Abstract
Bay region diol epoxides are recognized ultimate carcinogens of polycyclic aromatic hydrocarbons (PAH), and in vitro studies have demonstrated that they can be detoxified by conjugation with glutathione, leading to the widely investigated hypothesis that individuals with low activity forms of glutathione-S-transferases are at higher risk of PAH induced cancer, a hypothesis that has found at most weak support in molecular epidemiology studies. A weakness in this hypothesis was that the mercapturic acids resulting from conjugation of PAH bay region diol epoxides had never been identified in human urine. We recently analyzed smokers’ urine for mercapturic acids derived from phenanthrene, the simplest PAH with a bay region. The only phenanthrene diol epoxide-derived mercapturic acid in smokers’ urine was produced from the reverse diol epoxide, anti-phenanthrene-3,4-diol-1,2-epoxide (11), not the bay region diol epoxide, anti-phenanthrene-1,2-diol-3,4-epoxide (10), which does not support the hypothesis noted above. In this study, we extended these results by examining the conjugation of phenanthrene metabolites with glutathione in human hepatocytes. We identified the mercapturic acid N-acetyl-S-(r-4,t-2,3-trihydroxy-1,2,3,4-tetrahydro-c-1-phenanthryl)-L-cysteine (14a), (0.33–35.9 pmol/mL at 10 µM 8, 24h incubation, N = 10) in all incubations with phenanthrene-3,4-diol (8) and the corresponding diol epoxide 11, but no mercapturic acids were detected in incubations with phenanthrene-1,2-diol (7) and only trace amounts were observed in incubations with the corresponding bay region diol epoxide 10. Taken together with our previous results, these studies clearly demonstrate that glutathione conjugation of a reverse diol epoxide of phenanthrene is favored over conjugation of a bay region diol epoxide. Since reverse diol epoxides of PAH are generally weakly or non-mutagenic/carcinogenic, these results, if generalizable to other PAH, do not support the widely held assumption that glutathione-S-transferases are important in the detoxification of PAH in humans.
Keywords: phenanthrene diol epoxides, mercapturic acids, human hepatocytes
Introduction
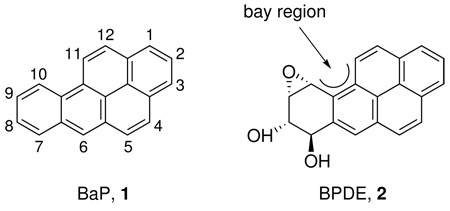
Bay region diol epoxides, such as anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE, 2), are well established ultimate carcinogenic metabolites of polycyclic aromatic hydrocarbons (PAH), a ubiquitous class of environmental carcinogens formed during the incomplete combustion of organic matter (1–4). PAH are universally accepted causes of cancer of the lung and skin in occupational settings involving exposure to combustion products, and are among the likely causes of lung cancer in smokers (5–8). Benzo[a]pyrene (BaP, 1), the parent compound of BPDE, is carcinogenic to humans, according to the International Agency for Research on Cancer (2).
Based completely on in vitro studies, BPDE and other bay and fjord region diol epoxides can be detoxified by conjugation with glutathione, catalyzed by glutathione-S-transferases (GSTs) including GST-P1-1, GST-M1-1, and GST-A1-1 (9–13). This leads to the logical hypothesis that individuals exposed to BaP and other PAH, such as smokers, should be at a higher risk for cancer if they have low activity or null variants of the GST genes. This hypothesis has been tested in many molecular epidemiology studies using genotyping methods but the results have been modest, showing only slightly elevated risks for some cancers in individuals with low GST activity genotypes (14–18). However, there is a potential flaw in this hypothesis: no studies had demonstrated the presence in human urine of the mercapturic acids that would result from glutathione conjugation of PAH bay region diol epoxides. Therefore, it was not known whether the proposed detoxification pathway even existed.
