Abstract
Mycobacteria are significant pathogens of laboratory zebrafish, Danio rerio (Hamilton). Stress is often implicated in clinical disease and morbidity associated with mycobacterial infections but has yet to be examined with zebrafish. The aim of this study was to examine the effects of husbandry stressors on zebrafish infected with mycobacteria. Adult zebrafish were exposed to Mycobacterium marinum or Mycobacterium chelonae, two species that have been associated with disease in zebrafish. Infected fish and controls were then subjected to chronic crowding and handling stressors and examined over an 8-week period. Whole-body cortisol was significantly elevated in stressed fish compared to non-stressed fish. Fish infected with M. marinum ATCC 927 and subjected to husbandry stressors had 14% cumulative mortality while no mortality occurred among infected fish not subjected to husbandry stressors. Stressed fish, infected with M. chelonae H1E2 from zebrafish, were 15-fold more likely to be infected than non-stressed fish at week 8 post-injection. Sub-acute, diffuse infections were more common among stressed fish infected with M. marinum or M. chelonae than non-stressed fish. This is the first study to demonstrate an effect of stress and elevated cortisol on the morbidity, prevalence, clinical disease and histological presentation associated with mycobacterial infections in zebrafish. Minimizing husbandry stress may be effective at reducing the severity of outbreaks of clinical mycobacteriosis in zebrafish facilities.
Keywords: cortisol, diffuse infections, husbandry stress, Mycobacteria, zebrafish
Introduction
Mycobacteriosis is a common disease of wild and cultured fish (Decostere, Hermans & Haesebrouck 2004). Among the more common species infecting fish are Mycobacterium marinum, Mycobacterium chelonae, Mycobacterium abscessus and Mycobacterium fortuitum (Chinabut 1999). Piscine mycobacteriosis is a chronic progressive disease. There are often no external signs until advanced stages of the disease during which non-specific signs are present including emaciation, haemorrhagic and dermal lesions, swelling of the abdomen due to large amounts of ascites, lethargy, and death (Gauthier & Rhodes in press). The chronic proliferative form of the disease is characterized by granulomas, while sub-acute forms of the disease are associated with necrosis and acid-fast bacilli scattered diffusely among affected tissues including the kidney, liver, spleen, and often all visceral organs (Ferguson 2006). Inflammatory responses are common in sub-acute forms but without the formation of true granulomas.
Aquarium fish are particularly susceptible to mycobacterial infections (Pate, Jenčič, Žolnir-Dovč & Ocepek 2005; Zanoni, Florio, Fioravanti, Rossi & Prearo 2008) and mycobacteria have been identified as significant pathogens of zebrafish, Danio rerio (Hamilton) (Astrofsky, Schrenzel, Bullis, Smolowitz & Fox 2000; Kent, Whipps, Matthews, Florio, Watral, Bishop-Stewart, Poort & Bermudez 2004; Seok, Koo, Kasuga, Kim, Lee, Lee, Park, Baek, Lee, Kim, Lee, Lee, Cho & Park 2006; Whipps, Dougan & Kent 2007). Zebrafish are excellent hosts for these bacteria and have been increasingly used to study the pathogenesis of mycobacteriosis (Prouty, Correa, Barker, Jagadeeswaran & Klose 2003; Tobin & Ramakrishnan 2008). The increasing popularity of zebrafish as a research model (Dahm & Geisler 2006; Lieschke & Currie 2007) has resulted in a significant increase in the number of laboratories rearing, breeding and transporting zebrafish. This increases the potential for dissemination and exacerbation of infectious diseases, such as mycobacteriosis (Kent, Feist, Harper, Hoogstraten-Miller, Law, Sánchez-Morgado, Tanguay, Sanders, Spitsbergen & Whipps 2009).
The pathogenesis of Mycobacterium spp. from zebrafish research facilities has recently been examined (Watral & Kent 2007). Infection with M. marinum isolates resulted in 100% prevalence with mortality between 30% and 100%. Infection with M. chelonae, M. peregrinum or M. abscessus isolates resulted in low to moderate infection prevalence with negligible mortality. Recently, Mycobacterium haemophilum has been identified as the causative agent of outbreaks resulting in high mortality in zebrafish facilities (Whipps et al. 2007). Conversely, in well-maintained laboratories M. chelonae usually causes minimal mortality, even when fish exhibit a relatively high prevalence of infection and histological changes (Whipps, Matthews & Kent 2008). Clearly differences in the virulence and pathogenicity of Mycobacterium spp. play a significant role in subsequent outbreaks of disease (Watral & Kent 2007), but the rearing environment may also be important.
Husbandry stress is often considered a factor in exacerbating diseases of fish. Stress is a physiological cascade of events that occurs when organisms attempt to maintain homeostatic balance after perception of a threat (Schreck, Contreras-Sánchez& Fitzpatrick 2001). The response to stress typically includes elevation of the stress hormone cortisol (Barton 2002). Fish may adapt to acute stressors but typically fail to adapt under conditions of chronic or repeated stress (Schreck 2000). Thus chronically stressed fish tend to be more susceptible to pathogens and diseases than non-stressed fish (Schreck 1996).
We have recently described increases in whole-body cortisol levels of zebrafish exposed to chronic crowding or acute handling stressors (Ramsay, Feist, Varga, Westerfield, Kent & Schreck 2006; Ramsay, Feist, Varga, Westerfield, Kent & Schreck in review). Chronic stress resulting in chronically elevated cortisol is generally immunosuppressive and often a factor contributing to increased disease prevalence and morbidity in fish populations (Kent & Hedrick 1987; Maule, Tripp, Kaattari & Schreck 1989; Saeij, Verburg-van Kemenade, van Muiswinkel & Wiegertjes 2003; Dror, Sinyakov, Okun, Dym, Sredni & Avtalion 2006), and stress has been implicated in exacerbating mycobacteriosis in zebrafish (Harriff, Bermudez & Kent 2007; Westerfield 2007).
