Abstract
Nasal trigeminal chemosensitivity in mice and rats is mediated in part by epithelial solitary chemoreceptor (chemosensory) cells (SCCs), but the exact role of these cells in chemoreception is unclear (Finger et al. 2003). Histological evidence suggests that SCCs express elements of the bitter taste transduction pathway including T2R (bitter taste) receptors, the G protein α-gustducin, PLCβ2, and TRPM5, leading to speculation that SCCs are the receptor cells that mediate trigeminal nerve responses to bitter taste receptor ligands. To test this hypothesis, we used calcium imaging to determine whether SCCs respond to classic bitter-tasting or trigeminal stimulants. SCCs from the anterior nasal cavity were isolated from transgenic mice in which green fluorescent protein (GFP) expression was driven by either TRPM5 or gustducin. Isolated cells were exposed to a variety of test stimuli to determine which substances caused an increase in intracellular Ca2+ ([Ca2+]i). GFP positive cells respond with increased [Ca2+]i to the bitter receptor ligand denatonium, and this response is blocked by the PLC inhibitor U73122. In addition GFP+ cells respond to the PLC activator 3M3FBS, the neuromodulators ATP and ACh, but only very rarely to other bitter-tasting or trigeminal stimuli. Our results demonstrate that TRPM5- and gustducin-expressing nasal SCCs respond to the T2R agonist, denatonium via a PLC-coupled transduction cascade typical of T2Rs in the taste system.
Keywords: Chemoreceptor, bitter, nasal, trigeminal, respiratory
Introduction
The nose is a complex organ that functions to monitor, condition, and filter inspired air (Baraniuk and Kaliner 1991; Harkema et al. 2006). Intranasal trigeminal fibers not only mediate somatosensory sensations such as touch, temperature, and pain but are also responsive to a wide variety of chemical stimuli (Bryant and Silver 2000). Stimulation of nasal trigeminal fibers by noxious chemical substances elicits respiratory, cardiovascular, and hormonal responses that may protect the airways from further exposure (Silver et al. 1986). Therefore, the primary function of this intranasal trigeminal system is to act as a sentinel of the airways, guarding the respiratory system from inhalation of noxious substances (Silver 1990).
Nasal detection of irritants has classically been thought to be mediated by trigeminal free nerve endings that reside in the nasal mucosa and terminate within a few micra of the epithelial surface, just below the level of tight junctions (Bryant and Silver 2000; Finger et al. 1990). Therefore, detection of chemical stimuli in the nasal cavity was thought to necessitate diffusion across this barrier to gain access to free nerve endings. However, a newly discovered population of modified epithelial cells termed solitary chemoreceptor cells or chemosensory cells (both abbreviated SCCs) also contributes to nasal trigeminal sensitivity (Finger et al. 2003; Sbarbati and Osculati 2005a). SCCs form synaptic contacts with afferent trigeminal nerve fibers and possess a transduction and signaling cascade similar to type II taste cells that includes T2R “bitter” taste receptors, the G protein α-gustducin, phospholipase C isoform β2 (PLCβ2), and the transient receptor potential melastatin channel subtype 5 (TRPM5) (Finger et al. 2003; Lin et al. 2007). In mice, SCCs are scattered throughout the nasal respiratory epithelium with the highest numbers positioned just posterior to the nasal vestibule (Finger et al. 2003; Lin et al 2007). Cells with signal transduction cascades similar to SCCs occur throughout the respiratory and digestive tracts (Bezencon et al. 2006; Finger et al. 2003; Hofer et al. 1996; Merigo et al. 2007; Merigo et al. 2005; Sbarbati et al. 2004a; Sbarbati et al. 2004b; Sbarbati and Osculati 2005b; Tizzano et al. 2006; Wu et al. 2002).
Since the discovery of SCCs in mammals, their functional significance has remained speculative. Expression of bitter-taste transduction machinery coupled with the finding by Finger et al. (2003) that bitter compounds in the nasal cavity cause respiratory depression and ethmoid nerve responses, has led to speculation that SCCs broaden trigeminal chemosensitivity by detecting bitter compounds. But do SCCs actually mediate trigeminal responses to bitter compounds or are the trigeminal responses to these compounds mediated by other mechanisms? Liu and Simon (1998) demonstrated that cultured trigeminal ganglion neurons are capable of responding to bitter substances so SCCs may not mediate the trigeminal response to these compounds. Only one study to date has attempted to isolate and record from nasal SCCs. Lin et al. (2007) observed that TRPM5-expressing cells isolated from the mouse nasal cavity respond to high concentrations of odorants which may or may not act via T2R receptors. To date, no study has investigated if isolated nasal SCCs actually respond to bitter tasting compounds.