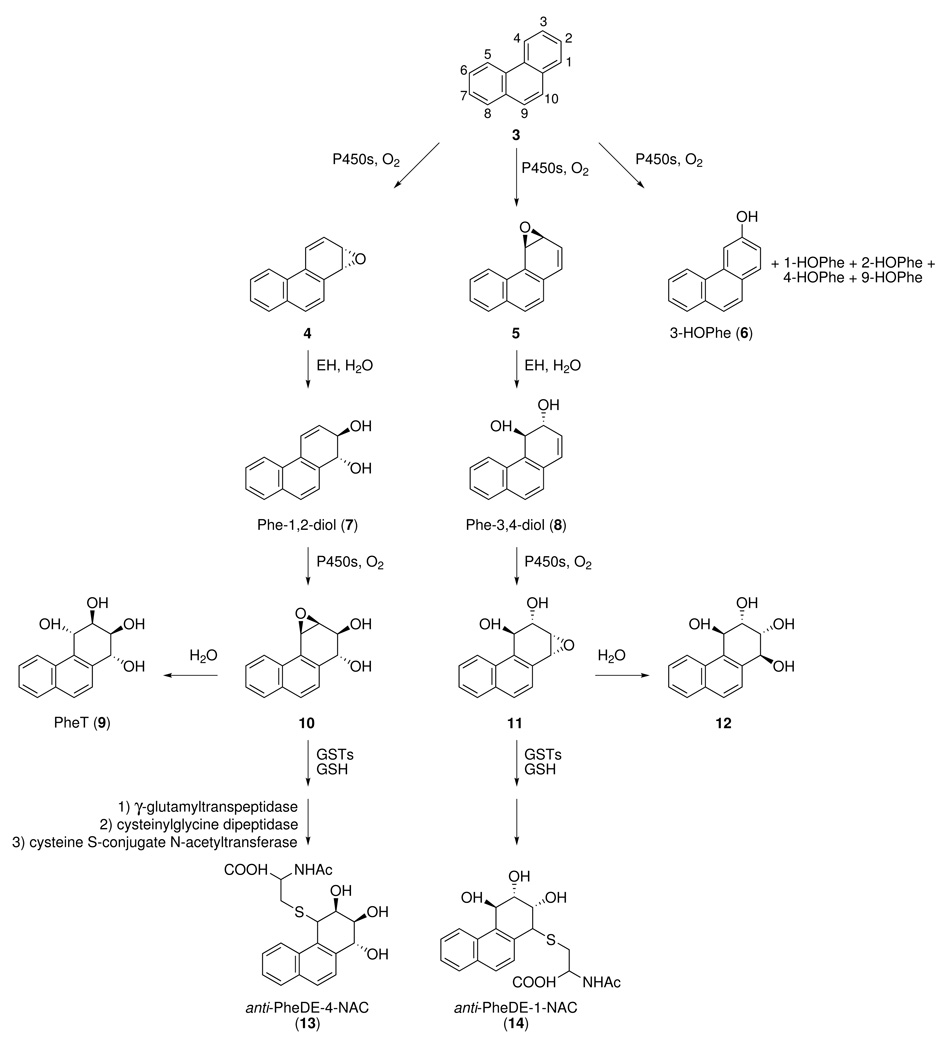
We recently investigated this question by focusing on phenanthrene (3, Scheme 1), the simplest PAH with a bay region, and a common environmental constituent. While phenanthrene is considered non-carcinogenic, it shares with BaP and other PAH many of the same metabolic pathways, catalyzed by the same enzymes (19–21). An overview of phenanthrene metabolism is presented in Scheme 1. trans, anti-PheT (9), formed by hydrolysis of the bay region diol epoxide 10, is present in human urine in readily detectable quantities and we have suggested its use as a biomarker of PAH exposure plus metabolic activation (22,23). Glutathione conjugation of bay region diol epoxide 10 would ultimately produce mercapturic acid 13, but we did not detect this metabolite in human urine (24). Rather, we detected 14, resulting from glutathione conjugation of the “reverse diol epoxide” 11 (24). Our conclusion from that study was that reverse diol epoxides, which are generally considered non-carcinogenic, are conjugated with glutathione in humans while potentially carcinogenic diol epoxides are not, thus weakening the hypothesis that GSTs are involved in PAH detoxification in humans.
Scheme 1.
Overview of phenanthrene metabolism to diol epoxides and mercapturic acids
In this study, we used human hepatocytes to further investigate the formation of glutathione conjugates of phenanthrene metabolites. Consistent with the results obtained from our analysis of human urine, we found that mercapturic acids formed via the reverse diol epoxide 11 were produced to a far greater extent than those from bay region diol epoxide 10. We propose that GSTs are unlikely to drive carcinogenic PAH diol epoxide detoxification in humans.
Materials and Methods
Chemicals, Enzymes, and Antibodies
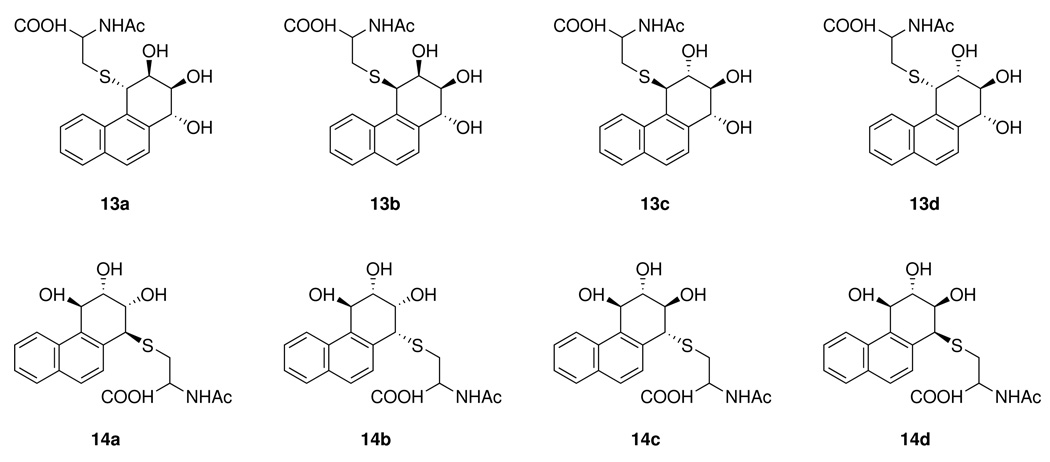
Racemic phenanthrene-1,2-diol (Phe-1,2-diol (7)), Phe-3,4-diol (8), diol epoxide 10, and tetraol 9 were generously provided by Drs. Donald Jerina and H. Yagi, National Institutes of Health. Diol epoxide 11 was synthesized from 8 (24). It contained 50% of the syn-isomer. The concentration of 11 in incubation mixtures was based on the amount of anti-isomer only, and was confirmed by HPLC analysis. Mercapturic acid standards 13a-d, 14a-d (Chart 1) and anti-[13C6]PheDE-4-NAC were prepared by reaction of N-acetylcysteine with the appropriate diol epoxides, as described previously (24). β-Glucuronidase and arysulfatase (from Helix pomatia) were obtained from Roche Diagnostics Corp, Indianapolis, IN. Anti-GSTA1 and anti-GSTM1 antibodies were purchased from EMD Biosciences (Gibbstown, NJ).
Chart 1.
Structures of mercapturic acids which would be produced by conjugation of diol epoxides: 13a,b from 10; 13c,d from the corresponding syn-diol epoxide; 14a,b from 11; 14c,d from the corresponding syn-diol epoxide.
Human Hepatocytes
Primary human hepatocytes were purchased from Cellzdirect (St. Louis, MO). In brief, freshly isolated hepatocytes were plated onto 12-well plates (7 × 105 cells/well) and overlaid with Matrigel® 24–48 h after attachment. Cells were shipped overnight on cold preservation media (Cellzdirect) and, upon receipt, media was replaced with serum-free Williams’ E media without phenol red, and cell maintenance supplement (Cellzdirect) containing 0.1 µM dexamethasone, 10 µg/mL gentamicin, 15 mM HEPES, 2mM L-glutamine, 6.25 µg/mL human recombinant insulin, 6.25 ng/mL selenous acid, 1.25 mg/mL bovine serum albumin, 6.25 µg/mL human transferrin, and 5.35 µg/mL linoleic acid. Cells were allowed to recover from shipping for 10 h at 37°C in an atmosphere containing 5% CO2. Prior to incubation with substrate, the media was exchanged with 2 mL fresh media per well.
