Abstract
Macrophages play a crucial role in the innate immune response against the human pathogen Streptococcus pyogenes, yet the innate immune response against the bacterium is poorly characterized. In the present study, we show that caspase-1 activation and IL-1β secretion were induced by live, but not killed S. pyogenes, and required expression of the pore-forming toxin streptolysin O. Using macrophages deficient in inflammasome components, we found that both Nlrp3 and Asc were crucial for caspase-1 activation and IL-1β secretion, but dispensable for pro-IL-1β induction, in response to S. pyogenes infection. Conversely, macrophages deficient in the essential TLR adaptors Myd88 and Trif showed normal activation of caspase-1, but impaired induction of pro-IL-1β and secretion of IL-1β. Notably, activation of caspase-1 by TLR2 and TLR4 ligands in the presence of SLO required Myd88/Trif whereas that induced by S. pyogenes was blocked by inhibition of NF-κB. Unlike activation of the Nlrp3 inflammasome by TLR ligands, the induction of caspase-1 activation by S. pyogenes did not require exogenous ATP or the P2X7R. In vivo experiments revealed that Nlrp3 was critical for the production of IL-1β but was not important for survival in a mouse model of S. pyogenes peritoneal infection. These results indicate that caspase-1 activation in response to S. pyogenes infection requires NF-κB and the virulence factor streptolysin O, but proceeds independently of P2X7R and TLR signaling.
Keywords: IL-1β, macrophages, bacterial infection, inflammation
Introduction
Recognition of invading microorganisms by multicellular organisms is pivotal for the induction of a rapid and effective immune response. Initial recognition of microorganisms is mediated by pattern recognition receptors (PRRs), ancient molecules of the innate immune system that can be found in plants, invertebrates and vertebrates (1). The most extensively studied PRRs are the TLRs, which comprise transmembrane proteins that recognize conserved bacterial components such as LPS, flagellin, lipoproteins, lipoteichoic acid and unmethylated CpG DNA (2). More recently, another class of PRRs called intracellular nucleotide-binding oligomerization domain (Nod)-like receptors (NLRs) have been identified. Whereas TLRs sense bacterial products present at the outer cell surface or in endosomes, NLRs mediate cytoplasmic recognition of bacterial products (3). The two best studied NLRs, nucleotide-binding oligomerization domain containing 1 and 2 (Nod1 and Nod2) sense bacterial molecules produced during the synthesis and/or degradation of peptidoglycan and mediate the activation of the transcription factor NF-κB and MAP kinases (4).
Another group of NLRs participates in the formation of a protein complex called the inflammasome which mediates the induction of caspase-1 activation in response to microbial and endogenous stimuli. The inflammasome includes a NLR protein and an adaptor protein called apoptosis associated speck-like protein (Asc) which can link the NLR to the pro-caspase-1. Several NLR proteins can form inflammasomes including NLR family pyrin domain- containing 3 (Nlrp3) (also known as Cryopyrin or Nalp3), NLR family CARD domain- containing 4 (Nlrc4) (also known as Ipaf) and Nlrp1 (also known as Nalp1) (5). Activation of the inflammasome results in self-cleavage and activation of pro-caspase-1 into the active protease. Caspase-1, in turn, mediates the processing of several targets including pro-IL-1β into its biological active form (6–8). IL-1β plays an important role in the induction of immune responses and the development of inflammatory conditions such as fever and septic shock (9). In addition, gain of function Nlrp3 mutations are associated with several autoinflammatory syndromes that are characterized by aberrant IL-1β processing and elevated IL-1β levels (10).
The inflammasomes are activated by different stimuli (8, 11). For example, mouse Nlrp1b is a sensor of lethal toxin produced by Bacillus anthracis (12); Nlrc4 detects cytosolic flagellin in cells infected with Salmonella (13, 14), Legionella (15) and Pseudomonas (16, 17). In addition, Nlc4 is activated by cytosolic Shigella in a flagellin-independent manner (18). The Nlrp3 inflammasome is activated by a variety of microbial ligands including muramyl dipeptide, bacterial and viral RNA (19), as well as endogenous signals such as urate crystals (20). In cells exposed to TLR-ligands, the activation of the Nlrp3 inflammasome requires a second signal that includes stimulation of the purinergic receptor P2X, ligand-gated ion channel 7 (P2X7R) by ATP (21), or addition of certain pore-forming toxins (22). Recent studies showed that TLR agonists promote activation of the Nlrp3 inflammasome via a priming effect that is mediated through TLR signaling and NF-κB activation (23, 24). In human monocytes, stimulation with TLR ligands induces the release of endogenous ATP that is thought to contribute to the production of IL-1β (25, 26). In addition, in macrophages exposed to TLR ligands both the P2X7R agonist ATP (27, 28) and certain bacterial pore-forming toxins (29) potentiate IL-1β production. However, the role of TLR, the P2X7R and pore-forming toxins in the regulation of IL-1β secretion in the context of more physiological conditions including bacterial infection remains largely unknown.