In animal models for mycobacteria, stress has been demonstrated to suppress the immune response. Swimming stress decreased the acute inflammatory response of autoimmune-prone mice infected with Mycobacterium avium (Martins & Águas 1995). Restraint stress impaired the activation of T-cells in mice with mycobacterial infections (Zwilling, Brown, Christner, Faris, Hilburger, McPeek, Van Epps & Hartlaub 1992). Host resistance to Mycobacterium bovis was reduced in hamsters with increased serum cortisol levels (Righi, Pinheiro, Guerra & Palermo-Neto 1999; Palermo-Neto, Santos, Guerra, Santos & Pinheiro 2001). The immune response to mycobacteria has been linked to B- and T-cells as evidenced by higher susceptibility of mutants lacking these cell types. Zebrafish rag1 mutants, lacking fully functional B- and T-cells, were hyper-susceptible to infection with M. marinum (strain M) because of a failure to control bacterial growth (Swaim, Connolly, Volkman, Humbert, Born & Ramakrishnan 2006). Similar impairments to adaptive immunity may result in chronic stress (Schreck 1996) suggesting that stress may increase susceptibility to mycobacterial infections in zebrafish.
Many Mycobacterium species are ubiquitous in the aquatic environment, making control by avoidance of these pathogens very difficult. Furthermore, there is no effective treatment for mycobacteriosis in zebrafish. A better understanding of the pathogenesis of Mycobacterium spp., including factors affecting host susceptibility, may enable zebrafish researchers to manage this pathogen and prevent potential disease outbreaks through effective husbandry practices. The aim of this study was to examine the role of husbandry stressors on the susceptibility of adult zebrafish to mycobacterial infections including the prevalence of infection and cumulative mortality. Therefore, we did not examine mycobacterial isolates causing severe clinical disease and high mortality. We chose the type strain of M. marinum ATCC 927 because it resulted in a high infection prevalence and low mortality in previous studies in our laboratory (Ostland, Watral, Whipps, Austin, St-Hilaire, Westerman & Kent 2008; Watral & Kent 2007) and M. chelonae H1E2 because it resulted in a moderate infection prevalence, negligible mortality, and is commonly found in apparently healthy fish in zebrafish facilities (Whipps et al. 2008).
Materials and methods
Bacteria and growth conditions
Mycobacterium marinum ATCC 927 was obtained from the American Type Culture Collection (Manassas, VA, USA) and was first isolated over 80 years ago from aquarium fish (Aronson 1926). Prior to infection of the fish in our study, M. marinum was passed through hybrid striped bass (Morone saxatilis × Morone chrysops; Ostland et al. 2008), re-isolated on Lowenstein-Jensen medium (Remel) and used for an in vivo pathogenesis study (Watral & Kent 2007; Ostland et al. 2008). The M. chelonae H1E2 was isolated from zebrafish (TU strain) during routine screening at a zebrafish facility (Whipps et al. 2008).
The growth conditions of each M. marinum and M. chelonae were identical. Fresh colonies of bacteria were suspended in Middlebrook 7H9 broth containing 1% albumin-dextrose-catalase (Becton Dickson) with 0.1% Tween (Sigma-Aldrich) to prevent clumping of the bacteria. The cultures were placed on a rocker at 28 °C for 5 days. The presence of mycobacteria in the cultures was verified by acid-fast staining via the Kinyoun method (Hardy Diagnostics). Concentrations of the inocula were adjusted using McFarlane’s standards and verified by plating on Middlebrook 7H10 media with 10% oleic acid-albumin dextrose-catalase (Becton Dickson).
Fish husbandry and experimental design
Zebrafish (AB strain; 15–19 months old; 2/3 males, 1/3 females) from the Zebrafish International Resource Center in Eugene (OR, USA) were randomly allocated to acrylic tanks (10 L) in a Bio-safety Level 2 Laboratory at Oregon State University in Corvallis (OR, USA). Tanks were filled with static de-chlorinated city water and fish were allowed to acclimatise for 2 weeks at a density of 1.5–2 fish per litre. Water temperature was maintained at 27–28 °C. Ammonia and nitrite levels were monitored daily using test kits and water changes were performed periodically. Additionally, box-type aquarium filters with porous lava rock were placed into each tank for biological filtration. Fish were fed twice daily (Zeigler adult zebrafish diet; Zeigler Bros Inc.) during the acclimatisation and experimental periods.
Mycobacterium marinum-stress study. Treatments were randomly assigned to 12 tanks each containing 15 fish (180 fish total; average wet weight: 541 ± 30 mg). Four tanks were assigned to be M. marinum-infected tanks; two of these tanks were assigned to be stressed (M. marinum-stress) and two were assigned to receive no stress (M. marinum-control). The remaining eight tanks were assigned to be sham-infected; four of these tanks were assigned to be stressed (sham-stress) and four were assigned to receive no stress (sham-control). Fish in all four of the M. marinum-infection tanks were processed for histology and cultured for bacteria. Fish in two sham-stress and two sham-control tanks were processed for histology and cultured for bacteria. Fish in the remaining two sham-stress and two sham-control tanks were examined for whole-body cortisol only. We recognized that infection with mycobacteria may have constituted a stressor to the fish and subsequently increased cortisol. However, it was beyond the scope of this study to determine the dynamics of the cortisol response to mycobacterial infection. Therefore, we did not measure cortisol in M. marinum-infected groups.
Mycobacterium chelonae-stress study. We repeated the protocol used for the M. marinum study using M. chelonae, but we did not measure whole-body cortisol during this study. We therefore used four tanks injected with M. chelonae (two M. chelonae-stress, two M. chelonae-control) and four sham-injected (two sham-stress, two sham-control). Each tank contained 17–20 fish; a total of 109 fish were used for this study. The average wet weight of fish was 484 mg (±20 mg).
Infection of fish and stress protocol
Exposure to the bacteria was performed as described by Watral & Kent (2007). Briefly, fish were anaesthetized with 150 mg L−1 buffered tricaine methane sulphonate (MS-222; Argent). Each fish from the infection tanks was intraperitoneally (i.p.) injected with 50 μL inoculum to achieve a target dose of 5 × 104 colony forming units (CFU) per fish. The actual dose received by fish in the M. marinum study was 1 × 102 CFU per fish while fish from the M. chelonae study received the target dose of 5 × 104 CFU. Fish from the infection-control tanks were sham-injected i.p. with sterile distilled water (50 μL).
