Abstract
Antibody microarrays have emerged as useful tools for high-throughput protein analysis and candidate biomarker screening. We describe here the development of a multiplexed microsphere-based antibody array capable of simultaneously measuring ten inflammatory protein mediators. Cytokine-capture microspheres were fabricated by covalently coupling monoclonal antibodies specific for cytokines of interest to fluorescently-encoded 3.1 μm polymer microspheres. An optical fiber bundle containing ∼50,000 individual 3.1 μm diameter fibers was chemically etched to create microwells in which cytokine-capture microspheres could be deposited. Microspheres were randomly distributed in the wells to produce an antibody array for performing a multiplexed sandwich immunoassay. The array responded specifically to recombinant cytokine solutions in a concentration-dependent fashion. The array was also used to examine endogenous mediator patterns in saliva supernatants from patients with pulmonary inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD). This array technology may prove useful as a laboratory-based platform for inflammatory disease research and diagnostics, and its small footprint could also enable integration into a microfluidic cassette for use in point-of-care testing.
Keywords: Antibody array, saliva diagnostics, fiber-optic array, pulmonary disease
Introduction
Protein and antibody microarrays have been used for a number of bioanalytical applications, including cancer and immune disease research, as well as protein expression profiling studies.1-4 These highly multiplexed analysis methods have the potential to greatly benefit clinical diagnostics and biomarker screening, as the protein pathways involved with many diseases are complex.5-8 Antibody microarrays are particularly promising tools for these applications, because they possess the capability to simultaneously detect numerous proteins in low sample volumes.6, 9-13
Pulmonary inflammatory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), involve a complex network of cytokines and chemokines that can be differentially expressed depending on the type of disease, the state of exacerbation, or the presence or absence of a concurrent bacterial or viral infection.7, 14-20 Antibody array technology is a potentially powerful technique for pulmonary inflammatory disease research and diagnostics, as it can be used to define individual cytokine profiles for patients at different levels of disease control, and to evaluate treatment options and efficacy. By routinely monitoring protein profiles of individuals with pulmonary inflammatory diseases, physicians may be able to make better informed decisions about treatment options, as different drugs could have different effects on different individuals, thereby creating the potential for personalized therapies.
Pulmonary inflammatory disease research is commonly conducted using induced sputum or bronchoalveolar lavage (BAL) fluid as sample matrices.21-28 The collection methods involved with obtaining these specimens are either invasive, as in the case of BAL fluid, or can cause discomfort or bronchoconstriction.29, 30 Saliva is being examined as an alternative diagnostic fluid to blood, because it contains many of the same analytes as blood. In addition, its collection is safe, noninvasive, and can be carried out by minimally trained medical personnel.31-35 We hypothesized that saliva could also be used as a window to the airways in pulmonary inflammatory diseases and could serve as a surrogate for sputum and nasal lavage fluid, due to the proximity and anatomic connection of the oral cavity to the upper and lower airways.35-37
Our strategy for developing a salivary diagnostics platform utilizes antibody array technology to examine multiple protein biomarkers simultaneously with a fluorescence readout. Antibody arrays are available in numerous formats, including planar chip microarrays,1, 38 semi-quantitative planar membrane arrays,39 and microsphere-based suspension arrays.40 The antibody array technology employed here combines the advantage of microsphere-based suspension array fabrication with the use of a fluorescence microscope for array analysis. Arrays were produced by chemically etching high-density optical fiber bundles to create microwells, in which antibody-functionalized microspheres are randomly deposited to make antibody arrays. Building on the duplexed antibody array technology previously created in our laboratory,41, 42 we now describe a highly multiplexed antibody array capable of simultaneously detecting ten protein mediators associated with pulmonary inflammatory diseases in saliva using a low sample volume (100 μL) and a total assay time of 2.5 hours. The specificity and sensitivity of the platform was characterized by testing solutions prepared with purified recombinant proteins. As a proof-of-concept, these arrays were utilized to measure endogenous analyte levels in saliva samples collected from patients with asthma and COPD, as well as from healthy control individuals. The microsphere-based antibody array is an attractive alternative to commercially available quantitative cytokine ELISAs that can require greater volumes and longer times to examine a single analyte.
Experimental Details
Materials
Hexagonal fiber-optic bundles of 1.5 mm diameter containing ∼50,000 individual 3.1 μm fibers were custom-drawn by Schott (Southbridge, MA). Amine-functionalized mono-disperse microspheres (3.1 μm diameter, 87% methylstyrene and 13% divinylbenzene copolymer) were purchased from Bangs Laboratories, Inc. (Fishers, IN). Europium (III) thenoyltrifluoroacetonate trihydrate (Eu-dye) was obtained from Acros Organics (Morris Plains, NJ) and 7-Amino-4-methylcoumarin (AMC) was obtained from Anaspec, Inc. (San Jose, CA). Methanol, tetrahydrofuran (THF), dimethyl sulfoxide (DMSO), glutaraldehyde (8% aqueous solution), phosphate-buffered saline (PBS) concentrate (10×), sodium azide, and tris-buffered saline (TBS) with Tween 20 (wash buffer), were purchased from Sigma-Aldrich. Distilled deionized water (Milli-Q Water Purification system Millipore, Billerica, MA) was used for reconstituting reagents. TBS StartingBlock, PBS StartingBlock, and PBS Protein-Free blocking buffers were obtained from Pierce Biotechnology, Inc. (Rockford, IL). Streptavidin-Alexa Fluor 448 conjugate (AF488) was purchased from Invitrogen (Carlsbad, CA). Black, flat-bottom 96-well microtiter plates were purchased from Costar (Corning, NY). An orbital shaker was purchased from Fisher Scientific.
