Chem. 2005;48:5243–5256. doi: 10.1021/jm050066a. [DOI] [PubMed] [Google Scholar]
- 88.Leong CO, Suggitt M, Swaine DJ, Bibby MC, Stevens MF, Bradshaw TD. Mol. Cancer Ther. 2004;3:1565–1575. [PubMed] [Google Scholar]
- 89.Mann J, Baron A, Opoku-Boahen Y, Johansson E, Parkinson G, Kelland LR, Neidle S. J. Med. Chem. 2001;44:138–144. doi: 10.1021/jm000297b. [DOI] [PubMed] [Google Scholar]
- 90.Phifer SS, Lee D, Seo EK, Kim N-C, Graf TN, Kroll DJ, Navarro HA, Izydore RA, Jimenez F, Garcia R, Rose WC, Fairchild CR, Soejarto DD, Farnsworth NR, Kinghorn AD, Oberlies NH, Wall ME, Wani MC. [Google Scholar]
- 91.Phifer SS, Lee D, Seo EK, Kim NC, Graf TN, Kroll DJ, Navarro HA, Izydore RA, Jimenez F, Garcia R, Rose WC, Fairchild CR, Wild R, Soejarto DD, Farnsworth NR, Kinghorn AD, Oberlies NH, Wall ME, Wani MC. Unublished results. [Google Scholar]
- 92.Jones WP, Lobo-Echeverri T, Mi Q, Chai H, Lee D, Soejarto DD, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. J. Pharm. Pharmacol. 2005;57:1101–1108. doi: 10.1211/jpp.57.9.0005. [DOI] [PubMed] [Google Scholar]
- 93.Braca A, Armenise A, Morelli I, Mendez J, Mi Q, Chai HB, Swanson SM, Kinghorn AD, De Tommasi N. Planta Med. 2004;70:540–550. doi: 10.1055/s-2004-827155. [DOI] [PubMed] [Google Scholar]
- 94.Rivero-Cruz JF, Lobo-Echeverri T, Mi Q, Chai H-B, Soejarto DD, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. [Google Scholar]
- 95.Kim N-C, Seo E-K, Mi Q, Navarro H, Burgess JP, Mukherjee R, Tan GT, Farnsworth NR, Pezzuto JM, Kinghorn AD, Wani MC, Wall ME. doi: 10.1021/np0103158. [DOI] [PubMed] [Google Scholar]
- 96.Roux D, Hadi HA, Thoret S, Guénard D, Thoison O, Païs M, Sévenet T. J. Nat. Prod. 2000;63:1070–1076. doi: 10.1021/np0000872. [DOI] [PubMed] [Google Scholar]