Abstract
We have used the well-accepted and easily available 2-[18F]Fluoro-2-deoxyglucose ([18F]FDG) positron emission tomography (PET) tracer as a prosthetic group for synthesis of 18F-labeled peptides. We herein report the synthesis of [18F]FDG-RGD (18F labeled linear RGD) and [18F]FDG-cyclo(RGDDYK) (18F labeled cyclic RGD) as examples of the use of [18F]FDG. We have successfully prepared [18F]FDG-RGD and [18F]FDG-cyclo(RGDDYK) in 27.5% and 41% radiochemical yields (decay corrected) respectively. The receptor binding affinity study of FDG-cyclo(RGDDYK) for integrin αvβ3 , using αvβ3 positive U87MG cells confirmed a competitive displacement with 125I-echistatin as a radioligand. The IC50 value for FDG-cyclo(RGDDYK) was determined to be 0.67 ± 0.19µM. High contrast small animal PET images with relatively moderate tumor uptake were observed for [18F]FDG-RGD and [18F]FDG-cyclo(RGDDYK) as PET probes in xenografts models expressing αvβ3 integrin. In conclusion, we have successfully used [18F]FDG as a prosthetic group to prepare 18F]FDG-RGD and [18F]FDG-cyclic[RGDDYK] based on a simple one step radiosynthesis. The one step radiosynthesis methodology consists of chemoselective oxime formation between an aminooxy functionalized peptide and [18F]FDG. The results have implications for radiolabeling of other macromolecules and would lead to a very simple strategy for routine pre-clinical and clinical use.
Currently, time-sensitive methods used for 18F-labeling of peptides for positron emission tomography (PET) imaging involve the development of unique 18F-containing prosthetic groups for attachment to the peptides that will likely require significant effort to pervasively implement for routine pre-clinical and clinical application. Therefore, the ability to utilize reagents currently well-accepted and easily available in the PET community at the final formulation step will likely be highly beneficial. The use of 2-[18F]Fluoro-2-deoxyglucose ([18F]FDG) is well-established in clinical PET centers (1) and we show here the potential use of this tracer, without further chemical modifications, to label peptides that can be used to report on one or more biomarkers in vivo.
We herein report the synthesis of a 18F-labeled linear trimeric-peptide, [18F]FDG-RGD, and 18F-labeled cyclic trimeric-peptide, [18F]FDG-cyclo(RGDDYK) by using [18F]FDG without further modification. We chose αvβ3 integrin and its peptide ligand because the trimeric-peptides sequences argenine-glycine-aspartic acid (RGD) recongnizes the αvβ3 integrin found on new blood vessels and tumor cells. RGD peptide binds to αvβ3 integrin and inhibits angiogenesis (2). The αvβ3 integrin is highly expressed in activated endothelial and solid tumor cells (3–4). The synthesis of the radiolabeled peptides utilizes the reaction between the aldehyde functionality in the linear, open form of [18F]FDG with an aminooxy functionality in the peptide to form an oxime bond. The resulting 18F-labeled RGD molecules were evaluated in vitro for their binding affinity and ultimately as PET probes in xenograft models expressing αvβ3 integrin to demonstrate the integrity of the binders post labeling.
Chemoselective oxime formation between an aminooxy group and a carbonyl functionality has recently been successfully utilized for [18F]-labeling of peptides (5–6), and we hypothesized that it can be used to directly attach [18F]FDG to biomarker-targeted peptides. The highly specific oxime formation between an aminooxy-functionalized peptide and an 4-[18F]fluorobenzaldehdey ([18F]FBA) has previously offered high-yield, two steps synthesis for 18F- labeled peptides (6–9). [18F]FBA can be obtained in a non-optimized radiochemical yield of 50 % in 30 min, with reported radiochemical yields of 60%–80% for the final 18F-peptides. The hypothesis is further supported by a recent report by Wuest et al., which reported the oxime formation between [18F]FDG and N-[6-(aminooxy)hexyl]maleimide to create a thiol reactive prosthetic group [18F]FDG-maleimidehexyloxime ([18F]FDG-MHO) in moderately high yield (45–69%) at 100 °C over 15 minutes (10). The basis of this chemistry lies in the fact that at 100 °C, FDG exists in a dynamic equilibrium between its cyclic form and a liner form, where the latter contains an aldehyde functionality at position 1 that can potentially react with the aminooxy functionality under appropriate conditions. We wanted to understand the feasibility of whether such conditions could be translated directly to a targeting peptide while maintaining the peptide’s biological efficacy in cell culture and small living subjects.