Monoclonal antibodies specific for human VEGF (clone No. 26503), EGF (clone No. 10827), IP-10 (clone No. 33036), IL-8 (clone No. 6217), MCP-1 (clone No. 23007), TIMP-1 (clone No. 63515), RANTES (clone No. 21418), MIP-1β (clone No. 24006), Eotaxin-2 (clone No. 61016), and IL-6 (clone No.6708); biotin-labeled polyclonal detection antibodies complementary to each cytokine; and purified recombinant proteins were obtained from R&D Systems, Inc. (Minneapolis, MN). ELISA kits used in preliminary saliva screening experiments were obtained from R&D Systems, Inc., and RayBiotech, Inc. (Norcross, GA).
Fiber arrays were imaged using a custom-built epi-fluorescence microscope (Olympus America Inc., Center Valley, PA), which included a 20× Olympus objective, excitation and emission filter wheels (Ludl Electronic Products Ltd., Hawthorne, NY), mercury light source, and charge-coupled device camera (Orca-ER, Hamamatsu, Japan). Optical filters for fluorescence imaging of AF488 (Set 31001), AMC (Set 31000), as well as excitation (365/10), dichroic (525DCLP), and emission (605/55) filters for Eu-dye were purchased from Chroma Technology Corp. (Rockingham, VT). Array images were acquired and analyzed using IPlab software obtained from Scanalytics (now BD Biosciences Bioimaging, Rockville, MD). Heatmaps of saliva screening data were created using software developed by Ashley et al. (http://medstaging.stanford.edu/ashleylab/2col_tools_scripts.html). Heatmaps are presented as dataset-normalized values.
Microsphere Encoding
Microspheres were encoded as follows: ten 50 μL (0.1 mg/μL) aliquots of microspheres were each suspended in 1.5 mL polypropylene microcentrifuge tubes with 200 μL of 1× PBS and then washed by vortex and centrifugation at 9,300 × g for 2 min. This washing procedure was performed three times with 1× PBS. The microsphere aliquots were then washed three times in 200 μL of THF, followed by suspension in 200 μL of THF containing a defined concentration of Eu-dye (either 1.0, 0.75, 0.5, 0.125, 0.0625, or 0.025 M, “Set A”) or combination of Eu-dye and AMC (either 0.8/0.4, 0.6/0.4, 0.4/0.4, or 0.05/0.4 M Eu-dye/AMC, “Set B”). Solutions containing both AMC and Eu-dye were prepared in 4 mL amber glass vials by initially dissolving AMC and Eu-dye in DMSO and THF, respectively, then combining the solutions to produce the dye combinations listed above. The microsphere aliquots were suspended in 1.5 mL polypropylene microcentrifuge tubes with the dye solutions, and then allowed to equilibrate on a rotation mixer for 2 h at room temperature (RT) in the dark. The microsphere aliquots were then washed six times each in 200 μL methanol and 200 μL of 1× PBS, followed by suspension in 500 μL of 1× PBS with 0.01% Tween 20 and 0.09% sodium azide for storage at 4°C, protected from light.
Cytokine-Capture Microsphere Preparation
Cytokine-capture microspheres were produced as follows: separate 100 μL aliquots of the ten different fluorescently-encoded microsphere types (∼1 mg of microspheres) were washed three times in 300 μL of 1× PBS by vortex and centrifugation at 9,300 × g for 2 min. The microsphere aliquots were then suspended in 1.5 mL polypropylene microcentrifuge tubes with 1 mL of 8% glutaraldehyde and placed on a rotation mixer at RT in the dark for 2 h.42, 43 Following the incubation, each microsphere aliquot was washed three times with 300 μL of 1× PBS, then 90 g/mL of monoclonal antibody (anti-VEGF, EGF, IP-10, IL-8, MCP-1, TIMP-1, RANTES, MIP-1β, Eotaxin-2, or IL-6) in 500 μL of 1× PBS was added to each encoded microsphere aliquot (1.0, 0.75, 0.5, 0.125, 0.0625, and 0.025 M Eu-dye, or 0.8/0.4, 0.6/0.4, 0.4/0.4, 0.05/0.4 M Eu-dye/AMC, respectively). The microsphere-antibody suspensions were allowed to react on a rotation mixer at RT in the dark for 4 h. The resulting antibody-conjugated microspheres were washed once with 300 μL TBS StartingBlock buffer by vortex and centrifugation at 2,300 × g for 2 min. The microspheres were then blocked with 300 μL TBS StartingBlock on a rotation mixer at RT in the dark for 30 min. Following this blocking step, each microsphere aliquot was washed again in 300 μL TBS StartingBlock buffer as discussed above, then stored in 100 μL TBS StartingBlock buffer with 0.05% sodium azide at 4°C, protected from light. When properly stored, the cytokine-capture microspheres maintained their binding activities for up to three months (data not shown).
Fiber-optic microsphere array preparation
Fiber bundles were hand-cut to ∼3 cm lengths and polished on a TechPrep polishing machine (Allied High Tech Products, Inc., Rancho Dominguez, CA) sequentially using 30-, 15-, 6-, 3-, 1-, 0.5-μm, and “final polishing” lapping films. The distal fiber bundle ends were then etched using 0.025 M hydrochloric acid to form microwell arrays, after which etching residue was removed from the microwells by sonicating the fiber bundle ends in deionized water. The stored cytokine-capture microspheres were pooled into a stock preparation by combining the various microsphere types. A 1 μL aliquot of this microsphere stock was deposited onto the etched end of each fiber bundle and allowed to dry at ambient conditions for 5 min. Excess microspheres were then removed from the fiber bundle end using a lint-free cotton swab moistened with wash buffer.
Sample collection and processing
Whole saliva samples were collected and processed at Boston University Medical Center (BUMC). All samples were de-identified and study participants gave written informed consent consistent with protocols approved by the Institutional Review Boards at BUMC and Tufts University. Participants were instructed to chew on a 1.0 g wax bolus (Parafilm) at a mastication rate of 30 strokes/min, and to expectorate every 30 s into 50 mL Falcon tubes on ice. A total of 15 mL of whole saliva was collected, aliquotted, and centrifuged at 13,150 × g for 20 min at 4°C to separate the cells and food debris (whole saliva sediment) from the liquid phase of whole saliva. Aliquotted samples comprising native whole saliva, whole saliva supernatant, and whole saliva sediment were placed on dry ice, transported to Tufts University, and remained in storage at −80°C until analysis. Whole saliva supernatant samples were used for all cytokine analyses.
