Abstract
Membrane transporters are now recognized as important determinants of the transmembrane passage of drugs. Organic anion transporting polypeptides (OATP) form a family of influx transporters expressed in various tissues important for pharmacokinetics. Of the 11 human OATP transporters, OATP1B1, OATP1B3 and OATP2B1 are expressed on the sinusoidal membrane of hepatocytes and can facilitate the liver uptake of their substrate drugs. OATP1A2 is expressed on the luminal membrane of small intestinal enterocytes and at the blood-brain barrier, potentially mediating drug transport at these sites. Several clinically used drugs have been identified as substrates of OATP transporters (e.g. many statins are substrates of OATP1B1). Some drugs may inhibit OATP transporters (e.g. cyclosporine) causing pharmacokinetic drug–drug interactions. Moreover, genetic variability in genes encoding OATP transporters can result in marked inter-individual differences in pharmacokinetics. For example, a single nucleotide polymorphism (c.521T > C, p.Val174Ala) in the SLCO1B1 gene encoding OATP1B1 decreases the ability of OATP1B1 to transport active simvastatin acid from portal circulation into the liver, resulting in markedly increased plasma concentrations of simvastatin acid and an enhanced risk of simvastatin-induced myopathy. SLCO1B1 polymorphism also affects the pharmacokinetics of many other, but not all (fluvastatin), statins and that of the antidiabetic drug repaglinide, the antihistamine fexofenadine and the endothelin A receptor antagonist atrasentan. This review compiles the current knowledge about the expression and function of human OATP transporters, their substrate and inhibitor specificities, as well as pharmacogenetics.
Keywords: organic anion transporting polypeptide, OATP, OATP1B1, SLCO1B1, repaglinide, statin, simvastatin, transporter, pharmacogenetics
Role of transporters in pharmacokinetics
After an orally administered drug is dissolved, it crosses the intestinal wall, reaches the liver via portal blood flow and subsequently enters the systemic circulation, which distributes the drug to the various tissues of the body (Tozer and Rowland, 2006). The drug is eliminated from the body by metabolism, which occurs mainly in the liver, and by excretion into bile or into urine. During these pharmacokinetic processes, a drug molecule passes through several biological membranes. The extent of drug movement through these membranes is generally affected by the physicochemical properties of a drug, namely size, lipophilicity and charge (or degree of ionization). In addition, membrane transporters have a significant role in facilitating or preventing drug movement (Ho and Kim, 2005).
Transporters may be classified as influx (uptake into cell) and efflux (out of cell) transporters, which are typically located either at the basolateral or apical membrane in polarized cells. Interplay of influx and efflux transporters together with phase I and II metabolism may be required for the sequential traverse of the basolateral and apical membranes (Giacomini and Sugiyama, 2006). For example, in the liver, an uptake transporter such as organic anion transporting polypeptide 1B1 (OATP1B1) may extract its drug substrates from the portal blood into hepatocytes. After metabolism, other drug transporters, such as multidrug resistance protein 1 (MDR1, also known as P-glycoprotein) may be important in efflux of the metabolite from the hepatocyte (Ho and Kim, 2005) (Figure 1). Drug transporters can therefore be regarded as completing the phase I and II enzyme-based detoxification system; drug uptake delivers the drug to the detoxification system to facilitate metabolism, whereas drug efflux decreases the load on detoxification enzymes (Nies et al., 2008).
Figure 1.

Role of transporters affecting hepatic uptake and excretion of drugs and the interplay of hepatic transporters with phase I and phase II metabolism in the hepatic elimination of drugs. BCRP, breast cancer resistance protein; BSEP, bile salt export pump; MDR, multidrug resistance protein; MRP, multidrug resistance-associated protein; NTCP, sodium taurocholate co-transporting polypeptide; OAT, organic anion transporter; OATP, organic anion transporting polypeptide; OCT, organic cation transporter.
Organic anion transporting polypeptides (OATPs)
OATPs are membrane influx transporters that regulate cellular uptake of a number of endogenous compounds and clinically important drugs (Niemi, 2007). The first discovered human member of the OATP/Oatp family was OATP1A2, which was cloned as an ortholog of rat Oatp1a1 (Kullak-Ublick et al., 1995). OATPs/Oatps within the same family share ≥40% amino acid sequence identity and are designated by Arabic numbering (e.g. OATP1) (Hagenbuch and Meier, 2004). Individual subfamilies include OATPs/Oatps with amino acid sequence identities ≥60% and are designated by letters (e.g. OATP1B). Individual gene products (proteins) within the same subfamily are designated by additional Arabic numbering (e.g. OATP1B1).
The human OATP family consists of 11 members: OATP1A2, 1B1, 1B3, 1C1, 2A1, 2B1, 3A1, 4A1, 4C1, 5A1 and 6A1 (Hagenbuch and Meier, 2003; Mikkaichi et al., 2004a; König et al., 2006) (Table 1). Of these, the roles of OATP1B1, 1A2, 1B3 and 2B1 in drug pharmacokinetics are best characterized. OATP1A2 may facilitate the entry of its substrates through the duodenal wall into circulation (Glaeser et al., 2007). OATP1B1, 1B3 and 2B1 are mainly located at the sinusoidal membranes of human hepatocytes and mediate the influx of their substrates from blood into the hepatocytes, and may thus represent an important step preceding elimination of drugs by metabolism or biliary excretion (Niemi, 2007).
Table 1.
Human OATP transporters, their gene names, chromosomal localization and tissue distribution. Modified from Niemi, 2007
| OATP | Gene name | Gene locus | Tissue distribution |
|---|---|---|---|
| OATP1A2 | SLCO1A2 | 12p12 | Brain, kidney, liver, intestine |
| OATP1B1 | SLCO1B1 | 12p12 | Liver |
| OATP1B3 | SLCO1B3 | 12p12 | Liver |
| OATP1C1 | SLCO1C1 | 12p12 | Brain, testis, ciliary body |
| OATP2A1 | SLCO2A1 | 3q21 | Ubiquitous |
| OATP2B1 | SLCO2B1 | 11q13 | Liver, placenta, intestine, heart, skin |
| OATP3A1 | SLCO3A1 | 15q26 | Ubiquitous |
| OATP4A1 | SLCO4A1 | 20q13.1 | Ubiquitous |
| OATP4C1 | SLCO4C1 | 5q21 | Kidney |
| OATP5A1 | SLCO5A1 | 8q13.1 | Unknown |
| OATP6A1 | SLCO6A1 | 5q21 | Testis |
OATP, organic anion transporting polypeptide.
According to computer-based hydropathy analysis, all OATPs share a very similar transmembrane domain organization, with 12 predicted transmembrane domains and a large fifth extracellular loop (Hagenbuch and Meier, 2003) (Figure 2; OATP1B1). Based on a comparative analysis of OATPs from multiple species, the transport of all OATPs/Oatps has been suggested to occur through a central, positively charged pore in a so-called rocker-switch type of mechanism (Meier-Abt et al., 2005). However, the exact transport mechanism of OATPs/Oatps has not been established (Mahagita et al., 2007).
Figure 2.
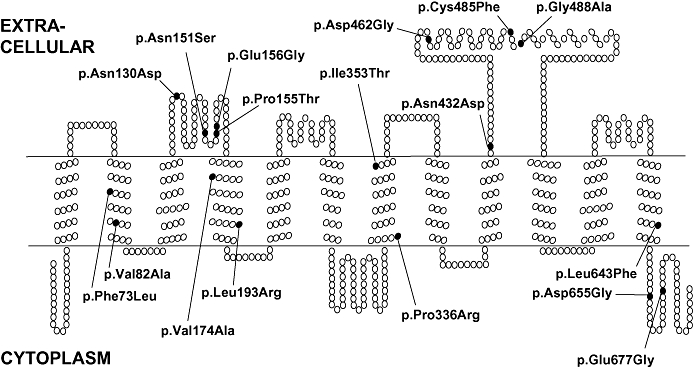
Schematic representation of the secondary structure of human organic anion transporting polypeptide 1B1, depicting the positions of known amino acid exchanges. Reprinted from Kalliokoski, 2008 with permission of the copyright holder.