GST Expression and Activity in Human Hepatocytes
Hepatocytes were lysed by sonication. Total protein concentrations were determined by the Bradford assay with Coomassie Plus protein reagent and BSA standard from Pierce (Rockford, IL). GST expression was evaluated by Western blotting. Hepatocyte lysates (15 µg protein) were subjected to electrophoresis through 10% polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and blocked with 3% milk in Tris-buffered saline and 0.05% Tween (TBST). The membranes were incubated with anti-GSTM1 antibody (1:3000 in 3% milk-TBST) for 1 h, washed 3 × 10 min with TBST, incubated with horseradish peroxidaseconjugated anti-rabbit secondary antibody (1:7500 in 3% milk-TBST) for 1 h, and washed 3 × 10 min. The immuno-reactive bands were visualized following addition of ECL detection reagent (Denville Scientific, Metuchen, NJ). The membranes were stripped using Restore Plus stripping buffer (Pierce, Rockford, IL) and immuno-blotting was performed as described above using anti-GSTA1 antibody.
GST activity was assessed by measuring conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) (25). In brief, hepatocyte lysates (75 µg protein) were incubated with 1 mM CDNB in the presence of 2.5 mM GSH. The rate of product formation was determined from the change in absorbance at 340 nm over 10 min.
Incubations of Human Hepatocytes with Phe Metabolites
Hepatocytes (approximately 0.12 mg protein per well) were incubated with Phe-1,2-diol (7), Phe-3,4-diol (8), 7 plus 8, or diol epoxides 10 or 11, dissolved in 20 µL DMSO, for a final substrate concentration of 1 or 10 µM. Aliquots (0.4 mL) of media were removed immediately following the addition of substrate, and 6h or 12h after addition. At 24 h after the addition of substrate, the remaining ∼800 µL was collected. Samples were stored at −20°C until analysis. Cell viability was assessed at 24 h by trypan blue exclusion staining.
Analysis for Mercapturic Acids 13a-d, 14a-d
A 0.2 mL aliquot of the human hepatocyte incubation media was combined with anti-[13C6]PheDE-4-NAC internal standard and loaded onto a Strata-X polymeric sorbent (33 µm, 30 mg/ 1 ml, Phenomenex) that was previously activated with 1 mL of CH3OH and 1 mL of H2O. The cartridge was washed with 1 mL of H2O and the analyte was eluted with 1 ml of 90% CH3OH. The eluant was collected in a 2 mL silanized vial and the solvents were removed on a Speedvac. The residue was taken up in 235 µL of CH3OH and 5 µL of 4% aqueous NH4OAc, transferred to an insert vial and concentrated to dryness. The residue was dissolved in 20 µL of 1% aqueous NH4OAc and 8 µL was analyzed by LC-ESI-MS/MS-SRM as described previously (24).
Analysis for Tetraols 9 plus 12
A 0.1 mL aliquot of the 2 mL human hepatocyte incubation media was added to a 1.5 mL polypropylene tube containing 0.4 mL 0.5M NaOAc buffer, pH 5, β-glucuronidase (3,500 units), arylsulfatase (28,000 units) and 500 pg [D10]PheT (9) (26) as internal standard, and the mixture was incubated overnight with shaking at 37°C. After incubation, the sample was desalted by loading onto a Strata-X polymeric sorbent that was previously activated using 1 mL of CH3OH and 1 mL of H2O. The cartridge was washed with 1 mL of H2O, 1 mL of 0.2 N KOH, twice with 1 mL volumes of H2O, and the analyte was eluted using 1 mL of 80% CH3OH. The analyte was collected in a 2 mL silanized vial and the solvents were removed on a Speedvac.
The residue was dissolved in 0.5 mL H2O and loaded onto an Oasis MCX cartridge (Waters) that was previously equilibrated using 1 mL of CH3OH and 1 mL of 2% aqueous HCOOH. The cartridge was washed with 1 mL of 2% aqueous HCOOH and the analyte was eluted using 1 mL of 80% CH3OH. The analyte was collected in a 2 mL silanized vial and the solvents were removed on a Speedvac. Analysis of 9 plus 12 as their trimethylsilyl derivatives was carried out by GC-MS, essentially as described (23).
Results
Characteristics of the 10 hepatocyte donors and GST activities as determined by the CDNB assay are summarized in Table 1. Activities were slightly lower than those reported previously (27–29). Western blotting demonstrated the presence of GSTA1 in all samples and GSTM1 in 4 of 10 samples, consistent with its deletion in approximately 50% of Caucasians (30).
Table 1.
Hepatocyte donor characteristicsa and GST activity
| Donor ID | Gender | Age | BMI | Smoker | CDNB Activityb |
|---|---|---|---|---|---|
| 1 | Female | 27 | 28.7 | No | 28 |
| 2 | Female | 46 | 21.9 | No | 104 |
| 3 | Female | 69 | 21.8 | Yes | 55 |
| 4 | Male | 68 | 37.0 | No | 32 |
| 5 | Female | 80 | 19.3 | No | 29 |
| 6 | Male | 26 | 38.7 | Yes | 34 |
| 7 | Female | 51 | 24.7 | Yes | 64 |
| 8 | Male | 50 | 30.0 | Yes | 30 |
| 9 | Female | 71 | 24.5 | Yes | 19 |
| 10 | Female | 76 | 20.1 | No | 35 |
Donors had no known history of hepatitis B, hepatits C, cirrhosis, or biliary disease
nmol CDNB conjugate / min / mg protein
In preliminary experiments, we incubated phenanthrene with human hepatocytes, but did not detect mercapturic acids. Therefore, in subsequent work we used Phe-1,2-diol (7) or Phe-3,4-diol (8), since both of these metabolites have been detected in human urine, with 2 −5 times more 7 than 8 (31). The mixtures were analyzed for mercapturic acids, using standards (13a-d and 14a-d, Chart 1) available from our previous work (24), in which N-acetylcysteine was allowed to react with either the anti-diol epoxides 10 and 11 or the corresponding syn-diol epoxides. All 8 standards were separable under our conditions. (Another set of 8 isomers is also possible by reaction with each opposite diol epoxide enantiomer, but these were not separable from those shown in Chart 1, at least in the case of 13a,b) (24).
