Abstract
For combination antihypertensive therapy with thiazide diuretics and β-blockers, the effect of the order of initiation of the drugs on the outcome has not been tested. Patients with uncomplicated hypertension were randomized to receive either hydrochlorothiazide (HCTZ) or atenolol monotherapy, followed by addition of the alternative drug. Blood pressure (BP) responses were evaluated by race and order of drug initiation. A total of 368 participants received combination therapy. Among the participants, blacks showed a greater BP-lowering effect than whites did with HCTZ monotherapy (−13.0/−7.4 mm Hg vs. −8.0/−4.2 mm Hg, P < 0.001) but a smaller BP-lowering effect than did whites with atenolol monotherapy (−1.1/−2.9 mm Hg vs. −9.9/−9.2 mm Hg, P < 0.0001). These differences were not evident during combination therapy. However, both groups showed greater response to HCTZ + atenolol than to atenolol + HCTZ (−19.1/−14.2 mm Hg vs. −15.6/−11.3 mm Hg, P < 0.0001). Despite optimal dosing of HCTZ + atenolol, only two-thirds of the participants achieved BP control. In HCTZ/atenolol combination antihypertensive therapy, the order in which the drugs are initiated affects total BP lowering during the first 4–6 months of therapy.
Hypertension affects approximately 73 million Americans and is a predominant risk factor for stroke, heart failure, ischemic heart disease/myocardial infarction, and chronic renal failure.1 Current Joint National Commission 7 consensus guidelines recommend thiazide diuretics as the preferred antihypertensive therapy in patients with uncomplicated hypertension, with β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers also considered appropriate first-line therapy.2 These guidelines also recommend that, in patients for whom a thiazide was not the initial therapy, it should be the second drug added when combination therapy is required.
Although the Joint National Commission 7 guidelines state a preference for thiazide diuretics as the initial therapy in all patients with uncomplicated hypertension, this is not universally accepted, as other guidelines suggest preference for a more patient-specific approach or simply disagree with the primacy of thiazide diuretics.3–5 There is less debate about the commission’s recommendation that, for any patient with uncomplicated hypertension requiring two drugs, a thiazide should be one of them. However, there are few data to provide guidance on whether the order in which thiazide is initiated (i.e., whether before or after the other drug) impacts the eventual lowering of blood pressure (BP). The concept of combination antihypertensive therapy is built partially on the assumption that antihypertensives work via different pharmacological mechanisms, and that if drugs addressing different BP-regulating pathways are combined, additional effects should be realized. Although the premise of logical drug combinations is accepted by most clinicians, we are aware of no studies that specifically investigated whether the order in which two antihypertensive drugs are initiated is important to the outcome.
We tested the hypothesis that addition of a β-blocker to a thiazide would be more effective than addition of a thiazide to a β-blocker. This hypothesis was based on evidence suggesting that thiazides activate the renin–angiotensin system (RAS),6 thus potentially “sensitizing” patients to a better response to drugs that affect the RAS (e.g., β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers).
RESULTS
Baseline demographic characteristics of the 368 participants included in this analysis are shown in Table 1. The table shows overall demographics as well as race and treatment strategy, with atenolol or hydrochlorothiazide (HCTZ) being the drug to which the patient was randomized for monotherapy. When considered by treatment strategy, there were significant differences at baseline in family history of hypertension and baseline home-recorded systolic BP (SBP) and diastolic BP (DBP) (but not clinic BP values). When considered by race, there were significant differences in age, percentage of females, estimated glomerular filtration rate, home-recorded DBP, heart rate, and adherence to monotherapy dosage as determined at the time of response assessment.
Table 1.