One week following injection, stressors were administered to the stress tanks. A continuous crowding stressor was administered for the duration of the studies. Fish were crowded in rectangular breeding cage inserts, with a false bottom, placed into the 10 L tanks so the edges of the breeding cage rested on the top edges of the tank; the crowding density was 30 fish L−1. In addition to continuous crowding, various handling stressors were administered randomly to ensure the fish did not acclimatise to the stressors. Fish were exposed to air by lifting the breeding cage out of the water for various durations from 30 s to 3 min. This was often repeated, either immediately or later in the day (within 2–6 h). Rapid repeated emersion and immersion of fish out of and into the water was also performed for periods up to 3 min. Low water handling was performed by holding the fish in the breeding cages so their backs were out of the water for periods up to 3 min. Handling of fish was performed at least once daily for the duration of the experiment. M. marinum-control and sham-control groups were maintained at the acclimatisation density (1.5–2 fish L−1) and not handled. Whole-body cortisol levels of sham-control and sham-stress groups were measured at 1 week (seven fish per tank) and two weeks (eight fish per tank) after stressors were initiated.
Cumulative mortality was measured over an 8 week experimental period which started after the fish were injected. Moribund fish were removed from the tank just prior to death. Mycobacterial infections were evaluated by histology and culture for M. marinum study and by histology alone for the M. chelonae study. The number of infected fish in each group was compared at week 4 (five fish per tank) post-injection (PI) and the remaining fish were sampled and evaluated at week 8 PI. Additionally, the numbers of fish with diffuse (subacute) infections were compared between groups at week 4 and 8 PI. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Oregon State University (ACUP #3144).
Histology
Upon sampling, fish were euthanized with buffered MS-222 (500 mg L−1), placed in Dietrich’s fixative (Gray 1954) and processed for histology. Prior to processing, preserved fish were de-calcified using 5% trichloroacetic acid in Dietrich’s fixative. Midsagittal sections were cut and stained using a modified Kinyoun’s cold acid-fast stain to identify mycobacteria. Briefly, slides were de-paraffinized, rehydrated and placed into carbol-fuchsin (8 g basic fuchsin HCl, 40 mL 95% ethanol, 200 mL deionized water, 16 mL liquefied phenol) for 15 min. De-staining was performed in a 1% hydrochloric acid solution in 70% ethanol for 2 min. Methylene blue was used to counter-stain (one dip immersion).
Bacterial culture
For the M. marinum study, prior to processing for histology, bacteria were cultured from each fish. The entire spleen and the distal third of the liver were removed from each fish and placed in 100 μL phosphate-buffered saline (PBS). A 1% cetylpyridinium chloride solution (100 μL) was added to this preparation. The tissues were manually homogenized and incubated overnight at room temperature to remove contaminants. The samples were rinsed twice with PBS, re-suspended with 100 μL PBS and plated on Middlebrook 7H10 plates. The plates were incubated for 7–10 days at 28 °C and bacterial growth (CFU) was recorded. Bacteria were not cultured for the M. chelonae study as a previous study with this same isolate in our laboratory showed an excellent correlation between culture and histology using acid fast staining (Whipps et al. 2008).
Whole-body cortisol
Whole-body cortisol was measured using the method of Ramsay et al. (2006). Whole zebrafish were homogenized and extracted for cortisol using diethyl ether. Samples were corrected for extraction efficiency and weight. Cortisol was measured in the extracted samples using a radioimmunoassay. Consistency between assays was verified by measuring cortisol in whole-body extracts spiked with known concentrations of cortisol. Intra-assay and inter-assay variation was accepted at no more than 10%.
Statistical analyses
Statistical analyses were performed using S-PLUS 7 (Insightful Corp. 2005). Whole-body cortisol levels of duplicate treatment tanks were compared using a Welch’s modified t-test and pooled if there was no significant difference between the duplicates. Pooled stressed and non-stressed groups at weeks 1 and 2 post-stress were then compared using an analysis of variance (ANOVA) with Fisher’s least significant difference (LSD).
Fisher’s exact tests were used to determine the strength of associations between treatment groups and prevalence of infection or prevalence of diffuse infection. An odds ratio (OR) was used to determine whether the odds of being infected were different between stressed and non-stressed groups. ORs were also used to determine if the odds of having a diffuse infection were greater between stressed and non-stressed groups. The odds were considered significant at OR > 2 (Ludbrook 2008). Comparisons were made within each study but not between studies. Significance differences between treatment groups were reported at ≤0.05.
Results
Background infection with Pseudoloma neurophilia
During the M. marinum study, the microsporidium, P. neurophilia, a parasite of the central nervous system of zebrafish, was found in many histological sections of the fish. The prevalence of P. neurophilia was 57% in the sham-injected tanks and 81% in the M. marinum-injected tanks (Table 1). There was a strong association between infection group (M. marinum-injected vs. sham-injected) and the prevalence of P. neurophilia infection (P = 0.0076). Pseudoloma neurophilia was not detected in the fish from the M. chelonae experiment.
Table 1.
Zebrafish were injected with Mycobacterium marinum or Mycobacterium chelonae or distilled water (DW; sham-injection) and some were exposed to stressors over an 8-week period
| Positive (culture or histology) |
Diffuse infections |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection | Tank | Inoculum (×102) | Stressor | Moribund | Week 4 PI | Week 8 PI | Moribund | Week 4 PI | Week 8 PI | Moribund | Pseudoloma positive |
| Mycobacterium marinum | A | 1 | Y | 1/15 | 5/5 | 8/9 | 1/1 | 3/5 | 5/9 | 1/1 | 13/15 |
| B | 1 | Y | 3/14 | 5/5 | 6/6 | 3/3 | 2/5 | 5/6 | 3/3 | 14/14 | |
| A | 1 | N | 0/14 | 5/5 | 9/9 | NA | 1/5 | 2/9 | NA | 10/14 | |
| B | 1 | N | 0/15 | 5/5 | 10/10 | NA | 1/5 | 2/10 | NA | 10/15 | |
| DW | A | 0 | Y | 1/15 | 0/5 | 0/5 | 0/1 | NA | NA | 0/1 | 5/15 |
| B | 0 | Y | 0/15 | 0/5 | 0/5 | NA | NA | NA | NA | 9/15 | |
| A | 0 | N | 1/15 | 0/5 | 0/5 | 0/1 | NA | NA | 0/1 | 10/15 | |
| B | 0 | N | 0/15 | 0/5 | 0/5 | NA | NA | NA | NA | 9/15 | |
| Mycobacterium chelonae | A | 500 | Y | 0/18 | 4/9 | 9/9 | NA | 3/9 | 9/9 | NA | 0/17 |
| B | 500 | Y | 0/16 | 4/7 | 8/9 | NA | 4/7 | 7/9 | NA | 0/18 | |
| A | 500 | N | 0/14 | 5/7 | 4/7 | NA | 5/7 | 2/7 | NA | 0/14 | |
| B | 500 | N | 0/18 | 5/8 | 5/10 | NA | 5/8 | 5/10 | NA | 0/18 | |
| DW | A | 0 | Y | 0/21 | 1/10 | 0/11 | NA | 0/10 | NA | NA | 0/21 |
| B | 0 | Y | 11/21* | 0/10 | 0/11 | NA | NA | NA | NA | 0/10 | |
| A | 0 | N | 0/21 | 0/10 | 0/11 | NA | NA | NA | NA | 0/21 | |
| B | 0 | N | 0/21 | 0/10 | 0/11 | NA | NA | NA | NA | 0/21 | |
Y, yes; N, no; NA, not applicable.