The Gram-positive bacterium Streptococcus pyogenes is an important human pathogen that causes various infections ranging from mild superficial skin and respiratory tract infections to life-threatening systemic diseases (30, 31). Recent studies showed an important role of macrophages in the host defense against S. pyogenes infection (32). However, the molecular interactions between S. pyogenes and macrophages are poorly understood. In the present study, we demonstrate that the production of IL-1β in macrophages infected with S. pyogenes is dependent on both TLR and Nlrp3 signaling. We further show that TLR signaling is required for induction of pro-IL-1β whereas activation of caspase-1 is mediated by the pore-forming toxin streptolysin O (SLO) and the host Nlrp3 inflammasome but proceeds independently of TLR signaling and the P2X7R.
Material and Methods
Mice
Mice deficient in Caspase-1, Nlrc4, Asc, Nlrp3, Myd88/Trif and P2X7R in C57BL/6J background have been previously described (13, 19, 24, 33, 34). All mice were backcrossed onto a C57BL/6 background for at least 6 times. Wild-type (WT) C57BL/6J mice were originally purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in our Animal Facility. The animal studies were conducted under approved protocols by the University of Michigan Committee on Use and Care of Animals. Mice were housed in a specific pathogen-free facility.
Bacteria
The Streptococcus pyogenes wild-type strain 950771 and its isogenic streptolysin O deficient mutant, a generous gift of Dr. Michael A. Wessels (Boston), have been described (35, 36). Bacteria were grown at 37°C in Todd-Hewitt broth (Difco) supplemented with 5% yeast extract (Difco). For stimulation, an overnight culture of the bacteria was diluted 1:10 in fresh broth and grown to late log-phase for 4 h. After centrifugation at 3000 × g for 5 min, bacteria were washed twice in PBS and resuspended in IMDM without FCS and antibiotics.
Stimulation of macrophages with bacteria or recombinant toxin
Mouse bone marrow-derived macrophages were obtained from femurs and tibia as described (37). For stimulation, macrophages were cultured in 48-well plates (2.5 × 105/well) or 6-well plates (2 × 106/well) and infected with S. pyogenes for 3.5 h and harvested for immunoblotting. For measurement of secreted cytokines, cells were washed twice with PBS and incubated for additional 16 h in IMDM containing 10% heat-inactivated fetal bovine serum and 300 μg/ml gentamycin to limit the growth of extracellular bacteria. Unless otherwise stated, experiments for cytokine secretion were performed at a bacterial/macrophage ratio of 4/1 and experiments for the evaluation of caspase-1 activation at a bacterial/macrophage ratio of 10/1. For experiments with recombinant streptolysin O, macrophages were stimulated with 10 μg/ml streptolysin O (Sigma) and 10 mM DTT for 30–60 min in PBS without Ca++ and Mg++ in the absence or presence of ligands, then rinsed, and incubated for 5 hrs in IMDM medium supplemented with heat inactivated serum and antibiotics. Ultrapure LPS from E. coli (10 μg/ml) and Pam3CSK (bacterial lipopeptide) at 10 μg/ml were from Invivogen.
Immunoblotting
Cells were lysed together with the cell supernatant by the addition of 1% NP-40, complete protease inhibitor cocktail (Roche, Mannheim, Germany) and 2 mM dithiothreitol. After centrifugation at 20000 × g for 15 min, the supernatant was mixed with 5 × fold SDS buffer and boiled for 5 min, and samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. Immunoblotting was performed with antibodies against caspase-1 (a gift of P. Vandenabeele, University of Ghent, Ghent, Belgium) or anti IL-1β antibody (R&D Systems) as described (37).
Mouse infection
Mice were injected i. p. with 5 × 105 CFU of S. pyogenes in PBS. After 20 h, serum was harvested and levels of IL-1β were determined by ELISA. Results were analyzed with Student’s t test and differences in data values were considered significant at a p value of <0.05. For the animal survival experimnets, comparisons between animal groups were performed by the Kaplan-Meier method using Prism software. Differences in data values were considered significant at a p value of less than 0.05.