To determine which component of nasal trigeminal chemoreception is mediated by SCCs, we used calcium imaging to examine the response of isolated SCCs to an array of bitter tasting and trigeminal irritant stimuli. We find that SCCs respond robustly and specifically to the bitter compound denatonium benzoate through a PLC-mediated signalling cascade, but do not respond to other tested bitter compounds or classical trigeminal stimuli.
Materials and Methods
Animals
All experiments referred to in this report were carried out in accordance with approved UCDHSC or Colorado State University IACUC animal protocols.
TRPM5-GFP mice were developed by R.F. Margolskee (Mt. Sinai School of Medicine, New York) and contained 5′ to 3′: 11 kb of mouse TRPM5 5′ flanking sequence, TRPM5 Exon 1 (untranslated), Intron 1, and the untranslated part of Exon 2, and eGFP (Clapp et al. 2006). Immunolabeling with an antibody against the TRPM5 protein by Lin et al. (2007) demonstrates nearly complete colocalization between GFP and the TRPM5 protein. Gustducin-GFP mice were also developed by R.F. Margolskee and the construct contained an 8.4 kb segment from the upstream region of the mouse α-gustducin gene (Huang et al. 1999; Wong et al. 1999). Expression of GFP for both mouse strains has been verified by comparison with immunocytochemistry for the relevant proteins (Lin et al. 2007; Wong et al. 1999).
Isolation of SCCs
SCCs were isolated from transgenic mice expressing GFP under control of promoters upstream of either the TRPM5 or α-gustducin promoter using an adaptation of the protocol described by Rawson et al. (1998). Briefly, mice were sacrificed by cervical dislocation and rapid exsanguination, the heads split down the midline, and the anterior respiratory nasal epithelium dissected in Tyrode's solution (pH 7.2-7.4). Tissue was transferred to calcium free Tyrode's and minced before being digested for 30 minutes in a Papain solution (20 units Papain (Worthington Biochemicals, Lakewood, NJ)/ml with 5 mM L-Cysteine). Enzyme activity was stopped by the addition of leupeptin (10 μg/ml) and normal Tyrode's. Cells were then gently triturated, the cell suspension plated on concanavalin-A coated coverglass and allowed to settle for 10 minutes. Cells were then loaded with ∼ 2μM fura-2 AM (Molecular Probes, Invitrogen Corporation, Carlsbad, CA) for 20 minutes, rinsed with Tyrode's, and allowed to sit for 20 minutes before imaging.
Ca2+ Imaging
Ca2+ imaging was carried out using the same experimental setup and protocol as in Clapp et al. (2006). Briefly, images were acquired every 5 seconds through the 40× oil immersion objective of an inverted Nikon Diaphot TMD microscope using Imaging Workbench 5.2 software (Indec Biosystems, Inc., Santa Clara, CA) and a CCD Sensicam QE camera (COOKE Corporation, Romulus, MI). Excitation wavelengths of 350nm and 380nm were used with an emission wavelength of ∼510nm. Stimuli were bath applied using a gravity flow perfusion system (Automate Scientific Inc., San Francisco, CA) and laminar flow perfusion chambers (RC-25F, Warner Scientific Inc., Hamden, CT). Data were graphed using OriginPro 7.5 software with intracellular Ca2+ levels being reported as F350/F380 versus time.
Solutions
Tyrode's solution contained (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 10 glucose, and 1 pyruvic acid adjusted to pH 7.4 with NaOH. All test stimuli were diluted in Tyrode's. Capsaicin and menthol were first suspended in a small amount (0.1% of final volume) of 95 % ethanol vehicle to allow them to go into solution. The final pH of stimulus solutions was 7.4.
Histology
Tissue preparation
TRPM5-GFP mice were deeply anaesthetized with 20% chloral hydrate and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Heads were post-fixed for 30 minutes and then rinsed in 0.1 M PB before being split down the midline to expose the nasal epithelium which was then dissected and stored in 0.1 M PB. Tissue was cryoprotected overnight in 0.1 M PB containing 20% sucrose before being cut on a cryostat. The 16 μm sections were mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at -20°C before being analyzed by immunocytochemistry.
Immunocytochemistry
Sections from TRPM5-GFP mice were rinsed in phosphate buffered saline (PBS) pH 7.2 and then blocked for 1 hour with 10% normal goat serum. Primary antibodies to gustducin (Rb anti-α-gustducin (1:1,000, Santa Cruz Biotech, Santa Cruz, CA) were then applied overnight at 4°C. The following day, sections were rinsed in PBS and then incubated with goat anti-Rb Alexa 568 (Molecular Probes, Invitrogen Corporation, Carlsbad, CA) secondary antibodies for 2 hours. Sections were then rinsed in PBS and mounted with Fluoromount G (Southern Biotech, Birmingham, AL).
