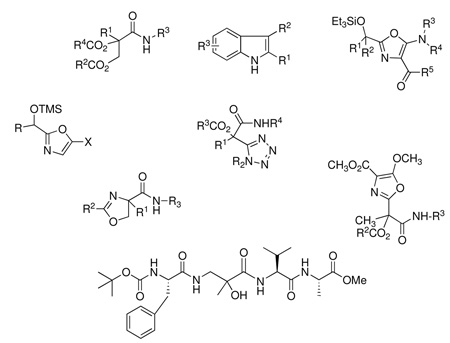
CONSPECTUS
By generating structural complexity in a single step from three or more reactants, multicomponent reactions (MCRs) make it possible to synthesize target compounds with greater efficiency and atom economy. The history of such reactions can be traced to the mid-nineteenth century when Strecker first produced α-aminonitriles from the condensation of aldehydes with ammonia and hydrogen cyanide.
Recently, academic chemists have renewed their interest in MCRs. In part, the pharmaceutical industry has fueled this resurgence because of the growing need to assemble libraries of structurally complex substances for evaluation as lead compounds in drug discovery and development programs. The application of MCRs to that increasingly important objective remains limited by the relatively small number of such reactions that can be broadly applied to prepare biologically relevant or natural-product-like molecular frameworks.
We were interested in applying logic-based approaches, such as our single reactant replacement (SRR) approach, as a way both to improve known MCRs and design new multiple-component routes to bioactive structures. This Account provides several examples that illustrate the use of SRR with known MCRs as starting points for synthetic innovation in this area.
As part of our working hypothesis, we initially explored strategies for engineering improvements into known MCRs, either by increasing the dimensionality—i.e. changing an n-component to an (n+1)-component reaction—or broadening the scope of useful input structures, or both. By exhaustively applying retrosynthetic analysis to the cognate MCR to identify and exploit alternative entry points into the overall reaction manifold, we have devised several such re-engineered MCRs. Serendipitous findings have also augmented the yield of useful developments from our logic-inspired approach. In some cases, we have identified surprising links between different compound families that provide useful new entry points for chemical library synthesis. In other cases, the same re-engineering logic made it possible (sometimes in unexpected ways) to transform certain non-elementary two-component reactions into higher order MCRs.
While logic may also inspire the search for new MCRs, the design process requires added chemical creativity, which cannot be reduced to a simple formula. The long-term goal of our research is to expand the useful repertoire of such reactions, which are important as complexity-generating tools in both combinatorial and diversity-oriented synthesis.
Keywords: Multicomponent Reactions, Diversity-Oriented Synthesis, Synthetic Methodology
INTRODUCTION
A multicomponent reaction (MCR) is generally defined as any process in which three or more reactants combine in one pot to form a product that incorporates structural features of each reagent.1 Besides generating structural complexity in a single step, MCRs offer the advantage of simplicity and synthetic efficiency over conventional chemical reactions. The most useful MCRs have the additional advantages of selectivity, synthetic convergency, and atom-economy.2
MCRs represent the cornerstones of both combinatorial chemistry and diversity-oriented synthesis, and thus have played a central role in the development of modern synthetic methodology for pharmaceutical and drug discovery research.3 Used in conjunction with target-oriented synthesis, combinatorial chemistry approaches can be employed to introduce or broaden structural variations in a lead compound of interest. Diversity-oriented synthesis is helpful in exploring large areas of chemical structure space in search of new bioactive small molecules that might not be identified by conventional natural product screening assays. While the two approaches are complementary, both benefit from the complexity-generating characteristics of MCRs.
Despite the spectacular investment in, and organic growth of, combinatorial chemistry as a platform technology within the pharmaceutical industry during the 1980s and 1990s, few new MCRs were discovered or developed by corporate research laboratories. Most combinatorial libraries were assembled using traditional, tried-and-true processes such as the Biginelli (1891), Hantzsch (1882), Mannich (1912), Passerini (1921), Strecker (1850) and Ugi (1959) reactions. However, judging from numerous recent reports,4–6 interest has now intensified within academic labs in the development of new MCRs as the basis for complexity-generating strategies for the synthesis of small molecules.
The earliest MCRs were almost certainly discovered by chance or serendipity. With the emergence of rational, well-defined reaction mechanisms guided by the concepts of structure and bonding in organic chemistry, opportunities arose for rational analysis and design of MCRs. For example, it seems likely (although uncertain) that the discovery of the Ugi four-component synthesis of α-aminoacid diamides was based on Ugi’s prior knowledge of the Passerini three-component synthesis of O-acylated α-hydroxyamides (vide infra). That link notwithstanding, the rational design (or improvement) of practical and versatile multicomponent reactions -- especially those that form medicinal or natural product-like frameworks -- remained, until recently, a largely unmined area of chemical research. This Account describes our laboratory’s efforts towards that goal.
IMPROVING AND INVENTING MCRs
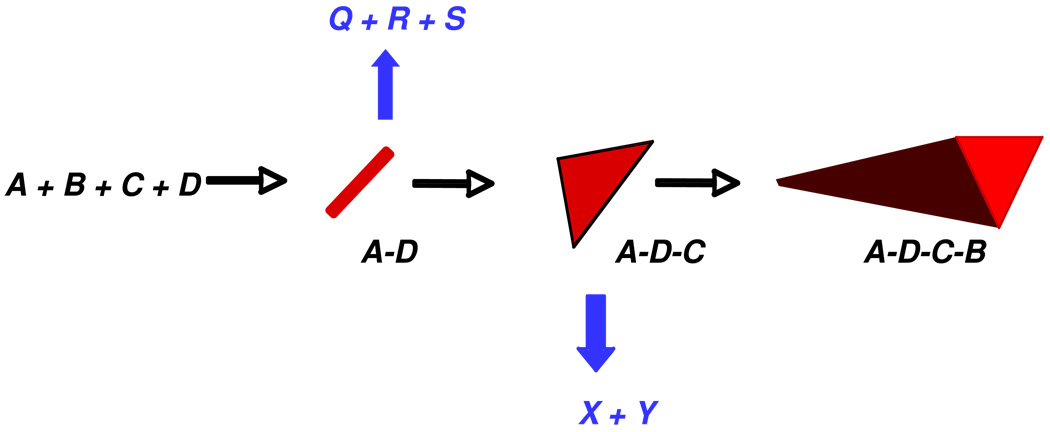
Figure 1 depicts a general approach to improving known MCRs that takes advantage of a detailed knowledge of reaction mechanism. A typical 4-component reaction of interest in combinatorial synthesis would employ inputs A, B, C, and D, each representing a family of compounds. The overall transformation might be visualized as a linear series of individual bimolecular reactions successively producing the symbolic intermediates A−D and A−D−C on the way to the final MCR product, A−D−C−B. [Note: The progression from line segment to triangle to pyramid is only meant to connote increasing molecular complexity, and is not meant to imply or designate specific connectivity between inputs.]
Figure 1.
Improving Known MCRs by Retrosynthetic Analysis
Applying retrosynthetic analysis to intermediates A−D−C and A−D might identify independent routes to those intermediates from X+Y or from Q+R+S, respectively, as indicated. It follows that combining X+Y with B (and likewise combining Q+R+S with C and B) would constitute new 3- and 5-component routes, respectively, to the same product of the cognate reaction of A+B+C+D, but from a more diverse set of reactants, thus broadening the potential scope of chemical library synthesis. If Q, R, and S are readily available commercial compounds, the enhanced dimensionality of the 5-component route exponentially increases the potential size of the synthetic library.