OATPs are encoded by genes of the SLCO family (previously SLC21) (Hagenbuch and Meier, 2004). The genes encoding human OATP1 family members are located in the short arm of chromosome 12, whereas genes encoding other OATPs are located in chromosomes 3, 5, 8, 11, 15 and 20. Numerous sequence variations, such as single nucleotide polymorphisms (SNPs), have been identified in SLCO genes (Tirona et al., 2001; Niemi et al., 2004; Lee et al., 2005; Niemi, 2007). These polymorphisms may lead to significant consequences on drug pharmacokinetics, for example by decreasing uptake activity of the corresponding OATP (König et al., 2006; Seithel et al., 2008).
Many studies investigating drug–drug interactions have focused on inhibition or induction of drug-metabolizing enzymes, most notably those of the cytochrome P450 (CYP) families. However, it has become evident that significant drug–drug interactions may result from inhibition and probably also from induction of transporter function (Ho and Kim, 2005). The estimation of the role of a single transporter in drug–drug interaction may be challenging, since, for example, many OATP1B1 substrates are also substrates of other drug transporters and often subject to metabolism by CYP enzymes (Smith et al., 2005a; Kivistö and Niemi, 2007).
OATP1B1
OATP1B1 (previously known as OATP2, OATP-C and LST-1) is mainly expressed on the sinusoidal membrane of human hepatocytes (Abe et al., 1999; Hsiang et al., 1999; König et al. 2000a). SLCO1B1 mRNA has been detected also in other tissues, including small intestinal enterocytes (Glaeser et al., 2007). In vitro, OATP1B1 has been shown to transport both unconjugated and conjugated bilirubin (Cui et al., 2001; Briz et al., 2003, 2006), although in one study, no differences in bilirubin transport existed between OATP1B1-transfected and non-transfected cells (Wang et al., 2003). Other endogenous OATP1B1 substrates include bile acids (cholate and taurocholate), conjugated steroids (estradiol-17β-glucuronide, estrone-3-sulfate and dehydroepiandrosterone-3-sulfate), eicosanoids (leukotrienes C4 and E4, prostaglandin E2 and thromboxane B2) and thyroid hormones (thyroxine and triiodothyronine) (Abe et al., 1999; Hsiang et al., 1999; König et al., 2000a; Tamai et al., 2000; Cui et al., 2001). Examples of in vitro OATP1B1 drug substrates include several HMG-CoA reductase inhibitors, or statins, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor antagonists (Table 2).
Table 2.
Drug substrates for OATP1B1, OATP1A2, OATP1B3 and OATP2B1
–, not provided; Km, Michaelis-Menten kinetic constant; OATP, organic anion transporting polypeptide.
SLCO1B1 (OATP1B1) pharmacogenetics
The OATP1B1 protein consisting of 691 amino acids is encoded by the SLCO1B1 gene (Abe et al., 1999; Hsiang et al., 1999) (Figure 2). A large number of SNPs and other sequence variations have been described in the SLCO1B1 gene, and their allele frequencies vary markedly between different populations (Tirona et al., 2001; Niemi, 2007; Pasanen et al., 2008). For example, the c.388A > G (p.Asn130Asp) SNP is quite common in all populations, with an allele frequency ranging from ∼40% in Europeans to ∼80% in Sub-Saharan Africans and East Asians, whereas the c.521T > C (p.Val174Ala) SNP, relatively common in Europeans and Asians (allele frequency ∼10–20%), is less frequent in Sub-Saharan Africans (∼2%) (Pasanen et al., 2008).
The two common SLCO1B1 SNPs, c.521T > C (p.Val174Ala) and c.388A > G (p.Asn130Asp), together form four functionally distinct haplotypes: SLCO1B1*1A (c.388A-c.521T, reference haplotype), *1B (c.388G-c.521T), *5 (c.388A-c.521C) and *15 (c.388G-c.521C) (Tirona et al., 2001; Nozawa et al., 2002; Nishizato et al., 2003; Niemi et al., 2004). The SLCO1B1*15 haplotype can be further subclassified on the basis of two promoter SNPs, g.-11187G > A and g.-10499A > C, forming the *15 (GAGC), *16 (GCGC), and *17 (AAGC) haplotypes (Niemi et al., 2004). The haplotype frequencies for the SLCO1B1*1A, *1B, *5, *15, *16 and *17 were 52, 27, 2.7, 2.4, 7.9 and 6.9%, respectively, in a population of 468 healthy Finns genotyped for 11 SLCO1B1 SNPs (Pasanen et al., 2006b).
SLCO1B1*5 and SLCO1B1*15 haplotypes have been associated with reduced transport activity of OATP1B1 in vitro in studies performed with several OATP1B1 substrates in different cell lines (Kameyama et al., 2005; Nozawa et al., 2005). The SLCO1B1*1B haplotype has, however, been associated with increased OATP1B1 transport activity in vitro in studies performed with bromosulfophthalein and estrone-3-sulfate (Michalski et al., 2002; Kameyama et al., 2005), whereas no change or reduced transport activity has been seen in other studies with different substrates (Tirona et al., 2003; Nozawa et al., 2005). The effect of the c.521T > C SNP appears to dominate over that of the c.388A > G SNP, as the SLCO1B1*15 haplotype (including both SNPs) has been consistently associated with reduced transport activity of OATP1B1 (Kameyama et al., 2005). In addition to the SLCO1B1 c.521T > C and c.388A > G SNPs, some of the other non-synonymous SLCO1B1 SNPs have been associated with altered (decreased) transport function of OATP1B1 in vitro (Tirona et al., 2001; Michalski et al., 2002), but their clinical significance is either negligible or unestablished, partly because most of them have low allele frequencies (Niemi, 2007; Pasanen et al., 2008).
Of the endogenous substrates of OATP1B1, only bilirubin transport has been reported to be altered (decreased) in vivo in subjects with the SLCO1B1 c.521T > C variant allele, but the findings have been controversial (Huang et al., 2004, 2005; Ieiri et al., 2004; Ho et al., 2007). More conclusive data are available on the effect of SLCO1B1 polymorphism on the pharmacokinetics of several drugs, notably the meglitinide analog oral antidiabetic drug repaglinide and HMG-CoA reductase inhibitors.
In one study in healthy volunteers, the AUC of 0.25 mg repaglinide was 188% larger in participants with the SLCO1B1 c.521CC than in those with the c.521TT genotype (Niemi et al., 2005b). In subsequent prospective genotype panel studies in healthy volunteers, the AUC of 0.5 mg repaglinide was ∼70% larger in SLCO1B1 c.521CC participants and ∼30% lower in SLCO1B1*1B/*1B (c.388GG-c.521TT) participants than in SLCO1B1*1A/*1A (c.388AA-c.521TT) participants (Kalliokoski et al., 2008a,c) (Figure 3A). In each study, after repaglinide administration, there was a tendency towards lower blood glucose concentrations in c.521CC participants than in SLCO1B1*1A/*1A participants and higher blood glucose concentrations in SLCO1B1*1B/*1B participants than in SLCO1B1*1A/*1A participants (Figure 2B), although these differences were not statistically significant. After ingestion of single repaglinide doses ranging from 0.25 to 2 mg, the AUC of repaglinide was 60–110% larger in participants with the c.521CC genotype than in those with the SLCO1B1*1A/*1A genotype (Kalliokoski et al., 2008b). Furthermore, the pharmacokinetics of repaglinide proved to be linear as a function of repaglinide dose in both SLCO1B1 genotype groups. Therefore, the effect of SLCO1B1 polymorphism persists over a wide dose range and patients with the c.521CC genotype may be more susceptible, than patients with the c.521TT or c.521TC genotype, for the blood glucose-lowering effect of repaglinide.
Figure 3.