Replicate tanks of the same treatment are indicated by the letters A and B. Morbidity (proportion of fish) was measured over an 8 week period. The prevalence of infection was evaluated by culture or histology at weeks 4 and 8 post-injection (PI). The proportion of fish with diffuse infections was measured at weeks 4 and 8 PI. The proportion of fish which were positive for Pseudoloma neurophilia via histology was measured.
Unexplained mortality of a sham-injected tank in Mycobacterium chelonae study at week 5 PI (no mycobacteria present).
Whole-body cortisol (Mycobacterium marinum study)
There were no significant differences between replicates of the same treatment; therefore the data were pooled. Whole-body cortisol was significantly higher in the stressed groups compared with the control groups (Fig. 1). There was no difference between the cortisol of the control groups from week 1 to 2 post-stress. However, among the stressed groups cortisol was significantly lower at week 2 compared with week 1 post-stress.
Figure 1.
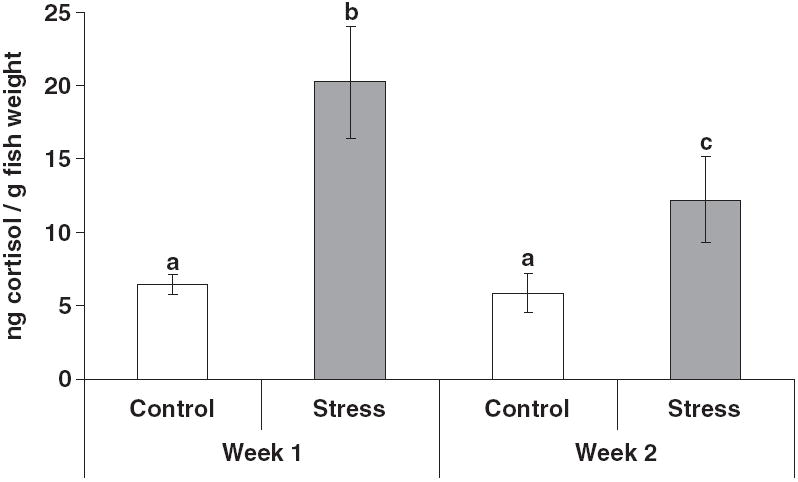
Mycobacterium marinum-stress experiment. Mean whole-body cortisol (ng g−1 fish; +SEM; n = 15) of control groups (no stressors) and stress groups (chronic crowding and random daily handling) 1 and 2 weeks after the stressors were initiated. Replicate tanks of the same treatment were pooled because they were not significantly different. Different letters above the standard error bars indicate a significant difference between treatment groups (P < 0.05).
Histopathology and culture
Infected fish from both the M. chelonae and M. marinum studies exhibited characteristic mycobacteriosis including granulomas containing acid-fast bacilli in the visceral organs and kidney (Fig. 2a,b,e,f). Additionally, diffuse infections associated with inflammation caused by acid-fast bacilli scattered in the kidney, liver and gastrointestinal tract were identified in numerous individuals, particularly among stressed fish (Fig. 2c,d,g,h).
Figure 2.
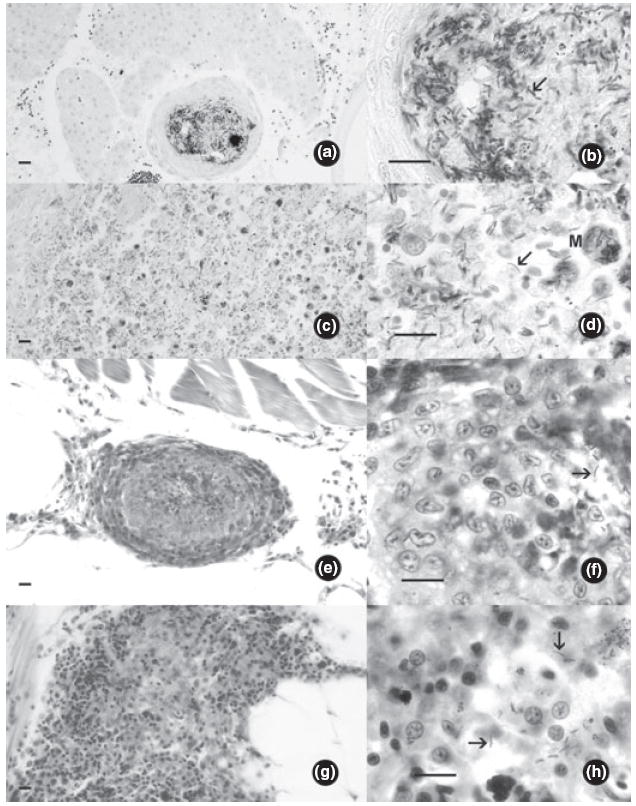
Histological sections of zebrafish infected with Mycobacterium spp. (acid-fast staining). Arrows indicate individual bacteria in lesions (bars = 10 μm). (a) Mycobacterium marinum. Granuloma in liver. (b) High magnification of A. (c) Mycobacterium marinum, diffuse infection in anterior kidney, with numerous bacteria within phagocytes. (d) High magnification of C, M = macrophage replete with bacteria. (e) Mycobacterium chelonae. Granuloma in mesenteries ventral to kidney. (f) High magnification of E showing bacteria. (g) Mycobacterium chelonae, diffuse infection in mesenteries. (h) High magnification of G showing bacteria.