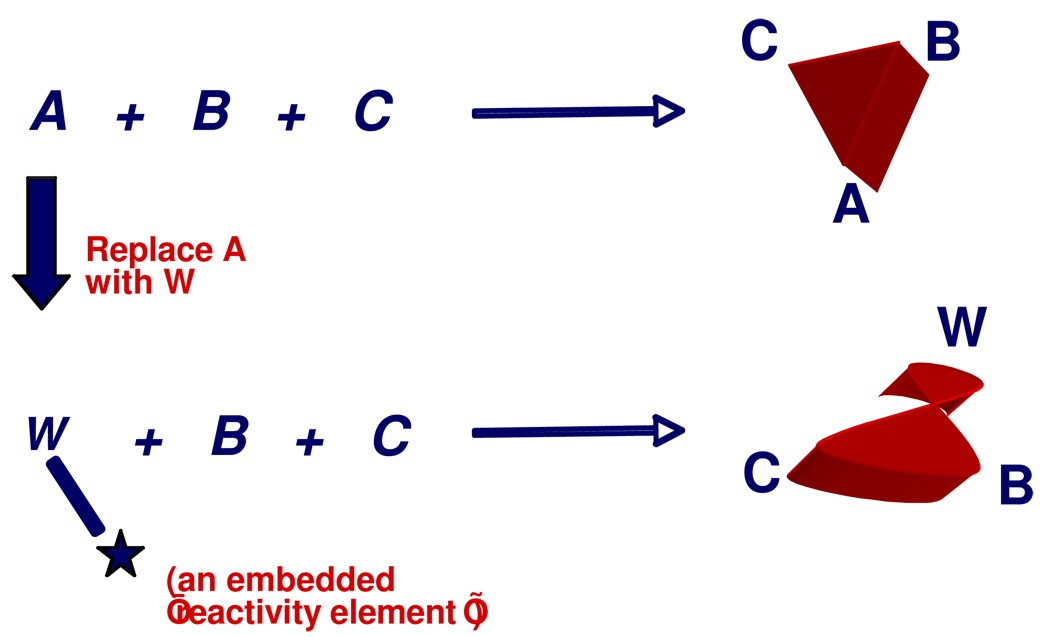
Thus, logical ways to reengineer (and thus improve) a known MCR can be described. However, the notion that the creative and/or serendipitous elements involved in inventing or discovering new MCRs might also be guided, somehow, by a rationally-designed process seems far less obvious. Nevertheless, Figure 2 depicts a general strategy whereby mechanistic insights into a known MCR might serve as an innovation platform for finding new MCRs using a process nicknamed the single reactant replacement (SRR) approach.
Figure 2.
The Single Reactant Replacement Approach to Finding New MCRs
This approach begins with a systematic assessment of the mechanistic and/or functional role of each reactant in a known MCR. Based on the resulting chemical insights, one input (A, in this case) is then replaced with a different input W that mimics the key chemical reactivity or property necessary for condensation to occur with B and C. By embedding additional reactivity or functionality (either explicit or latent) into W, the resulting MCR might be directed to a different outcome -- for example, either a new structural framework or ring system. Thus, the chemist’s mechanistic insight into a known MCR can serve as an innovation platform from which to design or create imaginative SRR substitutions.
While the newly-developed MCR might resemble the cognate reaction, the SRR process is iterative, and can be applied again, this time replacing one of the other components in the same fashion. After one or two SRR cycles, the new MCRs that emerge are likely to be quite distinctive, and bear little resemblance to their progenitors.
EARLY EXAMPLES OF THE SRR APPROACH
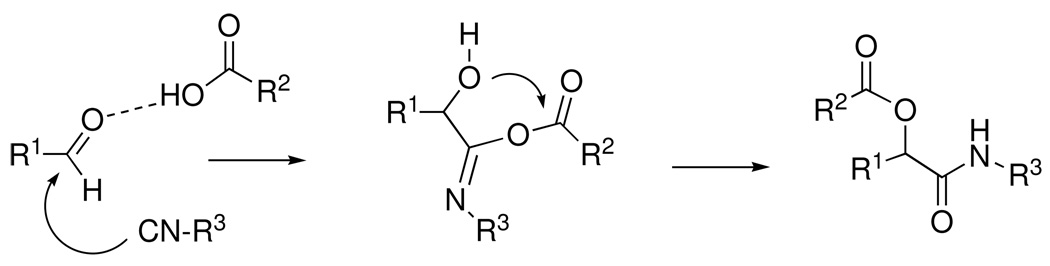
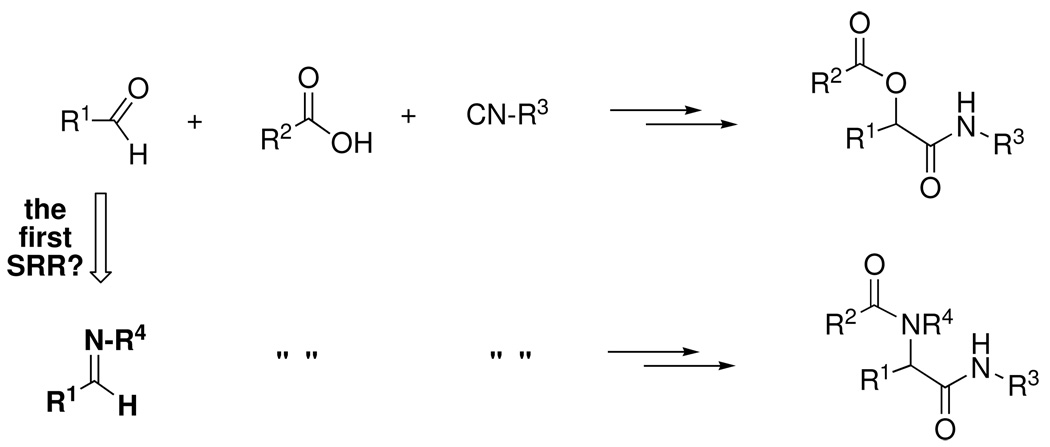
The Passerini reaction serves as a useful example (Scheme 1), since it has been thoroughly studied over the past 80 years.7 According to the widely accepted mechanism, the key reactivity element of the carbonyl component is its electrophilicity, which is sufficiently enhanced by H-bonding to (or protonation by) the carboxylic acid reactant to trigger nucleophilic attack by the isonitrile component. A subsequent O-to-O rearrangement affords the final α-acyloxycarboxamide shown.
Scheme 1.
Informed by such mechanistic knowledge, a clever chemist (in this instance Ivar Ugi) might recognize that replacing the simple carbonyl component with an imine of comparable electrophilicity could conceivably result in a mechanistically similar condensation with a carboxylic acid and isonitrile, in this instance to afford an α-acylaminocarboxamide (Scheme 2).8
Scheme 2.
Although we cannot now know the extent to which logic, and not serendipity, played a role in the discovery of the famous Ugi 4-component peptide synthesis, the authors of his recent obituary9 noted that “Ugi studied both chemistry and mathematics at Tübingen (1949–52), but later concentrated solely on chemistry. Nevertheless his love of mathematics remained influential during his subsequent scientific career.”
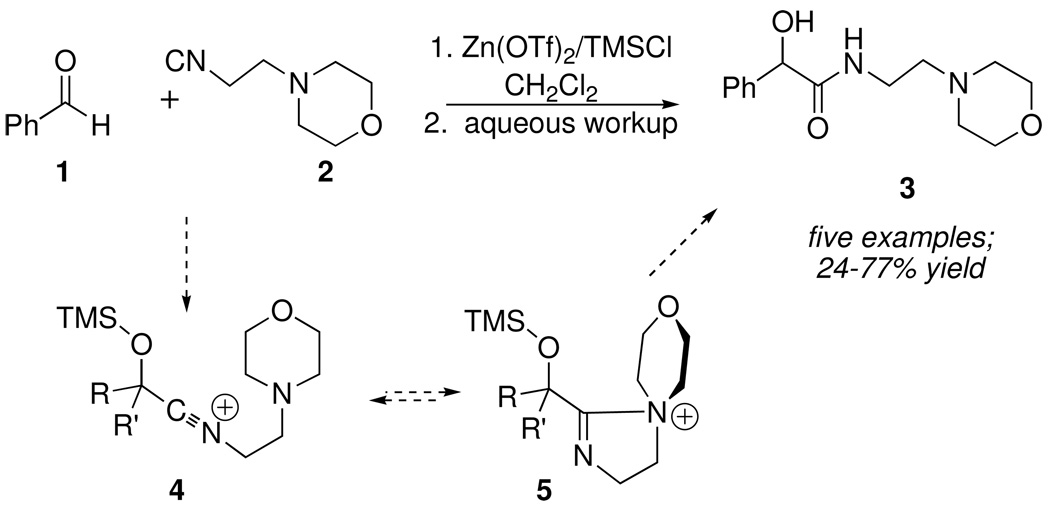
Following in Ugi’s footsteps, and guided by the SRR approach, we investigated the effect of replacing the carboxylic acid component of the Passerini reaction with Lewis acids that might also activate (by coordination instead of H-bonding) simple carbonyl compounds towards nucleophilic addition of the isonitrile component. Using the Passerini condensation of benzaldehyde 1 with morpholinoethylisonitrile 2 (Scheme 3) as a test reaction, we discovered that formation of 3 was promoted by trimethylsilyltriflate, which could be generated in situ by the combination of Zn(OTf)2 and TMSCl. Under these mild conditions, isonitrile 2 formed adducts with several representative carbonyl compounds.10
Scheme 3.
