Abstract
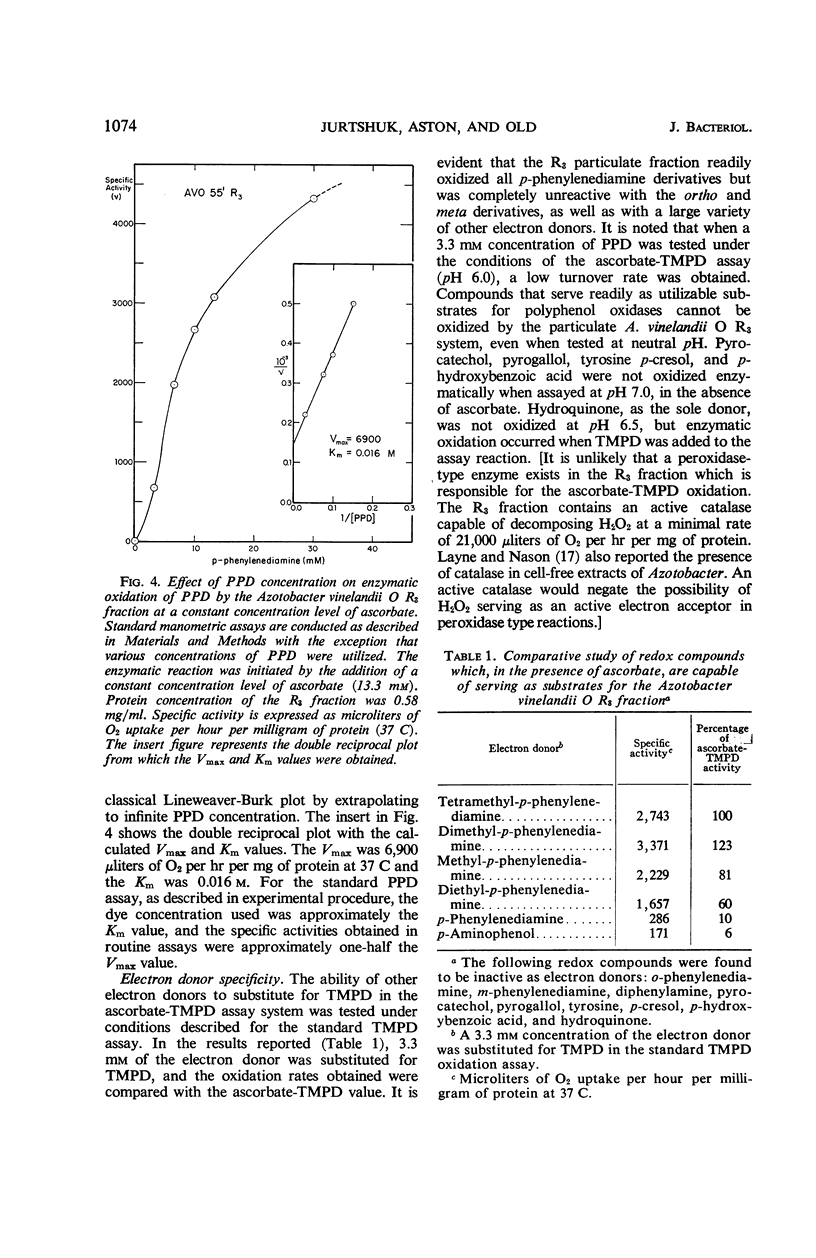
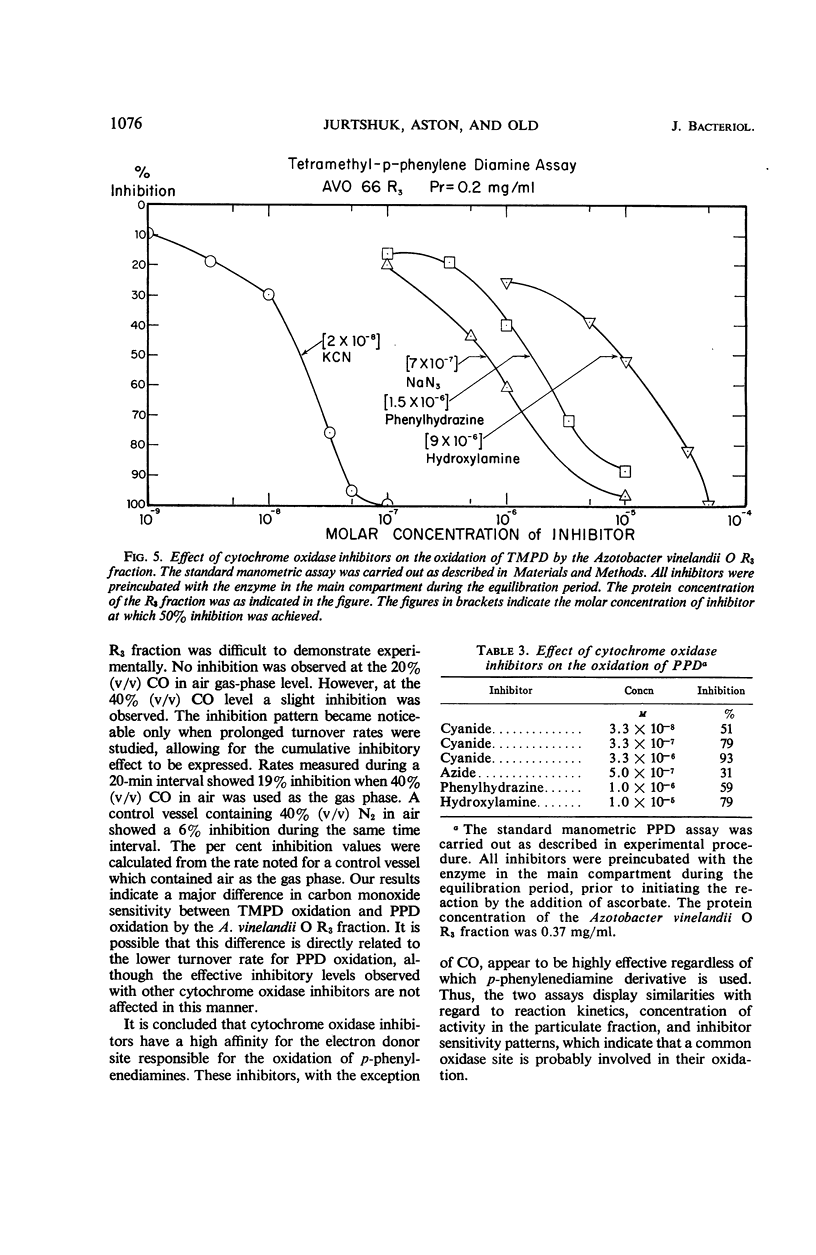
The ability of the electron transport particulate fraction of Azotobacter vinelandii strain O to oxidize tetramethyl-p-phenylenediamine (TMPD) and p-phenylenediamine (PPD) was examined in detail. The highest specific activity for TMPD and PPD oxidation concentrated in the A. vinelandii O R3 fraction. The A. vinelandii O R3 fraction was used to develop a standard manometric assay which gave optimal oxidation rates for both of these dyes. The conditions of the assay and all essential related enzymatic kinetic parameters are presented. Other para derivatives of phenylenediamines also were oxidized readily, whereas ortho and meta derivatives were not. Hydroquinone, p-hydroxybenzoic acid, p-cresol, tyrosine, pyrogallol, pyrocatechol, and diphenylamine were not able to serve as electron donors for the A. vinelandii O R3 system. The probable involvement of a particle-bound cytochrome oxidase is indicated by the marked sensitivity of both TMPD and PPD oxidation to cyanide, axide, phenylhydrazine, hydroxylamine, and, to a lesser degree, carbon monoxide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER M., WILSON P. W. Intracellular distribution of tricarboxylic acid cycle enzymes in Azotobacter vinelandii. J Bacteriol. 1956 Feb;71(2):252–253. doi: 10.1128/jb.71.2.252-253.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M., Wilson P. W. ENZYME LOCALIZATION IN Azotobacter Vinelandii. Proc Natl Acad Sci U S A. 1955 Nov 15;41(11):843–848. doi: 10.1073/pnas.41.11.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOREI H. G., BJORKLUND U. Oxidation through the cytochrome system of substituted phenylenediamines. Biochem J. 1953 Jun;54(3):357–362. doi: 10.1042/bj0540357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUEMMER J. H., WILSON P. W., GLENN J. L., CRANE F. L. Electron transporting particle from Azotobacter vinelandii. J Bacteriol. 