[18F]FDG-Peptide Conjugation
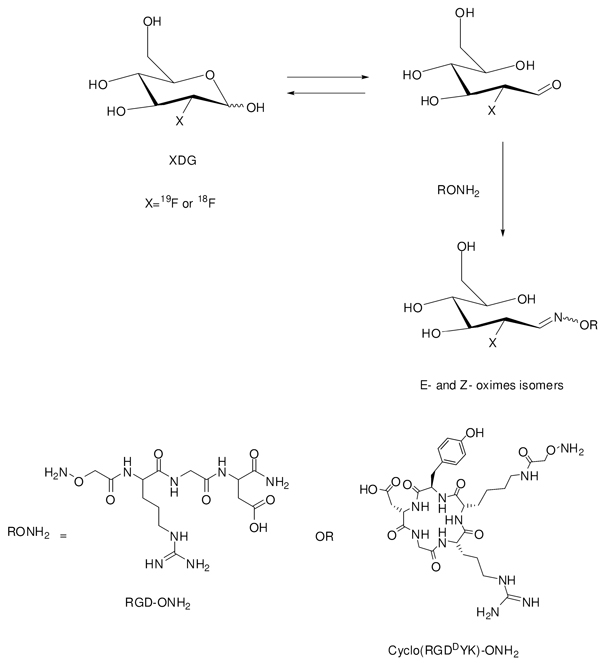
The synthesis of FDG-RGD and [18F]FDG-RGD were carried out as outlined in scheme 1. The linear aminooxy-RGD (RGD-ONH2, 2 mg) was incubated with 3.7 equivalents cold FDG in 16 % ethanol in saline (120 µl) in the presence of 0.4 % TFA at 100 °C for 40 min to afford FDG-RGD (E- and Z-oximes) in 39.5 % yield. The formation of E- and Z-oximes was expected. It has been reported that in a similar case, the reaction of glucose with O-(4-nitrobenzyl)-hydroxylamine afforded E and Z-oximes isomers (11). The mass spectrometry (ESI-MS) analysis of the isolated product showed two identical mass peaks of 584.2 ([M+H]+) which correspond to FDG-RGD (E- and Z-oximes) (cal. [M+H] for RGD-FDG is 584.5.). Similarly, aminooxy-RGD (2 mg) was reacted with 4–6 mCi of [18F]FDG in the same condition as above but in 30 minutes to afford [18F]FDG-RGD (E- and Z- oximes) in 27.5 % radiochemical yields based on [18F]FDG (decay corrected). [18F]RGD-FDG oximes were purified by HPLC and used for biological studies. Similar stereochemistry has been observed in the synthesis of 18F-lableled peptide Gluc-s-Dpr ([18F]BOAT)TOACF by using an 18F-benzaldehyde (6).
Scheme 1.
Schematic synthesis of FDG-RGD and FDG-cyclo(RGDDYK) (X=19F) from FDG or [18F]FDG- RGD and [18F]FDG-cyclo(RGDDYK) ( X=18F) from [18F]FGD.