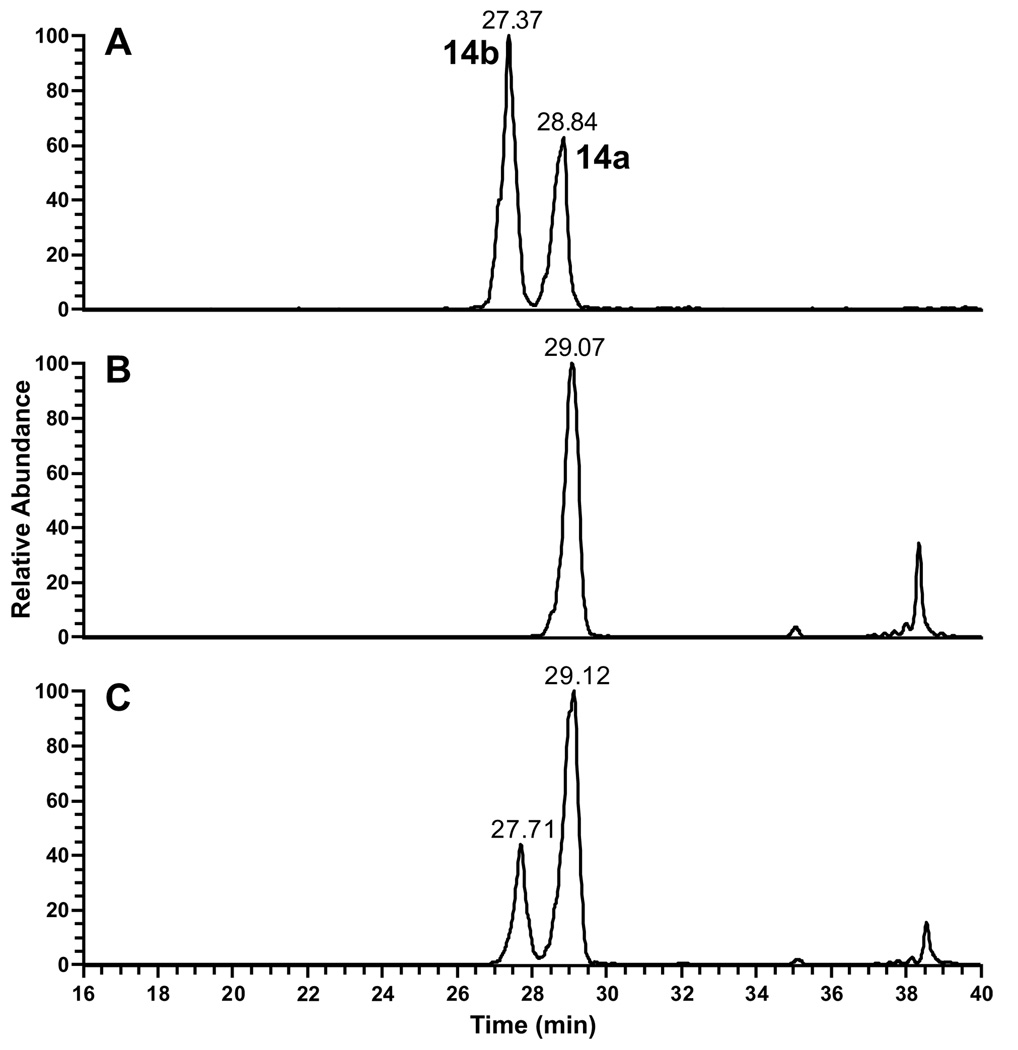
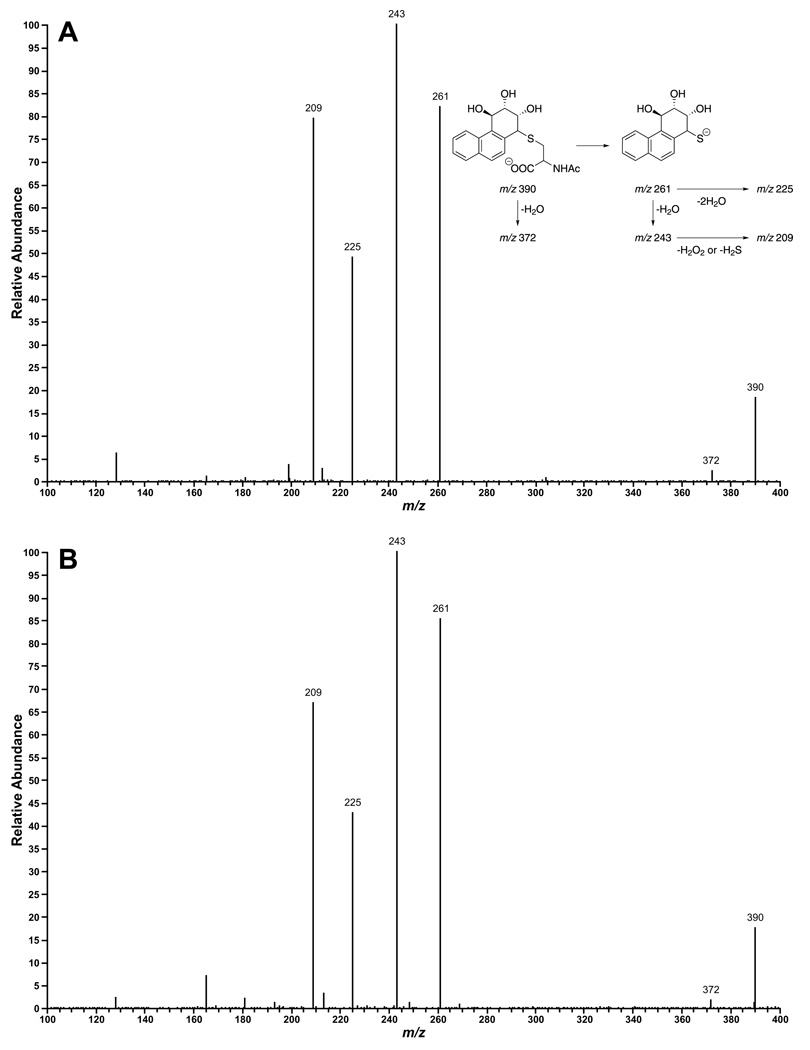
Analysis by LC-ESI-MS/MS-SRM of incubation mixtures of Phe-3,4-diol (8) and human hepatocytes produced chromatograms such as that shown in Figure 1B. The retention time of the peak illustrated and its MS/MS transitions (m/z 390 → 261; m/z 390 → 243; m/z 390 → 225; and m/z 390 → 209) matched that of standard 14a, as demonstrated by co-injection (Figure 1C), but not any of the other standards. No peak corresponding to standards 13a-d was observed in any incubation of Phe-1,2-diol (7) with these human hepatocyte samples. The results are summarized in Table 2. Amounts of 14a per incubation (mean ± S.D.) were 0.35 ± 0.91 pmol and 4.07 ± 7.27 pmol at 12 and 24h, respectively, for the 1 µM incubations and 0.96 ± 2.16 pmol and 15.6 ± 21.4 pmol at 12 and 24h for the 10 µM incubations.
Figure 1.
Chromatogram obtained upon LC-ESI-MS/MS-SRM analysis of (A) standard 14a and 14b obtained by the reaction of diol epoxide 11 with N-acetylcysteine. (Mercapturic acids 14c and 14d from syn-11 elute 5 – 8 min later than 14a under these conditions (24)); (B) a 24h incubation mixture of human hepatocytes and 10 µM Phe-3,4-diol (8), and (C) Coinjection of A and B demonstrating an increase in the peak corresponding to 14a. SRM was performed at m/z390 → m/z (209 + 225 + 243 + 261).
Table 2.
Formation of mercapturic acid 14a from Phe-3,4-diol (8) in human hepatocytesa
| pmol 14a per incubation |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human hepatocyte sample and incubation time (h) | ||||||||||||||||||||
| Substrate Concentration |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||||||||
| µM | 12 | 24 | 12 | 24 | 12 | 24 | 12 | 24 | 12 | 24 | 12 | 24 | 12 | 24 | 12 | 24 | 12 | 24 | 12 | 24 |
| 1 | NDb | 0.49 | 0.12 | 4.13 | ND | 1.06 | 0.06 | 1.44 | ND | 0.13 | ND | ND | ND | 0.30 | 0.30 | 5.79 | 2.92 | 24.0 | 0.09 | 3.33 |
| 10 | 0.12 | 3.75 | 0.28 | 20.7 | 0.21 | 7.89 | 0.09 | 5.16 | ND | 1.30 | ND | 0.65 | 0.24 | 4.06 | 1.67 | 24.5 | 6.59 | 71.8 | 0.36 | 16.3 |