1957 Jan;73(1):113–116. doi: 10.1128/jb.73.1.113-116.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- CHANCE B. The carbon monoxide compounds of the cytochrome oxidases. III. Molecular extinction coefficients. J Biol Chem. 1953 May;202(1):407–416. [PubMed] [Google Scholar]
- COTA-ROBLES E. H., MARR A. G., NILSON E. H. Submicroscopic particles in extracts of Azotobacter agilis. J Bacteriol. 1958 Mar;75(3):243–252. doi: 10.1128/jb.75.3.243-252.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFF J. T., WYSS O. Isolation and classification of a new series of Azotobacter bacteriophages. J Gen Microbiol. 1961 Feb;24:273–289. doi: 10.1099/00221287-24-2-273. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., MATSUBARA H., KUSAI K., NAKAI M., OKUNUKI K. High purification and properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1958 Aug;29(2):297–302. doi: 10.1016/0006-3002(58)90188-4. [DOI] [PubMed] [Google Scholar]
- JACOBS E. E. Phosphorylation coupled to electron transport initiated by substituted phenylenediamines. Biochem Biophys Res Commun. 1960 Nov;3:536–539. doi: 10.1016/0006-291x(60)90170-4. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- LAYNE E. C., NASON A. Cytochrome c oxidase from Azotobacter vinelandii. J Biol Chem. 1958 Apr;231(2):889–898. [PubMed] [Google Scholar]
- MACHINIST J. M., CRANE F. L., JACOBS E. E. TETRACHLOROHYDROQUINONE OXIDASE ACTIVITY IN BEEF HEART MITOCHONDRIA. J Biol Chem. 1965 Apr;240:1788–1795. [PubMed] [Google Scholar]
- PACKER L., JACOBS E. E. Coupling of phosphorylation to terminal segments of the mitochondrial respiratory chain. Biochim Biophys Acta. 1962 Feb 26;57:371–373. doi: 10.1016/0006-3002(62)91132-0. [DOI] [PubMed] [Google Scholar]