Wholemount immunocytochemistry
Mice expressing GFP under control of the TRPM5 promoter were perfused with 4% PFA as described above and the heads post-fixed for 30 minutes. The head was then split down the midline and the anterior nasal epithelium dissected in 0.1 M PB. Nasal tissue was then rinsed well with PBS, blocked for 45 minutes with 10% NGS, and incubated with the primary antibody (Rb anti-PGP9.5 [1:1,000, AbD Serotec, Raleigh, NC]) overnight at room temperature on a shaker table. The following day tissue was rinsed well with PBS and then incubated with the secondary antibody goat anti-Rb Alexa 568 (1:400, Molecular Probes, Invitrogen Corporation, Carlsbad, CA). Tissue was then rinsed well in PBS, spread flat on slides, and mounted with Fluoromount G (Southern Biotech, Birmingham, AL).
Confocal Microscopy
Immunofluorescence of labeled SCCs was imaged on an Olympus Fluoview laser scanning confocal microscope (Olympus America Inc., Melville, NY) using 20× (0.80 n.a.) and 60× (1.4 n.a.) oil immersion lenses. Optical sections (0.8 μm) were acquired through each field of view and then compiled into a z-stack. Sequential scanning of the two separate fluorescence channels avoided “bleed through” of signal from the inappropriate channel.
Analysis
Analysis and generation of traces was performed using OriginPro 7.5 software. Responses were defined as increases in intracellular calcium greater than two standard deviations (SD) from baseline (calculated as the average ratio during the 50-100 seconds preceding stimulus application) during stimulus presentation. Off responses were defined as responses elicited upon removal of the stimulus.
Responses were considered completely blocked if they were reduced to below 2 SD from baseline. Responses reduced by the block but still above 2 SD from baseline are reported as the percentage of the original response left after block.
For the most part, traces show all points and are unaltered. However, in some traces single points greater than two standard deviations from baseline due to fluctuations in the light source were omitted. Omitted points had to be noted as light fluctuation during the experiment, occur during a non-stimulation time, and be at least 2 SD from baseline to be considered for omission. Only points that significantly detracted from the visual quality of the trace were omitted. No points were omitted during stimulus application.
To assess if the number of GFP+ cells responding to a particular stimulus was significantly different from the number of non-GFP cells responding to that stimulus, a chi-square test was used. The GraphPad online statistical calculator (http://www.graphpad.com/quickcalcs/index.cfm) was used to perform this analysis.
Results
Identification of Nasal SCCs
To identify SCCs, we utilized two strains of transgenic mice in which GFP expression is driven by promoters upstream of two components of the transduction cascade; gustducin or TRPM5. Dissociation of the anterior nasal epithelium (area inside dotted line in Fig.1A) from either strain yielded numerous isolated non-GFP epithelial cells, and a smaller number of GFP+ cells. Acutely isolated GFP+ cells were located either in small clumps of tissue or were completely isolated (Fig.2). In agreement with Lin et al. (2007) and Kaske et al. (2007) we find numerous TRPM5-GFP+ cells in the nasal epithelium of mice (Fig.1B). Also in agreement with Lin et al. (2007) we find that gustducin-expressing cells constitute a subset of the TRPM5-expressing population of cells in the nasal cavity. In sections of the area of nasal epithelium from the vestibule to the posterior end of the vomeronasal organ (the area of respiratory epithelium isolated in this study), we find that approximately two-thirds (417/624) of the TRPM5-GFP+ cells are immunoreactive for α-gustducin while over 90% (417/446) of α-gustducin immunoreactive cells are TRPM5-GFP+ (Fig.1C-E). Lin et al (2008) reported that in the nasal cavity as a whole, approximately 20% of TrpM5 cells express gustducin. Accordingly, the population at the anterior end of the nasal cavity that we sampled for physiological recordings represent a specialized subset of the entire TrpM5+ population of SCCs. In keeping with these findings, more GFP+ cells could be isolated from TRPM5-GFP mice than from gustducin-GFP mice, reflecting the larger population of TRPM5+ chemosensory cells in the nasal cavity (Kaske et al. 2007; Lin et al. 2007).
Figure 1. Solitary chemoreceptor cells (SCCs) in the anterior nasal cavity of mice are identified by expression of TRPM5 or gustducin.