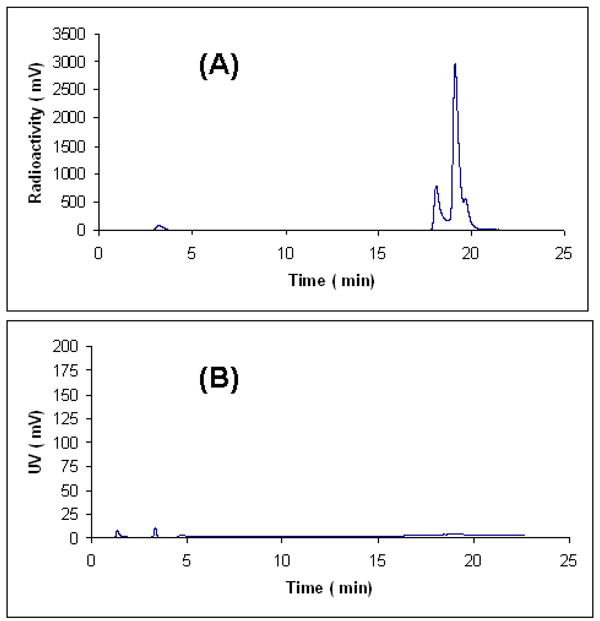
Likewise, conjugation of FDG and [18F]FDG to cyclo(RGDDYK)-ONH2 were achieved according to scheme 1. Cyclo(RGDDYK)-ONH2 (2 mg) was incubated with 5.7 equivalents of FDG in 16 % ethanol in saline (120 µl) and 0.4 % TFA at 100 °C for 60 min to give FDG-cyclo( RGDDYK) (E- and Z-oximes) in 41.4 % yield. The purified FDG-cyclo(RGDDYK) showed two identical mass peaks of 857.35 ([M+H]+) in ESI-MS which are corresponding to FDG-cyclo(RGDDYK) (E- and Z-oximes) (cal. M+H for FDG-cyclo(RGDDYK) is 857.47.). Conjugation of 18FDG to cyclo(RGDDYK)-ONH2 (2 mg) afforded [18F]FDG-cyclo(RGDDYK) (E- and Z-oximes) in 41% radiochemical yield based on [18F]FDG (decay corrected). [18F]FDG-cyclo(RGDDYK) (E- and Z-) oximes were purified but not separated by HPLC and the two 18F-labeled products (E- or Z-oximes) were isolated as a mixture and analyzed by analytical HPLC (Figure 1). The exact stereochemistry (E- and Z-) of these two products has not been determined yet. We found that under our experimental conditions maximum radiochemical yields were obtained at pH values of 1.5–2.5. When the reaction was performed at pH 4 in ammonium acetate buffer no significant products were produced. In our previous report (9) we addressed the selectivity of aldehyde towards the aminooxy groups over the amine groups in conjugation of RGD peptides with a simple aldehyde such as 4-flurobenzaldehyde. We found that when for example, two RGD aminooxy-functionalized peptides NH2OCH2CO-Arg-Gly-Asp-NH2 and NH2OCH2CO-Lys-Arg-Gly-Asp-NH2 were reacted at the same