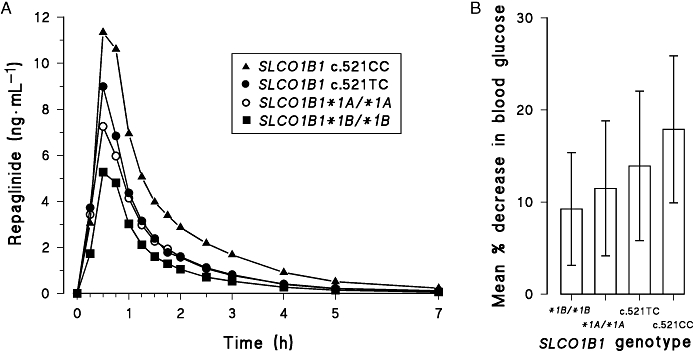
Mean plasma concentrations (A) and mean % decrease (±s.d.) in blood glucose 0–3 h (B) after ingestion of a single oral 0.5 mg dose of repaglinide in healthy participants with the SLCO1B1 c.521CC (n= 4, solid triangles), c.521TC (n= 12, solid circles), *1A/*1A (c.388AA-c521TT; n= 16, open circles) and *1B/*1B (c.388GG-c.521TT; n= 8, solid squares) genotypes. Modified from Kalliokoski et al., 2008a,c;.
Although repaglinide appears to be transported via OATP1B1 in vivo, direct in vitro evidence of this is lacking. In vitro data on the role of OATP1B1 in hepatic uptake are also lacking for nateglinide, the concentrations of which were increased in individuals with the SLCO1B1 c.521TC and c.521CC genotypes when investigated in a small group of healthy Chinese subjects (Zhang et al., 2006). These results could, however, not be reproduced in a larger group of healthy Caucasian volunteers (Kalliokoski et al., 2008c). Moreover, the SLCO1B1*1B/1B genotype did not significantly affect pharmacokinetics of nateglinide (Kalliokoski et al., 2008a). The pharmacokinetics of rosiglitazone and pioglitazone also remained unaffected by the SLCO1B1 c.521T > C SNP in healthy Caucasian subjects (Kalliokoski et al., 2008d), although these drugs are potential OATP1B1 substrates (Nozawa et al., 2004b; Chang et al., 2005).
One of the most studied drugs with respect to the SLCO1B1 polymorphism in vivo in humans is pravastatin, the plasma concentrations of which have been markedly increased in individuals of different ethnic backgrounds carrying one or especially two SLCO1B1 c.521C allelles (Mwinyi et al., 2004; Niemi et al., 2004; Igel et al., 2006; Ho et al., 2007; Deng et al., 2008). In one study, SLCO1B1 c.521CC participants had a 91 and 74% larger pravastatin AUC than those with the c.521TT or c.521TC genotype respectively (Niemi et al., 2006a). Furthermore, the plasma concentrations of active simvastatin acid, pitavastatin, atorvastatin and rosuvastatin have been 221, 162, 144 and 65% higher in c.521CC homozygotes than in c.521TT homozygotes (Pasanen et al., 2006a, 2007; Deng et al., 2008). However, the SLCO1B1 genotype had no significant effect on the pharmacokinetics of fluvastatin (Niemi et al., 2006a).
In addition, the AUCs of atrasentan and fexofenadine have been higher and the non-renal clearance of torsemide has been lower in healthy volunteers with the SLCO1B1 c.521CC genotype than in those with the c.521TT genotype (Niemi et al., 2005a; Katz et al., 2006; Vormfelde et al., 2008). SLCO1B1 genotype may also affect the pharmacokinetics of ezetimibe glucuronide (Oswald et al., 2008).
Data on the relevance of SLCO1B1 polymorphism in the pharmacokinetics of different drugs in patients are emerging. For example, in Asian patients, the SLCO1B1*15 haplotype has been associated with increased concentrations of SN-38, an active metabolite of anticancer drug irinotecan, which may be reflected in a higher risk of toxicity (Xiang et al., 2006; Takane et al., 2007, 2009;Han et al., 2008). The most convincing data on the clinical significance of the SLCO1B1 polymorphism comes from a recent genome-wide association study investigating simvastatin-induced myopathy (Link et al., 2008). More than 300 000 genetic markers were determined in 85 patients who had developed myopathy while receiving 80 mg simvastatin daily and in 90 control patients without myopathy. A non-coding SLCO1B1 SNP, which is in nearly complete linkage disequilibrium with the SLCO1B1 c.521T > C SNP, was the only strong marker associated with myopathy. More than 60% of the myopathy cases could be attributed to the c.521C variant, and the odds ratio for myopathy was 4.5 per copy of the c.521C allele. This association was replicated in a study of 20 000 patients receiving 40 mg simvastatin daily. Furthermore, the c.521C allele was associated with a slightly reduced and the c.388G allele with a slightly increased cholesterol-lowering effect of simvastatin. Therefore, knowledge on the patient's SLCO1B1 genotype might be useful in tailoring of drug therapy.
Role of OATP1B1 in drug–drug interactions
Many drugs have been identified in vitro as OATP1B1 inhibitors (Table 3). There are some in vivo interactions where OATP1B1 inhibition can be regarded as an important mechanism (Table 4). Cyclosporine has increased the plasma concentrations of atorvastatin and several other statins, probably partly due to OATP1B1 inhibition (Neuvonen et al., 2006). Other mechanisms are likely also involved in these interactions since cyclosporine inhibits several influx and efflux transporters, such as OATP1B3, OATP2B1, MDR1 and MRP2, and CYP3A4. However, fluvastatin, pitavastatin, pravastatin and rosuvastatin are not significantly metabolized by CYP3A4, and cyclosporine has increased their mean AUC ∼4–20-fold in organ transplant patients (Regazzi et al., 1993; Park et al., 2001; Hasunuma et al., 2003; Simonson et al., 2004). In addition to statins, cyclosporine has increased the mean AUC of repaglinide ∼2.5-fold in healthy subjects (Kajosaari et al., 2005b). Interestingly, the increase was 42% lower in subjects with the SLCO1B1 c.521TC genotype than in those with the c.521TT genotype. This finding may be explained by the reduced activity of OATP1B1 in carriers of the variant SLCO1B1 c.521C allele. Cyclosporine has also modestly (up to twofold) increased the AUCs of other OATP1B1 substrates, including bosentan, caspofungin and methotrexate (Binet et al., 2000; Fox et al., 2003; Niemi, 2007).
Table 3.
Selected OATP1B1, OATP1A2, OATP1B3 and OATP2B1 inhibitors
Ki provided instead of IC50.
–, not provided; IC50, inhibitor concentration producing 50% inhibition of transporter activity; OATP, organic anion transporting polypeptide; SN-38, an active metabolite of irinotecan.
Table 4.
Examples of the possible involvement of OATP1B1 inhibition in clinical drug-drug interactions
CYP3A4 inhibition also involved.
CYP2C8 inhibition is considered the main mechanism of interaction.
600 mg rifampicin administered as a 30-min iv infusion immediately before atorvastatin administration.
AUC, area under the plasma concentration-time curve; CYP, cytochrome P450; OATP, organic anion transporting polypeptide.
Gemfibrozil has also increased the plasma concentrations of several OATP1B1 substrates (Niemi, 2007). Although gemfibrozil and its 1-O-β-glucuronide metabolite both inhibit OATP1B1 in vitro (Shitara et al., 2004), the glucuronide metabolite is a very potent mechanism-based inhibitor of CYP2C8 in vitro and in vivo (Backman et al., 2002; Wang et al., 2002; Ogilvie et al. 2006; Tornio et al. 2008). Gemfibrozil has increased the AUC of repaglinide, which is metabolized by CYP2C8 and CYP3A4, up to eightfold in healthy subjects (Niemi et al., 2003a), and this interaction has been observed even when the last dose of gemfibrozil was ingested 12 h before repaglinide (Tornio et al., 2008). In a prospective genotype panel study, the mean increase in the repaglinide AUC by gemfibrozil has been ∼50% larger in SLCO1B1 c.521CC participants than in c.521TT participants, but the relative (seven- to eightfold) increases in the repaglinide AUC did not differ significantly between the genotype groups (Kalliokoski et al., 2008e). Gemfibrozil has also increased the AUC of drugs that are not at all or only partly metabolized by CYP2C8, including pravastatin, rosuvastatin and simvastatin, ∼2–3-fold in healthy subjects (Backman et al., 2000; Kyrklund et al., 2003; Schneck et al., 2004).