Multiplexed assay principle and procedure
The multiplexed microsphere-based sandwich assay procedure is summarized in Figure 1. The distal end of the fiber array containing cytokine-capture microspheres was incubated in 100 μL of sample for 2 h at RT on an orbital shaker at 500 rpm. Following sample incubation, the array was washed three times by immersion in 200 μL of wash buffer. The array was then incubated in 100 μL of a detection antibody cocktail containing 3 μg/mL each of anti-VEGF, EGF, IP-10, IL-8, MCP-1, TIMP-1, RANTES, MIP-1β, Eotaxin-2, and IL-6 biotinylated detection antibodies in PBS Protein-Free blocking buffer, for 30 min at RT on an orbital shaker at 500 rpm. Following another washing step, the fiber was mounted onto the epi-fluorescence microscope stage, then microsphere registration and background signal images were acquired. While the fiber array remained mounted on the microscope, the distal fiber end was then immersed in 200 μL of PBS Protein-Free blocking buffer containing 20 μg/mL streptavidin-AF488 conjugate for ∼1 min and then washed three times. Subsequently, a final AF488 signal image and another microsphere registration image were acquired. Microsphere fluorescence intensities for the background and final signal images were determined by obtaining the mean intensity of 32 pixels that filled the central area of each microsphere using IPLab software. The final signal for multiple microspheres (15−30) was averaged and background-subtracted to obtain the net response for each microsphere type.
Figure 1.
Principle of the fiber-optic microsphere-based antibody array. a) Capture antibodies are linked to fluorescently encoded, amine-functionalized microspheres using glutaraldehyde chemistry to produce cytokine-capture microspheres. A mixture of microspheres with different specificities is loaded into the wells of a high-density fiber-optic array to create an antibody array. b) When the array is incubated in a sample, the cytokine-capture microspheres bind their respective target analytes. The array is then incubated with a mixture of biotinylated detection antibodies. Finally, the detection antibodies are labeled with streptavidin-AF488. Note that the different microsphere types are illustrated by different colors, when they are actually encoded with different amounts of one or two fluorescent dyes.
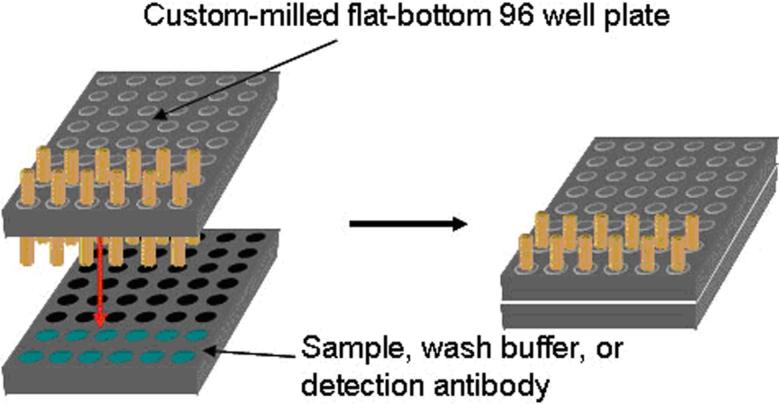
When analyzing multiple samples, fibers were mounted in a custom-milled 96-well microtiter plate to permit high-throughput and reproducible sample incubation (Figure 2). Using this holder, each fiber is exposed to the same experimental conditions such as temperature, agitation, incubation time, and washing. Following the detection antibody incubation and washing steps in the protocol, each fiber array can be sequentially mounted on the fluorescence microscope stage, incubated with streptavidin-AF488 conjugate, and imaged as discussed above.
Figure 2.
Illustration depicting the experimental setup for parallel fiber array incubation. The wells of a microtiter plate were milled such that multiple fiber arrays could be mounted for simultaneous incubation in separate sample solutions, while being exposed to identical experimental conditions.
Results and Discussion
Analyte selection and preliminary screening
There are numerous cytokines implicated in the pathogeneses of pulmonary inflammatory diseases,5, 14, 19, 44 many of which have been extensively investigated in BAL fluid24, 28, 45 and induced sputum.46-48 Although the measurement of cytokines in saliva is well-documented in the literature,49-57 there are few examples of salivary analysis in obstructive pulmonary diseases.36, 58 To construct the most useful array for monitoring pulmonary inflammatory diseases using saliva, preliminary analyte screening was required (see Supporting Information). We screened saliva from pulmonary patients and healthy individuals using a panel of cytokine ELISAs. This initial screening allowed us to examine a number of candidate biomarkers in saliva that have been implicated in pulmonary inflammation. Ultimately, analytes were selected for array development based on a combination of factors: their clinical relevance as described in the literature, their abundance in saliva, and/or their potential correlations with disease observed in the initial screening.
