Abstract
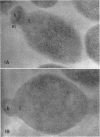
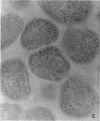
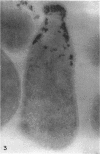
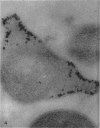
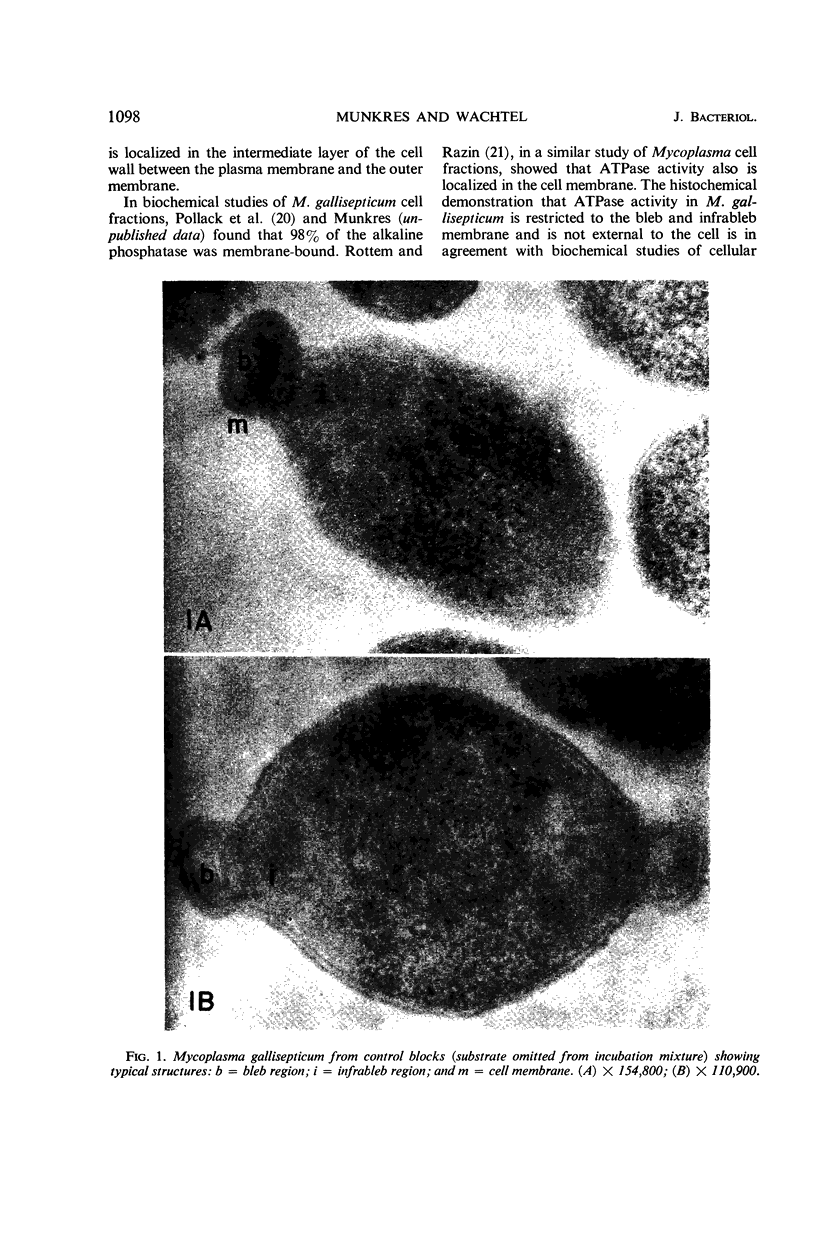
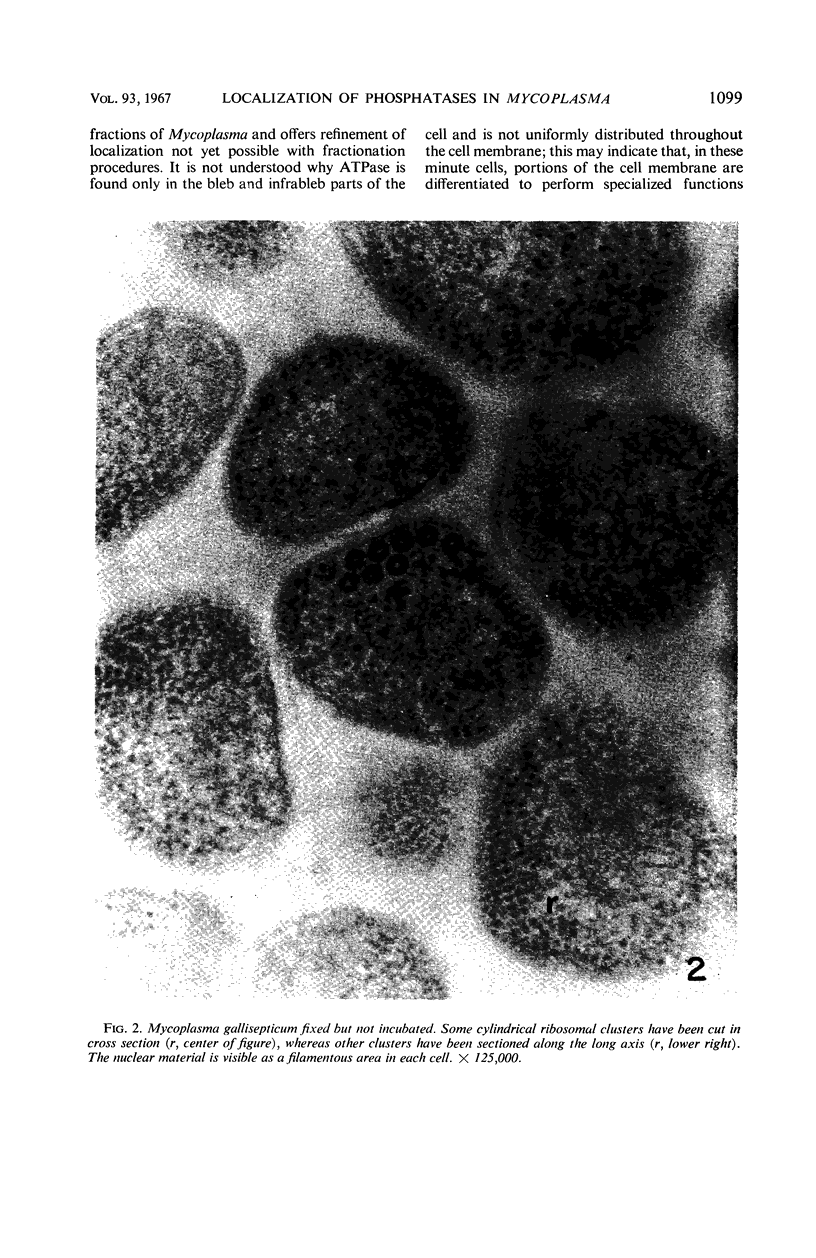
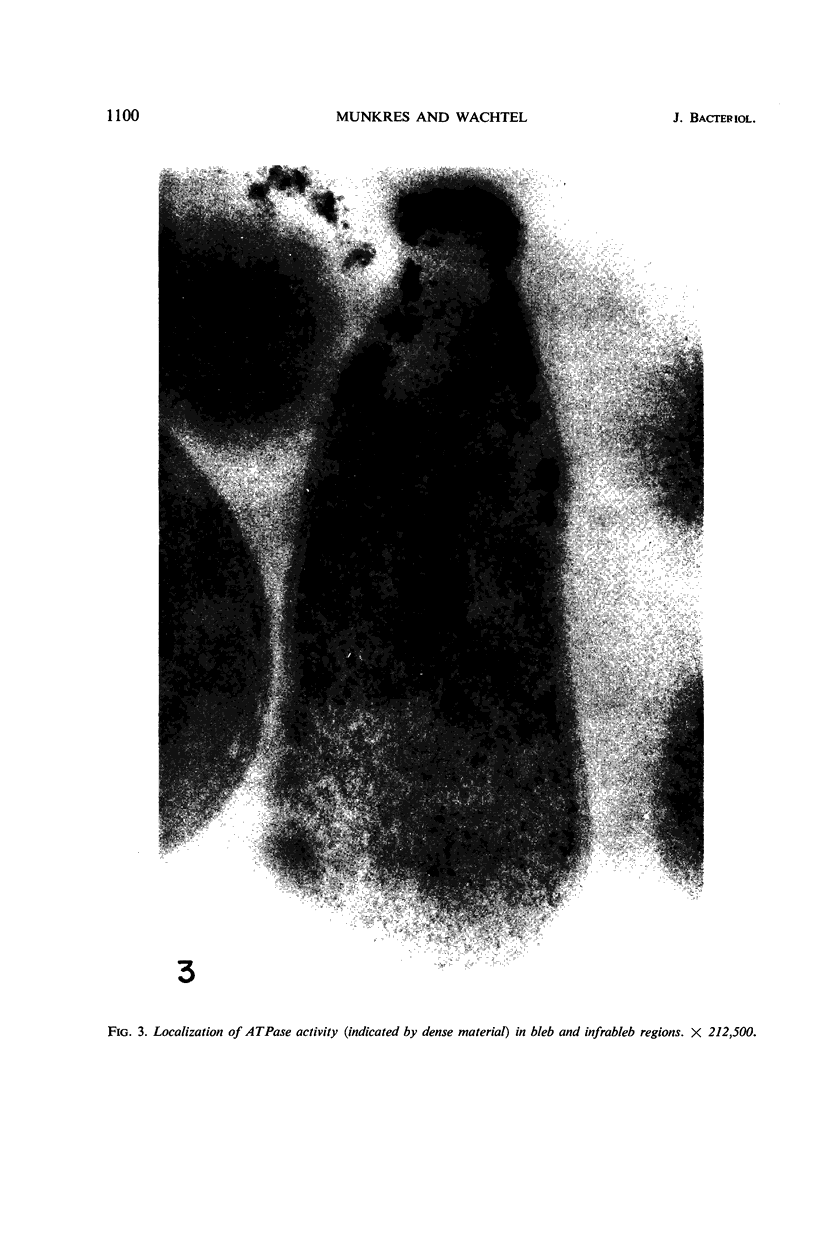
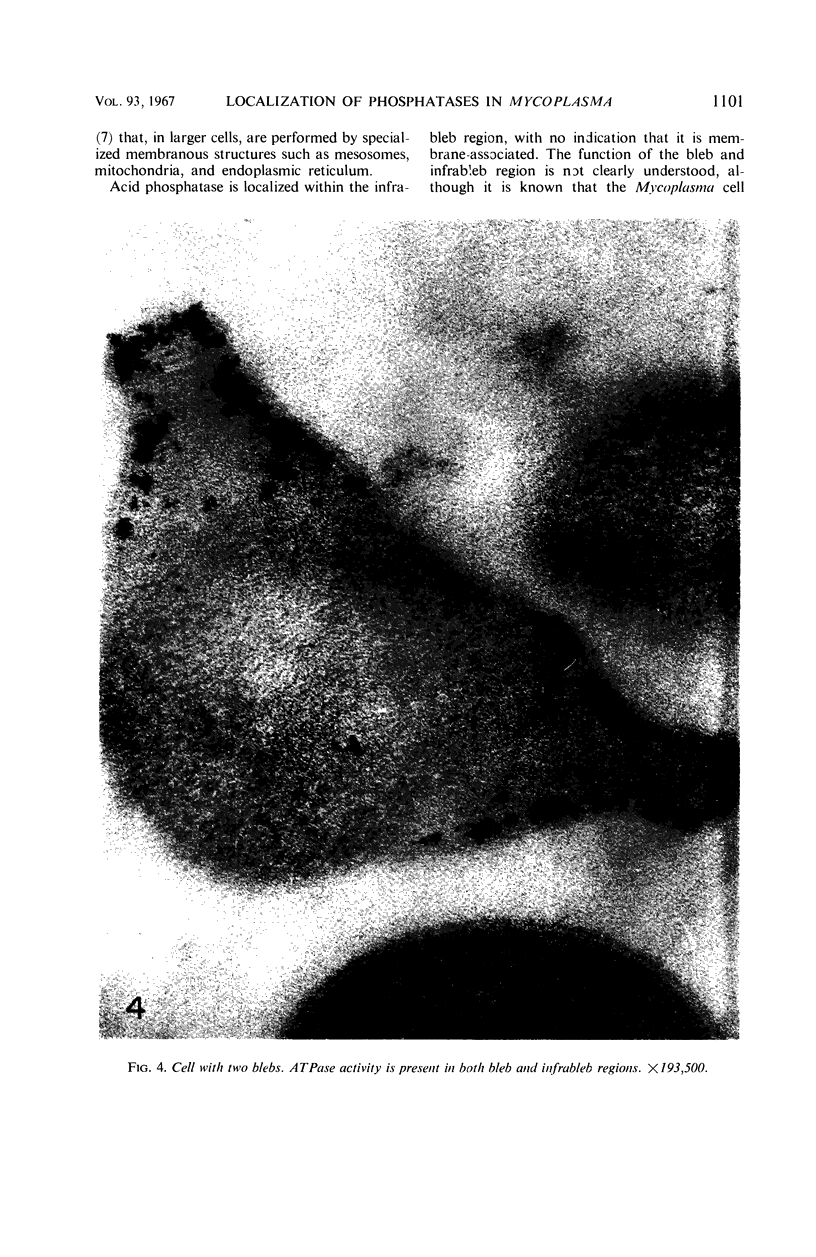
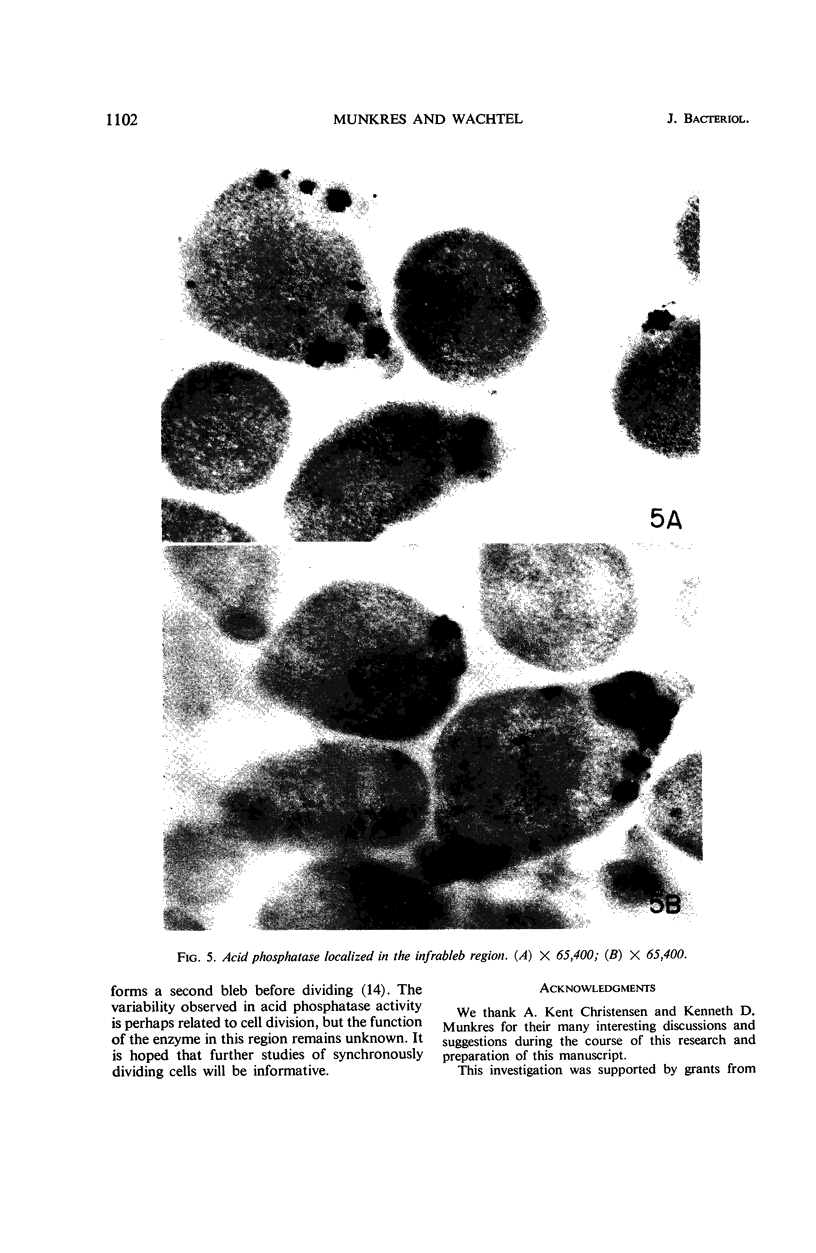
Histochemical studies of adenosine triphosphatase and acid phosphatase activity were performed on Mycoplasma gallisepticum. The adenosine triphosphatase activity appears to be localized in the bleb and infrableb regions exclusively and is associated with the cell membrane; acid phosphatase activity is localized in the infrableb region and does not appear to be membrane-associated. These findings are consistent with data from biochemical studies of Mycoplasma cell fractions but, unlike them, reveal that adenosine triphosphatase activity is restricted to a particular part of the cell membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Anderson D. R., Barile M. F. Ultrastructure of Mycoplasma hominis. J Bacteriol. 