Measurement of cytokines
Mouse cytokines were measured in culture supernatants or serum by ELISA kits from R&D Systems (Minneapolis, MN). Assays were performed in triplicate for each independent experiment. Student’s t test was used to determine statistical significance. A p value of <0.05 was considered significant.
Results
Live, but not heat-killed S. pyogenes induces IL-1β production
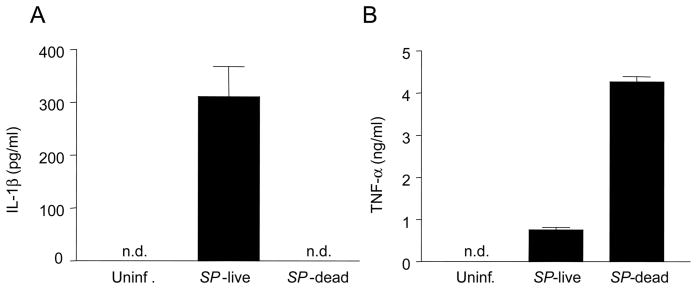
We first tested the secretion of IL-1β in bone-marrow derived macrophages infected with S. pyogenes. Live, but not heat-inactivated, S. pyogenes induced IL-1β production (Fig. 1A). Macrophage responses to heat-killed S. pyogenes were not generally impaired because heat-killed bacteria induced higher amounts of TNF-α than live bacteria (Fig. 1B). These results indicate that live bacteria specifically trigger IL-1β secretion.
Figure 1.
Live, but not heat-killed S. pyogenes, induces IL-1β production in macrophages. Macrophages were infected with viable S. pyogenes or heat-killed S. pyogenes and the release of IL-1β (A) and TNF-α (B) was determined by ELISA. Data shown are mean ± SD of triplicate samples of one experiment representative of three independent experiments, n.d. indicates not detectable.
S. pyogenes induces IL-1β production through caspase-1
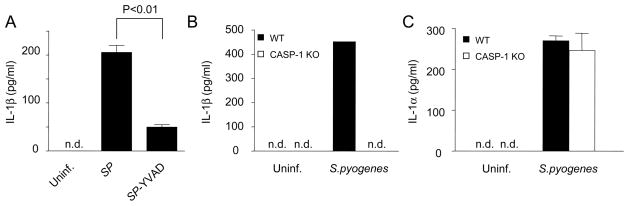
We next investigated the role of caspase-1 in S. pyogenes-induced IL-1β secretion using YVAD-cmk, a specific caspase-1 inhibitory peptide. Incubation of S. pyogenes-infected macrophages with YVAD-cmk reduced the release of IL-1β by 70–80% (Fig. 2A). To determine more conclusively the role of caspase-1 in S. pyogenes-induced IL-1β production, we infected macrophages deficient in caspase-1. IL-1β induced by S. pyogenes was undetectable in macrophages deficient in caspase-1 (Fig. 2B). In contrast, the production of IL-1α was comparable in WT and caspase-1-null macrophages (Fig. 2C). These results indicate that caspase-1 is essential for the secretion of IL-1β, but not IL-1β, in macrophages infected with S. pyogenes.
Figure 2.
Secretion of IL-1β upon S. pyogenes infection depends on caspase-1. A, Macrophages were infected with S. pyogenes in the presence or absence of the chemical caspase-1 inhibitor ac-YVAD-cmk and the release of IL-1β was determined by ELISA. B–C, Macrophages derived from WT and caspase-1 deficient mice were infected with S. pyogenes and the release of IL-1β (A) and IL-1α (C) was determined by ELISA. Data shown are mean ± SD of triplicate samples of one experiment representative of three independent experiments, n.d. indicates not detectable.