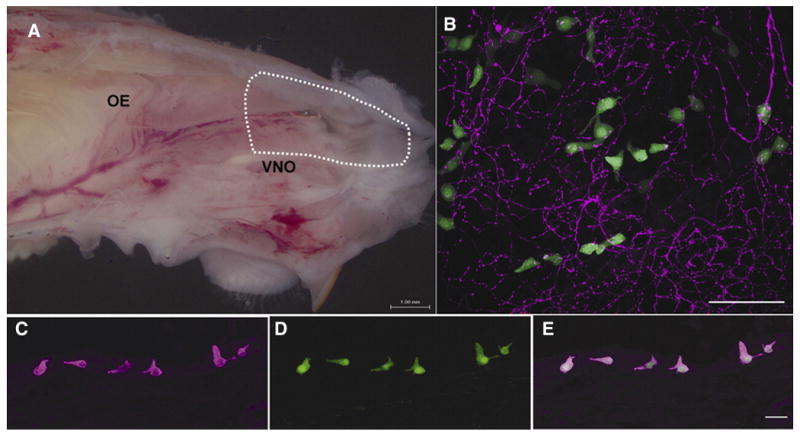
A. Hemisection of a mouse head (septum removed) revealing the nasal cavity. The area of epithelium dissected and dissociated to isolate GFP+ cells is indicated by the dotted line. OE = olfactory epithelium. VNO = vomeronasal organ. B. Whole mount preparation of nasal respiratory epithelium from a TRPM5-GFP transgenic mouse showing TRPM5-GFP+ cells in green and PGP9.5-immunoreactive trigeminal nerve fibers in red. Scale bar = 50 μm. C. In the anterior nasal cavity most gustducin immunoreactive cells (magenta) also express TRPM5-GFP (green)(D.). E. Overlay. Scale bar = 20 μm.
Figure 2. While all isolated cells load with Fura-2, only SCCs display GFP fluorescence.
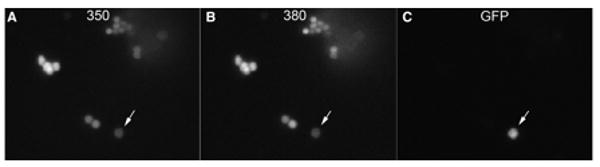
A. All isolated epithelial cells show Fura-2 fluorescence at 350 nm. B. Fura-2 fluorescence is detected in all isolated cells at 380 nm. C. Only SCCs express GFP fluorescence (arrow). Images taken with at 40× oil immersion lens.
Ca2+ responses in TRPM5-GFP cells
To investigate which component of nasal trigeminal chemosensitivity is mediated through SCCs we applied a variety of classic bitter and trigeminal stimuli and recorded relative intracellular Ca2+ levels ([Ca2+]i) in TRPM5- and gustducin-GFP+ cells (Tables 1 and 2). For most stimuli tested, TRPM5-GFP+ cells do not increase [Ca2+]i greater than two standard deviations above baseline (see Table 1). Rare TRPM5-GFP+ cells respond to the bitter compounds cycloheximide (1/38 GFP+ cells) and salicin (1/18 GFP+ cells).
Table 1. List of stimuli tested on cells from TRPM5-GFP mice.
Total numbers of TRPM5-GFP+ and non-GFP cells tested are indicated as well as the fraction responding. Responses to denatonium and phenylthiocarbamide (PTC) were suspected to be mediated through a T2R signaling cascade involving PLC. Therefore, the ability of responses to denatonium and PTC to be blocked with U73122 (a PLC inhibitor) was tested and the resulting fraction blocked is indicated. Abbreviations: PTC = phenylthiocarbamide, ATP = adenosine 5′-triphosphate, 3M3FBS = N-(3-trifluoromethylphenyl)-2,4,6-trimethylbenzenesulfonamide, PROP = 6-propyl-2-thiouracil.
| Chemical | Total Number TRPM5- GFP Cells Tested |
Number TRPM5- GFP Responding |
Percentage TRPM5-GFP Responding |
Fraction Blocked by U73122 |
Total Number Non-GFP Cells Tested |
Number Non-GFP Responding |
Percentage Non-GFP Responding |
Fraction Blocked by U73122 |
|---|---|---|---|---|---|---|---|---|
| TC, 15 mM | 30 | 27 | 90.0 | 0/6 | 12 | 11 | 91.7 | 1/3 |
| Denatonium, 15 mM | 125 | 66 | 52.8 | 14/14 | 56 | 9 | 16.1 | 1/1 |
| Cycloheximide, 0.1–20 mM | 38 | 1 | 2.6 | 7 | 0 | 0.0 | ||
| Salicin, 15 mM | 18 | 1 | 5.6 | 5 | 0 | 0.0 | ||
| Caffeine, 50 mM | 20 | 0 | 0.0 | 9 | 0 | 0.0 | ||
| Nicotine, 1–10 mM | 14 | 0 | 0.0 | 16 | 5 | 31.3 | ||
| Capsaicin, 10–100 μM | 15 | 1 | 6.7 | 18 | 3 | 16.7 | ||
| Menthol, 0.1–1 mM | 15 | 2 | 13.3 | 1 | 0 | 0.0 | ||
| Acetylcholine, 10 μM | 30 | 8 | 26.7 | 6 | 0 | 0.0 | ||
| ATP, 10–20 μM | 41 | 12 | 29.3 | 11 | 4 | 36.4 | ||
| PROP, 15 mM | 5 | 2 | 40.0 | 0 | 0 | 0.0 |
Table 2. Percent of GFP+ cells responding to denatonium in the different mouse strains used.