condition (8.5–150 equivalents, 45–60 min, 70 °C, pH 4) with 4-flourobenzaldehyde, in each case only one oxime product was formed. It strongly suggests that in conjugation of RGD-ONH2 or cyclo(RGDDYK)-ONH2 with FDG oximes are the major products. Similar selectivity was observed by Poethko et al. (6) where they tested the selectivity of 4-[18F]flurobenzaldehyde for aminooxy groups vs. amine groups in amino acids argentine serine, histidine, and lysine both in the presence and the absence of 2-aminooxyacetic acid. The major limitations of our 18F-labeling methodology are the high temperature (100 °C) and acidic pH conditions. High temperature and acidic pH are tolerated for the small unprotected peptides but might not be suitable for the large peptides.
Figure 1.
Analytical HPLC profiles of [18F]FDG-cyclo(RGDDYK) (E-and Z-oximes) after semiprep HPLC. A is radioactive trace and B is UV trace.
Cell Culture Binding Studies
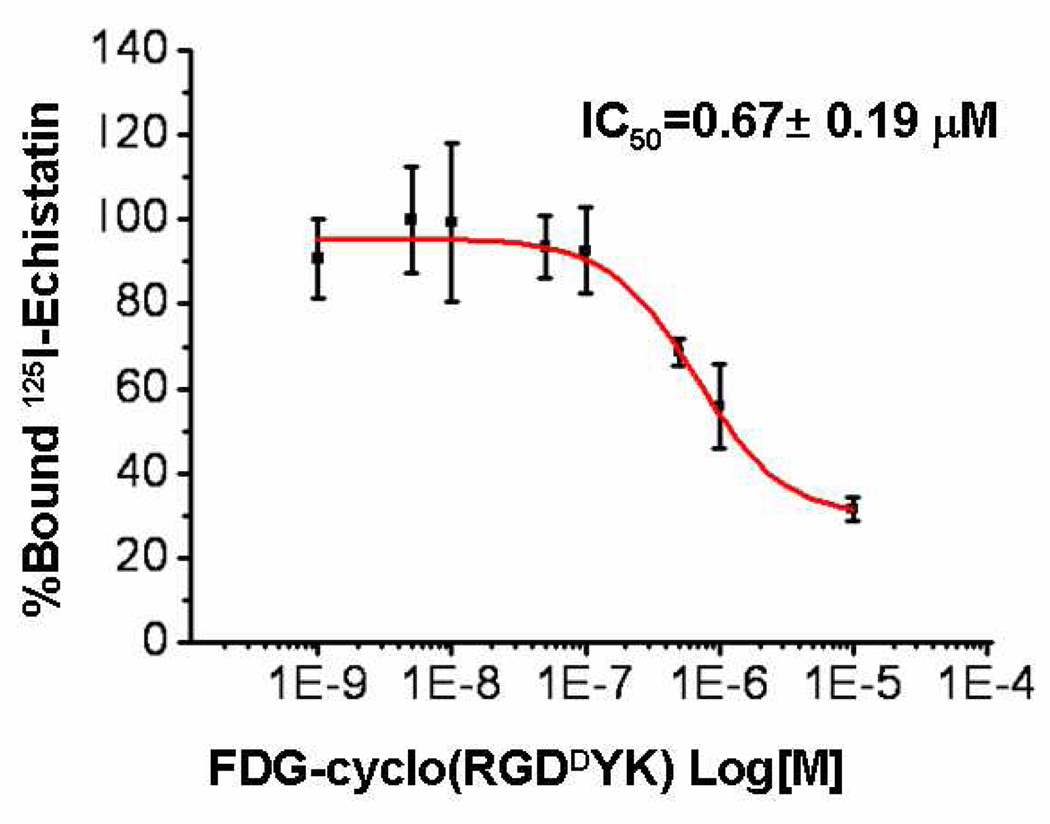
To determine whether FDG-cyclo(RGDDYK) has similar receptor binding affinity for integrin αvβ3 as cyclo(RGDDYK), we studied the receptor binding affinity of FDG-cyclo(RGDDYK) for integrin αvβ3 , using αvβ3 positive U87MG cells. The receptor binding affinity study of FDG-cyclo(RGDDYK) for integrin αvβ3 , using αvβ3 positive U87MG cells confirmed a competitive displacement with 125I-echistatin as a radioligand (Figure 2). The IC50 value for FDG-cyclo(RGDDYK) was determined to be 0.67 ± 0.19 µM. The corresponding IC50 value of cyclo(RGDDYK) is 0.23 µM (12).This result suggests that fluorine-labeling and the conditions (100 °C and acidic pH) did not have a dramatic affect on its binding affinity.