Rifampicin is a potent inducer of drug-metabolizing enzymes (Niemi et al., 2003b). Rifampicin 600 mg daily administered orally for 5 days has decreased the AUC of repaglinide by ∼60% (Niemi et al., 2000). At high concentrations, rifampicin also inhibits the elimination of repaglinide in vitro and in vivo (Bidstrup et al., 2004; Kajosaari et al., 2005a). In addition to inhibiting CYP enzymes, rifampicin is an inhibitor of OATP1B1 and OATP1B3 in vitro (Vavricka et al., 2002) and also in vivo, if administered intravenously immediately before the ‘victim’ drug. Intravenous rifampicin has increased the AUC of atorvastatin ∼7-fold (Lau et al., 2007), whereas oral treatment with rifampicin over 5 days has decreased the AUC of atorvastatin by ∼80%, probably due to induction of CYP3A4 (Backman et al., 2005). Although rifampicin is a substrate of OATP1B1 (Tirona et al., 2003), the SLCO1B1 genotype has had no effect on the induction of CYP3A4 by rifampicin, as measured by the plasma concentrations of 4β-hydroxycholesterol, an endogenous marker of CYP3A4 activity (Niemi et al., 2006b). These data suggest that OATP1B3 or other uptake transporters can compensate for reduced uptake of rifampicin by OATP1B1. Rifampicin also appears to have a modest inductive effect on OATP1B1 in vitro (Jigorel et al., 2006; Sahi et al., 2006).
In healthy subjects, atorvastatin has slightly increased the AUC of repaglinide in SLCO1B1*1A/*1A participants (by ∼20%) and the Cmax of repaglinide in SLCO1B1*1A/*1A (∼40%) and SLCO1B1 c.521TC (by ∼30%) participants (Kalliokoski et al., 2008e). Atorvastatin had no statistically significant effect on the blood glucose-lowering response to repaglinide, although some subjects experienced low blood glucose concentrations when repaglinide was administered together with atorvastatin. Based on the repaglinide metabolite findings of the study, atorvastatin is not a potent inhibitor of CYP3A4 or CYP2C8 in vivo and the most likely explanation for the effect of atorvastatin on repaglinide pharmacokinetics is inhibition of the OATP1B1-mediated hepatic uptake of repaglinide.
OATP1A2
OATP1A2 (previously known as OATP-A) is expressed in various tissues, including the brain, liver, kidneys and intestine (Kullak-Ublick et al., 1995; Glaeser et al., 2007), although one study found no detectable levels of SLCO1A2 mRNA in the duodenum (Meier et al., 2007). Endogenous substrates of OATP1A2 include bile acids, thyroid hormones, and steroid hormones and their conjugates (Kullak-Ublick et al., 1995, 1998; Bossuyt et al., 1996; Fujiwara et al., 2001). Moreover, OATP1A2 also transports several drugs (Table 2). Some SLCO1A2 (encoding OATP1A2) SNPs have shown decreased in vitro transport activity towards the OATP1A2 substrate estrone-3-sulfate (Lee et al., 2005), but the significance of these findings in vivo in humans is unknown. Several drugs, such as saquinavir, lovastatin, verapamil, dexamethasone and naloxone, have inhibited OATP1A2-mediated substrate uptake in vitro (Kullak-Ublick et al., 1998; Cvetkovic et al., 1999; Gao et al., 2000). Interestingly, also the flavonoids naringin, found in grapefruit juice, and hesperidin, found in orange juice, as well as these juices (at 5% soft drink strength) have inhibited OATP1A2-mediated fexofenadine uptake in vitro (Dresser et al., 2002; Bailey et al., 2007). Moreover, in studies in healthy subjects, the AUC of fexofenadine has been decreased by 25% by ingestion of naringin, and by 40–70% due to ingestion of grapefruit or orange juice (Dresser et al., 2002, 2005; Bailey et al., 2007), consistent with inhibition of OATP1A2 at the apical membrane of enterocytes.
OATP1B3
OATP1B3 (previously known as OATP8 and LST-2) was cloned based on sequence homology to the OATP1B1 (80% amino acid homology), and, similar to OATP1B1, it is mainly expressed on the sinusoidal membrane of human hepatocytes (König et al., 2000b; Abe et al., 2001). Endogenous substrates of OATP1B3 are similar to those of OATP1B1: bilirubin, bile acids, conjugated steroids, eicosanoids and thyroid hormones (König et al., 2000b; Abe et al., 2001; Cui et al., 2001; Kullak-Ublick et al., 2001), but the gastrointestinal peptide cholecystokinin is exclusively transported by OATP1B3 (Ismair et al., 2001). Moreover, the drug substrates of OATP1B3 overlap those of OATP1B1, but OATP1B3 seems to be the only hepatic OATP transporting digoxin, docetaxel and paclitaxel (Table 2). OATP1B3, in contrast to OATP1B1 and OATP2B1, has also been identified in vitro as capable of transporting amanitin, a toxin present in Amanita mushrooms (Letschert et al., 2006). The SLCO1B3 gene (encoding OATP1B3) is polymorphic (Iida et al., 2001), and some sequence variations have been associated with decreased transport activity of OATP1B3 in vitro (Letschert et al., 2004; Schwarz et al., 2006; Smith et al., 2007). In addition to inhibiting OATP1B1, cyclosporine is also an inhibitor of OATP1B3 in vitro (Ho et al., 2006b) and might thus interact with OATP1B3 substrates.
OATP2B1
OATP2B1 (previously OATP-B) is expressed at the sinusoidal membrane of hepatocytes in the liver, but also in other tissues, for example intestine and heart (Tamai et al., 2000; Kullak-Ublick et al., 2001; Kobayashi et al., 2003; Grube et al., 2006). Endogenous substrates of OATP2B1 include dehydroepiandrosterone-3-sulfate, estrone-3-sulfate and prostaglandin E2 (Tamai et al., 2000; Kullak-Ublick et al., 2001). Several drugs are substrates of OATP2B1 (Table 2). There is currently no data on the clinical relevance of SLCO2B1 polymorphism, although some SLCO2B1 sequence variations have been associated with altered transport activity of the protein in vitro (Nozawa et al., 2002; Ho et al., 2006a). Cyclosporine and gemfibrozil inhibit OATP2B1 in vitro (Ho et al., 2006b). In a study where glibenclamide was identified in vitro as a substrate for OATP2B1, grapefruit juice (at a concentration of 5%) significantly inhibited the OATP2B1-mediated uptake of estrone-3-sulfate by 80% (Satoh et al., 2005). However, grapefruit juice has had no effect on the pharmacokinetics of glibenclamide in healthy subjects (Lilja et al., 2007).
Other OATPs
OATP1C1 (previously OATP-F) is expressed in the human brain, testis and ciliary body, and shows a high affinity for thyroid hormones (Pizzagalli et al., 2002; Gao et al., 2005). OATP2A1 (hPGT) is broadly expressed in different tissues and acts as a prostaglandin transporter (Lu et al., 1996), but currently no drugs have been identified as its substrates. OATP3A1 (OATP-D) is expressed in two splice variants, and the shorter variant lacking 18 amino acids in the carboxyl terminus appears to be expressed only in the testis and brain, whereas the longer variant is ubiquitously expressed (Adachi et al., 2003; Huber et al., 2007). OATP3A1 transports estrone-3-sulfate, prostaglandin E2, thyroxine, vasopressin and benzylpenicillin (Tamai et al., 2000; Huber et al., 2007).