Histological analysis shows that about 2/3 of the TRPM5+ cells express gustducin, so the values in the table are to be expected if only the gustducin+ cells are the ones responding to denatonium (2/3 × 76.6% = 51.2%).
| Mouse Type | Percentage of Cells Responding to Denatonium | Number of Cells Tested |
|---|---|---|
| TRPM5-GFP | 52.8 | 125 |
| Gustducin-GFP | 76.6 | 47 |
Likewise, rare TRPM5-GFP+ cells respond to the trigeminal stimulants capsaicin (1/15 GFP+ cells) (see Fig.3) and menthol (2/15 GFP+ cells). These rare responses are small (just above 2 SD from baseline), slow (gradual increase in Ca2+), and cells did not usually recover (return to the baseline level before stimulus application). The classic bitter compound phenylthiocarbamide (PTC) elicits larger increases in intracellular Ca2+ in TRPM5-GFP+ cells (Fig.3). However, these increases in Ca2+ are relatively gradual and are not blocked by the PLC inhibitor U73122 (2 μM) (Fig.3) suggesting a non T2R mechanism. The number of TRPM5-GFP+ cells responding to the above stimuli is not significantly different from the number of non-GFP cells responding (P>0.05, chi-square test). Therefore, responses to these compounds are not specific to TRPM5-GFP+ cells and do not act through a PLC cascade. TRPM5-GFP+ cells also respond to acetylcholine (ACh) (8/30 GFP+ cells) and ATP (12/41 GFP+ cells) (Fig.4). However, the fraction of TRPM5-GFP+ cells responding to ATP and ACh is not significantly different than the fraction of non-GFP cells responding (P>0.05, chi-square test), indicating that ATP and ACh are not specifically detected by SCCs.
Figure 3. Responses to 15 mM PTC in TRPM5-GFP+ cells are more gradual than responses to 15 mM denatonium.
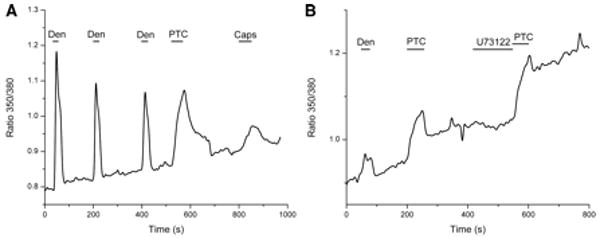
A. A TRPM5-GFP+ cell responds robustly and quickly to 15 mM denatonium and although the cell also responds to 15 mM PTC, the response appears slower and more gradual. In this cell the first denatonium response peaked within 6.21 sec. after application of denatonium. However, the PTC response in this cell took 50.68 sec. to peak. This particular cell was a rare case since it also gave a small response to 100 μM capsaicin. The capsaicin response is also more gradual than the response to denatonium. B. PTC responses are not blocked by the PLC inhibitor U73122.
Figure 4. ATP responses in TRPM5-GFP+ cells.
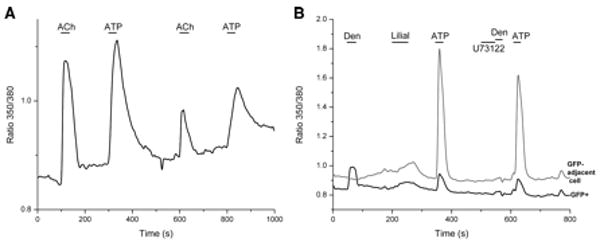
A. A TRPM5-GFP+ cell responds with increased intracellular Ca2+ to 10 μM ACh and 10 μM ATP. B. A TRPM5-GFP+ cell responsive to 15 mM denatonium also responds to 10 μM ATP but not to 500 μM (an irritatingly high concentration) lilial. The adjacent cell (GFP-, gray trace) only responded to ATP. The PLC inhibitor U73122 (2 μM) blocked the denatonium response in the GFP+ cell completely but did not block the ATP responses in either cell.
When exposed to the bitter compound denatonium benzoate, TRPM5-GFP+ cells rapidly (sharp increase in [Ca2+]i within 5 seconds of application) and robustly increase [Ca2+]i levels (66/125 GFP+ cells) (Fig.5). Denatonium-evoked Ca2+ responses reach a peak more rapidly (P<0.05, t-test) than PTC-evoked Ca2+ responses in SCCs (11.52 ± 2.22 sec. for denatonium vs. 61.88 ± 10.68 sec. for PTC when time of response was measured for the two compounds in the same cell (n = 4)). Denatonium-evoked Ca2+ responses in TRPM5-GFP+ cells range in size from 4% to 50% increases in relative Ca2+ levels over baseline. The fraction of TRPM5-GFP+ cells responding to denatonium is significantly higher than the fraction of non-GFP cells responding (9/56) (P<0.0001, chi-squared test). Denatonium responses in TRPM5-GFP+ cells are blocked (reduced Ca2+ increases to below 2 SD from baseline) by the PLC inhibitor U73122 (2 μM) (complete blocks in 14/14 GFP+ cells) (Fig.5A) indicating that denatonium is most likely acting through a T2R-PLC signaling cascade. The inactive analog of this drug (U73343) did not block denatonium responses (data not shown). Denatonium increases intracellular Ca2+ in TRPM5-GFP+ cells in a dose dependent manner with an EC50 of approximately 5.15 mM (Fig.5C).