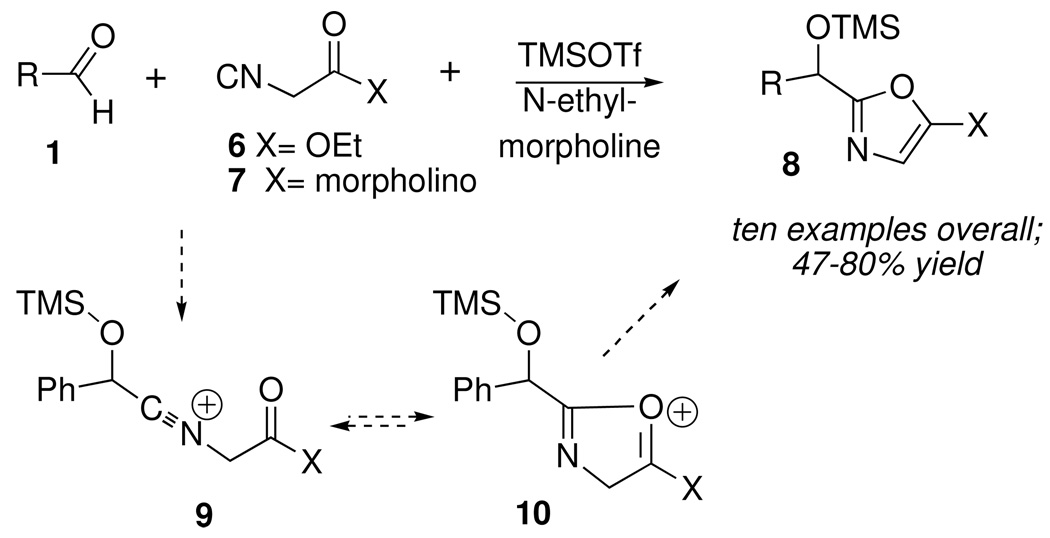
Interestingly, however, none of the corresponding condensations occurred when 2 was replaced with a simple, unfunctionalized isonitrile such as cyclohexyl or n-butyl isonitrile, suggesting that the morpholine ring in 2 contributed to the stabilization of intermediate nitrilium ion 4 (e.g. as 5) prior to hydrolytic workup. In support of that hypothesis, other isonitriles containing good donor groups, such as isocyanoacetate 6 or isocyanoacetamide 7 (Scheme 4) afforded the corresponding ethoxy or morpholinooxazoles 8 in good yield.10 Substituted oxazoles are active pharmacophores, and are also found in a variety of medicinally significant natural products. A plausible mechanism invokes a similar neighboring group effect, leading to stabilization of the initial nitrilium ion 9 as oxonium ion 10.11 Deprotonation of 10 by N-ethylmorpholine led to 8.
Scheme 4.
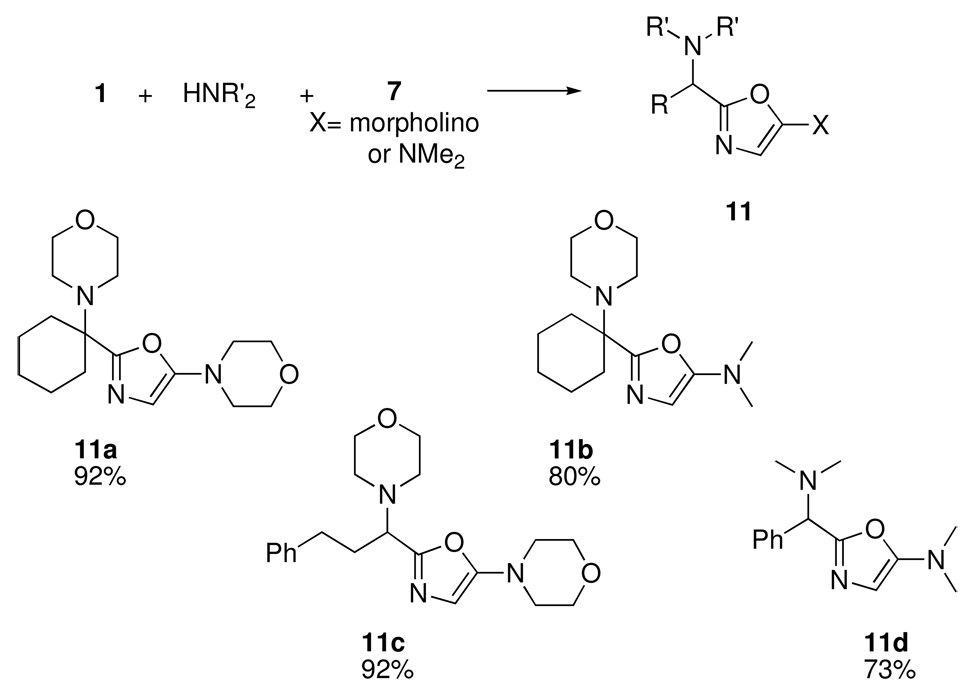
Having shown that a single SRR operation (substituting a Lewis acid for the Bronsted acid component in a Passerini reaction) led to a new synthesis of biologically important heterocycles, we decided to test the iterative SRR approach by subsequently replacing the carbonyl component 1 in the oxazole-forming process (Scheme 4) with the corresponding “iminium” species.12 In fact, premixing the carbonyl component with either morpholine or dimethylamine in a range of solvents (CH3OH, CH2Cl2, toluene) led to iminium analogs of 1 that reacted smoothly with isocyanoacetamides 7 (X= morpholino or NMe2) to afford bis-amino-oxazoles 11 (Scheme 5).12 In this instance, it turned out that no Lewis acid promoter was necessary, although yields were significantly higher in the presence of pyridine hydrochloride or triethylamine hydrochloride. An assortment of aldehydes and ketones, represented by some of the examples shown in Scheme 5, successfully underwent this transformation.
Scheme 5.
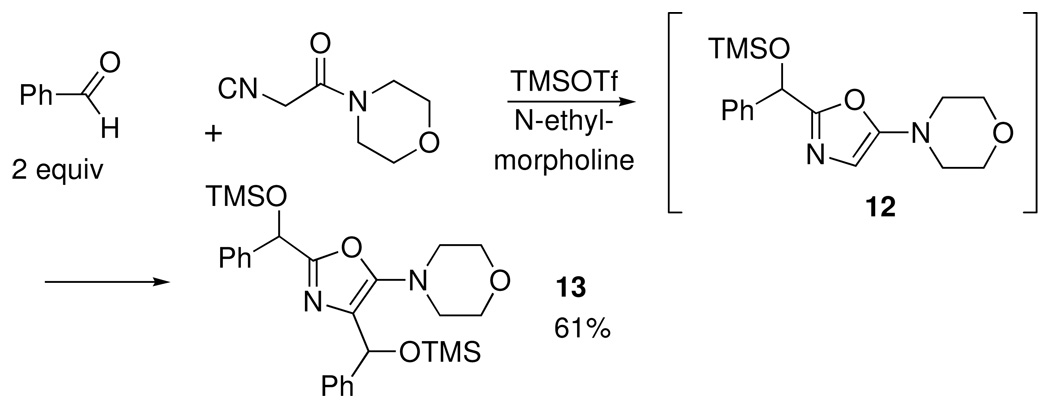
Besides being amenable to iterative SRR, the oxazole-forming process in Scheme 4 gave an unexpected result when benzaldehyde was reacted with isocyanoamide 7. The anticipated (and desired) adduct 12 (Scheme 6) was accompanied by minor amounts of a 2:1 adduct, identified as 13, which likely arose by a second condensation of benzaldehyde at the 4-position of the oxazole ring in 12. As expected. 13 became the dominant product using two equiv of benzaldehyde.
Scheme 6.
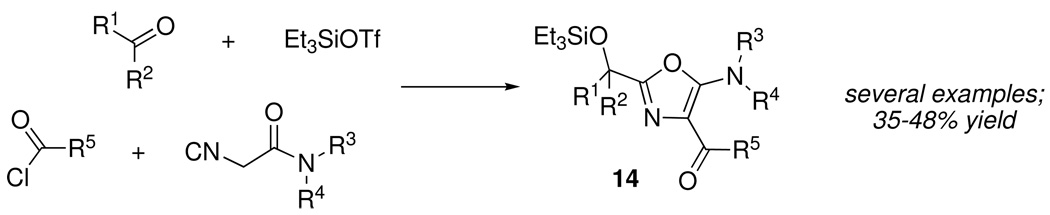
Such unexpected findings can represent useful opportunities for further exploration. In this instance, formation of the side product 13, which was general with aromatic aldehydes, provided a key insight into the elevated reactivity at C-4 of 5-aminooxazoles, which could be exploited in the form of a new, one-pot, 4-component condensation of aldehydes, silyltriflates, isocyanoacetamides and acid chlorides to afford 2,4,5-trisubstituted oxazoles 14 (Scheme 7).14 The process was compatible with a range of aliphatic and aromatic acid chlorides, including crotonyl, cinnamoyl, phenoxyacetyl, and phenacyl chlorides.