The production of IL-1β induced by S. pyogenes infection is independent of the P2X7R
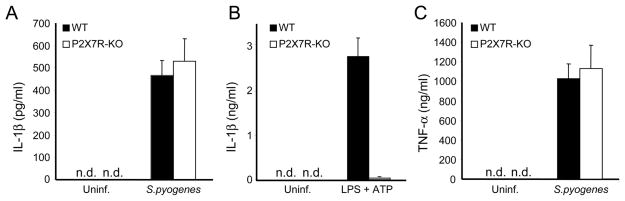
Human monocytes stimulated with microbial ligands secrete IL-1β, which is in part mediated by the release of endogenous ATP and stimulation of the P2X7R (25, 26) In contrast, human and mouse macrophages stimulated with TLR ligands or certain extracellular bacteria do not secrete IL-1β unless ATP is added exogenously (33). To investigate the role of P2X7R in the secretion of IL-1β induced by S. pyogenes, we infected macrophages deficient in the P2X7R and assessed the production of IL-1β. WT and P2X7R-deficient macrophages secreted comparable amounts of IL-1β (Fig. 3A) and TNF-α (Fig. 3C) in response to S. pyogenes infection. In contrast, stimulation of WT macrophages with TLR ligands and ATP induced the production of IL-1β which was abrogated in macrophages deficient in the P2X7R (Fig. 3B). These results indicate that IL-1β production in macrophages infected with S. pyogenes is independent of the P2X7R.
Figure 3.
The production of IL-1β in S. pyogenes infected macrophages is independent of the P2X7R. Macrophages derived from WT and P2X7R-deficient mice were infected with S. pyogenes and the release of IL-1β (A) and TNF-α (B) was determined by ELISA.
S. pyogenes- induced caspase-1 activation and IL-11β release is dependent on streptolysin O
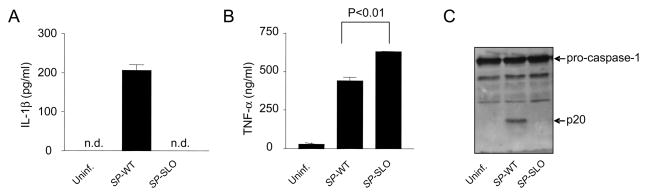
The addition of recombinant SLO to cells exposed to bacterial molecules such as muramyl dipeptide or flagellin can induce the activation of caspase-1 (21). To test the role of SLO under the more physiological context of bacterial infection, macrophages were incubated with WT bacteria and an isogenic mutant S. pyogenes strain deficient in SLO. Notably, the S. pyogenes lacking SLO did not induce detectable IL-1β secretion but elicited even higher amounts of TNF-α than the WT bacterium (Fig. 4B) Consistently, S. pyogenes induced the activation of caspase-1, as determined by the induction of the p20 subunit which is generated by autoproteolytic processing of pro-caspase-1 into the active enzyme (Fig. 4C). In contrast, the mutant S. pyogenes strain deficient in SLO did not activate caspase-1 (Fig. 4C). Together, these results indicate that infection of macrophages with S. pyogenes induces caspase-1-dependent release of IL-1β, a process that requires the expression of SLO.
Figure 4.
S. pyogenes- induced caspase-1 activation and IL-1β secretion is dependent on streptolysin O. Macrophages were infected with WT S. pyogenes (SP-WT) or S. pyogenes deficient in SLO (SP-SLO). The release of IL-1β (A) and TNF-α (B) was determined by ELISA and activation of caspase-1 was analyzed by immunoblotting (C). (A and B) Data shown are mean ± SD of triplicate samples of one experiment representative of three independent experiments, n.d. indicates not detectable.
S. pyogenes infection activates the Nlrp3 inflammasome
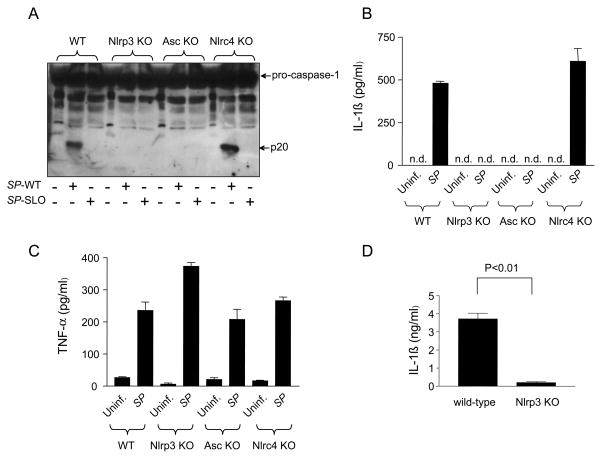
We assessed next the role of the different inflammasomes in S. pyogenes-induced caspase-1 activation. To this end, we infected WT or macrophages deficient in Nlrp3, Nlrc4 and Asc and analyzed the activation of caspase-1. Infection of WT and Nlrc4-deficient macrophages, but not macrophages deficient in Nlrp3 or Asc, with WT bacterium induced caspase-1 activation as revealed by the detection of the p20 subunit of caspase-1 (Fig. 5A). In agreement with these results, the secretion of IL-1β in S. pyogenes-infected macrophages was abrogated in macrophages deficient in Nlrp3 or Asc, but not Nlrc4 (Fig. 5B). In contrast, Nlrp3 and Asc as well as Nlrc4 were dispensable for the production of TNF-α in response to S. pyogenes (Fig. 5C). To further assess the role of the Nlrp3 inflammasome in S. pyogenes infection, we investigated the production of IL-1β in mice infected i. p. with S. pyogenes. Infection of WT mice with S. pyogenes induced the production of IL-1β in serum which was impaired in mice deficient in Nlrp3 (Fig. 5D). However, we found that WT and Nlrp3-deficient mice were equally susceptible to S. pyogenes infection as assessed by i. p. administration of 102 and 104 CFU (supplemental Fig. 1). These results indicate that the Nlrp3 inflammasome is critical for caspase-1 activation and IL-1β secretion in response to S. pyogenes but does not play an important role in the susceptibility to infection.