Acid-fast bacterial colonies were recovered from 20 infected fish from both the M. marinum-stress and M. marinum-control groups. Colony counts ranged from 1 × 102 to 7 × 104 CFU (Fig. 3). Individuals with the highest bacterial counts were from the stressed groups. Histopathology was more sensitive at identifying M. marinum infections than culture (Table 2).
Figure 3.
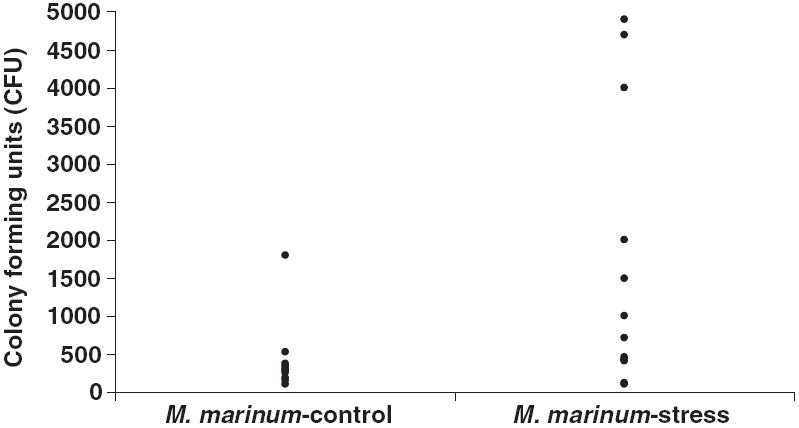
Bacterial colony counts from individual zebrafish from the Mycobacterium marinum study. Counts ranged from 100 to 70 000 colony-forming units (CFU). One individual in the M. marinum-stress group had a colony count 70 000 CFU (not shown on figure).
Table 2.
Proportion of zebrafish positive for mycobacteria by culture (acid-fast colonies) and histology (acid-fast bacterial rods) at weeks 4 and 8 post-injection (PI)
| Week 4 PI |
Week 8 PI |
|||
|---|---|---|---|---|
| Culture | Histopathology | Culture | Histopathology | |
| Mycobacterium marinum-stress tank | ||||
| A | 2/5 | 2/5 | 3/9 | 5/9 |
| B | 1/5 | 4/5 | 3/5 | 3/5 |
| Mycobacterium marinum-control tank | ||||
| A | 0/5 | 4/5 | 2/9 | 6/9 |
| B | 0/5 | 4/5 | 8/10 | 7/10 |
Cumulative mortality over time
During the M. marinum-study, there was no morbidity among the M. marinum-control tanks. Cumulative mortality was 14% (four of 29 died) among the M. marinum-stress tanks (Fig. 4; Table 1). Interestingly, there was 3% mortality (one of 29 died) in both the sham-control and sham-stress tanks but there was no evidence of mycobacterial infection in any individuals from these groups. However, many of the individuals in these sham-infected tanks were infected with P. neurophilia (Table 1).
Figure 4.
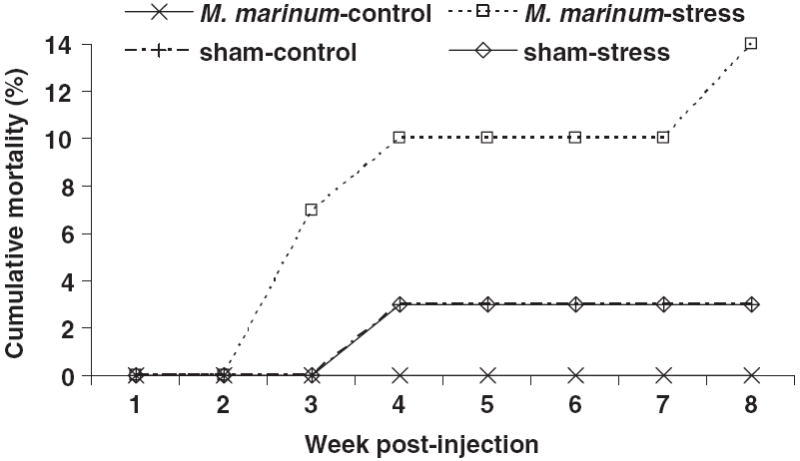
Cumulative mortality (%) of Mycobacterium marinum-injected zebrafish subjected to stress and not stressed (control) and sham-injected fish subjected to stress and not stressed (control) over time (week post-injection).
For the M. chelonae-stress study, all fish in one of the control-stress tanks unexpectedly died at week 5 PI (n = 11). There were no mycobacteria or other pathogens identified from this group. There was no additional mortality among any of the other groups.
Prevalence of mycobacterial infections
None of the control fish in the M. marinum-stress study became infected with mycobacteria (0/60 fish infected). Conversely, almost all the fish injected with M. marinum were infected at both week 4 (20/20 fish infected) and 8 PI (33/34 fish infected; Table 1). Therefore, OR were not compared for the M. marinum-stress study because the odds of being infected, among the M. marinum groups, were the same.
For the M. chelonae-stress study there was a single fish in one of the sham-stress tanks that had acid-fast mycobacterial rods in the swim bladder (1/21 fish infected). There was no further histological evidence of mycobacteria in any visceral tissues and no internal or external signs of mycobacteriosis.
Among the M. chelonae-infected groups, at week 4 PI, there was no association between stress treatment and infection (P = 0.473); 8/16 fish were infected in the stressed group and 10/15 infected in the non-stressed group. However, at week 8 PI, there was a positive association between stress treatment and infection (P = 0.03); the stressed group (17/18 fish infected) was over 15-fold more likely to be infected than the non-stressed group (9/17 fish infected; OR = 15.11).
Prevalence of diffuse infections
There was a strong association between stress treatment and diffuse infections for fish infected with M. marinum (P = 0.0012; Table 1). Stressed fish infected with M. marinum were almost sevenfold more likely to have diffuse infections compared to fish that were not stressed (OR = 6.97). At week 4 PI, there was no association between stress treatment and diffuse infections for M. chelonae-infected fish (P = 0.285). However, by week 8 PI, there was a strong association between stress treatment and M. chelonae-infection (P = 0.0045); stressed fish were over 11-fold more likely to have diffuse infection than non-stressed fish (OR = 11.43).