Scheme 7.
RE-ENGINEERING AND IMPROVING KNOWN MCRs
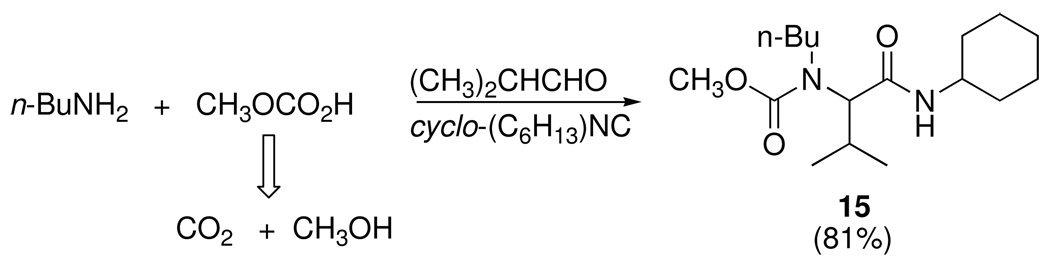
We decided to illustrate the approach depicted in Figure 1 by applying it to Ugi’s well-known four-component diamide synthesis, whose genesis is suggested in Scheme 2. As indicated in Figure 1, the pool of available products from MCRs can be expanded by increasing the number of reacting components -- a notion that was perhaps first implemented by the development, in Ugi’s laboratory, of a 5-component route to amidourethanes such as 15 (Scheme 8).15 Ugi recognized that one class of carboxylic acids, monoalkylcarbonic acids, could be prepared independently by the reversible reaction of CO2 with alcohols, and subsequently function as the acid component in Ugi condensations. Although the reaction was limited to low MW liquid alcohols, the result nicely illustrated the concept of increasing the dimensionality of MCRs.
Scheme 8.
After a comprehensive review of all the known reactive intermediates implicated in the Ugi reaction, retrosynthetic analysis of the first-formed imine 18 (Scheme 9) identified an alternative approach to this intermediate based on the addition of organometallic reagents to nitriles. In principle, two complementary combinations of nitriles with either Grignard of organolithium reagents might provide a direct, 4-component route to N-unsubstituted ketimines 17 (usually difficult to prepare by condensations of ketones with ammonia) by in situ protonation of the corresponding metalloimines 16. Besides implementing this route to N-unsubstituted diamides 19 (R3= H), we further demonstrated that imine 17 underwent amine/imine exchange with primary amines R3NH2 to afford N-substituted imines 18, thus facilitating a one-pot, 5-component Ugi reaction that formed N-substituted α-aminoacid diamides 19.16
Scheme 9.
Ugi’s contributions, as well as those from our lab at Cornell and elsewhere, showed that boosting the dimensionality of MCRs could generate larger, more diverse chemical libraries. However, as has already been noted, relatively few useful (i.e. widely adopted) MCRs are known, making it apparent that the re-engineering approach would encounter certain natural limitations. Absent a more general pool of new MCRs with which to tinker, opportunities for improvement would soon be depleted.
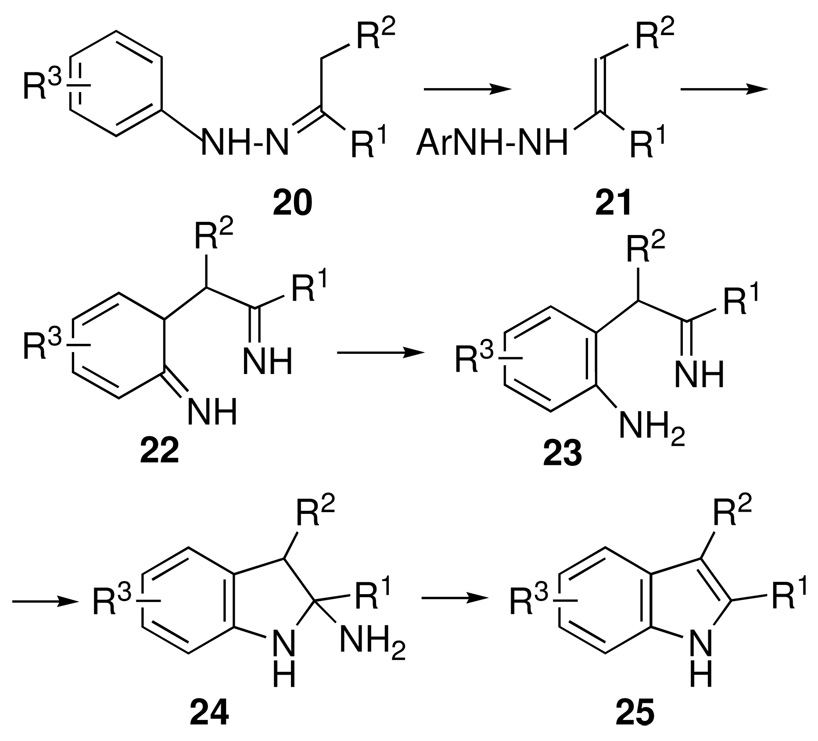
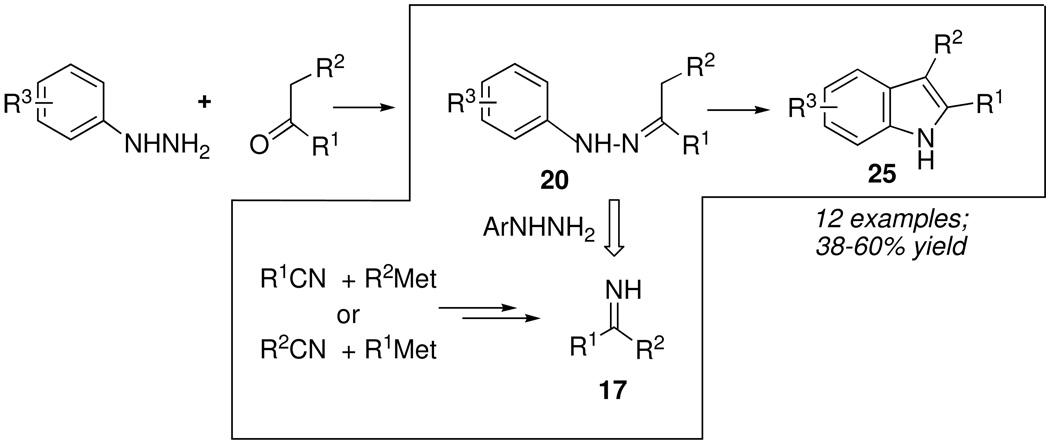
Since multicomponent reactions are defined as processes involving a minimum of three reactants, the possibility of enhancing the dimensionality of non-elementary two-component (A+ B → C) chemical reactions looked like an attractive prospect, especially when such processes involved one or more intermediates that could be subjected to retrosynthetic analysis. For example, the well-known Fischer indole reaction (Scheme 10) involves an initial condensation of arylhydrazines with enolizable ketones to form hydrazone 20, followed by a series of acid-catalyzed tautomerizations, rearrangements and eliminations, leading to substituted indoles 25. A detailed mechanistic analysis reveals no fewer than five discreet intermediates (20–24, Scheme 10) that might be targeted for independent synthesis, of which the initial arylhydrazone 20 was a particularly attractive candidate.
Scheme 10.
We reasoned that the same condensation of nitriles with Grignard or organolithium reagents that previously furnished a route to N-unsubstituted imines 17 (after protonation) for the Ugi reaction might also be adapted to a 3-component Fischer indole synthesis (Scheme 11). Thus, imine 17 should react rapidly with arylhydrazines to form hydrazones 20, which, after rearrangement into the corresponding indoles 25 would incorporate structural diversity from three, instead of two, reactants.
Scheme 11.
In the event, the new 3-component Fischer synthesis formed indoles as efficiently as the traditional 2-component route.17 Of particular interest was the use of arylhydrazine hydrochloride salts instead of the free arylhydrazines, first reported by Dave,18 to transform the initial metalloimines 16 into 17 while also liberating the hydrazine component that transformed 17 into 20. Besides replacing air and light-sensitive arylhydrazines with stable, crystalline hydrazine precursors, the Dave variation also made it possible to circumvent the use of higher temperatures and more corrosive Lewis acid catalysts simply by employing 2.1 equiv of the arylhyrazine hydrochloride salt in glacial acetic acid (90 °C) to promote indole formation.