Baseline demographics
| Overall (N = 368) | Atenolola | HCTZa | P value | ||||
|---|---|---|---|---|---|---|---|
| Black (n = 72) | White (n = 103) | Black (n = 80) | White (n = 101) | ATEN vs. HCTZ | Black vs. White | ||
| Age | 50.2 (8.7) | 48.0 (8.5) | 52.0 (8.5) | 48.1 (7.8) | 52.0 (8.8) | 0.94 | <0.0001 |
| Sex (% female) | 55.71 | 79.2 | 44.66 | 65.0 | 43.6 | 0.28 | <0.0001 |
| Duration of hypertension (years) | 8.3 (7.6) | 8.2 (7.8) | 10.0 (8.1) | 6.9 (6.9) | 8.4 (7.6) | 0.10 | 0.06 |
| Family history of hypertensionb | 77.93% | 86.11% | 80.58% | 79.75% | 70.30% | 0.03 | 0.25 |
| Never taken an antihypertensive drug | 9.24% | 6.94% | 8.74% | 6.25% | 11.88% | 0.79 | 0.22 |
| Taking antihypertensive drug at entry | 78.26% | 76.39% | 84.47% | 75.00% | 76.24% | 0.27 | 0.28 |
| Smoking status | |||||||
| Current smoker (%) | 10.87 | 9.72 | 6.80 | 17.50 | 10.89 | 0.06 | 0.13 |
| Number of cigarettes per day | 13.6 (9.5) | 9.9 (8.7) | 14.6 (8.3) | 12.6 (8.8) | 15.6 (10.8) | 0.30 | 0.02 |
| Ex-smoker (%) | 23.10 | 23.61 | 24.27 | 13.75 | 30.69 | 0.96 | 0.05 |
| Ever smoker (%) | 33.97 | 33.33 | 31.07 | 31.25 | 41.58 | 0.23 | 0.43 |
| BMI (kg/m2) | 31.0 (5.7) | 31.6 (6.9) | 30.8 (6.2) | 31.3 (5.4) | 30.6 (4.7) | 0.74 | 0.27 |
| Estimated GFR (ml/min/1.73 m2)c | 95.3 (20.3) | 104.4 (26.9) | 90.2 (14.8) | 101.3 (16.3) | 90.2 (19.9) | 0.63 | <0.0001 |
| Baseline clinic BP | |||||||
| Systolic (mm Hg) | 152.7 (13.0) | 151.6 (12.8) | 153.0 (13.0) | 152.6 (13.8) | 154.0 (12.5) | 0.53 | 0.33 |
| Diastolic (mm Hg) | 98.7 (6.7) | 98.8 (7.9) | 98.4 (6.3) | 99.7 (6.7) | 98.2 (6.3) | 0.61 | 0.16 |
| Baseline average home-recorded BP | |||||||
| Systolic (mm Hg) | 146.4 (10.8) | 145.5 (11.0) | 144.9 (9.4) | 147.7 (12.3) | 148.0 (10.7) | 0.02 | 0.87 |
| Diastolic (mm Hg) | 93.6 (6.3) | 93.9 (6.5) | 91.9 (5.6) | 95.3 (7.2) | 93.9 (5.7) | 0.006 | 0.01 |
| Baseline HR (from home-recorded data), beats/min | 76.6 (8.7) | 78.8 (8.5) | 75.7 (8.3) | 77.8 (9.3) | 75.3 (8.3) | 0.56 | 0.002 |
| Adherenced | |||||||
| Monotherapy assessment | 94.3% | 91.67% | 97.09% | 90.00% | 97.98% | 0.78 | 0.005 |
| Combination therapy assessmente | 95.6% | 95.65% | 98.94% | 91.89% | 94.38% | 0.07 | 0.20 |
Twelve participants are Asian or other. Values are mean ± SD unless otherwise noted.
ATEN, atenolol; BMI, body mass index; BP, blood pressure; GFR, glomerular filtration rate; HCTZ, hydrochlorothiazide; HR, heart rate; MDRD, Modification of Diet in Renal Disease.
Indicates initial randomized monotherapy.
Family history of hypertension defined as hypertension in a parent or sibling.
Estimated GFR calculated using the four-variable MDRD equation: 186 × serum creatinine(−1.154) × age−0.203 × (1.210 for black participants) × (0.742 for female participants).
Percentage of participants who were 100% adherent to dosage in the week prior to the visit.
Of 337 patients with adherence data at combination therapy assessment.
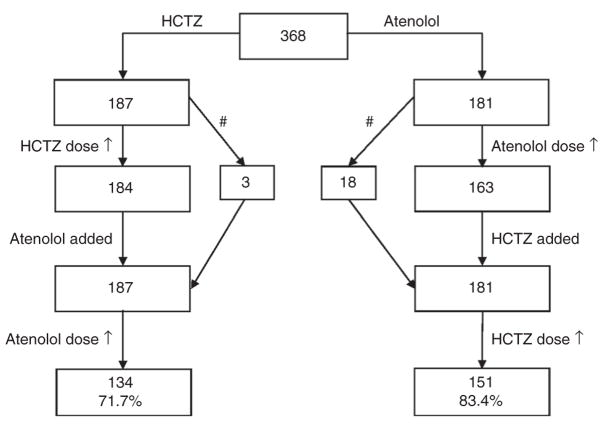
Figure 1 shows the progression of the first 368 participants who completed all aspects of the study and were treated with both study drugs. Among these 368 participants, the maximum doses of both drugs were taken by 273 participants (139 in the atenolol arm and 134 in the HCTZ arm).