Figure 2.
Inhibition of 125I-Echistatin (integrin αvβ3-specific) binding to αvβ3 intgrin on U87MG cells by FDG-cyclo(RGDDYK).
Biodistribution and Imaging
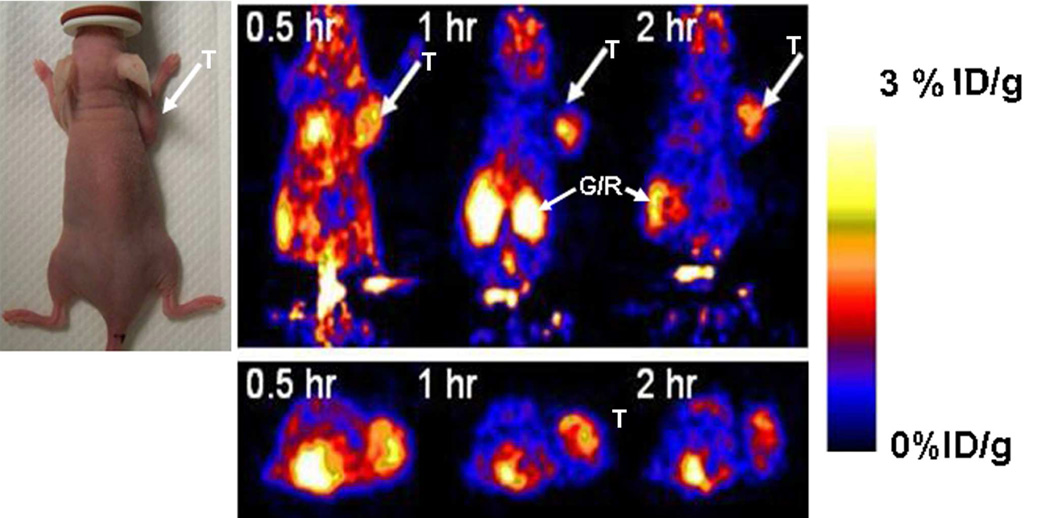
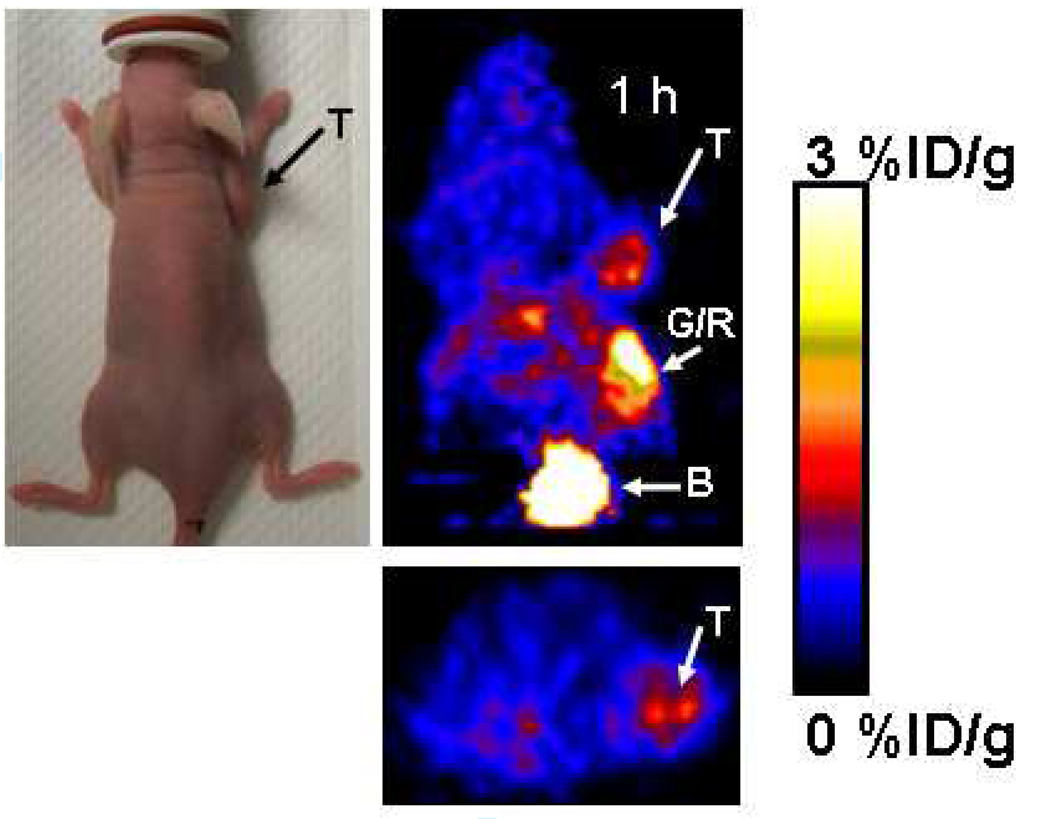
In order to determine the in vivo imaging performance of [18F]-labeled FDG-RGD over alternatively 18F-labeled RGD analogs, we evaluated [18F]FDG-RGD in U87MG tumor bearing nude mice which express αvβ3 integrin on both tumor vasculature and tumor cells. In vivo biodistribution of [18F]FDG–RGD in U87MG tumor bearing nude mice (n=6) expressing αvβ3 integrin is shown in Table 1. The ratios of tumor to blood, tumor to liver and tumor to kidney at 2 h post injection of the probe are comparable to [18F]Galacto-RGD analog that has been used for biodistribution of [18F]Galacto-RGD in osteosarcoma tumor bearing nude mice which express αvβ3 integrin (13). MicroPET images of U87MG tumor bearing nude mice (n=6), 0.5, 1 and 2 hr after tail vein injection of 18F labeled [18F]FDG–RGD probe were obtained. Figure 3 shows the microPET image of one representative mouse. The subcutaneous U87MG mice tumors could be clearly visualized from surrounding background tissue.
Table 1.
Biodistribution of [18F]FDG- RGD in mice bearing U87MG xenografts. Data are expressed as of percentage of injected radioactivity per gram of organ or tissue (% ID/g) after intravenous injection of [18F]FDG- RGD (10–30 µCi) at different time post injection (N=3 for each group).