Microsphere Decoding and Registration
Microsphere encoding was used to group ten microsphere types into two sets. The six microsphere types in Set A were encoded with six different concentrations of Eu-dye, such that the types could be distinguished simply by observing their Eu-dye fluorescence emission intensities (Figure 3b).59 The four microsphere types in Set B were encoded with four different concentrations of Eu-dye and a fixed concentration of AMC. Observing AMC fluorescence allowed the Set B microspheres to be distinguished from Set A, because only microspheres from Set B showed fluorescence from AMC (Figure 3a). The four microsphere types within Set B were sub-classified by determining their Eu-dye fluorescence intensities (Figure 3c). Eu-dye registration images were taken at two different exposure times because the Set B microspheres were less intense than the Set A microspheres. A longer exposure time was thus required to distinguish the fluorescence differences in the four Set B Eu-dye encoding levels. Six microsphere types were used in Set A and four in Set B. The presence of the second dye (AMC) in Set B adds complexity so we limited this set to four bead types. Using the aforementioned registration images, the identity of each microsphere type in the array could be decoded by manually analyzing each image. Each microsphere type was determined and designated on the image by its distinct level of Eu-dye and/or AMC fluorescence. These decoded microsphere registration images for each array were overlaid onto subsequent AF488 signal images to determine the fluorescence from each microsphere in the sandwich assay. Microsphere encoding levels were maintained for several months if stored protected from light (data not shown).59
Figure 3.
a) AMC registration image acquired with a 500 ms exposure to distinguish Set A and Set B microspheres (Set A microspheres are identified by colored squares, Set B microspheres are circled). b) Eu-dye registration image acquired with a 100 ms exposure to distinguish the six Set A encoding levels (Highest fluorescence intensity = blue, second highest = white, third highest = red, fourth highest = green, fifth highest = cyan, sixth highest = yellow). c) Eu-dye registration image acquired with a 500 ms exposure to distinguish the four Set B encoding levels (Highest fluorescence intensity = blue, second highest = white, third highest = red, fourth highest = cyan).
Array Characterization
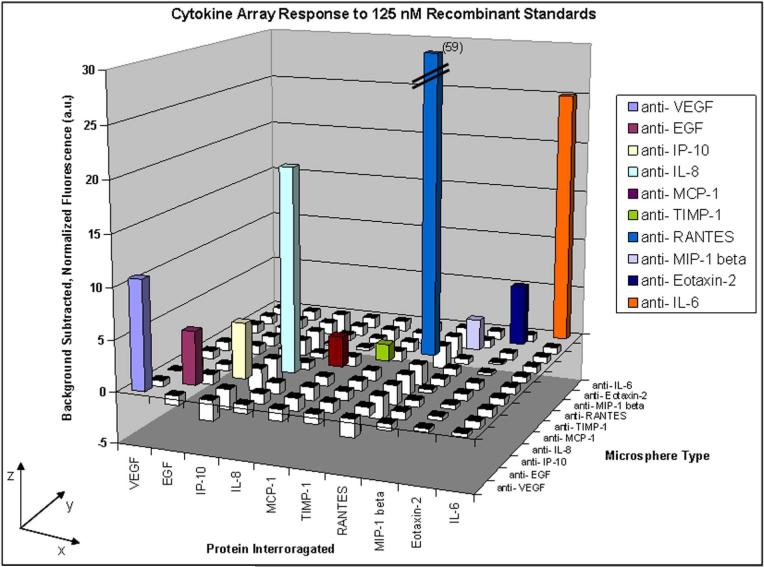
To evaluate the ability of the array to simultaneously determine multiple cytokine concentrations in saliva, we first examined the responses generated for each specific analyte binding to its complementary cytokine-capture microsphere, and the extent to which any cross-reactivity occurred for the other microsphere types on the array. We incubated ten separate microsphere arrays containing all ten cytokine-capture microsphere types with ten solutions containing each individual cytokine (125 nM). This molar concentration ranged from 30 pg/ml to 10.5 μg/ml in our 10-analyte panel. Following sample incubation, the fibers were incubated in a detection antibody cocktail containing complementary biotinylated detection antibodies for all ten cytokines (Figure 4). A threshold was set for each fiber to the background signal plus three times the standard deviation of the background. Any net signal from a microsphere type that was less than this threshold was considered a negative response while any net signal from a microsphere type greater than this threshold was considered a positive response. We observed that each microsphere type produced a positive response only when incubated with its complementary cytokine.
Figure 4.
Cross-reactivity testing results using single recombinant cytokine solutions and a detection antibody cocktail. Solutions of each cytokine (125 nM) were tested using separate arrays containing all ten microsphere types (x-axis), followed by incubation with a detection antibody cocktail containing complementary antibodies for all ten cytokines. Each data point is the average fluorescence of 15−30 microspheres of each type. The net responses for all microsphere types (y-axis) were determined and normalized to three times the standard deviation of the background signals (z-axis). Responses greater than zero, shown as colored bars, indicate a positive response from a microsphere type. Responses less than zero are shown as white bars.