Figure 5.
S. pyogenes infection activates caspase-1 via the Nlrp3 inflammasome. A, WT macrophages and macrophages deficient in Nlrp3, Nlrc4 or Asc were infected with WT S. pyogenes (SP-WT) or S. pyogenes deficient in SLO (SP-SLO) and the activation of caspase-1 was analyzed by immunoblotting. B, C WT and indicated mutant macrophages were left uninfected or were infected with S. pyogenes (SP). The secretion of IL-1β (B) or TNF-α (C) was determined by ELISA. Values shown are means ± SD of triplicate samples of one experiment representative of three independent experiments. n.d. indicates not detectable. D, WT and Nlrp3-deficient mice (n=5 per group) were injected i. p. with 5 × 105 CFU of S. pyogenes. After 20 h, serum levels of IL-1β were determined by ELISA. Values shown are mean ± SD of five mice. One experiment representative of three independent experiments is shown.
TLR signaling, but not Nlrp3, is required for pro-IL-1β induction in response to S. pyogenes infection
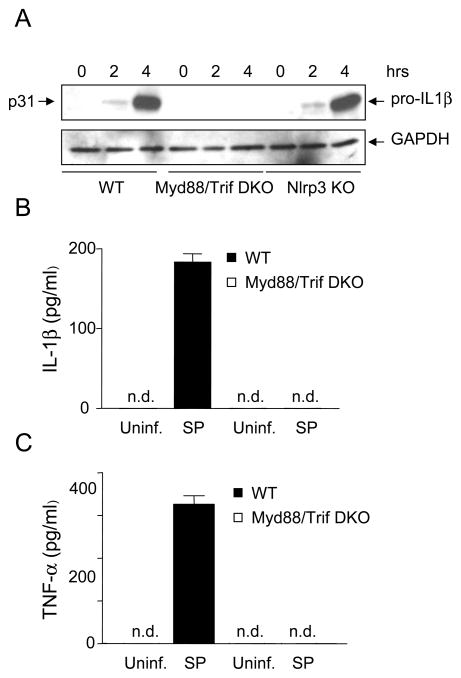
Induction of pro-IL-1β is required for IL-1β secretion in response to inflammatory stimuli. We examined next whether TLR signaling was involved in the production of pro-IL-1β in macrophages infected with S. pyogenes. We found that the induction of pro-IL-1β in response to S. pyogenes infection was abrogated in macrophages deficient in Myd88 and Trif, the adaptor molecules required for TLR signaling whereas Nlrp3 was dispensable (Fig. 6A). Consistently, secretion of IL-1β and TNF-α induced by S. pyogenes was impaired in Myd88/Trif-deficient macrophages (Fig. 6B and 6C). These results indicate that TLR signaling, but not Nlrp3, is essential for pro-IL-1β induction in response to S. pyogenes.
Figure 6.
TLR signaling, but not Nlrp3, is required for pro-IL-1β induction in response to S. pyogenes infection. A, WT and mutant macrophages deficient in Myd88 and Trif (DKO) or Nlrp3 (Nlrp3 KO) were left uninfected or infected with S. pyogenes for indicated time and extracts were immunoblotted with antibodies for IL-1β or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a loading control. B–C, WT and mutant macrophages were left uninfected or infected with S. pyogenes (SP). The secretion of IL-1β (B) or TNF-α (C) was determined by ELISA. Data shown are mean ± SD of triplicate samples of one experiment representative of three independent experiments, n.d. indicates not detectable.