OATP4A1 (OATP-E) is ubiquitously expressed and it transports oestrogens, prostaglandins, thyroid hormones, taurocholate, benzylpenicillin and unaprostone (Tamai et al., 2000; Fujiwara et al., 2001; Gao et al., 2005). OATP4C1 (OATP-H) is localized at the basolateral membrane of human proximal tubule cells, and therefore it may mediate the uptake of its substrates from the blood into the kidney (Mikkaichi et al., 2004b). OATP4C1 transports thyroid hormones, digoxin, methotrexate and the antidiabetic drug sitagliptin (Mikkaichi et al., 2004b; Chu et al., 2007). OATP5A1 (OATP-J) is known only at the cDNA level (Hagenbuch and Meier, 2004), and mRNA of SLCO6A1 (OATP-I) has been detected in the testis (Lee et al., 2004).
Conclusion
OATP transporters, especially OATP1B1, 1A2, 1B3 and 2B1, may play important roles in the pharmacokinetics of several drugs. Therefore, genetic variation in OATP-encoding genes and inhibition of OATP function may have clinically significant consequences on drug therapy. The SLCO1B1 c.521T > C (p.Va.174Ala; *5 or *15 haplotype) variant allele is associated with reduced hepatic uptake and increased plasma concentrations of several OATP1B1 substrates, including repaglinide and several statins. The SLCO1B1*1B haplotype appears to be associated with enhanced hepatic uptake and reduced plasma concentrations of OATP1B1 substrates. SLCO1B1 c.521T > C SNP is strongly associated with simvastatin-induced myopathy. Inhibition of OATP1B1 is involved in the drug–drug interactions of cyclosporine, gemfibrozil and rifampicin with OATP1B1 substrates. OATP1A2 may facilitate the intestinal absorption of drugs, such as fexofenadine, and OATP1B3 and 2B1 may be important for hepatic uptake of their substrates drugs.
Glossary
Abbreviations:
- AUC
area under the plasma concentration-time curve
- BCRP
breast cancer resistance protein
- BSEP
bile salt export pump
- Cmax
peak plasma concentration
- CYP
cytochrome P450
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- hPGT
human prostaglandin transporter
- LST
liver-specific transporter
- MDR1
multidrug resistance protein 1
- MRP
multidrug resistance-associated protein
- NTCP
sodium taurocholate co-transporting polypeptide
- OAT
organic anion transporter
- OATP
organic anion transporting polypeptide
- OCT
organic cation transporter
- SLC
solute carrier family
- SLCO
solute carrier organic anion transporter family
- SN-38
active metabolite of irinotecan
- SNP
single nucleotide polymorphism
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120:1689–1699. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M, et al. Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol. 2003;285:F1188–F1197. doi: 10.1152/ajprenal.00402.2002. [DOI] [PubMed] [Google Scholar]
- Arnadottir M, Eriksson LO, Thysell H, Karkas JD. Plasma concentration profiles of simvastatin 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitory activity in kidney transplant recipients with and without ciclosporin. Nephron. 1993;65:410–413. doi: 10.1159/000187521. [DOI] [PubMed] [Google Scholar]
- Åsberg A, Hartmann A, Fjeldsa E, Bergan S, Holdaas H. Bilateral pharmacokinetic interaction between cyclosporine A and atorvastatin in renal transplant recipients. Am J Transplant. 2001;1:382–386. doi: 10.1034/j.1600-6143.2001.10415.x. [DOI] [PubMed] [Google Scholar]
- Bachmakov I, Glaeser H, Fromm MF, König J. Interaction of oral antidiabetic drugs with hepatic uptake transporters: focus on organic anion transporting polypeptides and organic cation transporter 1. Diabetes. 2008;57:1463–1469. doi: 10.2337/db07-1515. [DOI] [PubMed] [Google Scholar]
- Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–691. doi: 10.1067/mcp.2002.128469. [DOI] [PubMed] [Google Scholar]
- Backman JT, Luurila H, Neuvonen M, Neuvonen PJ. Rifampin markedly decreases and gemfibrozil increases the plasma concentrations of atorvastatin and its metabolites. Clin Pharmacol Ther. 2005;78:154–167. doi: 10.1016/j.clpt.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–129. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, et al. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther. 2006;318:521–529. doi: 10.1124/jpet.106.104364. [DOI] [PubMed] [Google Scholar]
- Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- Bidstrup TB, Stilling N, Damkier P, Scharling B, Thomsen MS, Brøsen K. Rifampicin seems to act as both an inducer and an inhibitor of the metabolism of repaglinide. Eur J Clin Pharmacol. 2004;60:109–114. doi: 10.1007/s00228-004-0746-z. [DOI] [PubMed] [Google Scholar]
- Binet I, Wallnöfer A, Weber C, Jones R, Thiel G. Renal hemodynamics and pharmacokinetics of bosentan with and without cyclosporine A. Kidney Int. 2000;57:224–231. doi: 10.1046/j.1523-1755.2000.00838.x. [DOI] [PubMed] [Google Scholar]
- Bossuyt X, Müller M, Meier PJ. Multispecific amphipathic substrate transport by an organic anion transporter of human liver. J Hepatol. 1996;25:733–738. doi: 10.1016/s0168-8278(96)80246-7. [DOI] [PubMed] [Google Scholar]
- Briz O, Serrano MA, Macias RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371:897–905. doi: 10.1042/BJ20030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz O, Romero MR, Martinez-Becerra P, Macias RI, Perez MJ, Jimenez F, et al. OATP8/1B3-mediated cotransport of bile acids and glutathione: an export pathway for organic anions from hepatocytes? J Biol Chem. 2006;281:30326–30335. doi: 10.1074/jbc.M602048200. [DOI] [PubMed] [Google Scholar]
- Campbell SD, de Morais SM, Xu JJ. Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem Biol Interact. 2004;150:179–187. doi: 10.1016/j.cbi.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Cancidas prescribing information Available at: http://www.cancidas.com (accessed June 4, 2008.