Figure 5. TRPM5-GFP+ cells from the anterior nasal cavity respond specifically to denatonium benzoate through a PLC-mediated pathway.
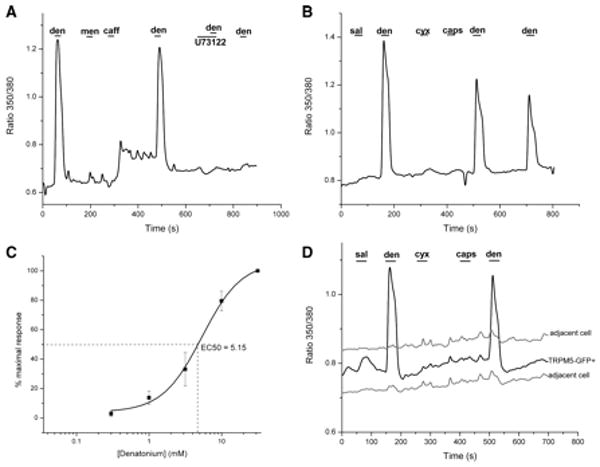
A. TRPM5-GFP+ cells respond with a rapid increase in intracellular calcium to 15 mM denatonium (den) but not to the trigeminal stimulants menthol (men) (0.1 mM) and caffeine (caff) (50 mM) (although this cell gave an off response to caff). Denatonium responses in TRPM5-GFP+ cells are completely blocked by the PLC inhibitor U73122 (2 μM). B. TRPM5-GFP+ cells respond to denatonium (den) but not to the bitter compounds salicin (sal) (15 mM) or cycloheximide (cyx) (1 mM). TRPM5-GFP+ cells also do not respond to capsaicin (caps) (10 μM). C. Dose response curve for denatonium in TRPM5-GFP+ cells. For each point n=5 and error bars show SEM. D. The detection of denatonium is specific to TRPM5-GFP+ cells as adjacent non-GFP epithelial cells do not respond.
Ca2+ responses in Gustducin-GFP cells
Gustducin-GFP+ cells also respond robustly and specifically to denatonium (Fig.6). Since gustducin-GFP+ cells express T2R receptors (Finger et al. 2003) and are a subset of the TRPM5 expressing population of nasal cells (Lin et al. 2007), it was expected that a larger percentage of gustducin-GFP+ cells would respond to denatonium than TRPM5-GFP+ cells. In agreement, 46.4% (66/125) of TRPM5-GFP cells respond to denatonium while 76.6% (36/47) gustducin-GFP+ cells respond to denatonium (Table 2). The number of gustducin-GFP+ cells responding to denatonium (36/47) is significantly higher (P<0.001, chi-squared test) than the number of non-GFP cells responding to denatonium (0/7). Denatonium responses in gustducin-GFP+ cells, like those in TRPM5-GFP+ cells, are blocked by the PLC inhibitor U73122 (2 μM) (Fig.6). In 9 of 13 GFP+ cells, the denatonium response was completely blocked. On average (all denatonium-responsive gustducin-GFP cells where U73122 was tested), U73122 reduced the denatonium response to 5.31 ± 4.04% of the original peak value. Thus denatonium activates SCCs via a PLC-mediated signalling cascade.
Figure 6. Responses to 15 mM denatonium in Gustducin-GFP+ cells.
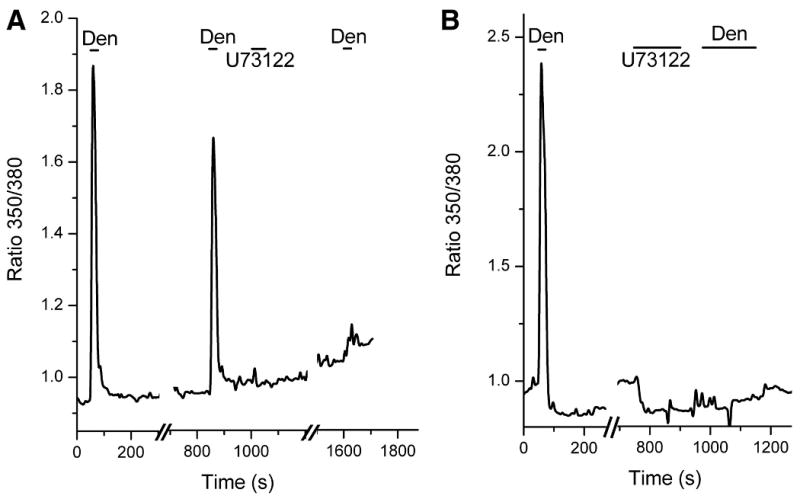
A. A gustducin-GFP+ cell responds with a robust increase in [Ca2+]i when challenged with 15 mM denatonium. The denatonium response in gustducin-GFP+ cells is mediated by a PLC signal cascade since it is blocked by 2 μM U73122. B. Another example of a denatonium response in a gustducin-GFP+ cell. The response in this cell was also blocked by 2 μM U73122.