Discussion
Mycobacteriosis is a significant disease of laboratory zebrafish (Astrofsky et al. 2000; Kent et al. 2004; Whipps et al. 2007), with no effective treatments currently available. Indeed, zebrafish are a highly susceptible species based on laboratory transmission studies comparing zebrafish to medaka, Oryzias latipes (Temminck & Schlegel) (Broussard & Ennis 2007), and hybrid striped bass, M. chrysops × M. saxatilis (Ostland et al. 2008). Better understanding the factors affecting dissemination and exacerbation of this disease may aid in controlling outbreaks in zebrafish colonies. We have demonstrated husbandry stress exacerbates mycobacterial infections in adult zebrafish. To our knowledge, this is the first study linking stress and disease in laboratory zebrafish.
We detected background infection with the microsporidium, P. neurophilia during the M. marinum study. Mycobacterium spp. and P. neurophilia are both common pathogens of laboratory zebrafish and co-infection is not uncommon (M.L. Kent, V. Watral & J.M. Ramsay, personal observations). Pseudoloma neurophilia infects the central nervous system and somatic muscle of zebrafish and is associated with emaciation, spinal deformity and low-level chronic mortality (Matthews, Brown, Larison, Bishop-Stewart, Rogers & Kent 2001; Kent & Bishop-Stewart 2003). The low-level mortality among the sham-control and sham-stress groups, which were negative for mycobacteria, may be attributable to P. neurophilia. Interestingly we found a higher prevalence of microsporidiosis in zebrafish infected with M. marinum. Microsporidiosis tends to be more prevalent in immune-suppressed individuals (Didier & Weiss 2006) and mycobacteriosis has been demonstrated to suppress immunity (Fenton & Vermeulen 1996; Moura & Mariano 1997). Loma salmonae (Microsporidia)-infected rainbow trout, Oncorhynchus mykis (Walbaum), treated with the immune-suppressing synthetic steroid dexamethasone, had increased parasitic infections compared to untreated fish (Lovy, Speare, Stryhn & Wright 2008). Interestingly, stress alone did not appear to exacerbate P. neurophilia infections, even though stress has been demonstrated to be immunosuppressive (Schreck 1996). However, the effects of stress in P. neurophilia infections have not been fully examined and we are reluctant to dismiss its role in pathogenesis. Further investigation on the effects of co-infection with mycobacteria and microsporidia may be useful in controlling these common pathogens of zebrafish.
In the M. chelonae study, we found one fish in the non-infected group with a mycobacterial infection of the swim bladder. Similar background levels of mycobacteria have been found in previous studies in our laboratory (Harriff et al. 2007). Environmental mycobacteria, such as M. chelonae and M. fortuitum, are common in freshwater aquaria (Zanoni et al. 2008), and M. fortuitum is commonly isolated from fish without causing disease (Beran, Matlova, Dvorska, Svastova & Pavlik 2006). Our laboratory has examined hundreds of histological sections of zebrafish stained with acid-fast through our diagnostic service of the National Institutes of Health Zebrafish International Resource Center (http://zebrafish.org/zirc/health/index.php), and we frequently see acid-fast bacteria only in the lumina of the intestine and swim bladder. The swim bladder can easily be colonized via the gut as zebrafish are physostomous. Moreover, infection of this organ is a common finding in zebrafish mycobacteriosis (Whipps et al. 2008).
Mycobacterial infections were more readily identified by histopathology than culture for the M. marinum study. Mycobacterium marinum is fastidious in culture making this method of identification less sensitive than histology for this particular species of mycobacteria (Kent et al. 2004). Conversely, there is a strong correlation between culture and histopathology with M. chelonae (Whipps et al. 2008). Mycobacterium chelonae exhibits more rapid growth than M. marinum which could account for the better correlation between culture and histopathology (Whipps et al. 2008).
Among the M. marinum-injected group we were able to achieve almost 100% infection after injection with 1 × 102 CFU with no mortality among groups not subjected to chronic stressors. Similarly, 100% of zebrafish were infected at a dose of 103 CFU, with no mortality (Prouty et al. 2003). Our dosage used in the present study was much lower than that used in previous virulence trials with zebrafish (Watral & Kent 2007; Ostland et al. 2008), where injection of 5 × 104 CFU of the same strain of M. marium caused 100% infection and about 30% mortality in non-stressed fish. Identifying the minimum and maximum dose required to achieve a chronic infection with no mortality may be useful for studying the effects of stress during chronic mycobacteriosis.
Whole-body cortisol levels were higher for stressed than non-stressed groups, and consistent with our other studies (Ramsay et al. 2006; Ramsay et al. in review). Stressed groups, infected with M. marinum, experienced 14% cumulative mortality while non-stressed groups experienced no mortality. In a previous study, zebrafish injected with 5 × 104 CFU of the same strain of M. marinum (ATCC 927) had 30% mortality over an 8 week period (Watral & Kent 2007) suggesting that chronic stress and elevated cortisol may lower the dose of M. marinum which will result in mortality. This agrees with studies on other species. Daily handling significantly increased plasma cortisol levels and reduced the survival of carp, Cyprinus carpio L., injected with Trypanoplasma borreli (Saeij et al. 2003). Goldfish infected with Aeromonas salmonicida and subjected to handling stress had significantly elevated plasma cortisol levels associated with decreased survival compared to non-stressed groups (Dror et al. 2006). Chronic cortisol injection of leopard frogs, Rana pipiens, injected with M. marinum (M) resulted in 70% mortality over a 19-week period while frogs not treated with cortisol experienced no mortality (Ramakrishnan, Valdivia, McKerrow & Falkow 1997). Mice injected with M. tuberculosis experienced similar increases in mortality when injected with corticosteroids (Batten & McCune 1957).