- Chang C, Pang KS, Swaan PW, Ekins S. Comparative pharmacophore modeling of organic anion transporting polypeptides: a meta-analysis of rat Oatp1a1 and human OATP1B1. J Pharmacol Exp Ther. 2005;314:533–541. doi: 10.1124/jpet.104.082370. [DOI] [PubMed] [Google Scholar]
- Chen C, Mireles RJ, Campbell SD, Lin J, Mills JB, Xu JJ, et al. Differential interaction of 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos. 2005;33:537–546. doi: 10.1124/dmd.104.002477. [DOI] [PubMed] [Google Scholar]
- Chu XY, Bleasby K, Yabut J, Cai X, Chan GH, Hafey MJ, et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007;321:673–683. doi: 10.1124/jpet.106.116517. [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- Deng JW, Song IS, Shin HJ, Yeo CW, Cho DY, Shon JH, et al. The effect of SLCO1B1*15 on the disposition of pravastatin and pitavastatin is substrate dependent: the contribution of transporting activity changes by SLCO1B1*15. Pharmacogenet Genomics. 2008;18:424–433. doi: 10.1097/FPC.0b013e3282fb02a3. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Kim RB, Bailey DG. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin Pharmacol Ther. 2005;77:170–177. doi: 10.1016/j.clpt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- Fox RI, Morgan SL, Smith HT, Robbins BA, Choc MG, Baggott JE. Combined oral cyclosporin and methotrexate therapy in patients with rheumatoid arthritis elevates methotrexate levels and reduces 7-hydroxymethotrexate levels when compared with methotrexate alone. Rheumatology. 2003;42:989–994. doi: 10.1093/rheumatology/keg277. [DOI] [PubMed] [Google Scholar]
- Franke RM, Baker SD, Mathijssen RH, Schuetz EG, Sparreboom A. Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin Pharmacol Ther. 2008;84:704–709. doi: 10.1038/clpt.2008.94. [DOI] [PubMed] [Google Scholar]
- Fujino H, Saito T, Ogawa S, Kojima J. Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. J Pharm Pharmacol. 2005;57:1305–1311. doi: 10.1211/jpp.57.10.0009. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, et al. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology. 2001;142:2005–2012. doi: 10.1210/endo.142.5.8115. [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- Gao B, Huber RD, Wenzel A, Vavricka SR, Ismair MG, Remé C, et al. Localization of organic anion transporting polypeptides in the rat and human ciliary body epithelium. Exp Eye Res. 2005;80:61–72. doi: 10.1016/j.exer.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Sugiyama Y. Membrane transporters and drug response. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the Pharmacological Basis Of Therapeutics. New York: McGraw-Hill; 2006. pp. 41–70. [Google Scholar]
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- Grube M, Köck K, Oswald S, Draber K, Meissner K, Eckel L, et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006;80:607–620. doi: 10.1016/j.clpt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, et al. Influence of the organic anion-transporting polypeptide 1B1 (OATP1B1) polymorphisms on irinotecan-pharmacokinetics and clinical outcome of patients with advanced non-small cell lung cancer. Lung Cancer. 2008;59:69–75. doi: 10.1016/j.lungcan.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Hasunuma T, Nakamura M, Yachi T, Arisawa N, Fukushima K, Iijima H, et al. The drug-drug interactions of pitavastatin (NK-104), a novel HMG-CoA reductase inhibitor and cyclosporine. J Clin Ther Med. 2003;19:381–389. [Google Scholar]
- Hedman M, Neuvonen PJ, Neuvonen M, Holmberg C, Antikainen M. Pharmacokinetics and pharmacodynamics of pravastatin in pediatric and adolescent cardiac transplant recipients on a regimen of triple immunosuppression. Clin Pharmacol Ther. 2004;75:101–109. doi: 10.1016/j.clpt.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Hermann M, Asberg A, Christensen H, Holdaas H, Hartmann A, Reubsaet JL. Substantially elevated levels of atorvastatin and metabolites in cyclosporine-treated renal transplant recipients. Clin Pharmacol Ther. 2004;76:388–391. doi: 10.1016/j.clpt.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos. 2006;34:1229–1236. doi: 10.1124/dmd.106.009290. [DOI] [PubMed] [Google Scholar]
- Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78:260–277. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Ho RH, Leake BF, Kim RB, Wang Y. OATP2B1 allelic variants differentially transport rosuvastatin in vitro [Abstract. Drug Metab Rev. 2006a;38(Suppl 2):240–241. [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006b;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, et al. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17:647–656. doi: 10.1097/FPC.0b013e3280ef698f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- Huang MJ, Kua KE, Teng HC, Tang KS, Weng HW, Huang CS. Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res. 2004;56:682–689. doi: 10.1203/01.PDR.0000141846.37253.AF. [DOI] [PubMed] [Google Scholar]
- Huang CS, Huang MJ, Lin MS, Yang SS, Teng HC, Tang KS. Genetic factors related to unconjugated hyperbilirubinemia amongst adults. Pharmacogenet Genomics. 2005;15:43–50. doi: 10.1097/01213011-200501000-00007. [DOI] [PubMed] [Google Scholar]
- Huber RD, Gao B, Sidler Pfändler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol. 2007;292:C795–C806. doi: 10.1152/ajpcell.00597.2005. [DOI] [PubMed] [Google Scholar]
- Ichimaru N, Takahara S, Kokado Y, Wang JD, Hatori M, Kameoka H, et al. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis. 2001;158:417–423. doi: 10.1016/s0021-9150(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Ieiri I, Suzuki H, Kimura M, Takane H, Nishizato Y, Irie S, et al. Influence of common variants in the pharmacokinetic genes (OATP-C, UGT1A1, and MRP2) on serum bilirubin levels in healthy subjects. Hepatol Res. 2004;30:91–95. doi: 10.1016/j.hepres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Igel M, Arnold KA, Niemi M, Hofmann U, Schwab M, Lütjohann D, et al. Impact of the SLCO1B1 polymorphism on the pharmacokinetics and lipid-lowering efficacy of multiple-dose pravastatin. Clin Pharmacol Ther. 2006;79:419–426. doi: 10.1016/j.clpt.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Iida A, Saito S, Sekine A, Mishima C, Kondo K, Kitamura Y, et al. Catalog of 258 single-nucleotide polymorphisms (SNPs) in genes encoding three organic anion transporters, three organic anion-transporting polypeptides, and three NADH:ubiquinone oxidoreductase flavoproteins. J Hum Genet. 2001;46:668–683. doi: 10.1007/s100380170019. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Maeda K, Kishimoto W, Saito A, Harada A, Ebner T, et al. Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006;34:1109–1115. doi: 10.1124/dmd.105.009175. [DOI] [PubMed] [Google Scholar]
- Ismair MG, Stieger B, Cattori V, Hagenbuch B, Fried M, Meier PJ, et al. Hepatic uptake of cholecystokinin octapeptide by organic anion-transporting polypeptides OATP4 and OATP8 of rat and human liver. Gastroenterology. 2001;121:1185–1190. doi: 10.1053/gast.2001.28704. [DOI] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34:1756–1763. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- Kajosaari LI, Laitila J, Neuvonen PJ, Backman JT. Metabolism of repaglinide by CYP2C8 and CYP3A4 in vitro: effect of fibrates and rifampicin. Basic Clin Pharmacol Toxicol. 2005a;97:249–256. doi: 10.1111/j.1742-7843.2005.pto_157.x. [DOI] [PubMed] [Google Scholar]
- Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. 2005b;78:388–399. doi: 10.1016/j.clpt.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A. Effects of SLCO1B1 polymorphism on the pharmacokinetics of the oral antidiabetic drugs repaglinide, nateglinide, rosiglitazone, and pioglitazone. Department of Clinical Pharmacology, University of Helsinki, Finland, PhD Thesis.