1965 Jul;90(1):180–192. doi: 10.1128/jb.90.1.180-192.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNUN A., DEPAHN E. M., STOPPANI A. O. SOME PROPERTIES OF PARTICLE-BOUND INTRACELLULAR ATPASE FROM BAKER'S YEAST. Biochim Biophys Acta. 1964 Sep 18;89:532–539. doi: 10.1016/0926-6569(64)90079-3. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Hughes D. E. The enzymic activity of the outer shell of Lactobacillus arabinosus. J Gen Microbiol. 1965 Jul;40(1):81–95. doi: 10.1099/00221287-40-1-81. [DOI] [PubMed] [Google Scholar]
- DOMERMUTH C. H., NIELSEN M. H., FREUNDT E. A., BIRCH-ANDERSEN A. ULTRASTRUCTURE OF MYCOPLASMA SPECIES. J Bacteriol. 1964 Sep;88:727–744. doi: 10.1128/jb.88.3.727-744.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLBERG P. A., HAUGE J. G. THE INVOLVEMENT OF MEMBRANES IN PROTEIN SYNTHESIS IN BACTERIUM ANITRATUM. Biochim Biophys Acta. 1965 Jan 11;95:80–85. doi: 10.1016/0005-2787(65)90213-3. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- MARR A. G. Enzyme localization in bacteria. Annu Rev Microbiol. 