Figure 1.
Progression of participants through the study protocol. #Dose of first drug not increased: hydrochlorothiazide (HCTZ) dose was held due to blood pressure (BP) <120/70 mm Hg at visit, but BP was above this cutoff on follow-up, so atenolol was added or atenolol dose was held due to heart rate <55 bpm, another dose-limiting side effect, or BP <120/70 mm Hg, but because BP was above this cutoff on follow-up, HCTZ was added.
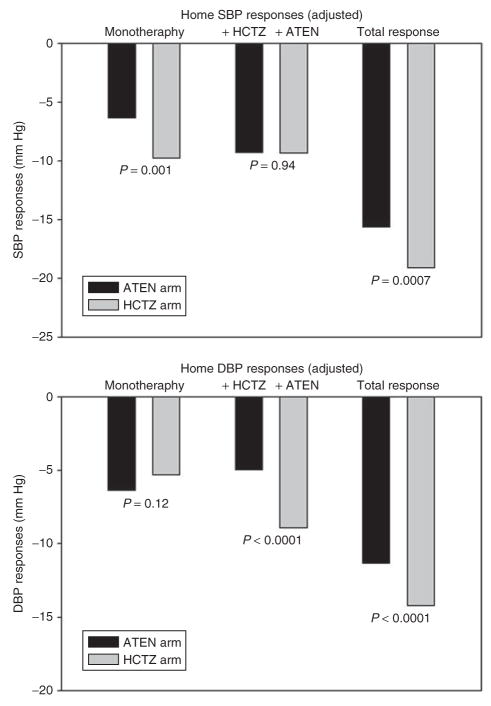
Figure 2 shows the changes in BP by the two treatment strategies. These data suggest that there are differences in BP response depending on the treatment approach adopted. Specifically, there was a greater response to atenolol when it was added to HCTZ (−9.6/−8.8 mm Hg) than when it was given as monotherapy (−6.6/−6.8 mm Hg). When analyzed by order of initiation of the two drugs, the response to HCTZ + atenolol was greater overall than that seen for atenolol + HCTZ (P = 0.0007/<0.0001).
Figure 2.
Adjusted home-recorded systolic blood pressure (SBP) and diastolic blood pressure (DBP) responses to atenolol, HCTZ, and the combination of the two drugs (adjusted for baseline BP, family history of high blood pressure, strategy arm, and final doses of HCTZ and atenolol). “ATEN arm” indicates those randomized to atenolol monotherapy (with addition of HCTZ); “HCTZ arm” indicates those randomized to HCTZ (with addition of atenolol). ATEN, atenolol; HCTZ, hydrochlorothiazide.
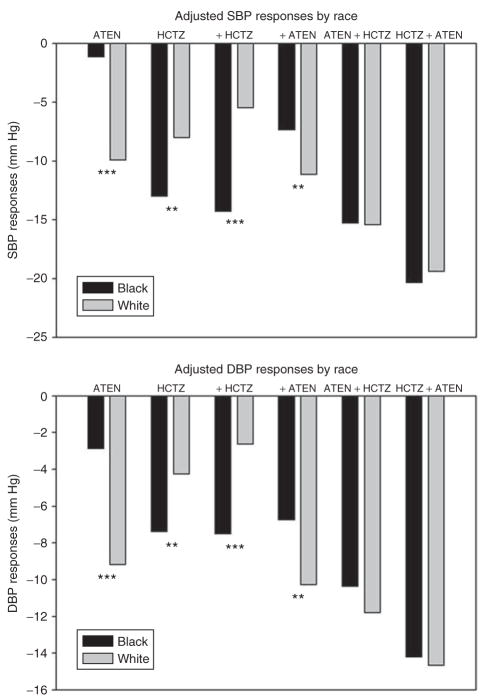
Table 2 depicts the BP values by treatment arm at baseline, after monotherapy, and after combination therapy in black and white participants. Figure 3 depicts the changes in BP by race and treatment strategy. There were striking differences between the two groups in the responses observed, both to HCTZ and to atenolol. White participants showed a greater response than blacks did to atenolol whether it was administered as the first drug or the second, whereas blacks showed a greater response to HCTZ than whites did, whether it was administered first or second. In addition, any differences in response by race to the individual drugs were no longer evident when evaluating the overall response to a combination of the two drugs.