Human hepatocytes were incubated with racemic 8 (1 or 10 µM) for 12 or 24h in a total volume of 2 ml. Under identical conditions with each hepatocyte sample at each time point, mercapturic acid products were not detected in incubations with racemic Phe-1,2-diol (7).
ND, not detected. Detection limit = 0.04 pmol, per 2 ml incubation.
We also carried out incubations containing both Phe-1,2-diol (7) and Phe-3,4-diol (8) and hepatocytes. These were performed with hepatocytes from 2 subjects (# 9 and #10 of Table 2), using mixtures containing concentrations of 1µM or 10 µM of each diol, and as in the studies summarized above, only mercapturic acid 14a from Phe-3,4-diol (8) was observed, in concentrations similar to those seen when 8 alone was the substrate.
One possible explanation for not detecting mercapturic acids derived from Phe-1,2-diol (7) in these experiments would be that diol epoxide 10 was not formed. Therefore, we quantified levels of racemic PheT (9 + 12) in some samples. The results, which are summarized in Table 3, clearly show that diols 7 and 8 were both converted to diol epoxides, and conversion of 8 exceeded that of 7.
Table 3.
Formation of tetraols (9 plus 12) from Phe-1,2-diol (7) and Phe-3,4-diol (8)a
| pmol 9 + 12 per incubation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human hepatocyte sample and incubation time (h) | ||||||||||
| Substrate | Substrate Concentration |
1 | 2 | 3 | ||||||
| µM | 6 | 12 | 24 | 6 | 12 | 24 | 6 | 12 | 24 | |
| Phe-1,2-diol (7) | 1 | 9.5 | 13.0 | 18.5 | 6.3 | 8.2 | 10.9 | 10.7 | 16.8 | 21.8 |
| 10 | 32.4 | 45.9 | 59.8 | 57.4 | 73.5 | 86.5 | 62.2 | 105 | 135 | |
| Phe-3,4-diol (8) | 1 | 23.3 | 49.1 | 131 | 23.1 | 48.8 | 128 | 31.8 | 87.4 | 296 |
| 10 | 97.3 | 239.8 | 853 | 159 | 546 | 2490 | 158 | 665 | 3240 | |
Human hepatocytes were incubated with racemic 7 or 8 (1 or 10 µM) for 6, 12, or 24h in a total volume of 2 ml. Tetraols 9 and 12 were analyzed together as a racemic mixture.