Discussion
SCCs were first characterized in the nasal cavity of rodents by Finger et al. (2003) who demonstrated that SCCs express T2R “bitter” taste receptors and that the ethmoid nerve responds when the bitter stimuli cyclocheximide, quinine, or denatonium is presented in the nasal cavity. Therefore it was hypothesized that SCCs mediate trigeminal responses to bitter tasting compounds. Our results partially support this hypothesis by demonstrating that isolated TRPM5-GFP+ or gustducin-GFP+ cells in the nasal cavity respond to the bitter compound denatonium. However, out of all the bitter stimuli tested, SCCs reliably responded only to denatonium through a PLC cascade. The ethmoid responses to denatonium in the rat nasal cavity recorded by Finger et al. (2003) were not as large as those for cycloheximide and quinine so it was surprising that denatonium evoked the best responses in SCCs. It is possible that trigeminal responses to cycloheximide and quinine are mediated by activation of trigeminal nerve fibers themselves. Liu and Simon (1998) demonstrated that cultured rat trigeminal ganglion cells are able to respond to several bitter stimuli; quinine and strychnine elicited calcium increases in 22% and 18% of cultured trigeminal ganglion cells respectively, while only 5% responded to denatonium. Therefore, denatonium responses may be more specific to SCCs and not mediated by broadly responsive nerve fibers.
Lin et al. (2007) recently demonstrated that the anterior nasal epithelium and TRPM5-expressing nasal SCCs respond to high concentrations of several odorants. We did not observe broad responsiveness of SCCs to multiple irritating stimuli. One possible explanation for the apparent discrepancy between the findings of Lin et al. (2007) and the current study is that the larger population of TRPM5-expressing SCCs exhibits a broad detection range for irritants while the gustducin-expressing subpopulation of SCCs is specifically tuned to detect a narrow range of relevant stimuli.
Our results demonstrate that SCCs are sensitive to denatonium benzoate in the same concentration range as taste receptor cells in mice (Boughter et al. 2005; Caicedo et al. 2003; Damak et al. 2006; Finger et al. 2005; Ruiz et al. 2003) and hamsters (Frank et al. 2004). Since mT2R8 is a denatonium receptor (Chandrashekar et al. 2000) and is expressed by SCCs (Finger et al. 2003), our results support the hypothesis that mT2R8 is mediating the response to denatonium in SCCs.
We find that cycloheximide does not stimulate most SCCs. Cycloheximide was ineffective at stimulating most SCCs with concentrations ranging from 0.1 mM to as high as 20 mM. At this extremely high concentration, cells showed slow large decreases in intracellular calcium or died. This correlates with in situ hybridization results by Finger et al. (2003) which failed to demonstrate the mouse T2R responsible for detecting cycloheximide (mT2R5) in nasal SCCs. Therefore it is possible that cycloheximide acts through some nonspecific mechanism that activates nerve fibers in the rat nasal cavity. It is also possible that rat SCCs express different T2R receptors than mice. Since the nerve recordings were made in rat and in situ hybridization performed on mouse tissue by Finger et al. (2003) it is not known if mice and rats should be expected to express similar nasal T2Rs.
Other T2R mediated compounds tested are also ineffective at stimulating SCC receptor pathways. Phenylthiocarbamide (PTC) and the related 6-propyl-2-thiouracil (PROP) cause increases in intracellular calcium in SCCs but this effect appears to be through some nonspecific mechanism since all epithelial cells respond in a similar manner and the response is not blocked by a PLC inhibitor. Responses to PTC and PROP are also slower than what is usually reported for receptor mediated events. Salicin, which is known to activate a human T2R, did not stimulate SCCs.
Since SCCs do not respond to many bitter compounds and seem to be relatively narrowly tuned, they may express a limited number of T2Rs. It is possible the T2Rs expressed by SCCs are not activated by many classical bitter tastants because they have evolved to detect other classes of compounds. For example, a human T2R, hTAS2R46, responds to a broad array of substances including lactones and diterpenoids, as well as denatonium (Brockhoff et al. 2007) but does not respond to unrelated bitter-tasting substances such as ouabain or salicin.