- Kalliokoski A, Backman JT, Neuvonen PJ, Niemi M. Effects of the SLCO1B1*1B haplotype on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. Pharmacogenet Genomics. 2008a;18:937–942. doi: 10.1097/FPC.0b013e32830d733e. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. The effect of SLCO1B1 polymorphism on repaglinide pharmacokinetics persists over a wide dose range. Br J Clin Pharmacol. 2008b;66:818–825. doi: 10.1111/j.1365-2125.2008.03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics and pharmacodynamics of repaglinide and nateglinide. J Clin Pharmacol. 2008c;48:311–321. doi: 10.1177/0091270007311569. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. No significant effect of SLCO1B1 polymorphism on the pharmacokinetics of rosiglitazone and pioglitazone. Br J Clin Pharmacol. 2008d;65:78–86. doi: 10.1111/j.1365-2125.2007.02986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski A, Backman J, Kurkinen K, Neuvonen P, Niemi M. Effects of Gemfibrozil and Atorvastatin on the Pharmacokinetics of Repaglinide in Relation to SLCO1B1 Polymorphism. Clin Pharmacol Ther. 2008e;84:488–496. doi: 10.1038/clpt.2008.74. [DOI] [PubMed] [Google Scholar]
- Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;15:513–522. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- Katz DA, Carr R, Grimm DR, Xiong H, Holley-Shanks R, Mueller T, et al. Organic anion transporting polypeptide 1B1 activity classified by SLCO1B1 genotype influences atrasentan pharmacokinetics. Clin Pharmacol Ther. 2006;79:186–196. doi: 10.1016/j.clpt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kivistö KT, Niemi M. Influence of drug transporter polymorphisms on pravastatin pharmacokinetics in humans. Pharm Res. 2007;24:239–247. doi: 10.1007/s11095-006-9159-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–708. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- Kopplow K, Letschert K, König J, Walter B, Keppler D. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol. 2005;68:1031–1038. doi: 10.1124/mol.105.014605. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, et al. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109:1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Fisch T, Oswald M, Hagenbuch B, Meier PJ, Beuers U, et al. Dehydroepiandrosterone sulfate (DHEAS): identification of a carrier protein in human liver and brain. FEBS Lett. 1998;424:173–176. doi: 10.1016/s0014-5793(98)00168-9. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–533. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther. 2003;73:538–544. doi: 10.1016/S0009-9236(03)00052-3. [DOI] [PubMed] [Google Scholar]
- Kyrklund C, Backman JT, Kivisto KT, Neuvonen M, Laitila J, Neuvonen PJ. Plasma concentrations of active lovastatin acid are markedly increased by gemfibrozil but not by bezafibrate. Clin Pharmacol Ther. 2001;69:340–345. doi: 10.1067/mcp.2001.115542. [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000a;278:G156–G164. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000b;275:23161–23168. doi: 10.1074/jbc.M001448200. [DOI] [PubMed] [Google Scholar]
- König J, Seithel A, Gradhand U, Fromm MF. Pharmacogenomics of human OATP transporters. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:432–443. doi: 10.1007/s00210-006-0040-y. [DOI] [PubMed] [Google Scholar]
- Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- Lee SY, Williamson B, Caballero OL, Chen YT, Scanlan MJ, Ritter G, et al. Identification of the gonad-specific anion transporter SLCO6A1 as a cancer/testis (CT) antigen expressed in human lung cancer. Cancer Immun. 2004;4:13. [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- Lemahieu WP, Hermann M, Asberg A, Verbeke K, Holdaas H, Vanrenterghem Y, et al. Combined therapy with atorvastatin and calcineurin inhibitors: no interactions with tacrolimus. Am J Transplant. 2005;5:2236–2243. doi: 10.1111/j.1600-6143.2005.01005.x. [DOI] [PubMed] [Google Scholar]
- Letschert K, Keppler D, König J. Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8) Pharmacogenetics. 2004;14:441–452. doi: 10.1097/01.fpc.0000114744.08559.92. [DOI] [PubMed] [Google Scholar]
- Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- Lilja JJ, Niemi M, Fredrikson H, Neuvonen PJ. Effects of clarithromycin and grapefruit juice on the pharmacokinetics of glibenclamide. Br J Clin Pharmacol. 2007;63:732–740. doi: 10.1111/j.1365-2125.2006.02836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, et al. SLCO1B1 variants and statin-induced myopathy – a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- Liu L, Cui Y, Chung AY, Shitara Y, Sugiyama Y, Keppler D, et al. Vectorial transport of enalapril by Oatp1a1/Mrp2 and OATP1B1 and OATP1B3/MRP2 in rat and human livers. J Pharmacol Exp Ther. 2006;318:395–402. doi: 10.1124/jpet.106.103390. [DOI] [PubMed] [Google Scholar]
- Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT) J Clin Invest. 1996;98:1142–1149. doi: 10.1172/JCI118897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, et al. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther. 2006;79:427–439. doi: 10.1016/j.clpt.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takahashi K, Ohtsu N, Oguma T, Ohnishi T, Atsumi R, et al. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007;4:85–94. doi: 10.1021/mp060082j. [DOI] [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSH-bile acid cotransport. Am J Physiol Gastrointest Liver Physiol. 2007;293:G271–G278. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- Mathew P, Cuddy MS, Tracewell WG, Salazar D. An open-label study on the pharmacokinetics (PK) of pitavastatin (NK-104) when administered concomitantly with fenofibrate or gemfibrozil in healthy volunteers [abstract. Clin Pharmacol Ther. 2004;75:33. [Google Scholar]
- Matsushima S, Maeda K, Ishiguro N, Igarashi T, Sugiyama Y. Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2008;36:663–669. doi: 10.1124/dmd.107.017814. [DOI] [PubMed] [Google Scholar]
- Meier Y, Eloranta JJ, Darimont J, Ismair MG, Hiller C, Fried M, et al. Regional distribution of solute carrier mRNA expression along the human intestinal tract. Drug Metab Dispos. 2007;35:590–594. doi: 10.1124/dmd.106.013342. [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Mokrab Y, Mizuguchi K. Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J Membr Biol. 2005;208:213–227. doi: 10.1007/s00232-005-7004-x. [DOI] [PubMed] [Google Scholar]
- Michalski C, Cui Y, Nies AT, Nuessler AK, Neuhaus P, Zanger UM, et al. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem. 2002;277:43058–43063. doi: 10.1074/jbc.M207735200. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Tanemoto M, Ito S, Abe T. The organic anion transporter (OATP) family. Drug Metab Pharmacokinet. 2004a;19:171–179. doi: 10.2133/dmpk.19.171. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci USA. 2004b;101:3569–3574. doi: 10.1073/pnas.0304987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. Evidence for inverse effects of OATP-C (SLC21A6) 5 and 1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther. 2004;75:415–421. doi: 10.1016/j.clpt.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Mück W, Mai I, Fritsche L, Ochmann K, Rohde G, Unger S, et al. Increase in cerivastatin systemic exposure after single and multiple dosing in cyclosporine-treated kidney transplant recipients. Clin Pharmacol Ther. 1999;65:251–261. doi: 10.1016/S0009-9236(99)70104-9. [DOI] [PubMed] [Google Scholar]
- Nakagomi-Hagihara R, Nakai D, Kawai K, Yoshigae Y, Tokui T, Abe T, et al. OATP1B1, OATP1B3, and MRP2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab Dispos. 2006;34:862–869. doi: 10.1124/dmd.105.008888. [DOI] [PubMed] [Google Scholar]
- Nakai D, Nakagomi R, Furuta Y, Tokui T, Abe T, Ikeda T, et al. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J Pharmacol Exp Ther. 2001;297:861–867. [PubMed] [Google Scholar]
- Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8:787–802. doi: 10.2217/14622416.8.7.787. [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495–500. doi: 10.1067/mcp.2000.111183. [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: potentially hazardous interaction between gemfibrozil and repaglinide. Diabetologia. 2003a;46:347–351. doi: 10.1007/s00125-003-1034-7. [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin : clinical relevance. Clin Pharmacokinet. 2003b;42:819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- Niemi M, Kivistö KT, Hofmann U, Schwab M, Eichelbaum M, Fromm MF. Fexofenadine pharmacokinetics are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1) Br J Clin Pharmacol. 2005a;59:602–604. doi: 10.1111/j.1365-2125.2005.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, et al. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther. 2005b;77:468–478. doi: 10.1016/j.clpt.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Niemi M, Pasanen MK, Neuvonen PJ. SLCO1B1 polymorphism and sex affect the pharmacokinetics of pravastatin but not fluvastatin. Clin Pharmacol Ther. 2006a;80:356–366. doi: 10.1016/j.clpt.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Niemi M, Kivistö KT, Diczfalusy U, Bodin K, Bertilsson L, Fromm MF, et al. Effect of SLCO1B1 polymorphism on induction of CYP3A4 by rifampicin. Pharmacogenet Genomics. 2006b;16:565–568. doi: 10.1097/01.fpc.0000215070.52212.0e. [DOI] [PubMed] [Google Scholar]