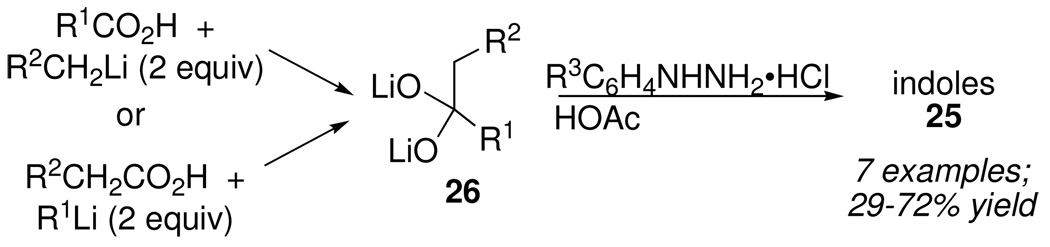
A related 3-component variant of Fischer’s method was also reduced to practice by taking advantage of Henry Gilman’s 1933 discovery that ketones resulted from the reaction of carboxylic acids with organolithium reagents (2 equiv) in ether.19 Two complementary routes to the intermediate dialkoxides 26 were possible (Scheme 12). Protonation of 26, hydrazone formation and transformation to the product indoles 25 could be accomplished in one pot using 3.1 equiv of ArNHNH2•HCl.
Scheme 12.
Our success in devising 3-component indole syntheses from nitriles and carboxylic acids via metalloimines 16 and dialkoxides 26, respectively, led us to consider other precursors to the key arylhydrazones 20, which, having two contiguous heteroatoms connected by three strategic bonds, invited extensive retrosynthetic analysis. As an example, Buchwald’s group at MIT used Pd-catalyzed cross-coupling of benzophenone hydrazones with aryl bromides to prepare arylhydrazones and thence indoles in excellent yield.20
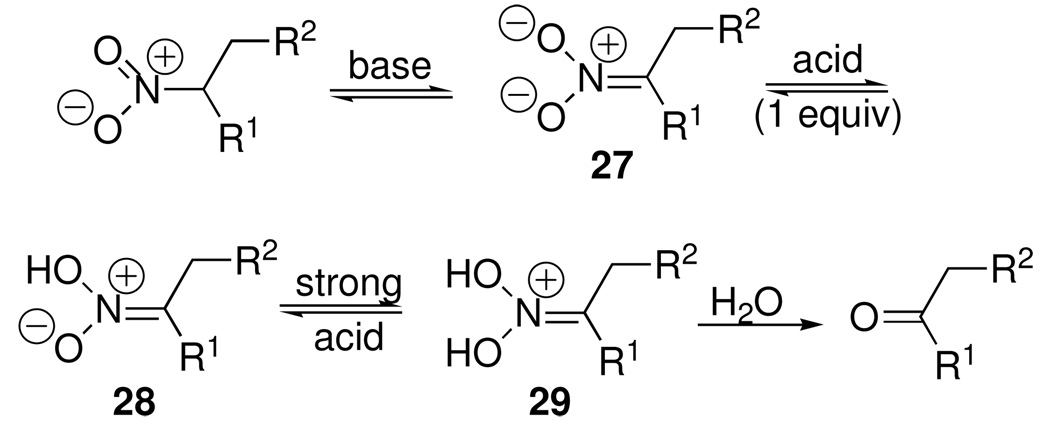
While searching for other functional groups that might be transformed into arylhydrazones by N/N interchange, we became particularly interested in nitroalkanes, which can be converted into aldehydes or ketones by treatment with base, then strong acid. In this process, known as the Nef reaction (Scheme 13), the initially formed nitronate anion 27 is protonated first to the aci-nitro species 28 and then to the iminium species 29, which undergoes hydrolysis to the carbonyl product. In principle, any of the intermediates 27–29 might be capable of arylhydrazone formation.
Scheme 13.
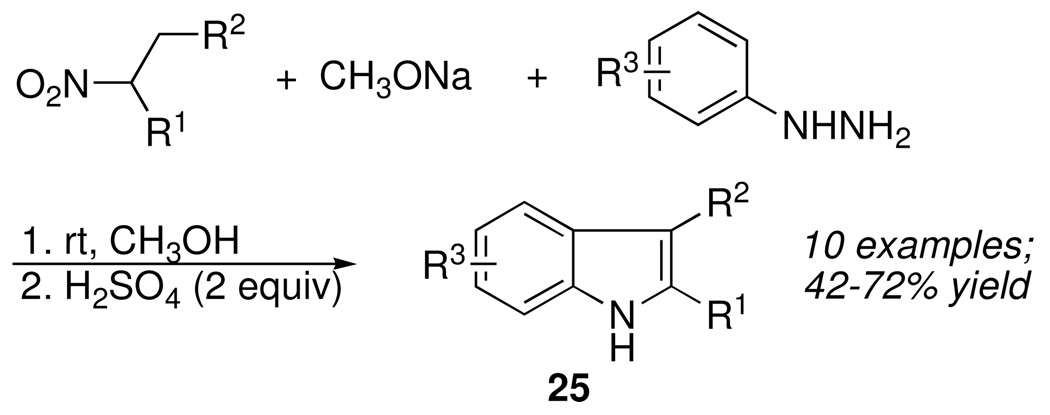
In fact, several cases have been reported of nitronates 27 reacting with thiols to form thiolhydroxamate esters.21 Although the same process could not be achieved using phenylhydrazine, it was possible to react nitroalkanes with a mixture of aryhydrazine and sodium methoxide, whereupon mild acidification and heating of the mixture afforded substituted indoles in good yield (Scheme 14).22 Control experiments demonstrated that the mechanism almost certainly involved the simultaneous convergent transformation of aci-nitro compound 27 as well as the corresponding ketone and oxime intermediates into arylhydrazone and thence to indole. Such multiplex channeling of products, together with the ease of assembling substituted nitroalkanes by well-known alkylation or conjugate addition strategies, provided another avenue to medicinally important indoles.
Scheme 14.
EVOLUTION OF MCRs BY SRR
Of particular interest in expanding the repertoire of useful MCRs has been the goal of developing practical, atom-economical ways to assemble new molecular frameworks that might display biological activity. As a rule-of-thumb guide, such frameworks typically embody all the complexity and functional group density found in bioactive natural products. Recent efforts in our laboratory have focused on the use of the SRR paradigm to discover such MCRs by the stepwise evolution of new processes using a known MCR as the starting point for innovation.
Despite eighty-odd years since its discovery, little is known about the scope of carbonyl-type electrophiles that undergo the Passerini reaction. The reaction works well with aldehydes and (to a lesser extent) ketones. At one end of the reactivity scale, carboxylic esters are inert, whereas at the other, acid halides react rapidly and independently with the isonitrile component. However, the effect of embedding nearby reactive groups within the carbonyl component has not been investigated. Mindful of the effect that even small functional changes might engender (simply substituting an imine for the carbonyl led to the Ugi reaction), we set out to vary the carbonyl component using the SRR approach.
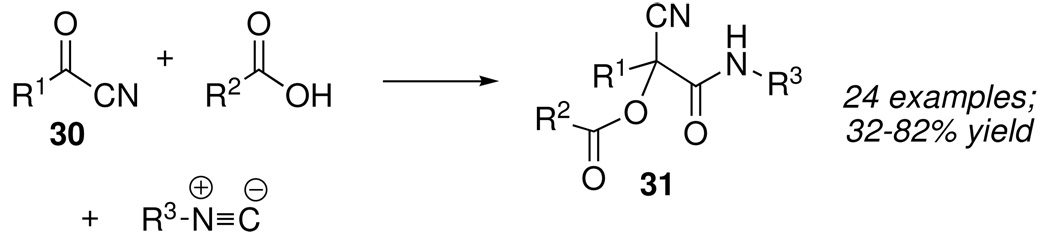
We turned our attention first to acyl cyanides, which are comparable to aldehydes and ketones in their reactivity towards nucleophiles. Besides being shelf-stable and readily prepared from acid chlorides, acyl cyanides 30 (Scheme 15) contain an embedded reactivity element (the asterisked feature in Figure 2) that we hoped to exploit in several different ways.
Scheme 15.