In conclusion, the principal finding of our study is that not all nasal SCCs are broad detectors of trigeminal irritants, but rather may be “tuned” to detect a narrow range of noxious compounds in the nasal cavity. The only tested compound that both TRPM5-GFP and Gustducin-GFP cells responded to through a PLC-mediated signalling cascade was denatonium benzoate. While SCCs likely mediate trigeminal nerve responses to denatonium, the mechanisms by which the trigeminal nerve responds to other bitter compounds, e.g. cycloheximide, remain enigmatic.
Acknowledgments
We would like to thank Dr. Robert F. Margolskee who generously permitted use of the transgenic mouse strains employed in this study. We would also like to thank Dr. Tatsuya Ogura who developed and demonstrated to us many of the techniques employed in this study.
Grants: NIDCD Grants NRSA DC008275-01, RO1 DC 006070 and P30 DC 04657
Footnotes
Current address for BDG: University of Calgary, Department of Physiology and Biophysics, 3330 Hospital Dr. NW, Calgary, Alberta, T2N 4N1, Canada
Contributor Information
Brian D Gulbransen, Email: bgulbran@ucalgary.ca.
Tod R Clapp, Email: Tod.Clapp@colostate.edu.
Sue C Kinnamon, Email: sckinna@lamar.colostate.edu.
References
- Baraniuk JN, Kaliner M. Neuropeptides and nasal secretion. Am J Physiol. 1991;261:L223–235. doi: 10.1152/ajplung.1991.261.4.L223. [DOI] [PubMed] [Google Scholar]
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2006 doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, Raghow S, Nelson TM, Munger SD. Inbred mouse strains C57BL/6J and DBA/2J vary in sensitivity to a subset of bitter stimuli. BMC Genet. 2005;6:36. doi: 10.1186/1471-2156-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhoff A, Behrens M, Massarotti A, Appendino G, Meyerhof W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J Agric Food Chem. 2007;55:6236–43. doi: 10.1021/jf070503p. [DOI] [PubMed] [Google Scholar]
- Bryant B, Silver WL. Chemesthesis: the common chemical sense. New York: Wiley-Liss; 2000. [Google Scholar]
- Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci. 2003;23:9947–9952. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Finger TE, St Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol. 1990;294:293–305. doi: 10.1002/cne.902940212. [DOI] [PubMed] [Google Scholar]
- Frank ME, Bouverat BP, MacKinnon BI, Hettinger TP. The distinctiveness of ionic and nonionic bitter stimuli. Physiol Behav. 2004;80:421–431. doi: 10.1016/j.physbeh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 2006;34:252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- Hofer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2007 doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Responses of cultured rat trigeminal ganglion neurons to bitter tastants. Chem Senses. 1998;23:125–130. doi: 10.1093/chemse/23.2.125. [DOI] [PubMed] [Google Scholar]
- Merigo F, Benati D, Di Chio M, Osculati F, Sbarbati A. Secretory cells of the airway express molecules of the chemoreceptive cascade. Cell Tissue Res. 2007;327:231–247. doi: 10.1007/s00441-006-0280-7. [DOI] [PubMed] [Google Scholar]
- Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A. alpha-Gustducin immunoreactivity in the airways. Cell Tissue Res. 2005;319:211–219. doi: 10.1007/s00441-004-1007-2. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Gomez G, Cowart B, Restrepo D. The use of olfactory receptor neurons (ORNs) from biopsies to study changes in aging and neurodegenerative diseases. Ann N Y Acad Sci. 1998;855:701–707. doi: 10.1111/j.1749-6632.1998.tb10648.x. [DOI] [PubMed] [Google Scholar]
- Ruiz CJ, Wray K, Delay E, Margolskee RF, Kinnamon SC. Behavioral evidence for a role of alpha-gustducin in glutamate taste. Chem Senses. 2003;28:573–579. doi: 10.1093/chemse/bjg049. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Crescimanno C, Osculati F. Identification and characterization of a specific sensory epithelium in the rat larynx. J Comp Neurol. 2004a;475:188–201. doi: 10.1002/cne.20172. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Osculati F. Laryngeal chemosensory clusters. Chem Senses. 2004b;29:683–692. doi: 10.1093/chemse/bjh071. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Progress in Neurobiology. 2005a;75:295–307. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Prog Neurobiol. 2005b;75:295–307. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Silver WL. Physiological Factors in Nasal Trigeminal Chemoreception. New York: Marcel Dekker, Inc.; 1990. [Google Scholar]
- Silver WL, Mason JR, Adams MA, Smeraski CA. Nasal trigeminal chemoreception: responses to n-aliphatic alcohols. Brain Res. 1986;376:221–229. doi: 10.1016/0006-8993(86)90183-6. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Merigo F, Sbarbati A. Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J Anat. 2006;209:333–337. doi: 10.1111/j.1469-7580.2006.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Ruiz-Avila L, Margolskee RF. Directing gene expression to gustducin-positive taste receptor cells. J Neurosci. 1999;19:5802–5809. doi: 10.1523/JNEUROSCI.19-14-05802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]