TLR signaling is required for caspase-1 activation induced by SLO and LPS or synthetic lipopeptide but not S. pyogenes infection
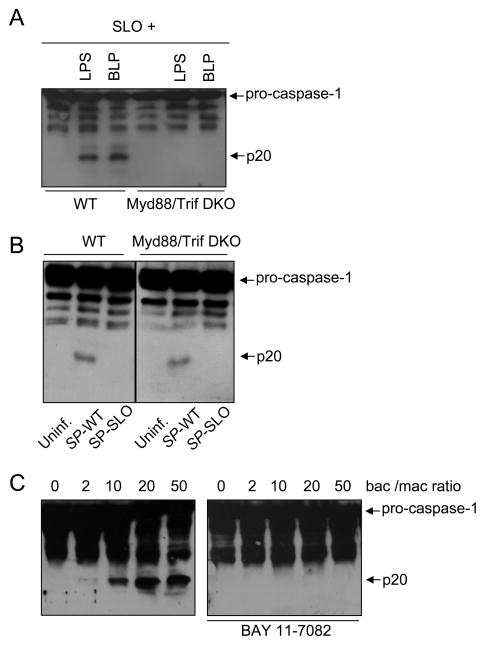
Stimulation of macrophages with ATP and TLR agonists including LPS (TLR4 agonist) and synthetic lipopeptide (TLR2 agonist) induces caspase-1 activation via the Nlrp3 inflammasome (23, 24). Furthermore, caspase-1 activation via Nlrp3 in response to TLR agonists and ATP requires a priming effect mediated through TLR signaling and NF-κB activation (23, 24). Consistently, activation of caspase-1 induced by LPS or synthetic lipopeptide in the presence of SLO was impaired in macrophages deficient in Myd88 and Trif (Fig. 7A). Notably, the activation of caspase-1 induced by infection with S. pyogenes was independent of Myd88 and Trif (Fig. 7B). However, caspase-1 activation triggered by infection with S. pyogenes was abrogated by pre-treatment of macrophages with BAY 11–7082 (Fig. 7C), a drug that inhibits NF-κB activation by targeting the Iκ-B kinase complex (38). These results suggest that TLR signaling is dispensable for S. pyogenes-mediated caspase-1 activation but this process relies on NF-κB activation.
Figure 7.
Caspase-1 activation induced by S. pyogenes is independent of TLR signaling but blocked by NF-κB inhibitor. A, WT and mutant macrophages deficient in both Myd88 and Trif (DKO) were left untreated or stimulated with LPS or bacterial lipopeptide (BLP) in the presence of SLO. Activation of caspase-1 was determined by immunoblotting. Pro-caspase-1 and the processed p20 subunit of active caspase-1 are indicated by arrows. B, WT and mutant macrophages in both Myd88 and Trif (DKO) were left uninfected or infected with WT S. pyogenes (SP-WT) or S. pyogenes deficient in SLO (SP-SLO). Activation of caspase-1 was determined by immunoblotting. C, Macrophages were left untreated or pretreated with the NF-κB inhibitor BAY 11-7082 (20 μM) for 1 hour, and then left uninfected or infected with S. pyogenes at the indicated bacteria/macrophage ratio. Activation of caspase-1 was determined by immunoblotting. Data shown are representative of three independent experiments.
Discussion
S. pyogenes is a highly pathogenic bacterium but its interaction with the innate immune system remains poorly characterized. In this study, we show that infection with S. pyogenes induces the secretion of IL-1β and this response is mediated by the coordinated interaction between TLR signaling and the Nlrp3 inflammasome. In addition, the results indicate that active production of the pore-forming toxin SLO is required for caspase-1 and IL-1β secretion induced by S. pyogenes as heat-inactivated bacteria and bacteria lacking SLO were impaired in stimulating the Nlrp3inflammasome.