Table 2.
Home-recorded BP values (in mm Hg) in relation to treatment arm
| Atenolol arm (n = 72 B, 103 W) | HCTZ arm (n = 80 B, 101 W) | P values SBP P/DBP P | |
|---|---|---|---|
| Baseline | |||
| Blacks | 145.5/93.9 ± 1.3/0.8 | 147.7/95.3 ± 1.4/0.8 | 0.25/0.23 |
| Whites | 144.9/91.9 ± 0.9/0.6 | 148.0/93.9 ± 1.1/0.6 | 0.03/0.01 |
| Monotherapya | |||
| Blacks | 146.0/91.7 ± 1.3/0.9 | 134.3/87.2 ± 1.0/0.7 | <0.0001/0.0001 |
| Whites | 136.4/84.0 ± 1.2/0.5 | 138.5/88.9 ± 0.8/0.5 | 0.068/<0.0001 |
| Combination therapya | |||
| Blacks | 132.1/84.2 ± 1.4/0.9 | 126.9/80.2 ± 1.3/0.7 | 0.005/0.002 |
| Whites | 130.3/80.8 ± 0.8/0.5 | 127.6/78.6 ± 0.8/0.6 | 0.02/0.009 |
Mean ± SEM. P value indicates comparison of atenolol arm vs. hydrochlorothiazide (HCTZ) arm.
BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Adjusted for baseline BP, age, gender, eGFR, adherence, treatment strategy, and final doses of HCTZ and atenolol.
Figure 3.
Blood pressure (BP) reduction in relation to treatment and race (adjusted for age, gender, baseline BP, adherence, treatment strategy, and final doses of HCTZ and atenolol). **P < 0.01, ***P < 0.0001 by race. ATEN, atenolol; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; SBP, systolic blood pressure.
Figure 3 depicts a difference in antihypertensive response depending on the order in which the drugs were initiated. In both groups there was a better overall response to combination therapy, in terms of both SBP and DBP, when HCTZ was initiated before atenolol. Specifically, in blacks there was a 3.8/3.1 mm Hg (results given throughout as SBP/DBP) better response to HCTZ-first (P values: 0.036/0.016), and in whites there was a 2.6/2.0 mm Hg better response to HCTZ-first (P values: 0.019/0.009). The data suggest that the effect of the order of initiation of the drugs is due primarily to differences in response to atenolol, as shown in Table 3. For example, the decline in BP in blacks taking atenolol monotherapy was −1.6/−3.5 mm Hg, vs. −7.5/−6.9 mm Hg when atenolol therapy was added to HCTZ monotherapy. Whites also showed more BP lowering with this order of therapy, but the differences in response were smaller than for blacks. In contrast, responses to HCTZ were nearly identical in blacks, whether given as monotherapy or as an add-on to atenolol, and for whites there are nominal but not significantly different responses to HCTZ as monotherapy vs. add-on therapy.
Table 3.
Comparison of BP response to atenolol or HCtZ as monotherapy vs. add-on therapy in whites and blacks
| n | Mean BP change | SE | P value | |
|---|---|---|---|---|
| SBP | ||||
| Atenolol in blacks | ||||
| Monotherapy | 72 | −1.6 | 1.3 | 0.0005 |
| As add-on | 80 | −7.5 | 1.2 | |
| Atenolol in whites | ||||
| Monotherapy | 103 | −9.6 | 0.8 | 0.08 |
| As add-on | 101 | −11.7 | 0.8 | |
| HCTZ in blacks | ||||
| Monotherapy | 80 | −12.6 | 1.3 | 0.65 |
| As add-on | 72 | −13.5 | 1.4 | |
| HCTZ in whites | ||||
| Monotherapy | 101 | −7.8 | 0.8 | 0.31 |
| As add-on | 103 | −6.7 | 0.7 | |
| DBP | ||||
| Atenolol in blacks | ||||
| Monotherapy | 72 | −3.5 | 0.8 | 0.0019 |
| As add-on | 80 | −6.9 | 0.8 | |
| Atenolol in whites | ||||
| Monotherapy | 103 | −8.8 | 0.5 | 0.03 |
| As add-on | 101 | −10.5 | 0.5 | |
| HCTZ in blacks | ||||
| Monotherapy | 80 | −7.7 | 0.9 | 0.65 |
| As add-on | 72 | −7.1 | 0.9 | |
| HCTZ in whites | ||||
| Monotherapy | 101 | −3.9 | 0.5 | 0.39 |
| As add-on | 103 | −3.2 | 0.5 | |
Adjusted for baseline BP, age, sex, eGFR, adherence, treatment strategy, and final doses of atenolol and HCTZ. P value indicates comparison within race of response to drug given as monotherapy vs. add-on therapy.
BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide; SBP, systolic blood pressure.
The preceding analyses were controlled for final HCTZ dose and final atenolol dose. However, as part of a sensitivity analysis, we also separately tested participants who received the maximum doses of both drugs. The findings in terms of results and P values were nearly identical, and there were no situations in which a test was significant in one analysis and not significant in the other analysis (data not shown). To ensure that the findings were not in some way influenced by previous treatment or by the washout procedure, we restricted the analysis to the 80 patients who entered the trial untreated (45 randomized to the HCTZ arm and 35 to the atenolol arm). In this sensitivity analysis, the findings were essentially identical to those for the full cohort, with a total BP decline with combination therapy of −19.1/−14.3 mm Hg for the HCTZ arm and −16.1/−11.8 mm Hg for the atenolol arm. These data suggest that the findings are not the result of previous therapy or of washout from previous therapy. Finally, we repeated the analysis using the clinic BP data rather than the home-recorded BP data. When considering the total BP response, differences in clinic BP readings based on the order of initiation of the drugs were significant (mean ± SE; atenolol + HCTZ: −21.2/−13.9 ± 0.9/0.7 mm Hg vs. HCTZ + atenolol: −24.0/−16.5 ± 0.9/0.7; P values 0.039/0.006). As with the home-recorded BP data, the effect was of a greater order of magnitude in blacks than in whites.
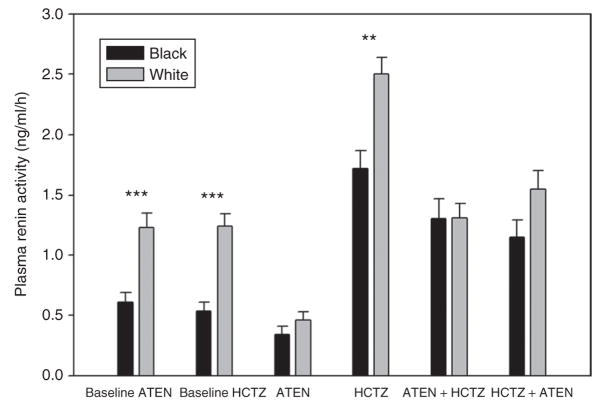
Figure 4 shows the plasma renin activity (PRA) data at baseline, following treatment with atenolol or HCTZ monotherapy, and with combination therapy with each drug as the first drug. White participants had significantly higher PRA at baseline, but atenolol caused a significantly greater drop in this group than in blacks, such that the PRA values in the two groups during atenolol treatment were similar. The greater PRA decline from baseline with atenolol therapy in whites is consistent with their greater decline in BP in response to the drug, and the possibly greater likelihood of their having high renin hypertension. In contrast, HCTZ caused similar increases in PRA in blacks and whites, such that the differences in PRA by race remained during HCTZ treatment. During combination therapy, PRA was similar between the two groups; however, this value was significantly higher than baseline in blacks but not different from baseline in whites.
Figure 4.
Plasma renin activity (PRA) in relation to race and treatment strategy. Baseline indicates the baseline values for the two randomized groups. “ATEN arm” indicates those randomized to atenolol monotherapy (with addition of HCTZ), “HCTZ arm” indicates those randomized to HCTZ (with addition of atenolol). **P < 0.01, ***P < 0.0001 for comparison between blacks and whites. ATEN, atenolol; HCTZ, hydrochlorothiazide.
The Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) protocol included dose titration and drug addition to reach a relatively low BP target of <120/70 mm Hg. Using a more traditional home-recorded BP target of 135/85 mm Hg,7 the differences in response to monotherapy by race remained evident, although there was less evidence of any effect caused by the order of drug initiation when evaluating those who achieved a controlled BP, defined as BP <135/85 mm Hg. These data, depicted in Supplementary Figure S1 online, show that significantly more blacks than whites achieve BP control with HCTZ monotherapy, and significantly more whites than blacks achieve control with atenolol monotherapy. It is of interest to note that, despite the very poor average response of blacks to atenolol monotherapy, more blacks (21%) achieved BP control with atenolol than whites did with HCTZ (13%). Importantly, in both groups, the majority (64% of blacks, 56% of whites) did not achieve BP control even with the optimal drug, reinforcing the observation that even for those with mild to moderate hypertension, two drugs will often be needed to achieve BP control. There were no significant differences with respect to the rate of control of BP achieved during combination therapy, either on the basis of race (Figure 4) or on the basis of order of initiation of the drugs (overall: atenolol + HCTZ = 61% vs. HCTZ + atenolol = 68%, P = 0.15; blacks: atenolol + HCTZ = 57% vs. HCTZ + atenolol = 69%, P = 0.13; whites: atenolol + HCTZ = 63% vs. HCTZ + atenolol = 68%, P = 0.43). It is worth noting that, even with the combination therapy, only approximately two-thirds of these patients with mild to moderate hypertension achieved BP control.
DISCUSSION
The novel finding is that the order in which antihypertensive drugs are initiated in combination therapy has an influence on BP response, particularly in black patients. Our assessment of drug-induced changes in BP during the first 4–6 months of therapy showed that there is a 3–4 mm Hg greater drop in BP overall when the treatment order is HCTZ + atenolol as compared with atenolol + HCTZ. This was also evident for absolute BP, with the HCTZ arm showing higher baseline home-recorded BP values (significantly so in whites) and lower BP values at the end of the therapy in both blacks and whites. This effect was observed after at least 6 weeks on combination therapy (and at least 15–18 weeks of total therapy), a duration of therapy usually considered to reflect the chronic response. Smaller differences in BP-lowering effects have been associated with differences in outcome in large hypertension clinical trials;8–10 therefore, the differences in BP lowering observed here, if sustained in the long term, could represent clinically important differences. It is important to note that the Valsartan Antihypertensive Long-term Use Evaluation trial suggested that BP responses at time points as early as 1 month after commencement of therapy were associated with long-term cardiovascular events and survival.10 Therefore, even if the differences in BP observed here were to moderate over a longer period of time, these early differences in BP control might still influence cardiovascular outcomes.
An evaluation of the data suggests that the difference in response depending on the order in which the drugs are initiated is explained by the fact that atenolol as background therapy does not lead to any potentiation of the HCTZ response. In contrast, the data suggest that HCTZ background therapy potentiates the response to atenolol. Our primary hypothesis for the mechanism underlying this finding is that the overall response represents the interaction of the two drug classes on BP control mechanisms. Specifically, thiazide diuretics are well known to activate the RAS, and this effect likely moderates the BP lowering produced by thiazide diuretic therapy. The effects of HCTZ on PRA are evident in Figure 4. Conversely, the BP-lowering effects of β-blockers are thought to derive largely from their indirect inhibitory effects on the RAS. at is, the β-blocker off-sets the RAS activation caused by the thiazide diuretic, leading to a greater response than would be predicted from the sum of responses to the two drugs. Although it might have been presumed that this would be true irrespective of the order of drug initiation, these data suggest that perhaps this effect is more pronounced when the thiazide can first activate the RAS in the absence of the β-blocker. This is also generally supported by the PRA data, which suggests that the rise in PRA induced by HCTZ is moderated by 25–50% by pretreatment with atenolol. It is possible, therefore, that additive effects are observed in the atenolol + HCTZ combination, whereas there are synergistic effects with the HCTZ + atenolol combination.
These data also add to the body of literature on the differences in antihypertensive drug response between blacks and whites. First, using the most commonly employed doses and drugs within the thiazide diuretic and β-blocker classes, we show significant differences in response between blacks and whites, with whites showing substantially larger responses to a β-blocker and blacks having larger responses to a thiazide diuretic. This was true whether BP response was assessed as the absolute treated BP, the drug-induced change in BP, or the percentage of patients achieving BP control as defined by a specific BP target. Interestingly, these data suggest that if a white patient is likely to achieve BP control with a single drug, then a β-blocker would be preferred over a thiazide diuretic. In fact, only 13% of the whites in this study achieved BP control with HCTZ monotherapy, vs. 44% with atenolol monotherapy. In direct contrast to this finding, the study data provide evidence supporting thiazide diuretics as the preferred initial therapy in blacks.