| Organ (%ID/g) | 0.5 h | 2 h |
|---|---|---|
| U87MG | 0.94 ± 0.25 | 0.27 ± 0.06 |
| Blood | 0.43 ± 0.05 | 0.02 ± 0.00 |
| Heart | 3.56 ± 1.11 | 3.67 ± 0.26 |
| Liver | 0.44 ± 0.03 | 0.22 ± 0.04 |
| Lungs | 0.78 ± 0.05 | 0.47 ± 0.37 |
| Muscle | 0.27 ± 0.07 | 0.09 ± 0.03 |
| Kidney | 1.95 ± 0.17 | 0.55 ± 0.51 |
| Spleen | 0.49 ± 0.10 | 0.64 ± 0.68 |
| Brain | 0.58 ± 0.04 | 0.16 ± 0.02 |
| Intestine | 0.32 ± 0.03 | 0.19 ± 0.09 |
| Stomach | 0.61 ± 0.50 | 0.30 ± 0.32 |
| Bone | 0.29 ± 0.03 | 0.23 ± 0.20 |
| Ratios | ||
| T/Blood | 2.19 ± 0.55 | 13.27 ± 1.05 |
| T/Muscle | 3.71 ± 1.49 | 3.50 ± 1.90 |
| T/Liver | 2.17 ± 0.76 | 1.25 ± 0.08 |
| T/Kidney | 0.49 ± 0.14 | 0.74 ± 0.43 |
Figure 3.
Decay–corrected microPET images (coronal top and trnsaxial bottom) of a nude mouse (photograph shown on left) bearing U87MG xenograft at 0.5, 1 and 2 h after tail injection of 100 µCi of [18F]FDG-RGD. T is for tumor, G/R is gastrointestinal/renal activities and color bar represents % ID/g.
To evaluate in vivo imaging performance of [18F]FDG-cyclo(RGDDYK) in small living subjects, we investigated biodistribution and microPET imaging of U87MG tumor bearing nude mice expressing αvβ3 integrin by using [18F]FDG-cyclo(RGDDYK) as a probe. Biodistribution of [18F]FDG-cyclo(RGDDYK) in U87MG tumor bearing nude mice (n=9) expressing αvβ3 integrin is shown in Table 2. The ratios of tumor to blood, tumor to muscle and tumor to liver at 2 h post injection of the probe were 3, 13, and 6 respectively. MicroPET images of U87MG tumor bearing nude mice (n=3), 60 min after tail vein injection of [18F]FDG]-cyclo(RGDDYK) were also obtained. Figure 4 shows the microPET image of one representative mouse. The subcutaneous U87MG mouse tumor could be clearly visualized from surrounding background tissue. Tracer activity within the tumor was determined to be 1.06 ± 0.20 % ID/g, by region of interest (ROI) analysis of the three mice which matches with the value of 0.95 ± 0.4 % ID/g determined by the biodistribution studies (Table 2) and muscle uptake was 0.20 ± 0.12 % ID/g. Although the reasons for the relatively fast clearance and moderate tumor uptake, after 60 minutes of injection of [18F]FDG-cyclo(RGDDYK) are not clear, the use of 18F labeled dimeric or tetrameric 18FDG labeled RGD should be explored. Recent reports (14–16) on the radiolabeled DOTA conjugated-cyclic-RGD, monomer, dimer and tetramer with 64Cu showed that tetrameric cyclic-RGD peptide tracer had about twice as much tumor uptake than the corresponding dimer. Also, the dimer had significantly higher uptake than the monomer counterpart. Likewise, It has been shown that 18F-labeled dimeric RGD peptide, [18F]FB-E[c(RGDYK)]2 has superior imaging characteristic than the corresponding monomer (17).
Table 2.
Biodistribution of [18F]FDG-cyclo(RGDDYK) in mice bearing U87MG xenografts. Data are expressed as of percentage of injected radioactivity per gram of organ or tissue (% ID/g) after intravenous injection of [18F]FDG- RGD (10–30 µCi) at different time post injection (N=3 for each group).
| Organ (%ID/g) |
0.5 hr | 1hr | 2 hr |
| Kidney | 4.75 ±5.60 | 3.75 ± 1.42 | 16.1 ± 13.0 |
| Liver | 1.32 ± 1.3 | 0.43 ± 0.40 | |
| Brain | 0.80 ± 1.09 | 0.13 ± 0.07 | |
| Heart | 1.11 ± 0.22 | 2.57 ± 2.53 | |
| Lung | 0.54 ± 45 | 0.60 ± 0.66 | |
| Muscle | 0.30 ± 0.10 | 0.13 ± 0.06 | |
| Stomach | 0.17 ± 0.03 | 0.46 ± 0.52 | |
| Intestine | 0.50 ± 0.02 | 0.28 ± 0.19 | |
| Bone | 0.71 ± 0.65 | 0.13 ± 0.21 | |
| Blood | 2.04 ± 2.28 | 0.84 ± 0.19 | 0.54 ± 0.31 |
| U87MG | 0.63 ± 0.13 | 0.95 ± 0.40 | 1.53 ± 1.59 |
| Spleen | 0.19 ± 0.16 | 0.17 ± 0.20 | |
| Ratios | |||
| T/Blood | 0.32 ± 0.17 | 1.19 ± 0.63 | 2.75 ± 1.62 |
| T/Muscle | 3.72 ± 2.16 | 12.9 ± 9.41 | |
| T/Liver | 0.49 ± 0.30 | 6.02 ± 6.08 | |
| T/Kidney | 0.15 ± 0.08 | 0.28 ± 0.17 | 0.17 ± 0.08 |
Figure 4.