S. pyogenes is known to secrete several virulence factors among them the cytotoxin SLO, a member of a conserved family of cholesterol-dependent pore-forming cytolysins (39). It has been demonstrated that various cytolysins such as nigericin (40) and maitotoxin (29) can induce caspase-1-dependent release of IL-1β in macrophages pre-stimulated with TLR ligands (22). In the present study, we provide evidence that SLO is critical for activation of the Nlrp3 inflammasome in response to S. pyogenes infection. Unlike TLR ligands that require exogenous ATP stimulation for activation of caspase-1 via Nlrp3, S. pyogenes triggered caspase-1 activation via Nlrp3 independently of the P2X7R. These results indicate that the Nlrp3 inflammasome can be activated via P2X7R-dependent and P2X7R-independent mechanisms in response to microbial stimuli. One possibility is that SLO acts by mimicking the function induced by ATP activation thereby bypassing the requirement for P2X7R stimulation. Because the pore-forming SLO can mediate the delivery of microbial molecules to the host cytosol and cytosolic escape of S. pyogenes (41), it is possible that infection with S. pyogenes results in cytosolic internalization of bacterial molecules or the bacterium via SLO to trigger Nlrp3-dependent caspase-1 activation. Because activation of caspase-1 induced by LPS or lipopeptide and SLO requires TLR signaling, the results suggest that MyD88/Trif-independent activation of the Nlrp3 inflammasome in response to S. pyogenes infection cannot be explained by SLO-mediated internalization of TLR ligands. However, SLO could promote caspase-1 activation by mediating internalization of microbial molecules distinct of TLR ligands.
Recent studies showed that S. pyogenes induces macrophage cell death, a process that requires SLO but the cellular demise was largely independent of caspase-1 (42). Failure to induce cell death was associated with higher survival rates of the internalized bacteria inside macrophages (42). Consistent with these results, we found that the mutant strain SLO induced higher amounts of TNF-α than WT bacteria which may be explained by the presence of higher number of mutant S. pyogenes inside macrophages. In addition, SLO can induce TLR-independent production of type-I interferons (43). Collectively, these results indicate that SLO can induce different processes in host cells including cell death, caspase-1 activation, IL-1β and interferon production which are likely to contribute to bacterial virulence and host defense.
Activation of the Nlrc4 inflammasome is dependent on the presence of a functional type III/IV secretion system, a feature of pathogenic bacteria (11). Here we show that the activation of the Nlrp3 inflammasome by S. pyogenes is similarly dependent on the expression of a virulence factor, the pore-forming toxin SLO. A common feature of these virulence factors is the formation of pores in host membranes or alteration of membrane permeability which may allow the cytosolic localization of microbial molecules. Consistent with this hypothesis, co-stimulation of macrophages with recombinant SLO and certain TLR ligands, but not each stimulus alone, triggered caspase-1 activation through the Nlrp3 inflammasome (21). We found that activation of caspase-1 with TLR ligands and SLO required TLR signaling as it was abolished in Myd88/Trif-deficient macrophages. These results are consistent with recent findings showing that TLR ligands promote activation of the Nlrp3 inflammasome, at least in part, through a priming effect mediated via TLR signalingand NF-κB activation (23, 24). Using macrophages that are deficient in the adaptor proteins Myd88 and Trif, and that therefore cannot signal via TLRs, we showed that S. pyogenes-induced caspase-1 activation is independent of TLR signaling. One possible model to explain these results is that S. pyogenes induces TLR-independent priming of the Nlrp3 inflammasome. This priming effect may be mediated via NF-κB as caspase-1 activation induced by S. pyogenes infection was blocked by treatment with a NF-κB inhibitor. In a non-excluding model, SLO may mediate internalization of bacterial molecules that are important for priming and/or activation of the Nlrp3 inflammasome independently of TLR signaling. Activation of Nlrp3 through SLO is likely to be indirect as a physical interaction between microbial molecules and NLR proteins has not yet been identified. Such an indirect mechanism has been proposed for the Nlrp3 inflammasome by TLR agonists and ATP or particulate matter such as silica or urate crystals (44). Indeed, there is evidence that reactive oxygen species and cathepsin B may contribute to the activation of the Nlrp3 inflammasome by a mechanism that remains undefined (45–48). Irrespective of the mechanism involved, the activation of inflammasome(s) is triggered by pathogenic bacteria via virulence factors such as pore-forming toxins or bacterial secretion systems. Further work is needed to understand the role of the inflammasome(s) in host defense against bacterial pathogens.
Supplementary Material
Acknowledgments
We thank Michael Wessels for generous supply of S. pyogenes strains, Shizuo Akira (Osaka University), Richard Flavell (Yale University) and Millennium Pharmaceuticals for mouse mutant strains, Joel Whitfield and Peter Kuffa for technical support and Sherry Koonse for excellent animal husbandry.