A careful review of the literature turned up a single example of an acyl cyanide, pyruvonitrile (30, R1= CH3), that had been subjected to Passerini conditions (formic acid, cyclohexylisonitrile), forming the corresponding α-acyloxy-α-cyanoamide 31 (R1= CH3, R2= H, R3= cyclohexyl) in 58% yield.23 In fact, the reaction proved to be general for aliphatic and alicyclic acyl cyanides and worked well using a broad range of carboxylic acids and isonitriles, including N-protected-α-aminoacids and α-isocyanocarboxylic esters.24 Of particular interest was the fact that reactions could be run neat, whereupon the products usually crystallized directly.
Without further purification of the initial product, it proved possible to “activate” the embedded nitrile functionality in 30 by a simple catalytic hydrogenation, whereupon the nucleophilic amine group released in 33 triggered a rearrangement leading to β-aminoacid diamides 34 (Scheme 16). The hydrogenation was best accomplished under acidic conditions (H2, 10% Pd/C, CH3OH, 5 equiv of HCl) to suppress any amine/imine exchange reactions of intermediate 32 during the nitrile reduction; however, simple hydrogenation of 30 in THF or EtOAc without acid also gave diamide 34, albeit in somewhat lower yield.
Scheme 16.
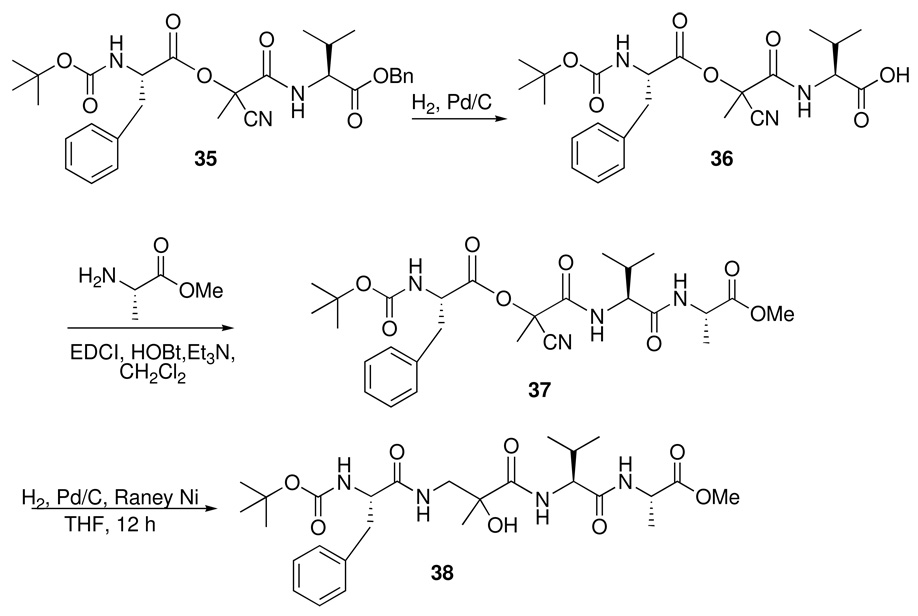
Overall, acyl cyanides could be transformed to β–aminoacid diamides in a one-pot, two-stage process. Using α-aminoacid-derived carboxylic acids and isonitriles, it also proved possible to synthesize the heterogeneous α/β/α tripeptide motif, examples of which have recently demonstrated antimicrobial properties, and which can be used to mimic turn elements in proteins. Moreover, by judicious choice of N- or C-protecting groups in the building blocks for the multicomponent reaction, the methodology can be used to incorporate the α/β/α motif into larger peptides by suitable coupling to either the N- or C-terminal.25 For example, Passerini product 35 (Scheme 17), prepared from pyruvonitrile, Boc-Phe, and the valine-derived isonitrile CNCH(i-Pr)CO2Bn in 40% yield, could be selectively debenzylated to acid 36 (96%), whereupon coupling with alanine methyl ester using a standard peptide methodology furnished 37 in 71% yield. Hydrogenation of the embedded nitrile group in 37 using palladium-activated Raney nickel26 triggered the expected acyl shift, leading to α/β/α//α/ tetrapeptide 38 in 30% yield.
Scheme 17.
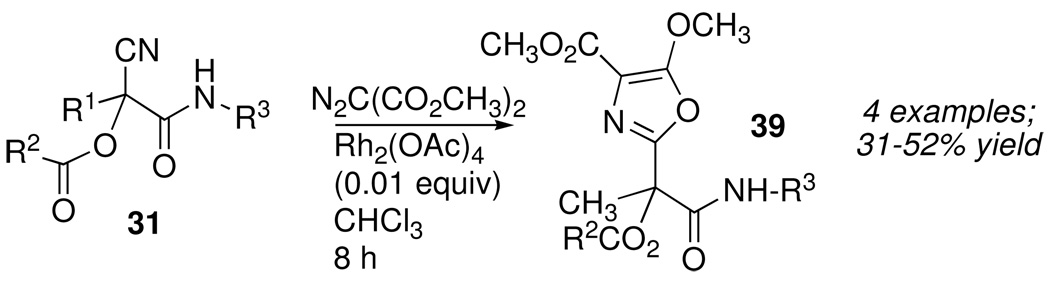
The embedded nitrile group in Passerini products of simple acyl cyanides like 31 can be further modified to generate new oxazole rings, a heterocycle that is widely distributed in natural products and has attracted interest as a pharmacophore in medicinal chemistry. For example, the rhodium acetate-catalyzed reaction of dimethyl diazomalonate with 31 afforded highly substituted oxazoles 39 (Scheme 18) in good yield.27
Scheme 18.
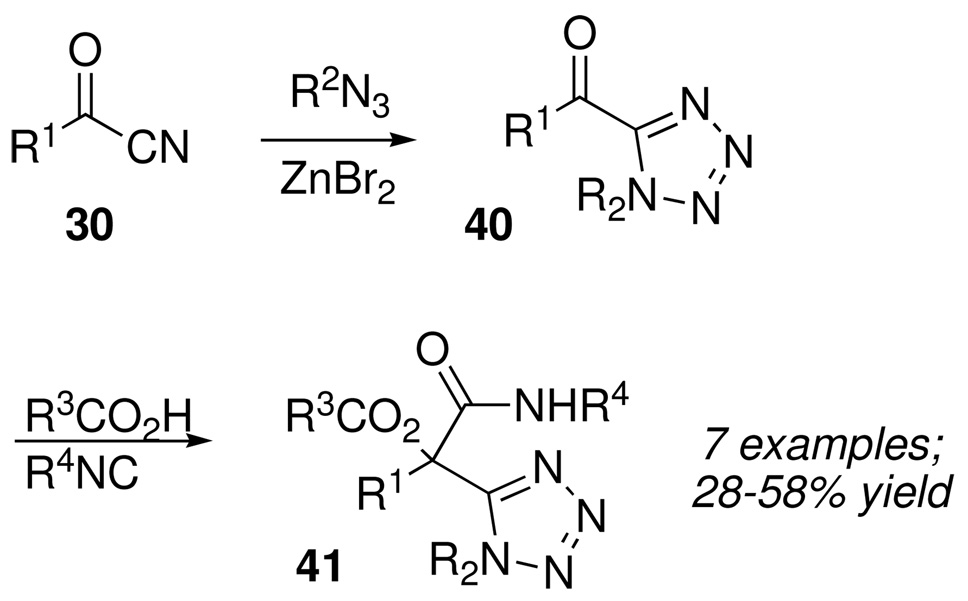
We also explored the cycloaddition of azides to 31 using Sharpless’s “click-chemistry” methodology, with the hope of preparing tetrazoles corresponding to 39, but without success. It did, however, prove possible to reverse the sequence of steps and synthesize densely functionalized tetrazoles 41 by successful Passerini condensations of acyl tetrazoles 40 (Scheme 19), which themselves were prepared by azide cycloadditions of acyl cyanides 30.
Scheme 19.
Besides functioning well as surrogates for simple carbonyl compounds in the Passerini reaction, acyl cyanides offered several additional reactivity elements (e.g. masked nucleophile, dipolarophile) that were successfully exploited in the construction of new β-peptide and heterocyclic frameworks. Having by no means exhausted the synthetic possibilities of the embedded nitrile group in 30 and its congeners, we nonetheless redirected our focus to the use of several other families of α-substituted ketones as carbonyl replacements, with the hope of uncovering, either intentionally or serendipitously, convergent, one-pot pathways to novel molecular skeletons.
We were particularly interested in MCRs involving α-diazoketones 42 because of the potential for the diazo group in the derived condensation product to undergo a broad range of insertion reactions. Moreover, α-diazoketones are readily accessible by the reaction of acid chlorides with diazomethane. To our surprise, however, mixtures of α-diazoketones with isonitriles and carboxylic acids (Passerini conditions) or with amines, isonitriles and carboxylic acids (Ugi conditions) proved unreactive, even when heated to 60–70 °C, an outcome we attributed to the enhanced resonance stabilization of the diazocarbonyl system.