Fish injected with M. chelonae did not experience any mortality over an 8-week period. Mycobacterium chelonae is typically found at background levels in zebrafish facilities and has been associated with low levels of mortality (<20%) which are often attributed to poor husbandry and stress (Astrofsky et al. 2000; Kent et al. 2004; Whipps et al. 2008). In our experiment stressed zebrafish infected with M. chelonae did not experience any mortality over the 8-week period, but stressed individuals were 15-fold more likely to be infected and show histological lesions compared to non-stressed individuals. Comparable results were obtained in catfish, Ictalurus punctatus (Rafinesque), where the percentage of fish infected with Edwardsiella ictaluri was higher in stressed than in non-stressed animals (Small & Bilodeau 2005). Although the infected-stressed fish did not die over the 8-week study, the increased prevalence of infection by this common, ubiquitous bacterium probably increases the likelihood of mortality associated with infection. Moreover, with fish used in research, underlying chronic conditions that cause low-grade or subclinical infections should be avoided because they may add non-protocol induced variables, including inadvertent alterations in the physiology of the host, which were not accounted for in the original experimental design; this may confound research results (Baker 2003; Kent et al. 2009).
Diffuse infections were greater in stressed groups than in non-stressed groups for both M. marinum and M. chelonae infected fish. This was likely due to impaired ability to sequester the bacteria in granulomas. For example, zebrafish (rag1) with impaired immunity failed to control mycobacterial growth resulting in increased diffuse infections (Swaim et al. 2006). In leopard frogs, injection with cortisol resulted in impaired formation of granulomas and increased replication of mycobacteria in tissues (Ramakrishnan et al. 1997). Stress generally reduces the host response (acute inflammatory, T-helper cell activation) to mycobacterial infections subsequently increasing host susceptibility to infection (Zwilling et al. 1992; Martins & Águas 1995; Palermo-Neto et al. 2001).
In conclusion, husbandry stress increased clinical disease associated with mycobacterial infections. With M. marinum, stressors increased mortality and diffuse infections. With M. chelonae (a less virulent bacterium), stressors increased the prevalence of infection and diffuse infections, but did not cause mortality. Mycobacteria are ubiquitous in the environment making eradication of this pathogen very difficult. Effective management of mycobacteria by minimizing husbandry stress in the zebrafish laboratory may be a reasonable means of controlling outbreaks of disease and ensuring continued success in zebrafish research.
Acknowledgments
The authors thank Martha Barr and Brenna Barr for assistance with fish care and Dr Christopher Whipps for advice on mycobacterial culture and strains. The authors thank Drs Monte Westerfield, John Leatherland and Dixon Landers for their review of the manuscript. This study was supported by grants from the National Institutes of Health (NIH NCRR 5R24RR017386-02 and NIH NCRR P40 RR12546-03S1).
References
- Aronson JD. Spontaneous tuberculosis in salt water fish. Journal of Infectious Diseases. 1926;39:315–320. [Google Scholar]
- Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comparative Medicine. 2000;50:666–672. [PubMed] [Google Scholar]
- Baker D. Natural Pathogens of Laboratory Animals: Their Effects on Research. ASM Press; Herdon, VA: 2003. [Google Scholar]
- Barton BA. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integrative and Comparative Biology. 2002;42:517–525. doi: 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- Batten JC, McCune RM., Jr The influence of corticotrophin and certain corticosteroids on populations of Mycobacterium tuberculosis in tissues of mice. British Journal of Experimental Pathology. 1957;38:413–423. [PMC free article] [PubMed] [Google Scholar]
- Beran V, Matlova L, Dvorska L, Svastova P, Pavlik I. Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment. Journal of Fish Diseases. 2006;29:383–393. doi: 10.1111/j.1365-2761.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- Broussard GW, Ennis DG. Mycobacterium marinum produces long-term chronic infections in medaka: a new animal model for studying human tuberculosis. Comparative Biochemistry and Physiology, Part C. 2007;145:45–54. doi: 10.1016/j.cbpc.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinabut S. Mycobacteriosis and nocardiosis. In: Woo PTK, Bruno DW, editors. Fish Diseases and Disorders. Vol. 3. CABI Publishing; New York, NY: 1999. pp. 319–340. [Google Scholar]
- Dahm R, Geisler R. Learning from small fry: the zebrafish as a genetic model organism for aquaculture fish species. Marine Biotechnology. 2006;8:329–345. doi: 10.1007/s10126-006-5139-0. [DOI] [PubMed] [Google Scholar]
- Decostere A, Hermans K, Haesebrouck F. Piscine mycobacteriosis: a literature review covering the agent and the disease it causes in fish and humans. Veterinary Microbiology. 2004;99:159–166. doi: 10.1016/j.vetmic.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Didier ES, Weiss LM. Microsporidiosis: current status. Current Opinion in Infectious Diseases. 2006;19:485–492. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror M, Sinyakov MS, Okun E, Dym M, Sredni B, Avtalion RR. Experimental handling stress as infection-facilitating factor for the goldfish ulcerative disease. Veterinary Immunology and Immunopathology. 2006;109:279–287. doi: 10.1016/j.vetimm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infection & Immunity. 1996;64:683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson HW. Systemic Pathology of Fish. Scotian Press; London: 2006. [Google Scholar]
- Gauthier DT, Rhodes MW. Mycobacteriosis in fishes: a review. The Veterinary Journal. 2008;180:33–47. doi: 10.1016/j.tvjl.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Gray P. The Microtomist’s Guide and Formulary. The Blakiston Company Inc.; New York, NY: 1954. [Google Scholar]