Decay–corrected microPET images (coronal top and trnsaxial bottom) of a nude mouse bearing a U87MG xenograft at 1 h after tail injection of 72 µCi of [18F]FDG-cyclo( RGDDYK). T, tumor; B, bladder; G/R, gastrointestinal/renal activities and color bar represents % ID/g.
Conclusion
We have successfully prepared [18F]FDG-RGD and [18F]FDG-cyclo(RGDDYK) based on a simple one step radiosynthesis by using [18F]FDG as a prosthetic group. The one step radiosynthesis methodology consists of chemoselective oxime formation between an easily synthesized aminooxy functionalized peptide and [18F]FDG. The [18F]FDG labeled RGD peptides can be used to image tumors expressing in living subjects. Imaging of integrin αvβ3 expression in living subjects would offer a potentially useful approach to diagnose tumors and their metastasis, to help us better understand tumor angiogenesis and to monitor target specific anti-angiogenesis treatment efficacy. The preliminary mice data support further investigation of the tracers and more comparisons to alternately labeled RGD peptides. The major limitations for generalization of our 18F-labeling methodology are the relatively high temperature (100°C) and acidic pH conditions required. High temperature and acidic pH are tolerated for the small unprotected peptides but might not be suitable for larger peptides. This approach may also be useful for labeling other biomolecules and would lead to a very simple strategy for routine pre-clinical and clinical use. The methodology additionally provides benefits such as the use of unprotected aminooxy precursor and the ability to form oximes in aqueous media, providing easy formulation for in vivo use. Indeed, the chemistry has been proven through many biological applications in the synthesis of glycopeptides (18–19), oligonucleotide-peptide conjugates (20–21), complex proteins (from peptide fragments) (22), and conjugation of oligo-ribonucleotides and proteins with metal chelates (23).
Supplementary Material
Detailed experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
ACKNOWLEDGMENT
This work was supported, in part, by Medical Diagnostics, GE Healthcare, National Cancer Institute (NCI) Small Animal Imaging Resource Program (SAIRP) grant R24 CA93862, and NCI In Vivo Cellular Molecular Imaging Center (ICMIC) grant P50 CA114747 (SSG). We also thank Dr. David Dick for [18F] production, Dr. Frederick T. Chin for modification of a GE TRACERlab FX-FN synthetic module for radiosynthesis, Dr. Alan Cuthbertson and Dr. Alex Gibson of GE Healthcare for their review of the manuscript.