The formation of mercapturic acids from diol epoxides 10 and 11 was then examined in 2 hepatocyte samples (# 2 and #3). The retention times of the mercapturic acids matched those of the appropriate standards (14a from 11 and 13a from 10). The daughter ion spectrum of 14a matched that of a standard (Figure 2). Diol epoxide 11 (10 µM) was converted to mercapturic acid 14a in amounts of 3.23, 34.8, and 142 pmol in sample #2 and 10.5, 73.0 and 257 pmol in sample #3 at 6, 12, and 24h, respectively, while the corresponding values for mercapturic acid 13a from diol epoxide 10 were 0.62, 1.27, and 1.10 pmol in sample #2 and 0.3, 0.5, and 0.7 pmol in sample #3, only 0.3 – 3.6% as great as 14a. Some 13b was also observed. These results demonstrate that conjugation of diol epoxide 11 with glutathione far exceeds that of 10, consistent with the results summarized in Table 2.
Figure 2.
MS/MS daughter ion spectra of m/z 390 of (A) standard 14a and (B) 14a formed from incubation of diol epoxide 11 with human hepatocytes.
Discussion
The results of this study are consistent with our previous analyses of human urine in which we detected only mercapturic acid 14a derived from Phe-3,4-diol (8) via reverse diol epoxide 11 in samples from 36 smokers (24). No evidence for the presence of mercpaturic acids 13a-d, derived from Phe-1,2-diol (7) via bay region diol epoxide 10 was found, in spite of the fact that overall GST activity was adequate to form more than 50 times the amounts of total mercapturic acids observed. These results demonstrate that reverse diol epoxides such as 11 are better substrates for GST conjugation in human hepatocytes than bay region diol epoxides such as 10. Although we did not study the fate of BPDE or other carcinogenic bay region diol epoxides here, previous metabolism studies demonstrate striking similarities between the metabolism of phenanthrene, the simplest PAH with a bay region, and other PAH (19–21). Since reverse diol epoxides are generally not associated with PAH mutagenicity or carcinogenicity, our results call into question the widely held assumption that bay region diol epoxides of carcinogenic PAH are detoxified by GSTs in humans, an assumption that underlies virtually all molecular epidemiology studies of GST genotypes, PAH exposure, and cancer.
Our analytical method was sensitive enough to detect mercapturic acids 13a-d in amounts approximately 10% as great as 14a from Phe-3,4-diol (8). This was calculated based on the amounts of tetraol formed from Phe-1,2-diol (7) and the detection limit of approximately 0.04 pmol of mercapturic acid per 2 ml incubation. In fact, the amount of mercapturic acid 13a produced from diol epoxide 10 was only 0.3–3.6% as great as that from reverse diol epoxide 11, in incubations starting with these substrates, consistent with the lack of detection of 13a-d from 7.
Sundberg and Jernstrom investigated the conjugation of bay and fjord region diol epoxides of several PAH in a series of studies (9–13). One observation with relevance to the present results was that, in studies of the (+)-enantiomer of BPDE, the actual fraction of this diol epoxide undergoing GST conjugation in Chinese hamster V79 cells stably expressing different human GSTs was only 2% or less than that observed with the pure enzymes (9). These results demonstrate that there are important competitive reactions, most likely with H2O or other cellular nucleophiles, which detoxify BPDE and other diol epoxides in cells. Indeed, the amounts of mercapturic acid 14a derived from Phe-3,4-diol (8) in our study were only about 0.2–3% as great as the amounts of tetraol produced at 24h (Table 2 and Table 3).
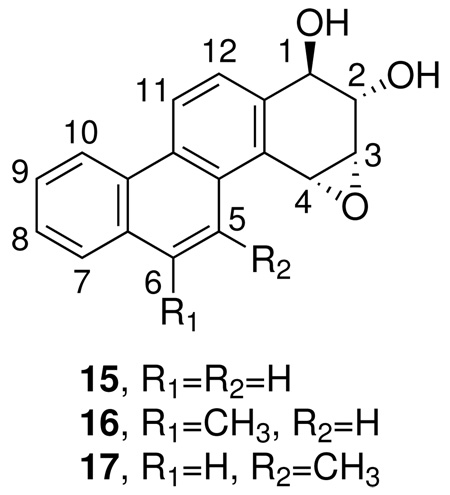
The studies of Sundberg and Jernstrom also demonstrated that conjugation of bay and fjord region diol epoxides with GSTs is highly dependent on steric characteristics of the diol epoxide, which were pertinent to the accommodation of these substrates in the GST active site (9–13). Although we did not investigate stereochemical details in this study, the benzylic oxirane carbon in bay region diol epoxide 10 is clearly more hindered than that of reverse diol epoxide 11, which is consistent with preferential conjugation of the latter. These results could be important in considering the high DNA binding and carcinogenic activities of sterically hindered bay region diol epoxides. For example, in unpublished work1, we have observed higher reactivities with glutathione, in the presence of rat liver cytosol, of diol epoxides 15 and 16 derived from the weak carcinogens chrysene and 6-methylchrysene compared to 17, an ultimate carcinogen of the strong carcinogen 5-methylchrysene. Further studies are required to investigate the contribution of glutathione conjugation to the biological activity of PAH diol epoxide metabolites.
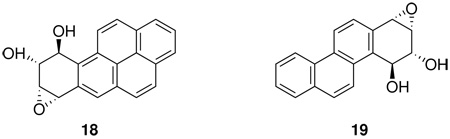
Most studies indicate that reverse diol epoxides are less mutagenic and carcinogenic than bay region diol epoxides, although the data base is somewhat limited (3,32). anti-BaP-9,10-diol-7,8-epoxide (18) and anti-chrysene-3,4-diol-1,2-epoxide (19) are generally less mutagenic than the corresponding bay region diol epoxides 1 and 15 in S. typhimurium strains and V79 cells, with the exception of 19 in strain TA98 (3,32).