- REPASKE R., JOSTEN J. J. Purification and properties of reduced diphosphopyridine nucleotide oxidase from Azotobacter. J Biol Chem. 1958 Aug;233(2):466–471. [PubMed] [Google Scholar]
- SMITH L. An investigation of cytochrome c oxidase activity in bacteria. Arch Biochem Biophys. 1954 Jun;50(2):315–321. doi: 10.1016/0003-9861(54)90046-6. [DOI] [PubMed] [Google Scholar]
- SMITH L. Reactions of cytochromes a and a3. I. Studies of oxidation and reduction of the pigments in a purified preparation. J Biol Chem. 1955 Aug;215(2):833–846. [PubMed] [Google Scholar]
- Slater E. C. The measurement of the cytochrome oxidase activity of enzyme preparations. Biochem J. 1949;44(3):305–318. doi: 10.1042/bj0440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A., HOVENKAMP H. G., SLATER E. C. The respiratory enzyme systems of Azotobacter vinelandii. Biochim Biophys Acta. 1957 Aug;25(2):336–347. doi: 10.1016/0006-3002(57)90477-8. [DOI] [PubMed] [Google Scholar]
- TISSIERES A. Purification, some properties and the specific biological activity of cytochromes c4 and c5 from Azotobacter vinelandii. Biochem J. 1956 Nov;64(3):582–589. doi: 10.1042/bj0640582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHARTON D. C., GRIFFITHS D. E. Studies on the electron transport system. XXXIX. Assay of cytochrome oxidase. Effect of phospholipids and other factors. Arch Biochem Biophys. 1962 Jan;96:103–114. doi: 10.1016/0003-9861(62)90458-7. [DOI] [PubMed] [Google Scholar]
- WILSON T. G., WILSON P. W. The terminal oxidation system of Azotobacter vinelandii. J Bacteriol. 1955 Jul;70(1):30–34. doi: 10.1128/jb.70.1.30-34.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YLER D. D., ESTABROOK R. W., SANADI D. R. ELECTRON AND ENERGY REQUIREMENTS FOR CYTOCHROME B REDUCTION DURING THE OXIDATION OF TETRAMETHYL-P-PHENYLENE DIAMINE. Biochem Biophys Res Commun. 1965 Jan 18;18:264–269. doi: 10.1016/0006-291x(65)90751-5. [DOI] [PubMed] [Google Scholar]