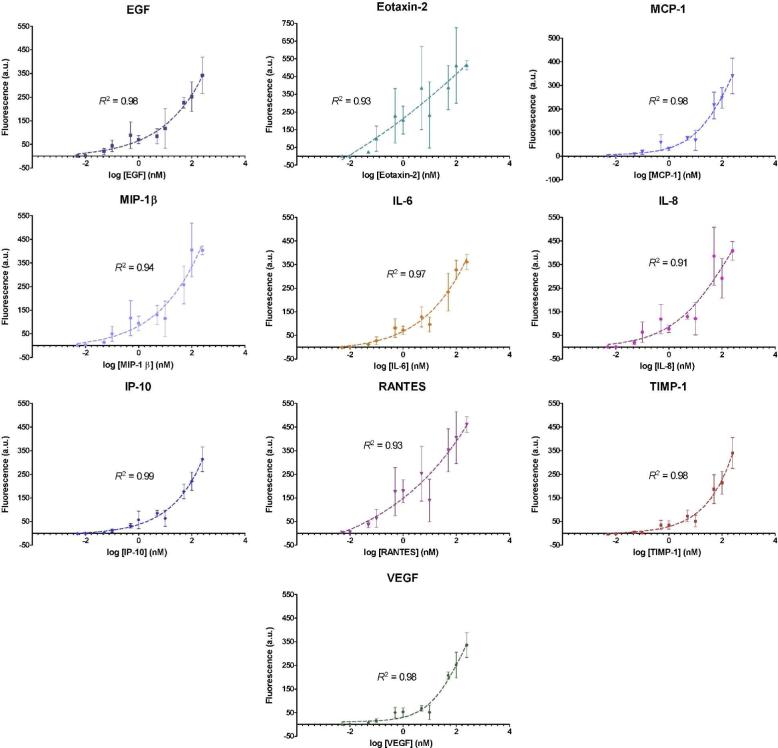
Standard curves for each cytokine-capture microsphere type were acquired by testing a serially diluted mixture containing all ten recombinant cytokines in PBS Protein-Free blocking buffer. The mean background-subtracted response for each microsphere type was determined and averaged for three separate array measurements (Figure 5). Four-parameter logistic regression trends were calculated using GraphPad Prism and curve fits were determined with R2 values, which ranged from 0.93 to 0.99. Similar concentration-dependent responses have been observed for other microsphere-based fluorescence immunoassays.60 The data demonstrate that the microspheres respond with increasing fluorescence at increasing concentrations of analyte, suggesting multiplexed quantitative measurements can be made on unknown samples. In addition, the results indicate that the different microsphere types have different responses to their respective cytokines (e.g. detection limits and sensitivities), presumably due to differences in monoclonal capture antibody binding affinities. We defined the theoretical limit of detection (LOD) for each microsphere type as the concentration corresponding to the mean fluorescence intensity of the blank measurement plus three times the standard deviation. The theoretical LODs for the ten microsphere types ranged from 8 to 470 pM (Table 1). Measurement reproducibility for calibration curve standards (100 pM to 250 nM) was evaluated by calculating the coefficient of variation (CV). For this proof of concept application, a single set of experimental parameters was used for all assays in the array. Additional refinement of the array protocol, such as detection antibody concentrations, incubation times, and incubation temperature, could provide improvements in LOD and precision to the individual assays. With further optimization, we hypothesize that the quantitative capabilities of the array will be more comparable to those of commercially available ELISAs.
Figure 5.
Standard curve analysis for each capture microsphere type on the multiplexed cytokine array.
Table 1.
Protein biomarkers selected as candidates for multiplexed microsphere-based array development.
| Cytokine Panel | ||||||
|---|---|---|---|---|---|---|
| Analyte | Common Name | UNIGENE Number | Molecular Weight (kDa) | Array Limit of Detection (LOD) (pM) | Coefficient of Variation Range (CV, in %) | ELISA Limit of Detection (LOD) (pM) |
| VEGF | Vascular endothelial growth factor | Hs.73793 | 42 | 469 | 7 − 60 | 0.119 |
| EGF | Epidermal growth factor | Hs.419815 | 6 | 11 | 10 − 72 | 0.117 |
| IP-10 | Interferon-inducible protein, 10 kDa (CXCL10) | Hs.632586 | 10 | 140 | 13 − 66 | 0.167 |
| IL-8 | Interleukin-8 (CXCL8) | Hs.624 | 8 | 8 | 8 − 68 | 0.438 |
| MCP-1 | Monocyte chemoattractant protein-1 (CCL2) | Hs.303649 | 8.7 | 90 | 10 − 64 | 0.574 |
| TIMP-1 | Tissue inhibitor of matrix metalloproteinase-1 | Hs.522632 | 28 | 167 | 19 − 57 | 2.86 |
| RANTES | Regulated upon activation, normal T cell expressed and secreted (CCL5) | Hs.514821 | 8 | 10 | 7 − 65 | 0.250 |
| MIP-1β | Macrophage inflammatory protein-β (CCL4) | Hs.75703 | 7.8 | 19 | 5 − 65 | 0.513 |
| Eotaxin-2 | MPIF-2, CCL24 | Hs.247838 | 10.6 | 15 | 5 − 80 | 0.236 |
| IL-6 | Interleukin-6 | Hs.654458 | 21 | 64 | 9 − 61 | 0.033 |
Saliva Testing
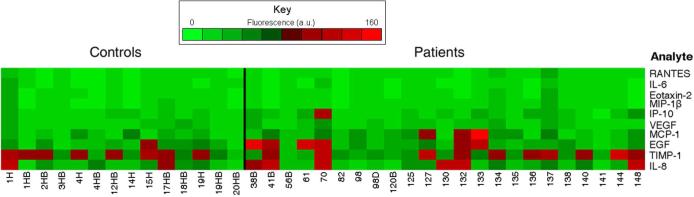
Saliva samples from patients with varying severity of asthma or COPD and healthy control individuals were analyzed using the fiber-optic microsphere-based antibody arrays. The mean response for each microsphere type was determined for each sample (Figure 6). These results demonstrate the importance of multiplexed biomarker measurements, as many of the patient samples exhibited distinct patterns of cytokines, with the elevated cytokines being expressed differently in different patients. For example, the IL-8, TIMP-1, EGF, MCP-1, VEGF, and IP-10 microsphere responses for patient 70 (moderate persistent asthma, in exacerbation) were approximately twice as high as the patient means for those cytokines. In contrast, the sample from patient 127 (moderate persistent asthma) exhibited elevated MCP-1 and TIMP-1 compared to the patient means, while the sample from patient 133 (mild asthma) exhibited elevated EGF, MCP-1, and IP-10. On average, elevated microsphere responses for IL-8, EGF, MCP-1, VEGF, and IP-10 were observed in patient samples compared to controls (Figure 7). In addition, we observed greater variability in patient samples compared to the healthy control samples, as illustrated by larger standard deviations for several of the mean microsphere responses (IL-8, TIMP-1, EGF, MCP-1, and IP-10). Finally, although some of the patient and control samples appear to have similar array responses, we hypothesize that after baseline biomarker levels can be established for each individual, changes will occur in the overall biomarker pattern concomitant with improvement or deterioration in disease. It is the individual's cytokine response pattern that will provide the most useful diagnostic and prognostic information.
Figure 6.
Fiber-optic microsphere array analyses for saliva samples from healthy control individuals (left) and patients with asthma or COPD (right). While the minimum fluorescence responses for each analyte were similar between the patient and control samples, the maximum responses for several of the analytes differed substantially between the two groups. The responses were as follows: IP-10 (patient = 83, control = 26), MCP-1 (patient = 144, control = 52), and EGF (patient = 127, control = 68) (Fluorescence reported as arbitrary units).