Furthermore, diol precursors to bay region diol epoxides are generally more carcinogenic than diol precursors to reverse diol epoxides (3). The mutagenicity and carcinogenicity of reverse diol epoxide 11 have not been studied, while the bay region diol epoxide 10 is a weak mutagen which exhibits virtually no carcinogenic activity (33,34). Probably detoxification of reverse diol epoxides by glutathione conjugation would have minimal impact on PAH carcinogenesis in exposed humans.
A limitation of this study was that we analyzed the human hepatocyte incubation mixtures for the mercapturic acids 13 and 14 but not their glutathione conjugate precursors. It is possible that differential metabolism of these precursors to the mercapturic acids in human hepatocytes could contribute to the results reported here.
In summary, the results of this study demonstrate that the reverse diol epoxide metabolite 11 of phenanthrene is conjugated far more effectively in human hepatocytes than the bay region diol epoxide 10. These results are consistent with analyses of human urine, in which mercapturic acids derived from reverse diol epoxide 11, but not 10, were detected (24). If these results are generalizable to carcinogenic PAH diol epoxides, which seems likely, they undermine the widely held assumption that glutathione conjugation of PAH diol epoxide ultimate carcinogens is an important protective mechanism against cancer in exposed humans.
Acknowledgement
This study was supported by grant number CA- 92025 from the National Cancer Institute. We thank Professor Sharon E. Murphy for her criticism and advice.
Footnotes
A.A. Melikian and S.S. Hecht, unpublished.
References
- 1.Luch A, Baird WM. Metabolic activation and detoxification of polycyclic aromatic hydrocarbons. In: Luich A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. London: Imperial College Press; 2005. pp. 19–96. [Google Scholar]
- 2.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet. Oncol. 2005;6:931–932. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- 3.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G.H.A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 4.Cooper CS, Grover PL, Sims P. The metabolism and activation of benzo[a]pyrene. Prog. Drug Metab. 1983;7:295–396. [Google Scholar]
- 5.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 32. Lyon, FR: IARC; 1983. Polynuclear Aromatic Compounds, Part 1. Chemical, Environmental and Experimental Data; pp. 419–430. [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 34. Lyon, FR: IARC; 1984. Polynuclear Aromatic Compounds, Part 3. Industrial Exposures in Aluminum Production, Coal Gasification, Coke Production, and Iron and Steel Founding; pp. 65–131. [Google Scholar]
- 7.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 35. Lyon, France: IARC; 1985. Polynuclear Aromatic Compounds, Part 4. Bitumens, Coal-Tars and Derived Products, Shale Oils and Soots; pp. 83–241. [PubMed] [Google Scholar]
- 8.Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nature Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 9.Sundberg K, Dreij K, Seidel A, Jernstrom B. Glutathione conjugation and DNA adduct formation of dibenzo[a,l]pyrene and benzo[a]pyrene diol epoxides in V79 cells stably expressing different human glutathione transferases. Chem. Res. Toxicol. 2002;15:170–179. doi: 10.1021/tx015546t. [DOI] [PubMed] [Google Scholar]
- 10.Dreij K, Sundberg K, Johansson AS, Nordling E, Seidel A, Persson B, Mannervik B, Jernstrom B. Catalytic activities of human alpha class glutathione transferases toward carcinogenic dibenzo[a,l]pyrene diol epoxides. Chem. Res. Toxicol. 2002;15:825–831. doi: 10.1021/tx025519i. [DOI] [PubMed] [Google Scholar]
- 11.Sundberg K, Dreij K, Berntsen S, Seidel A, Jernstrom B. Expression of human glutathione transferases in V79 cells and the effect on DNA adduct-formation of diol epoxides derived from polycyclic aromatic hydrocarbons. Chem.-Biol. Interact. 2001;133:91–94. [Google Scholar]
- 12.Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, Jernstrom B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19:433–436. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- 13.Sundberg K, Widersten M, Seidel A, Mannervik B, Jernstrom B. Glutathione conjugation of bay-and fjord-region diol epoxides of polycyclic aromatic hydrocarbons by glutathione transferase M1-1 and P1-1. Chem. Res. Toxicol. 1997;10:1221–1227. doi: 10.1021/tx970099w. [DOI] [PubMed] [Google Scholar]
- 14.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers & Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 15.Benhamou S, Lee WJ, Alexandrie AK, Boffetta P, Bouchardy C, Butkiewicz D, Brockmoller J, Clapper ML, Daly A, Dolzan V, Ford J, Gaspari L, Haugen A, Hirvonen A, Husgafvel-Pursiainen K, Ingelman-Sundberg M, Kalina I, Kihara M, Kremers P, Le Marchand L, London SJ, Nazar-Stewart V, Onon-Kihara M, Rannug A, Romkes M, Ryberg D, Seidegard J, Shields P, Strange RC, Stucker I, To-Figueras J, Brennan P, Taioli E. Meta-and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23:1343–1350. doi: 10.1093/carcin/23.8.1343. [DOI] [PubMed] [Google Scholar]
- 16.Hung RJ, Boffetta P, Brockmoller J, Butkiewicz D, Cascorbi I, Clapper ML, Garte S, Haugen A, Hirvonen A, Anttila S, Kalina I, Le Marchand L, London SJ, Rannug A, Romkes M, Salagovic J, Schoket B, Gaspari L, Taioli E. CYP1A1 and GSTM1 genetic polymorphisms and lung cancer risk in Caucasian nonsmokers: a pooled analysis. Carcinogenesis. 2003;24:875–882. doi: 10.1093/carcin/bgg026. [DOI] [PubMed] [Google Scholar]