Abbreviations in this paper
- Asc
protein called apoptosis associated speck-like protein
- BM
bone marrow
- KO
knockout
- NLR
Nod-like receptors
- Nlrp3
NLR family pyrin domain- containing 3
- Nlrc4
NLR family CARD domain- containing 4
- SLO
streptolysin O
- SP
Streptococcus pyogenes
- WT
wild type
Footnotes
This work was supported by grants AI063331 and AI064748. J.H. was supported by the Heisenberg-program of the Deutsche Forschungsgemeinschaft, L. F by a Fellowship from the Arthritis Foundation and T.R. by a Fellowship from the Swiss National Science Foundation
Disclosures
The authors declare that no competing interest exist
References
- 1.Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008;14:86–92. doi: 10.1007/s10156-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 2.Leulier F, Lemaitre B. Toll-like receptors--taking an evolutionary approach. Nat Rev Genet. 2008;9:165–178. doi: 10.1038/nrg2303. [DOI] [PubMed] [Google Scholar]
- 3.Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol. 2008;20:3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 5.Lamkanfi M, Kanneganti TD, Franchi L, Nunez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol. 2007;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- 6.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 8.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 10.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 11.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 12.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 13.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 14.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 15.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 16.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 17.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 20.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 21.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Freche B, Reig N, van der Goot FG. The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin Immunopathol. 2007;29:249–260. doi: 10.1007/s00281-007-0085-0. [DOI] [PubMed] [Google Scholar]
- 23.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, Van der Meer JW, Dinarello CA. Blood. 2008. Differential requirement for the activation of the inflammasome for processing and release of IL-1{beta} in monocytes and macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 28.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 30.Musser JM, Hauser AR, Kim MH, Schlievert PM, Nelson K, Selander RK. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci U S A. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldmann O, Rohde M, Chhatwal GS, Medina E. Role of macrophages in host resistance to group A streptococci. Infect Immun. 2004;72:2956–2963. doi: 10.1128/IAI.72.5.2956-2963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 34.Ozoren N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Erturk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Nunez G. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 35.Bricker AL, Cywes C, Ashbaugh CD, Wessels MR. NAD+-glycohydrolase acts as an intracellular toxin to enhance the extracellular survival of group A streptococci. Mol Microbiol. 2002;44:257–269. doi: 10.1046/j.1365-2958.2002.02876.x. [DOI] [PubMed] [Google Scholar]
- 36.Michos A, Gryllos I, Hakansson A, Srivastava A, Kokkotou E, Wessels MR. Enhancement of streptolysin O activity and intrinsic cytotoxic effects of the group A streptococcal toxin, NAD-glycohydrolase. J Biol Chem. 2006;281:8216–8223. doi: 10.1074/jbc.M511674200. [DOI] [PubMed] [Google Scholar]
- 37.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Nunez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 38.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 39.Braun V, Focareta T. Pore-forming bacterial protein hemolysins (cytolysins) Crit Rev Microbiol. 1991;18:115–158. doi: 10.3109/10408419109113511. [DOI] [PubMed] [Google Scholar]
- 40.Cheneval D, Ramage P, Kastelic T, Szelestenyi T, Niggli H, Hemmig R, Bachmann M, MacKenzie A. Increased mature interleukin-1beta (IL-1beta) secretion from THP-1 cells induced by nigericin is a result of activation of p45 IL-1beta-converting enzyme processing. J Biol Chem. 1998;273:17846–17851. doi: 10.1074/jbc.273.28.17846. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 42.Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, Karin M, Salvesen GS, Nizet V. Streptolysin o promotes group a streptococcus immune evasion by accelerated macrophage apoptosis. J Biol Chem. 2009;284:862–871. doi: 10.1074/jbc.M804632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gratz N, Siller M, Schaljo B, Pirzada ZA, Gattermeier I, Vojtek I, Kirschning CJ, Wagner H, Akira S, Charpentier E, Kovarik P. Group A streptococcus activates type I interferon production and MyD88-dependent signaling without involvement of TLR2, TLR4, and TLR9. J Biol Chem. 2008;283:19879–19887. doi: 10.1074/jbc.M802848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walev I, Klein J, Husmann M, Valeva A, Strauch S, Wirtz H, Weichel O, Bhakdi S. Potassium regulates IL-1 beta processing via calcium-independent phospholipase A2. J Immunol. 2000;164:5120–5124. doi: 10.4049/jimmunol.164.10.5120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.