Figure 7.
Mean microsphere responses for pulmonary inflammatory disease patients (red) and control samples (blue) tested with microsphere-based antibody arrays. Greater variability was observed in pulmonary patient samples for several of the biomarkers (IL-8, TIMP-1, EGF, MCP-1, and IP-10), as is illustrated by larger standard deviations.
We observed consistently low responses from several microspheres in saliva testing, namely RANTES, IL-6, Eotaxin-2, and MIP-1β. Although these low responses were likely due to the low concentration of these proteins in saliva, they also demonstrate that nonspecific signal from the sample matrix was negligible. By comparing the microsphere array results with preliminary screening data (see Supporting Information), we observed that the cytokines that appeared at high concentrations using commercially available ELISAs were frequently detected by the array (IL-8, VEGF, TIMP-1, EGF, MCP-1, and IP-10), while cytokines that appeared at low concentrations in preliminary screening elicited minimal responses from the array (Eotaxin-2, IL-6, RANTES, and MIP-1β). While the endogenous concentrations of certain cytokines in saliva could be below the working detection limit of their respective microspheres, decreasing the number of microspheres in the array could make the measurement of these cytokines more favorable, as the number of analyte binding events per microsphere will increase, thereby increasing the signal per microsphere.60, 61 Decreasing the number of microspheres distributed in an array can be accomplished by using smaller optical fiber bundles or by diluting the microsphere pool prior to array loading.61 Maximal microsphere loading for each fiber was used for these experiments, however, to ensure that a sufficient number of microspheres of each type could be examined in each array.
Our observations suggest that candidate protein biomarkers associated with pulmonary inflammation can be detected in saliva using antibody arrays. In addition, the array information can be used to create individual patient profiles that may elucidate disease pathogenesis. We hypothesize that each type of pulmonary inflammation or state of disease control produces a different cytokine profile. Analyzing cytokine profile variations for several patients over time using data mining techniques could aid in determining how improvement or deterioration in disease control affects the profiles. Clinical trends associated with each cytokine can also be investigated using a larger sample set that includes patients exhibiting a variety of disease conditions with different causes and degrees of severity.
The antibody array platform we describe has a notable advantage over traditional ELISAs in that it can be used to simultaneously measure ten proteins with a comparable sample volume and assay time to an ELISA where only one protein is measured. In addition, immunoassays incorporated into microfluidic platforms can be performed with even lower reagent volumes and assay times than immunoassays conducted outside of the microfluidic regime.62-64 Owing to its small footprint, the array technology we present could be readily adapted into a microfluidic cassette. Another advantage of this antibody array is its simple and flexible fabrication. Theoretically, the number of analytes that can be examined on a microsphere-based array is limited by the number of different microsphere types that can be encoded and the availability of specifically-binding antibody pairs. Array content can thus be easily modified as new candidate biomarkers are targeted.65 In addition, the flexibility of capture microsphere preparation and detection antibody labeling permits other biological recognition elements, such as recombinant single-chain antibody fragments (scFv),66 and signal reporters, such as rolling circle amplification67-69 or quantum dots,70 to be utilized.
Conclusion
The results presented here demonstrate a proof-of-concept for an antibody microarray that can simultaneously measure ten cytokines implicated in pulmonary inflammatory diseases. The array was used to analyze saliva from patients suffering from asthma and COPD. This report is the first demonstration of these proteins being simultaneously examined in saliva from patients with obstructive pulmonary diseases. This platform will be evaluated for clinical applications by completing validation experiments with additional saliva samples and comparing the results to standardized commercial ELISAs. Examination of a larger patient cohort can also permit correlations between cytokine profiles and the disease states to be examined. In addition, saliva samples can be tested in parallel with other matrices such as sputum, BAL fluid, or nasal lavage to further evaluate the use of saliva as a diagnostic medium for obstructive pulmonary diseases.
Supplementary Material
Acknowledgements
The authors thank the National Institutes of Health/National Institute of Dental and Craniofacial Research (NIH/NIDCR) (Grants U01 DE14950 and U01 DE017788) for their continued financial support. T.M.B. was supported by a GAANN fellowship from the Tufts University Department of Chemistry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR or the NIH.