- 17.Hashibe M, Brennan P, Strange RC, Bhisey R, Cascorbi I, Lazarus P, Oude Ophuis MB, Benhamou S, Foulkes WD, Katoh T, Coutelle C, Romkes M, Gaspari L, Taioli E, Boffetta P. Meta-and pooled analyses of GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes and risk of head and neck cancer. Cancer Epidemiol. Biomarkers Prev. 2003;12:1509–1517. [PubMed] [Google Scholar]
- 18.Vineis P, Veglia F, Anttila S, Benhamou S, Clapper ML, Dolzan V, Ryberg D, Hirvonen A, Kremers P, Le Marchand L, Pastorelli R, Rannug A, Romkes M, Schoket B, Strange RC, Garte S, Taioli E. CYP1A1, GSTM1 and GSTT1 polymorphisms and lung cancer: a pooled analysis of gene-gene interactions. Biomarkers. 2004;9:298–305. doi: 10.1080/13547500400011070. [DOI] [PubMed] [Google Scholar]
- 19.Nordquist M, Thakker DR, Vyas KP, Yagi H, Levin W, Ryan DE, Thomas PE. Metabolism of chrysene and phenanthrene to bay-region diol epoxides by rat liver enzymes. Mol. Pharmacol. 1981;19:168–178. [PubMed] [Google Scholar]
- 20.Thakker DR, Yagi H, Levin W, Wood AW, Conney AH, Jerina DM. Polycyclic aromatic hydrocarbons: metabolic activation to ultimate carcinogens. In: Anders MW, editor. Bioactivation of Foreign Compounds. New York: Academic Press, Inc; 1985. pp. 177–242. [Google Scholar]
- 21.Shou M, Korzekwa KR, Krausz KW, Crespi CL, Gonzalez FJ, Gelboin HV. Regio-and stereo-selective metabolism of phenanthrene by twelve cDNAexpressed human, rodent, and rabbit cytochromes P-450. Cancer Lett. 1994;83:305–313. doi: 10.1016/0304-3835(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 22.Hecht SS, Chen M, Yoder A, Jensen J, Hatsukami D, Le C, Carmella SG. Longitudinal study of urinary phenanthrene metabolite ratios: effect of smoking on the diol epoxide pathway. Cancer Epidemiol. Biomarkers & Prev. 2005;14:2969–2974. doi: 10.1158/1055-9965.EPI-05-0396. [DOI] [PubMed] [Google Scholar]
- 23.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol. Biomarkers & Prev. 2003;12:1501–1508. [PubMed] [Google Scholar]
- 24.Hecht SS, Villalta PW, Hochalter JB. Analysis of phenanthrene diol epoxide mercapturic acid detoxification products in human urine: relevance to molecular epidemiology studies of glutathione-S-transferase polymorphisms. Carcinogenesis. 2008;29:937–943. doi: 10.1093/carcin/bgn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 26.Carmella SG, Yoder A, Hecht SS. Combined analysis of r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in smokers' plasma. Cancer Epidemiol. Biomarkers & Prev. 2006;15:1490–1494. doi: 10.1158/1055-9965.EPI-06-0199. [DOI] [PubMed] [Google Scholar]
- 27.Soderdahl T, Kuppers-Munther B, Heins N, Edsbagge J, Bjorquist P, Cotgreave I, Jernstrom B. Glutathione transferases in hepatocyte-like cells derived from human embryonic stem cells. Toxicol. in Vitro. 2007;21:929–937. doi: 10.1016/j.tiv.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Cmarik JL, Inskeep PB, Meredith MJ, Meyer DJ, Ketterer B, Guengerich FP. Selectivity of rat and human glutathione S-transferases in activation of ethylene dibromide by glutathione conjugation and DNA binding and induction of unscheduled DNA synthesis in human hepatocytes. Cancer Res. 1990;50:2747–2752. [PubMed] [Google Scholar]
- 29.Donato MT, Gomez-Lechon MJ, Jover R, Nakamura T, Castell JV. Human hepatocyte growth factor down-regulates the expression of cytochrome P450 isozymes in human hepatocytes in primary culture. J Pharmacol Exp Ther. 1998;284:760–767. [PubMed] [Google Scholar]
- 30.Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P, Bouchardy C, Breskvar K, Brockmoller J, Cascorbi I, Clapper ML, Coutelle C, Daly A, dell'Omo M, Dolzan V, Dresler CM, Fryer A, Haugen A, Hein DW, Hildesheim A, Hirvonen A, Hsieh LL, Ingelman-Sundberg M, Kalina I, Kang D, Kihara M, Kiyohara C, Kremers P, Lazarus P, Le Marchand L, Lechner MC, van Lieshout EM, London S, Manni JJ, Maugard CM, Morita S, Nazar-Stewart V, Noda K, Oda Y, Parl FF, Pastorelli R, Persson I, Peters WH, Rannug A, Rebbeck T, Risch A, Roelandt L, Romkes M, Ryberg D, Salagovic J, Schoket B, Seidegard J, Shields PG, Sim E, Sinnet D, Strange RC, Stucker I, Sugimura H, To-Figueras J, Vineis P, Yu MC, Taioli E. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- 31.Jacob J, Grimmer G, Dettbarn G. Profile of urinary phenanthrene metabolites in smokers and non-smokers. Biomarkers. 1999;4:319–327. doi: 10.1080/135475099230705. [DOI] [PubMed] [Google Scholar]
- 32.Glatt H, Wameling C, Elsberg S, Thomas H, Marquardt H, Hewer A, Phillips DH, Oesch F, Seidel A. Genotoxicity characteristics of reverse diol-epoxides of chrysene. Carcinogenesis. 1993;14:11–19. doi: 10.1093/carcin/14.1.11. [DOI] [PubMed] [Google Scholar]
- 33.Buening MK, Levin W, Karle JM, Yagi H, Jerina DM, Conney AH. Tumorigenicity of bay-region epoxides and other derivatives of chrysene and phenanthrene in newborn mice. Cancer Res. 1979;39:5063–5068. [PubMed] [Google Scholar]
- 34.Wood AW, Chang RL, Levin W, Ryan DE, Thomas PE, Mah HD, Karle JM, Yagi H, Jerina DM, Conney AH. Mutagenicity and tumorigenicity of phenanthrene and chrysene epoxides and diol epoxides. Cancer Res. 1979;39:4069–4077. [PubMed] [Google Scholar]