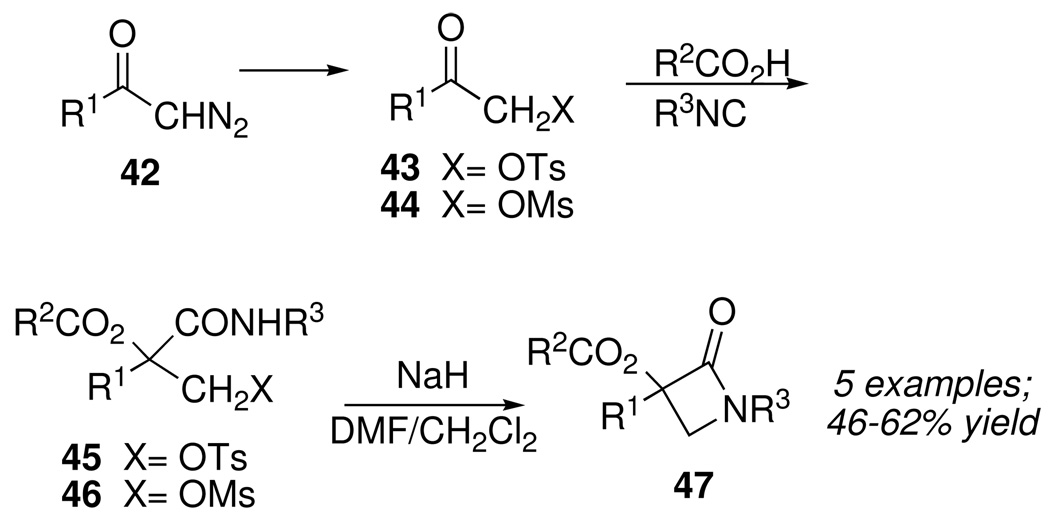
In an alternative approach we decided first to “disarm” the diazo group, while retaining a high level of electrophilic reactivity in alternative functionality, by taking advantage of the known insertion reaction of diazoketones with sulfonic acids to form β-ketotosylates 43 or ketomesylates 44 (Scheme 20).28 In fact, Passerini reactions of 43 and 44 successfully produced the corresponding MCR products 45 and 46. Such compounds were easily transformed into substituted acyloxy-β-lactams 47 following a procedure developed for cyclizations of the corresponding chloro compounds.29
Scheme 20.
Four-component Ugi reactions of sulfonyloxyketones 43 and 44 provided an unexpected entry into substituted 2-oxazolines 49 (Scheme 21), a key structural subunit in several novel classes of bioactive natural products. Condensation of either 43 or 44 with ammonia as the amine component furnished 49 by way of the intermediate diamides 48.30 Substituted oxazoline amides of type 49 are important pharmacophores in numerous bioactive natural products such as the cytotoxic agent brasilibactin 50,31 the antitumor agents B32030A and B32030D 51,32 and the recently discovered T-cell antigen didehydroxymycobactin 52.33
Scheme 21.
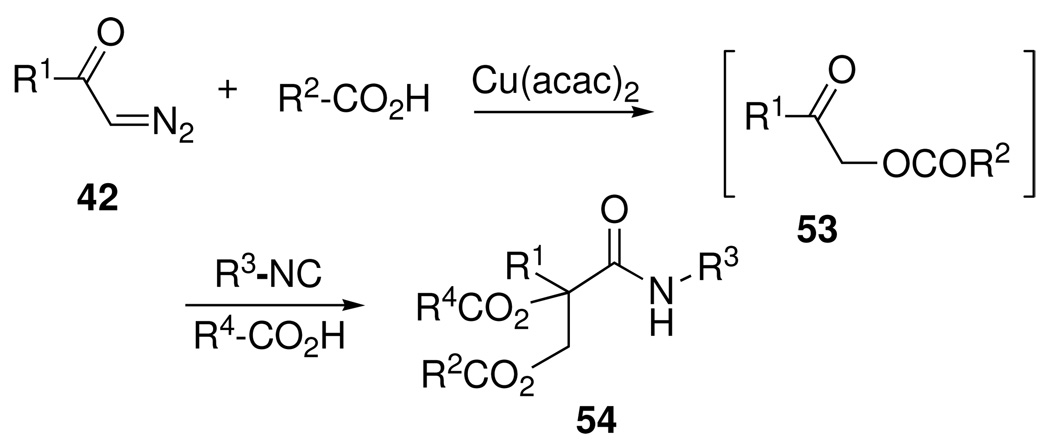
We also devised two-stage, one-pot, four-component condensations of diazoketones 42 that could be used to assemble a broad range of di-O-acylglyceric acid damide structures possessing amphiphilic properties.34 After an initial metal-catalyzed insertion of 42 into a carboxylic acid, the first-formed α-acyloxyketone 53 (Scheme 22) then underwent an in situ Passerini condensation to furnish the desired glyceramide 54. This MCR approach represented an efficient and convergent route to novel synthetic and facially amphiphilic compounds. By contrast, the more traditional stepwise acylation approach to glycerides usually resulted in ester interchange by O/O acyl migration reactions, thus complicating product purification.
Scheme 22.
CONCLUSION
As was noted in the Introduction, the origins of multicomponent reaction chemistry can be traced to the mid-nineteenth century. Its evolution since then, which continues in numerous laboratories,35 has involved both chance and serendipity, as well as more systematic approaches such as rational design or combinatorial strategies. As has already been amply demonstrated with retrosynthetic analysis in complex total synthesis, it is currently possible to deploy logic-based and/or computer-assisted approaches that enable chemists to design organic reactions36 and optimize their use of known multicomponent reactions in streamlining solutions to synthetic problems.37 We believe it is reasonable to investigate whether similar heuristic-based approaches can help define, identify and develop new (and/or improved) MCRs that will expand the useful repertoire of such transformations for both the academic and industrial scientific communities.
ACKNOWLEDGMENT
It gives me great pleasure to acknowledge a very hard-working and productive group of postdoctoral associates graduate students and undergraduate research assistants whose names appear in the Cornell references. This Account would not have been possible without their numerous, significant contributions. Initial funding of the work by the National Institutes of Health, and subsequent support from the Johnson & Johnson Focused Giving Program, is gratefully acknowledged.
Biography
Bruce Ganem was born in Boston, MA in 1948. He received degrees from Harvard College (B.A., 1969) and Columbia University (Ph.D., 1972). After postdoctoral work at Stanford University, he joined the faculty at Cornell in 1974, where he is currently Franz and Elisabeth Roessler Professor of Chemistry. Beyond his broad research and teaching activities in organic and synthetic chemistry, Ganem is also interested in the interface of science and business, and holds a joint faculty appointment in Cornell’s Johnson Graduate School of Management.
REFERENCES
- 1.(a) Ugi I, Dömling A, Hörl W. Multicomponent Reactions in Organic Chemistry. Endeavour. 1994;18:115–112. [Google Scholar]; (b) Armstrong RM, Combs AP, Tempest PA, Brown SD, Keating TA. Multiple-Component Condensation Strategies for Combinatorial Library Synthesis. Acc. Chem. Res. 1996;29:123–131. [Google Scholar]
- 2.Bienaymé H, Hulme C, Oddon G, Schmitt P. Maximizing Synthetic Efficiency: Multicomponent Transformations Lead the Way. Chem. Eur. J. 2000;6:3321–3329. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Hulme C, Gore V. Multi-component Reactions: Emerging Chemistry in Drug Discovery. From Xylocaine to Crixivan. Curr. Medicinal Chem. 2003;10:51–80. doi: 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]
- 4.(a) Dömling A. Recent Developments in Isocyanide Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; (b) Dömling A, Ugi I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000;39:3169–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (c) Doemling A. The Discovery of New Multicomponent Reactions. Curr. Opin. Chem. Biol. 2000;4:318–323. doi: 10.1016/s1367-5931(00)00095-8. [DOI] [PubMed] [Google Scholar]
- 5.Ramon DJ, Yus M. Asymmetric Multicomponent Reactions: The New Frontier. Angew. Chem. Int. Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]
- 6.Burke MD, Schreiber SL. A Planning Strategy for Diversity-oriented Synthesis. Angew. Chem. Int. Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- 7.Banfi L, Rivaq R. The Passerini Reaction. Org. React. 2005;65:1–140. [Google Scholar]
- 8.Ugi I, Meyr R, Fetzer U, Steinbrückner C. Versuche mit Isonitrilen. Angew. Chem. 1959;71:386–388. [Google Scholar]
- 9.Lemmen P, Fontain E, Bauer J, Ivar K. Ugi (1930–2005): Multi-component Reactions, Computer and Phorphorous Chemistry. Angew. Chem. Int. Ed. 2006;45:193. [Google Scholar]
- 10.Xia Q, Ganem B. Metal-Promoted Variants of the Passerini Reaction Leading to Functionalized Heterocycles. Org. Lett. 2002;4:1631–1634. doi: 10.1021/ol025877c. [DOI] [PubMed] [Google Scholar]
- 11.A related mechanism has been proposed for the cyclization of α-ketoimidoylchlorides to 2-acyl-5-ethoxyoxazoles: Huang W-S, Zhang Y-X, Yuan C-Y. Base Induced Intramolecular Cyclization of α-Ketoimidoyl Chloride - An Efficient Preparation of 2-Acyl-5-ethoxyoxazoles. Syn. Commun. 1996;26:1149–1154.