Footnotes
Supporting Information Available
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Urbanowska T, Mangialaio S, Zickler C, Cheevapruk S, Hasler P, Regenass S, Legay F. J. Immunol. Methods. 2006;316:1–7. doi: 10.1016/j.jim.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Angenendt P. Drug Discov. Today. 2005;10:503–511. doi: 10.1016/S1359-6446(05)03392-1. [DOI] [PubMed] [Google Scholar]
- 3.Gao W-M, Kuick R, Orchekowski RP, Misek DE, Qiu J, Greenberg AK, Rom WN, Brenner DE, Omenn GS, Haab BB, Hanash SM. BMC Cancer. 2005;5 doi: 10.1186/1471-2407-5-110. No pp. given. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen UB, Geierstanger BH. J. Immunol. Methods. 2004;290:107–120. doi: 10.1016/j.jim.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Holgate ST, Polosa R. Nat. Rev. Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 6.Wang CC, Huang R-P, Sommer M, Lisoukov H, Huang R, Lin Y, Miller T, Burke J. J. Proteome Res. 2002;1:337–343. doi: 10.1021/pr0255203. [DOI] [PubMed] [Google Scholar]
- 7.Groneberg D,A, Chung KF. Respir. Res. 2004;5:18. doi: 10.1186/1465-9921-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liotta L, Petricoin E. Nat. Rev. Genet. 2000;1:48–56. doi: 10.1038/35049567. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Nedelkov D, Corn RM. Anal. Chem. 2006;78:6504–6510. doi: 10.1021/ac060881d. [DOI] [PubMed] [Google Scholar]
- 10.Tam SW, Wiese R, Lee S, Gilmore J, Kumble KD. J. Immunol. Methods. 2002;261:157–165. doi: 10.1016/s0022-1759(01)00572-5. [DOI] [PubMed] [Google Scholar]
- 11.Haab BB. Curr. Opin. Biotech. 2006;17:415–421. doi: 10.1016/j.copbio.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Perlee LT, Christiansen J, Dondero R, Grimwade B, Lejnine S, Mullenix M, Shao W, Sorette M, Tchernev VT, Patel DD, Kingsmore SF. Proteome Sci. 2004;2 doi: 10.1186/1477-5956-2-9. No pp. given. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaras K, Ressine A, Nilsson E, Malm J, Marko-Varga G, Lilja H, Laurell T. Anal. Chem. 2007;79:5817–5825. doi: 10.1021/ac0709955. [DOI] [PubMed] [Google Scholar]
- 14.Kips JC. Eur. Respir. J. Suppl. 2001;34:24s–33s. doi: 10.1183/09031936.01.00229601. [DOI] [PubMed] [Google Scholar]
- 15.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Annu. Rev. Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 16.Schaller M, Hogaboam CM, Lukacs N, Kunkel SL. J. Allergy Clin. Immunol. 2006;118:295–302. doi: 10.1016/j.jaci.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Garra A, Murphy K. Curr. Opin. Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 18.Holt PG, Macaubas C, Stumbles PA, Sly PD. Nature. 1999;402:B12–B17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ. Respir. Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Look DC, Chin CL, Manzel LJ, Lehman EE, Humlicek AL, Shi L, Starner TD, Denning GM, Murphy TF, Sethi S. Proc. Am. Thorac. Soc. 2006;3:482–483. doi: 10.1513/pats.200603-060MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konno S.-i., Gonokami Y, Kurokawa M, Kawazu K, Asano K, Okamoto K.-i., Adachi M. Int. Arch. Allergy Immunol. 1996;109:73–78. doi: 10.1159/000237234. [DOI] [PubMed] [Google Scholar]
- 22.Xiao W, Hsu Y-P, Ishizaka A, Kirikae T, Moss RB. Chest. 2005;128:2316–2326. doi: 10.1378/chest.128.4.2316. [DOI] [PubMed] [Google Scholar]
- 23.Dente FL, Carnevali S, Bartoli ML, Cianchetti S, Bacci E, Di Franco A, Vagaggini B, Paggiaro P. Ann. Allerg. Asthma Im. 2006;97:312–320. doi: 10.1016/S1081-1206(10)60795-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim CK, Kim SW, Park CS, Kim BI, Kang H, Koh YY. J. Allergy Clin. Immunol. 2003;112:64–71. doi: 10.1067/mai.2003.1618. [DOI] [PubMed] [Google Scholar]
- 25.Gibson PG, Simpson JL. In: Methods in Molecular Medicine. O'Neill LAJ, Bowie A, editors. Vol. 60. Humana Press, Inc.; Totowa, NJ: 2001. pp. 305–312. [Google Scholar]
- 26.Erpenbeck VJ, Schmidt R, Guenther A, Krug N, Hohlfeld JM. Allergy. 2006;61:598–604. doi: 10.1111/j.1398-9995.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 27.Gibson PG. J. Allergy Clin. Immunol. 1998;102:S100–101. doi: 10.1016/s0091-6749(98)70039-9. [DOI] [PubMed] [Google Scholar]
- 28.Woodman L, Sutcliffe A, Kaur D, Berry M, Bradding P, Pavord ID, Brightling CE. Chest. 2006;130:371–378. doi: 10.1378/chest.130.2.371. [DOI] [PubMed] [Google Scholar]
- 29.Pizzichini E, Pizzichini MMM, Leigh R, Djukanovic R, Sterk PJ. Eur. Respir. J. Suppl. 2002;37:9s–18s. doi: 10.1183/09031936.02.00000902. [DOI] [PubMed] [Google Scholar]
- 30.Paggiaro PL, Chanez P, Holz O, Ind PW, Djukanovic R, Maestrelli P, Sterk PJ. Eur. Respir. J. Suppl. 2002;37:3s–8s. doi: 10.1183/09031936.02.00000302. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay R. Anal. Chem. 2006;78:4255–4259. doi: 10.1021/ac069420i. [DOI] [PubMed] [Google Scholar]
- 32.Wong DT. J. Am. Dent. Assoc. 2006;137:313–321. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman E, Lamster Ira B. Crit. Rev. Oral. Biol. Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 34.Herr AE, Hatch AV, Throckmorton DJ, Tran HM, Brennan JS, Giannobile WV, Singh AK. P. Natl. Acad. Sci. USA. 2007;104:5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bentur L, Mansour Y, Brik R, Eizenberg Y, Nagler RM. Respir. Med. 2006;100:1195–1201. doi: 10.1016/j.rmed.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Schmekel B, Ahlner J, Malmstrom M, Venge P. Respir. Med. 2001;95:670–675. doi: 10.1053/rmed.2001.1123. [DOI] [PubMed] [Google Scholar]