- Harriff MJ, Bermudez LE, Kent ML. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: a potential model for environmental mycobacterial infection. Journal of Fish Diseases. 2007;30:587–600. doi: 10.1111/j.1365-2761.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton) Journal of Fish Diseases. 2003;26:423–426. doi: 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Hedrick RP. Effects of cortisol implants on the PKX myxosporean causing proliferative kidney disease in rainbow trout, Salmo gairdneri. Journal of Parasitology. 1987;73:455–461. [PubMed] [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comparative Biochemistry and Physiology, Part C, Pharmacology, Toxicology & Endocrinology. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sánchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comparative Biochemistry and Physiology, Part C. 2009;140:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nature Reviews Genetics. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lovy J, Speare DJ, Stryhn H, Wright GM. Effects of dexamethasone on host innate and adaptive immune responses and parasite development in rainbow trout Oncorhynchus mykiss infected with Loma salmonae. Fish & Shellfish Immunology. 2008;24:649–658. doi: 10.1016/j.fsi.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Ludbrook J. Analysis of 2 × 2 tables of frequencies: matching test to experimental design. International Journal of Epidemiology. 2008;37:1430–1435. doi: 10.1093/ije/dyn162. [DOI] [PubMed] [Google Scholar]
- Martins TC, Águas AP. Stress modulates acute inflammation triggered by mycobacteria in autoimmunity-prone and normal mice. Inflammation Research. 1995;44:393–399. doi: 10.1007/BF01797867. [DOI] [PubMed] [Google Scholar]
- Matthews JL, Brown AMV, Larison K, Bishop-Stewart JK, Rogers P, Kent ML. Pseudoloma neurophilia n. g., n sp., a new microsporidium from the central nervous system of the zebrafish (Danio rerio) Journal of Eukaryotic Microbiology. 2001;48:227–233. doi: 10.1111/j.1550-7408.2001.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Maule AG, Tripp RA, Kaattari SL, Schreck CB. Stress alters immune function and disease resistance in Chinook salmon (Oncorhynchus tshawytscha) Journal of Endocrinology. 1989;120:135–142. doi: 10.1677/joe.0.1200135. [DOI] [PubMed] [Google Scholar]
- Moura ACN, Mariano M. Lipids from Mycobacterium leprae cell wall suppress T-cell activation in vivo and in vitro. Immunology. 1997;92:429–436. doi: 10.1046/j.1365-2567.1997.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostland VE, Watral V, Whipps CM, Austin FW, St-Hilaire S, Westerman ME, Kent ML. Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass Morone chrysops × M saxatilis and zebrafish Danio rerio. Diseases of Aquatic Organisms. 2008;79:107–118. doi: 10.3354/dao01891. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J, Santos FA, Guerra JL, Santos GO, Pinheiro SR. Glue solvent inhalation impairs host resistance to Mycobacterium bovis-induced infection in hamsters. Veterinary and Human Toxicology. 2001;43:1–5. [PubMed] [Google Scholar]
- Pate M, Jenčič V, Žolnir-Dovč M, Ocepek M. Detection of mycobacteria in aquarium fish in Slovenia by culture and molecular methods. Diseases of Aquatic Organisms. 2005;64:29–35. doi: 10.3354/dao064029. [DOI] [PubMed] [Google Scholar]
- Prouty MG, Correa NE, Barker LP, Jagadeeswaran P, Klose KE. Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiology Letters. 2003;225:177–182. doi: 10.1016/S0378-1097(03)00446-4. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Valdivia RH, McKerrow JH, Falkow S. Mycobacterium marinum causes both long-term subclinical infection and acute disease in the leopard frog (Rana pipiens) Infection & Immunity. 1997;65:767–773. doi: 10.1128/iai.65.2.767-773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Rapid cortisol response of zebrafish to acute net handling stress. Aquaculture. doi: 10.1016/j.aquaculture.2009.08.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture. 2006;258:565–574. [Google Scholar]
- Righi DA, Pinheiro SR, Guerra JL, Palermo-Neto J. Effects of diazepam on Mycobacterium bovis-induced infection in hamsters. Brazilian Journal of Medical and Biological Research. 1999;32:1145–1153. doi: 10.1590/s0100-879x1999000900014. [DOI] [PubMed] [Google Scholar]
- Saeij JP, Verburg-van Kemenade LB, van Muiswinkel WB, Wiegertjes GF. Daily handling stress reduces resistance of carp to Trypanoplasma borreli: in vitro modulatory effects of cortisol on leukocyte function and apoptosis. Developmental and Comparative Immunology. 2003;27:233–245. doi: 10.1016/s0145-305x(02)00093-9. [DOI] [PubMed] [Google Scholar]
- Schreck CB. Immunomodulation: endogenous factors. In: Iwama G, Nakanishi T, editors. The Fish Immune System: Organism, Pathogen, and Environment. Academic Press; London: 1996. pp. 311–337. [Google Scholar]
- Schreck CB. Accumulation and long-term effects of stress in fish. In: Moberg GP, Mench JA, editors. The Biology of Animal Stress: Assessment and Implications for Animal Welfare. CAB International; Wallingford: 2000. pp. 147–158. [Google Scholar]
- Schreck CB, Contreras-Sánchez W, Fitzpatrick MS. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture. 2001;197:3–24. [Google Scholar]
- Seok SH, Koo HC, Kasuga A, Kim Y, Lee EG, Lee H, Park JH, Baek MW, Lee HY, Kim DJ, Lee BH, Lee YS, Cho SN, Park JH. Use of PCR-restriction fragment length polymorphism for the identification of zoonotic mycobacteriosis in zebrafish caused by Mycobacterium abscessus and Mycobacterium chelonae. Veterinary Microbiology. 2006;114:292–297. doi: 10.1016/j.vetmic.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Small BC, Bilodeau AL. Effects of cortisol and stress on channel catfish (Ictalurus punctatus) pathogen susceptibility and lysozyme activity following exposure to Edwardsiella ictaluri. General and Comparative Endocrinology. 2005;142:256–262. doi: 10.1016/j.ygcen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, Ramakrishnan L. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infection & Immunity. 2006;74:6108–6117. doi: 10.1128/IAI.00887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Ramakrishnan L. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cellular Microbiology. 2008;10:1027–1039. doi: 10.1111/j.1462-5822.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comparative Biochemistry and Physiology, Part C. 2007;145:55–60. doi: 10.1016/j.cbpc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5. University of Oregon Press; Eugene, OR: 2007. [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiology Letters. 2007;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish (Danio rerio) Diseases of Aquatic Organisms. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni RG, Florio D, Fioravanti ML, Rossi M, Prearo M. Occurrence of Mycobacterium spp. in ornamental fish in Italy. Journal of Fish Diseases. 2008;31:433–441. doi: 10.1111/j.1365-2761.2008.00924.x. [DOI] [PubMed] [Google Scholar]
- Zwilling BS, Brown D, Christner R, Faris M, Hilburger M, McPeek M, Van Epps C, Hartlaub BA. Differential effect of restraint stress on MHC class II expression by murine peritoneal macrophages. Brain, Behavior and Immunity. 1992;4:330–338. doi: 10.1016/0889-1591(90)90036-p. [DOI] [PubMed] [Google Scholar]