- 12.For related findings with alkyl-substituted isocyanoacetamides, see Janvier P, Sun X, Bienyamé H, Zhu J. A Novel Multicomponent Synthesis of Polysubstituted 5-Aminooxazole and Its New Scaffold-Generating Reaction to Pyrrolo[3,4-b]pyridine. Org. Lett. 2001;3:877–880. doi: 10.1021/ol007055q. Janvier P, Sun X, Bienyamé H, Zhu J. Ammonium Chloride-Promoted Four-Component Synthesis of Pyrrolo[3,4-b]pyridin-5-one. J. Am. Chem. Soc. 2002;124:2560–2567. doi: 10.1021/ja017563a.
- 13.Xia Q, Ganem B. A General Synthesis of 2-Substituted-5-aminooxazoles: Building Blocks for Multifunctional Heterocycles. Tetrahedron Lett. 2003;44:6825–6827. [Google Scholar]
- 14.Wang Q, Ganem B. New Four-Component Condensations Leading to 2,4,5-Trisubstituted Oxazoles. Tetrahedron Lett. 2003;44:6829–6832. [Google Scholar]
- 15.Ugi I. The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions. Angew. Chem. Int. Ed. 1962;4:8–21. [Google Scholar]
- 16.Simoneau CA, George BA, Ganem B. A New Approach to 4- and 5-Component Ugi Condensations Starting from Nitriles. Tetrahedron Lett. 2006;47:1205–1207. [Google Scholar]
- 17.Simoneau CA, Ganem B. Multiple Component Fischer Indole Reactions. Tetrahedron. 2005;61:11374–11379. [Google Scholar]
- 18.Dave V. Convenient Preparation of 2,3-Dialkylindoles. Org Prep. Proced. Intl. 1976;8:41–44. [Google Scholar]
- 19.(a) Gilman H, van Ess PR. The Preparation of Ketones by the Carbonation of Organolithium Compounds. J. Am. Chem. Soc. 1933;55:1258–1261. [Google Scholar]; (b) Review: Jorgenson M. J. Preparation of Ketones from the Reaction of Organolithium Reagents with Carboxylic Acids. Org. React. 1970;18:1–97. [Google Scholar]
- 20.Wagaw S, Yang BH, Buchwald SL. A Palladium-Catalyzed Method for the Preparation of Indoles via the Fischer Indole Synthesis. J. Am. Chem. Soc. 1999;121:10251–10263. [Google Scholar]
- 21.(a) Keller TH, Yelland LJ, Benn MH. A New Glucosinolate Synthesis. Can. J. Chem. 1984;62:437–440. [Google Scholar]; (b) Buchanan JB. 3,787,470. U.S. Patent. 1974 Chem Abs. 1974, 80, 82059; Preparation of Thiolhydroxamate Esters from Nitroalkanes.; (c) Copenhaver JW. 2,786,865. U.S. Patent. 1957 Chem Abs. 1957, 51, 77105; Alpha-oximino Sulfides and their Preparation.
- 22.Simoneau CA, Strohl AM, Ganem B. One-pot Synthesis of Polysubstituted Indoles from Aliphatic Nitro Compounds under Mild Conditions. Tetrahedron Lett. 2007;48:1809–1811. doi: 10.1016/j.tetlet.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neidlein R. Heterocyclische Isonitrile. Arch. Pharm. 1966;299:603–605. [PubMed] [Google Scholar]
- 24.Oaksmith JM, Peters U, Ganem B. Three-Component Condensation Leading to β-Aminoacid Diamides; Convergent Assembly of β-Peptide Analogues. J. Am. Chem. Soc. 2004;125:13606–13607. doi: 10.1021/ja0450152. [DOI] [PubMed] [Google Scholar]
- 25.Oaksmith JM. Ph.D. Thesis. Cornell University; 2006. [Google Scholar]
- 26.Klenke B, Gilbert IH. Nitrile Reduction in the Presence of Boc-Protected Amino Groups by Catalytic Hydrogenation over Palladium-Activated Raney-Nickel. J. Org. Chem. 2001;66:2480–2483. doi: 10.1021/jo005637f. [DOI] [PubMed] [Google Scholar]
- 27.Clémençon IF, Ganem B. Tandem Multicomponent/Click Reactions: Synthesis of Functionalized Oxazoles and Tetrazoles from Acyl Cyanides. Tetrahedron. 2007;63:8665–8669. [Google Scholar]
- 28.Nogrady T, Doyle TW, Morris L. Diazo, Bromo, and Mesyloxy Ketones as Biological Alkylating Agents. J. Med. Chem. 1965;8:656–659. [Google Scholar]
- 29.Sebti S, Foucaud A. A Convenient Conversion of 2-Acyloxy-3-chlorocarboxamides to 3-Acyloxy-2-azetidinones in Heterogeneous Media. Synthesis. 1983:546–549. [Google Scholar]
- 30.Fan L, Lobkovsky E, Ganem B. Bioactive 2-Oxazolines: A New Approach via One-Pot, Four Component Reaction. Org. Lett. 2007;9:2015–2017. doi: 10.1021/ol070694h. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda M, Yamakawa M, Oka S, Tanaka Y, Hoshino Y, Mikami Y, Sato A, Fujiwara H, Ohizumi Y, Kobayashi J, Brasilibactin A. A Cytotoxic Compound from Actinomycete Nocardia. J. Nat. Prod. 2005;68:462–464. doi: 10.1021/np0496385. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto M, Murooka K, Nakajima S, Abe S, Suzuki H, Hirano K, Kondo H, Kojiri K, Suda H. BE-32030 A, B, C, D, and E, New Antitumor Substances Produced by Nocardia sp. A32030. J. Antibiot. 1997;50:815–821. doi: 10.7164/antibiotics.50.815. [DOI] [PubMed] [Google Scholar]
- 33.Moody DB, Young DC, Cheng T-Y, Rosat J-P, Roura-mir C, O’Connor PB, Zajonc DM, Walz A, Miller MJ, Levery SB, Wilson IA, Costello CE, Brenner MB. T Cell Activation by Lipopeptide Antigens. Science. 2004;303:527–531. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- 34.Fan L, Adams AM, Ganem B. Multicomponent Reaction Design: A One-Pot Route to Substituted Di-O-acylglyceric Acid Diamides from α-Diazoketones. Tetrahedron Lett. 2008;49:5983–5985. [Google Scholar]
- 35.(a) Mironov M. Design of Multi-Component Reactions: From Libraries of Compounds to Libraries of Reactions. QSAR Comb. Sci. 2006;25:423–431. [Google Scholar]; (b) Weber L. Algorithm-based Methods for the Discovery of Novel Multicomponent Reactions. Multicomponent Reactions. 2005:300–310. [Google Scholar]; (c) Jacobi von Wangelin A, Neumann H, Gordes D, Klaus S, Strübing D, Beller M. Multicomponent Coupling Reactions for Organic Synthesis: Chemoselective Reactions with Amide–Aldehyde Mixtures. Chem. Eur. J. 2003;9:4286–4294. doi: 10.1002/chem.200305048. [DOI] [PubMed] [Google Scholar]; (d) Weber L, Illgen K, Almstetter M. Discovery of New Multi Component Reactions with Combinatorial Methods. Synlett. 1999;3:366–374. [Google Scholar]
- 36.Zefirov NS. An Approach to Systematization and Design of Organic Reactions. Acc. Chem. Res. 1987;20:237–243. [Google Scholar]
- 37.Fontain E, Bauer J, Ugi I. Computer Assisted Bilateral Generation of Reaction Networks from Educts and Products. Chem. Lett. 1987:37–40. [Google Scholar]