1960;14:241–260. doi: 10.1146/annurev.mi.14.100160.001325. [DOI] [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- MOROWITZ H. J., TOURTELLOTTE M. E., GUILD W. R., CASTRO E., WOESE C. The chemical composition and submicroscopic morphology of Mycoplasma gallisepticum, avian PPLO 5969. J Mol Biol. 1962 Feb;4:93–103. doi: 10.1016/s0022-2836(62)80041-2. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J., Barrnett R. J. Ultrastructure and Ribosomes of Mycoplasma gallisepticum. J Bacteriol. 1965 Jul;90(1):193–204. doi: 10.1128/jb.90.1.193-204.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A., Barrnett R. J. Fine structural demonstration of phosphatase activity at pH 9. Nature. 1965 Jun 5;206(988):1001–1003. doi: 10.1038/2061001a0. [DOI] [PubMed] [Google Scholar]
- Morowitz H. J., Maniloff J. Analysis of the life cycle of Mycoplasma gallisepticum. J Bacteriol. 1966 Apr;91(4):1638–1644. doi: 10.1128/jb.91.4.1638-1644.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B., DE THE G., BEARD D., BEARD J. W. Electron microscopic study of the ATPase activity of the BAI strain A (myeloblastosis) avian tumor virus. J Cell Biol. 1962 Dec;15:451–462. doi: 10.1083/jcb.15.