- 37.Hyyppa T. J. Clin. Periodontol. 1981;8:500–507. doi: 10.1111/j.1600-051x.1981.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 38.Kersten B, Wanker EE, Hoheisel JD, Angenendt P. Expert Rev. Proteomic. 2005;2:499–510. doi: 10.1586/14789450.2.4.499. [DOI] [PubMed] [Google Scholar]
- 39.Huang RP. J. Immunol. Methods. 2001;255:1–13. doi: 10.1016/s0022-1759(01)00394-5. [DOI] [PubMed] [Google Scholar]
- 40.Prabhakar U, Eirikis E, Davis HM. J. Immunol. Methods. 2002;260:207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- 41.Szurdoki F, Michael KL, Walt DR. Anal. Biochem. 2001;291:219–228. doi: 10.1006/abio.2001.5041. [DOI] [PubMed] [Google Scholar]
- 42.Rissin DM, Walt DR. Anal. Chim. Acta. 2006;564:34–39. doi: 10.1016/j.aca.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Walt DR, Agayn VI. Trac-Trend. Anal. Chem. 1994;13:425–430. [Google Scholar]
- 44.Barnes PJ. Nat. Rev. Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 45.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. J. Allergy Clin. Immunol. 2002;110:899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Rahman AMO, El-Sahrigy SAF, Bakr SI. Chest. 2006;129:266–271. doi: 10.1378/chest.129.2.266. [DOI] [PubMed] [Google Scholar]
- 47.Kurashima K, Mukaida N, Fujimura M, Schroeder J-M, Matsuda T, Matsushima K. J. Leukocyte Biol. 1996;59:313–316. doi: 10.1002/jlb.59.3.313. [DOI] [PubMed] [Google Scholar]
- 48.Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, Liu J, Yagi S, Boushey HA. Am. J. Respir. Crit. Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 49.Leigh JE, Steele C, Wormley F, Fidel PL., Jr. Oral Microbiol. Immunol. 2002;17:311–314. doi: 10.1034/j.1399-302x.2002.170508.x. [DOI] [PubMed] [Google Scholar]
- 50.Chiappelli F, Iribarren Francisco J, Prolo P. Bioinformation. 2006;1:331–334. doi: 10.6026/97320630001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minetto M, Rainoldi A, Gazzoni M, Terzolo M, Borrione P, Termine A, Saba L, Dovio A, Angeli A, Paccotti P. Eur. J. Appl. Physiol. 2005;93:679–686. doi: 10.1007/s00421-004-1241-z. [DOI] [PubMed] [Google Scholar]
- 52.Katakura A, Kamiyama I, Takano N, Shibahara T, Muramatsu T, Ishihara K, Takagi R, Shouno T. Bull. Tokyo Dent. Coll. 2007;48:199–203. doi: 10.2209/tdcpublication.48.199. [DOI] [PubMed] [Google Scholar]
- 53.Vastardis S, Leigh JE, Wozniak K, Yukna R, Fidel PL., Jr. Oral. Microbiol. Immunol. 2003;18:88–91. doi: 10.1034/j.1399-302x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 54.Streckfus C, Bigler L, Navazesh M, Al-Hashimi I. Clin. Oral Investig. 2001;5:133–135. doi: 10.1007/s007840100104. [DOI] [PubMed] [Google Scholar]
- 55.Streckfus CF, Bigler LR. Oral. Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 56.Black KP, Merrill KW, Jackson S, Katz J. Oral Microbiol. Immunol. 2000;15:74–81. doi: 10.1034/j.1399-302x.2000.150202.x. [DOI] [PubMed] [Google Scholar]
- 57.Winkler O, Hadnagy W, Idel H. Int. J. Hyg. Environ. Health. 2001;204:181–184. doi: 10.1078/1438-4639-00092. [DOI] [PubMed] [Google Scholar]
- 58.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. J. Allergy Clin. Immunol. 2007;119:328–335. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Song L, Ahn S, Walt DR. Anal. Chem. 2006;78:1023–1033. doi: 10.1021/ac051417w. [DOI] [PubMed] [Google Scholar]
- 60.Derveaux S, Stubbe BG, Roelant C, Leblans M, De Geest BG, Demeester J, De Smedt SC. Anal. Chem. 2008;80:85–94. doi: 10.1021/ac071212i. [DOI] [PubMed] [Google Scholar]
- 61.Epstein JR, Lee M, Walt DR. Anal. Chem. 2002;74:1836–1840. doi: 10.1021/ac0156619. [DOI] [PubMed] [Google Scholar]
- 62.Zhou L, Wang K, Tan W, Chen Y, Zuo X, Wen J, Liu B, Tang H, He L, Yang X. Anal. Chem. 2006;78:6246–6251. doi: 10.1021/ac060598e. [DOI] [PubMed] [Google Scholar]
- 63.Schulte TH, Bardell RL, Weigl BH. Clin. Chim. Acta. 2002;321:1–10. doi: 10.1016/s0009-8981(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 64.Hayes MA, Polson NA, Phayre AN, Garcia AA. 2001. pp. 5896–5902. [DOI] [PubMed]
- 65.Weckmann M, Collison A, Simpson JL, Kopp MV, Wark PAB, Smyth MJ, Yagita H, Matthaei KI, Hansbro N, Whitehead B, Gibson PG, Foster PS, Mattes J. Nat. Med. 2007;13:1308–1315. doi: 10.1038/nm1660. [DOI] [PubMed] [Google Scholar]
- 66.Warren DJ, Bjerner J, Paus E, Bormer OP, Nustad K. Clin. Chem. 2005;51:830–838. doi: 10.1373/clinchem.2004.046979. [DOI] [PubMed] [Google Scholar]
- 67.Zhou H, Bouwman K, Schotanus M, Verweij C, Marrero JA, Dillon D, Costa J, Lizardi P, Haab BB. Genome Biol. 2004;5:R28. doi: 10.1186/gb-2004-5-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shao W, Zhou Z, Laroche I, Lu H, Zong Q, Patel DD, Kingsmore S, Piccoli SP. J. Biomed. Biotechnol. 2003:299–307. doi: 10.1155/S1110724303209268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schweitzer B, Roberts S, Grimwade B, Shao W, Wang M, Fu Q, Shu Q, Laroche I, Zhou Z, Tchernev VT, Christiansen J, Velleca M, Kingsmore SF. Nat. Biotechnol. 2002;20:359–365. doi: 10.1038/nbt0402-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geho D, Lahar N, Gurnani P, Huebschman M, Herrmann P, Espina V, Shi A, Wulfkuhle J, Garner H, Petricoin E, III, Liotta LA, Rosenblatt KP. Bioconjugate Chem. 2005;16:559–566. doi: 10.1021/bc0497113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.