LITRATURE CITED
- 1.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 2.Brooks PC, Clark R. Requirement of vascular integrine αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 3.Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr. Mol. Med. 2003;3:659–671. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- 4.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin. Exp. Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 5.Toyokuni T, Walsh JC, Dominguez A, Phelps ME, Barrio JR, Gambhir SS, Satyamurthy N. Synthesis of a New Hetrobifunctional Linker, N-[4-(Aminooxy)butyl]maleimide, for Facile Access to a Thiol-Reactive 18FLabeling Agent. Bioconjugate Chem. 2003;14:1253–1259. doi: 10.1021/bc034107y. [DOI] [PubMed] [Google Scholar]
- 6.Poethko T, Schottelius M, Thumshirn G, Hersel U, Herz M, Henriksen G, Kessler H, Schwaiger M, Wester H-J. Two-step methodology for high yield routine radiohalogenation of peptides, 18F-labeled RGD and octreotide analogs. J. Nucl. Med. 2004;45:892–902. [PubMed] [Google Scholar]
- 7.Berndt M, Pietzsch J, Wuest F. Labeling of low –density lipoproteins: 18F-labeled thio-reactive reagent N-[6-[18F]fluorobenzylidine)aminooxyhexyl]maeimide. Nucl. Med. and Biol. 2007;34:5–25. doi: 10.1016/j.nucmedbio.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Z, De Jesus OP, Namavari M, De A, Levi J, Webster JM, Zhang R, Lee B, Syud FA, Gambhir SS. Small-Animal PET Imaging of Human Epidermal Growth Factor Receptor Type 2 Expression with Site-Specific 18F-Labeled Protein Scaffold. J Nucl Med. 2008;49:804–813. doi: 10.2967/jnumed.107.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namavari M, De Jesus OP, Cheng Z, De A, Kovacs E, Levi J, Zhang R, Joshua K, Hoerner JK, Grade H, Syud FA, Gambhir SS. Direct Site-Specific Radiolabeling of an Affibody Protein with 4-[18F]Fluorobenzaldehyde via Oxime Chemistry. Molecular Imaging & Biology. 2008;10:177–181. doi: 10.1007/s11307-008-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berndt M, Pietzsch J, Bergmann R, Wuest F. A Novel role for [18F]FDG: Synthesis and application of a [18F]FDG-based prosthetic group for Peptides and protein labeling. J Nucl Med. 2006;47 Supplement 1:29. [Google Scholar]
- 11.Ramsy SL, Freeman C, Grace PB, Remond JW, MacLeod JK. Mild tagging procedures for the structural analysis of glycans. Carbohydrate. Res. 2001;333:59–71. doi: 10.1016/s0008-6215(01)00115-x. [DOI] [PubMed] [Google Scholar]
- 12.Cai w, Chen K, Li Z-B, Gambhir SS, Chen X. Dual-Function Probe for PET and Near-Infrared Fluorescence Imaging of Tumor Vasculature. J Nucl. Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 13.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptides and positron emission tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 14.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug. Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, Conti PS. MicroPET imaging of breast cancer αv-integrin expression with 64Culabeled dimeric RGD peptides. Mol. Imaging Biol. 2004;6:350–359. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X. MicroPET Imaging of Glioma αv-Integrin Expression Using 64Cu Labeled Tetrameric RGD Peptide. J. Nucl. Med. 2005;46:1707–1718. [PubMed] [Google Scholar]
- 17.Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. MicroPET imaging of αvβ3-Integrine Expression with 18F-Flabeled dimeric RGD peptide. Mol. Imaging. 2004;3:96–104. doi: 10.1162/15353500200404109. [DOI] [PubMed] [Google Scholar]
- 18.Renaudet O, Dumy P. Chemoselectively Template-Assembled Glycoconjugates as Mimics for Multivalent Presentation of Carbohydrates. Org. Lett. 2003;5:243–246. doi: 10.1021/ol0270935. [DOI] [PubMed] [Google Scholar]
- 19.Dey S, Sheppard TL. Ketone-DNA: A versatile Postsynthetic DNA decoration Platform. Org. Lett. 2001;3:3983–3986. doi: 10.1021/ol016626r. [DOI] [PubMed] [Google Scholar]
- 20.Hamma T, Miller PS. 4-(2-Aminooxy)-2-(ethylureido)quinoline-Oligonucleotide Conjugates: Synthesis , Binding Interactions and Derivatization with Peptides. Bioconjug. Chem. 2003;14:320–330. doi: 10.1021/bc025638+. [DOI] [PubMed] [Google Scholar]
- 21.Zatsepin TS, Stetsenko DA, Arzumanov AA, Romonova EA, Gait MJ, Oretskaya TS. Synthesis of Peptide- Oligonucleotide Conjugates with Single and Multiple Peptide Attached to 2′- Aldehydes through Thiazolidine, Oxime, and Hydrazine Linkages. Biocnjug Chm. 2002;13:822–830. doi: 10.1021/bc020016+. [DOI] [PubMed] [Google Scholar]
- 22.Bure C, Leliever D, Delmas A. Identification of by-products from an orthogonal peptide ligation by oxime bonds using spectrometry and tandem mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:2158–2164. doi: 10.1002/1097-0231(20001215)14:23<2158::AID-RCM147>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Peuralahti J, Puukka K, Hakala H, Mukkala V-M, Mulari Q, Hurskainen P, Hovinen J. Synthesis of Nonluminescent Lanthanide (III) Chelates Tethered to an Aminooxy Group and their Applicability to Biomolecules Derivatization. Bioconjug. Chem. 2002;13:876–880. doi: 10.1021/bc025502b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.