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Pollack M. E., Cleverdon R. C. Fractionation of mycoplasma cells for enzyme localization. Life Sci. 1965 May;4(9):973–977. doi: 10.1016/0024-3205(65)90200-6. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Adenosine triphosphatase activity of mycoplasma membranes. J Bacteriol. 1966 Sep;92(3):714–722. doi: 10.1128/jb.92.3.714-722.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT G., BARTSCH G., LAUMONT M. C., HERMAN T., LISS M. Acid phosphatase of bakers' yeast: an enzyme of the external cell surface. Biochemistry. 1963 Jan-Feb;2:126–131. doi: 10.1021/bi00901a022. [DOI] [PubMed] [Google Scholar]
- SEDAR A. W., BURDE R. M. LOCALIZATION OF THE SUCCINIC DEHYDROGENASE SYSTEM IN ESCHERICHIA COLI USING COMBINED TECHNIQUES OF CYTOCHEMISTRY AND ELECTRON MICROSCOPY. J Cell Biol. 1965 Feb;24:285–295. doi: 10.1083/jcb.24.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Barrett K. The reversible dissociation of the alkaline phosphatase of Escherichia coli. I. Formation and reactivation of subunits. J Biol Chem. 1965 Nov;240(11):4284–4292. [PubMed] [Google Scholar]
- Sedar A. W., Burde R. M. The demonstration of the succinic dehydrogenase system in Bacillus subtilis using tetranitro--blue tetrazolium combined with techniques of electron microscopy. J Cell Biol. 1965 Oct;27(1):53–66. doi: 10.1083/jcb.27.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONINO G. J., STEYN-PARVE E. P. Localization of some phosphatases in yeast. Biochim Biophys Acta. 1963 Mar 12;67:453–469. doi: 10.1016/0006-3002(63)91851-1. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-NEGATIVE BACTERIUM. TELLURITE REDUCTION IN PROTEUS VULGARIS. J Cell Biol. 1964 Mar;20:377–387. doi: 10.1083/jcb.20.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOELZ H. SITES OF ADENOSINE TRIPHOSPHATASE ACTIVITY IN BACTERIA. J Bacteriol. 1964 Oct;88:1196–1198. doi: 10.1128/jb.88.4.1196-1198.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. EVIDENCE FOR AN EXOCELLULAR SITE FOR THE ACID PHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1964 Dec;88:1743–1754. doi: 10.1128/jb.88.6.1743-1754.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]