Also consistent with the published literature is the finding that differences in response between blacks and whites are no longer evident during combination therapy. This highlights the fact that, although the response of blacks to β-blocker monotherapy may be poor, this is no longer so when the β-blocker is administered with a thiazide diuretic.
There are limitations of this study that deserve comment. First, thiazide/β-blocker combination products are commercially available, and we have no data on whether initiation of both drugs simultaneously would result in a response similar to HCTZ + atenolol or atenolol + HCTZ. Our study also does not provide insight into whether these differences in BP lowering would persist over longer-term therapy (i.e., many months to years). Historically, 4 weeks has been the duration of therapy after which one expects the response to reflect the chronic, stable response. However, it is possible that the differences in response by treatment strategy observed over the 4- to 6-month duration of this study would not persist with longer-term therapy. Finally, as noted in Methods, the primary objective of this trial is to test pharmacogenetic hypotheses. Therefore, our original proposal did not include this specific hypothesis relating to duration of the response effects, although it was a hypothesis that was developed prior to the decision to evaluate the BP response data at the approximate halfway point in enrollment.
In conclusion, this study suggests that initiation of HCTZ followed by atenolol results in superior BP lowering as compared with initiation in the reverse order, with differences in BP lowering that are potentially clinically important. The PEAR study is continuing enrollment, and we will seek to replicate this finding in the second half of the study population. Whether the observed BP differences with these two treatment strategies would persist over many months to years, and whether they would result in different outcomes, is unknown and would require further study.
METHODS
Study population
PEAR is an ongoing hypertension pharmacogenomics trial, and the trial design, inclusion and exclusion criteria, and pharmacogenomic aims have been described in detail elsewhere.11 PEAR is registered at ClinicalTrials.gov (NCT00246519; http://clinicaltrials.gov/ct2/show/NCT00246519).
This is an analysis of BP responses in the first 368 participants for whom responses were assessed, to both monotherapy and combination therapy. Briefly, primary care patients with mild to moderate essential hypertension, of any race or ethnicity, aged 17–65 years, are being enrolled at the University of Florida (Gainesville, FL), Emory University (Atlanta, GA), and the Mayo Clinic (Rochester, MN). The study was approved by the institutional review boards at each institution, and all participants have provided informed, written consent prior to being screened for participation. Race and ethnicity are self-defined by study participants, following the National Institutes of Health guidelines in which “race” indicates continental ancestry (e.g., “white” indicates European ancestry; “black” indicates African ancestry).
Enrolled participants had newly diagnosed, untreated, or treated hypertension. Exclusion criteria have been described in full11 and include DBP >110 mm Hg or SBP >180 mm Hg, secondary hypertension, cardiovascular disease, diabetes, renal insufficiency (serum creatinine >1.5 in male patients and >1.4 in female patients), liver enzymes >2.5 times the upper limit of normal, and treatment with BP-raising drugs, among others. Patients with hypertension who had been receiving treatment had their antihypertensive drugs discontinued for a washout period of a minimum of 18 days. At study entry, all participants were provided with a Microlife model 3AC1-PC home BP monitor (BP Microlife, Minneapolis, MN). The device met quality standards for accuracy12 and was set to measure BP in triplicate with each activation, recording the average SBP, DBP, and heart rate along with a date/time stamp. The participants were instructed to record their BP twice daily, in the morning soon after getting up from bed and again just before retiring for the night. The monitor stores up to 99 readings, and data were downloaded to a study computer at each study visit. Participants were required to have at least five morning readings and five evening readings during the previous 7 days. Clinic BP measurements were made using the home BP monitor to eliminate any device-related differences in the BP values. As an enrollment criterion, the average (previous week) home-recorded DBP while seated had to be >85 mm Hg, and the average (>5 min) clinic DBP while seated had to be >90 mm Hg.
Study protocol
Patients who met the inclusion criteria pertaining to BP levels were randomized in an unblinded fashion to HCTZ 12.5 mg daily or atenolol 50 mg daily, with randomization stratified by study site. Throughout the protocol, those with a previous-week average home-recorded or clinic SBP >120 mm Hg or DBP >70 mm Hg continued to move through the titration protocol, whereas those with BPs ≤120/70 mm Hg were maintained at their current drug/dose regimen. All BP-related protocol decisions were based on both home-recorded and clinic BP readings. The dosing protocol has been described in detail elsewhere.11 In most of the participants BP remained at >120/70 mm Hg throughout the protocol. Under these conditions, they were given the initial dose for 3 weeks and twice the initial dose for a further 6 weeks, after which response was assessed. The second drug was then added, and the dose of the second drug was doubled after 3 weeks; the participant took the combination therapy at this final dosage for at least 6 weeks. Therefore, the data presented here represent at least 15–18 weeks of therapy. Any participant with heart rate <55 bpm was precluded from receiving a higher atenolol dose but could still have HCTZ added, based on the BP readings. Fasting plasma samples were collected at baseline and at each visit during which BP response was assessed.
Study medications were provided in blister packs marked with the days of the week. Participants were instructed to leave the pill in the blister pack if they missed a dose. Data on adherence to therapy were collected by recording the doses taken, and in the week prior to each study visit, the specific days on which a dose was missed were recorded.
PRA was determined by radioimmunoassay of angiotensin-I in the presence of reagents that inhibit angiotensin I–converting enzyme and angiotensinases (DuPont, Boston, MA).
Statistical analyses
The primary analyses focus on home-recorded BP data because a number of factors suggest the superiority of home-recorded BP values over clinic-recorded ones, including lack of a placebo effect, significantly better prognostic value, and greater reproducibility.13–15
All statistical analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC). Baseline characteristics were compared by treatment strategy and race using a χ2-test or t-test, as appropriate. The SAS PROC GLM procedure was used to calculate the adjusted BP and BP responses, adjusting for the baseline BP, age, gender, estimated glomerular filtration rate, adherence to treatment strategy, and final atenolol and HCTZ doses. A two-sided P < 0.05 was considered significant for all analyses.
Supplementary Material
Acknowledgments
This work is supported by a grant from the National Institutes of Health (Bethesda, MD), U01 GM074492, funded as part of the Pharmacogenetics Research Network. In addition, the work is supported by the following grants from the National Institutes of Health, National Center for Research Resources: M01 RR00082 to the University of Florida, UL1 RR025008 and M01 RR00039 to Emory University, and UL1 RR024150 to the Mayo Clinic. We acknowledge the valuable contributions of the study participants, support staff, and study physicians. We also thank George Baramidze, Carmen Bray, R. Whit Curry, Karen Hall, Frederic Rabari-Oskoui, and Dan Rubin.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics—2008 Update. American Heart Association; Dallas, TX: 2008. [Accessed 18 June 2008]. http://www.americanheart.org/downloadable/heart/1200078608862HS_Stats%202008.final.pdf. [Google Scholar]
- 2.Chobanian AV, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Houston MC. ALLHAT debate: diuretics are not preferred, first-line initial therapy for hypertension. Arch Intern Med. 2004;164:570–571. doi: 10.1001/archinte.164.5.570-b. author reply 571–572. [DOI] [PubMed] [Google Scholar]
- 4.Scott I, Stowasser M. Are thiazide diuretics preferred as first-line therapy for hypertension? An appraisal of The Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Intern Med J. 2003;33:327–330. doi: 10.1046/j.1445-5994.2003.00431.x. [DOI] [PubMed] [Google Scholar]
- 5.Sica DA. Diuretics should continue to be one of the preferred initial therapies in the management of hypertension: the argument against. J Clin Hypertens (Greenwich) 2005;7:117–120. quiz 121–112. [PubMed] [Google Scholar]
- 6.Ernsberger P, Koletsky RJ. Metabolic effects of antihypertensive agents: role of sympathoadrenal and renin-angiotensin systems. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:245–258. doi: 10.1007/s00210-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 7.Pickering TG, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 8.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 9.Dahlöf B, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 10.Weber MA, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA, et al. Pharmacogenomics of antihypertensive drugs: rationale and design of the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study. Am Heart J. 2009;157:442–449. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkubo T, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 14.Stergiou GS, et al. Reproducibility of home, ambulatory, and clinic blood pressure: implications for the design of trials for the assessment of antihypertensive drug efficacy. Am J Hypertens. 2002;15:101–104. doi: 10.1016/s0895-7061(01)02324-x. [DOI] [PubMed] [Google Scholar]
- 15.Imai Y, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19:179–185. doi: 10.1097/00004872-200102000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.