- Nies AT, Schwab M, Keppler D. Interplay of conjugating enzymes with OATP uptake transporters and ABCC/MRP efflux pumps in the elimination of drugs. Expert Opin Drug Metab Toxicol. 2008;4:545–568. doi: 10.1517/17425255.4.5.545. [DOI] [PubMed] [Google Scholar]
- Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73:554–565. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- Noe J, Portmann R, Brun ME, Funk C. Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007;35:1308–1314. doi: 10.1124/dmd.106.012930. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004a;308:438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005;33:434–439. doi: 10.1124/dmd.104.001909. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, et al. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther. 2002;302:804–813. doi: 10.1124/jpet.302.2.804. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Sugiura S, Nakajima M, Goto A, Yokoi T, Nezu J, et al. Involvement of organic anion transporting polypeptides in the transport of troglitazone sulfate: implications for understanding troglitazone hepatotoxicity. Drug Metab Dispos. 2004b;32:291–294. doi: 10.1124/dmd.32.3.291. [DOI] [PubMed] [Google Scholar]
- Ogilvie BW, Zhang D, Li W, Rodrigues AD, Gipson AE, Holsapple J, et al. Glucuronidation converts gemfibrozil to a potent, metabolism-dependent inhibitor of CYP2C8: implications for drug-drug interactions. Drug Metab Dispos. 2006;34:191–197. doi: 10.1124/dmd.105.007633. [DOI] [PubMed] [Google Scholar]
- Olbricht C, Wanner C, Eisenhauer T, Kliem V, Doll R, Boddaert M, et al. Accumulation of lovastatin, but not pravastatin, in the blood of cyclosporine-treated kidney graft patients after multiple doses. Clin Pharmacol Ther. 1997;62:311–321. doi: 10.1016/S0009-9236(97)90034-5. [DOI] [PubMed] [Google Scholar]
- Oswald S, Konig J, Lutjohann D, Giessmann T, Kroemer HK, Rimmbach C, et al. Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharmacogenet Genomics. 2008;18:559–568. doi: 10.1097/FPC.0b013e3282fe9a2c. [DOI] [PubMed] [Google Scholar]
- Park JW, Siekmeier R, Lattke P, Merz M, Mix C, Schuler S, et al. Pharmacokinetics and pharmacodynamics of fluvastatin in heart transplant recipients taking cyclosporine A. J Cardiovasc Pharmacol Ther. 2001;6:351–361. doi: 10.1177/107424840100600404. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Neuvonen PJ, Niemi M. Global analysis of genetic variation in SLCO1B1. Pharmacogenomics. 2008;9:19–33. doi: 10.2217/14622416.9.1.19. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006a;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Backman JT, Neuvonen PJ, Niemi M. Frequencies of single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide 1B1 SLCO1B1 gene in a Finnish population. Eur J Clin Pharmacol. 2006b;62:409–415. doi: 10.1007/s00228-006-0123-1. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–733. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol. 2002;16:2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- Regazzi MB, Iacona I, Campana C, Raddato V, Lesi C, Perani G, et al. Altered disposition of pravastatin following concomitant drug therapy with cyclosporin A in transplant recipients. Transplant Proc. 1993;25:2732–2734. [PubMed] [Google Scholar]
- Sahi J, Sinz MW, Campbell S, Mireles R, Zheng X, Rose KA, et al. Metabolism and transporter-mediated drug-drug interactions of the endothelin-A receptor antagonist CI-1034. Chem Biol Interact. 2006;159:156–168. doi: 10.1016/j.cbi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Sandhu P, Lee W, Xu X, Leake BF, Yamazaki M, Stone JA, et al. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab Dispos. 2005;33:676–682. doi: 10.1124/dmd.104.003244. [DOI] [PubMed] [Google Scholar]
- Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, et al. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–523. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- Schneck DW, Birmingham BK, Zalikowski JA, Mitchell PD, Wang Y, Martin PD, et al. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2004;75:455–463. doi: 10.1016/j.clpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Schwarz UI, Dixit SG, Leake BF, Kim RB. Identification and functional characterization of OATP1B3 allelic variants using transfected HeLa cells [Abstract. Drug Metab Rev. 2006;38(Suppl 2):237. [Google Scholar]
- Seithel A, Glaeser H, Fromm MF, König J. The functional consequences of genetic variations in transporter genes encoding human organic anion-transporting polypeptide family members. Expert Opin Drug Metab Toxicol. 2008;4:51–64. doi: 10.1517/17425255.4.1.51. [DOI] [PubMed] [Google Scholar]
- Seithel A, Eberl S, Singer K, Auge D, Heinkele G, Wolf NB, et al. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos. 2007;35:779–786. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, et al. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–1481. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Hirano M, Sato H, Sugiyama Y. Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: analysis of the mechanism of the clinically relevant drug-drug interaction between cerivastatin and gemfibrozil. J Pharmacol Exp Ther. 2004;311:228–236. doi: 10.1124/jpet.104.068536. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Itoh T, Sato H, Li AP, Sugiyama Y. Inhibition of transporter-mediated hepatic uptake as a mechanism for drug-drug interaction between cerivastatin and cyclosporin A. J Pharmacol Exp Ther. 2003;304:610–616. doi: 10.1124/jpet.102.041921. [DOI] [PubMed] [Google Scholar]
- Simonson SG, Raza A, Martin PD, Mitchell PD, Jarcho JA, Brown CD, et al. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004;76:167–177. doi: 10.1016/j.clpt.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Smith NF, Figg WD, Sparreboom A. Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination. Expert Opin Drug Metab Toxicol. 2005a;1:429–445. doi: 10.1517/17425255.1.3.429. [DOI] [PubMed] [Google Scholar]
- Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005b;4:815–818. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- Smith NF, Marsh S, Scott-Horton TJ, Hamada A, Mielke S, Mross K, et al. Variants in the SLCO1B3 gene: interethnic distribution and association with paclitaxel pharmacokinetics. Clin Pharmacol Ther. 2007;81:76–82. doi: 10.1038/sj.clpt.6100011. [DOI] [PubMed] [Google Scholar]
- Spence JD, Munoz CE, Hendricks L, Latchinian L, Khouri HE. Pharmacokinetics of the combination of fluvastatin and gemfibrozil. Am J Cardiol. 1995;76:80A–83A. doi: 10.1016/s0002-9149(05)80024-4. [DOI] [PubMed] [Google Scholar]
- Su Y, Zhang X, Sinko PJ. Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of Saquinavir in Hep G2 cells. Mol Pharm. 2004;1:49–56. doi: 10.1021/mp0340136. [DOI] [PubMed] [Google Scholar]
- Takane H, Kawamoto K, Sasaki T, Moriki K, Kitano H, Higuchi S, et al. Life-threatening toxicities in a patient with UGT1A1*6/*28 and SLCO1B1*15/*15 genotypes after irinotecan-based chemotherapy. Cancer Chemother Pharmacol. 2009;63:1165–1169. doi: 10.1007/s00280-008-0864-x. [DOI] [PubMed] [Google Scholar]
- Takane H, Miyata M, Burioka N, Kurai J, Fukuoka Y, Suyama H, et al. Severe toxicities after irinotecan-based chemotherapy in a patient with lung cancer: a homozygote for the SLCO1B1*15 allele. Ther Drug Monit. 2007;29:666–668. doi: 10.1097/FTD.0b013e3181357364. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276:35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther. 2003;304:223–228. doi: 10.1124/jpet.102.043026. [DOI] [PubMed] [Google Scholar]
- Tornio A, Niemi M, Neuvonen M, Laitila J, Kalliokoski A, Neuvonen PJ, et al. The effect of gemfibrozil on repaglinide pharmacokinetics persists for at least 12 h postdose: evidence for mechanism-based inhibition of CYP2C8 in vivo. Clin Pharmacol Ther. 2008;84:403–411. doi: 10.1038/clpt.2008.34. [DOI] [PubMed] [Google Scholar]
- Tozer TN, Rowland M. Introduction to Pharmacokinetics and Pharmacodynamics. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Treiber A, Schneiter R, Häusler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos. 2007;35:1400–1407. doi: 10.1124/dmd.106.013615. [DOI] [PubMed] [Google Scholar]
- van Montfoort JE, Hagenbuch B, Fattinger KE, Muller M, Groothuis GM, Meijer DK, et al. Polyspecific organic anion transporting polypeptides mediate hepatic uptake of amphipathic type II organic cations. J Pharmacol Exp Ther. 1999;291:147–152. [PubMed] [Google Scholar]
- Wang JS, Neuvonen M, Wen X, Backman JT, Neuvonen PJ. Gemfibrozil inhibits CYP2C8-mediated cerivastatin metabolism in human liver microsomes. Drug Metab Dispos. 2002;30:1352–1356. doi: 10.1124/dmd.30.12.1352. [DOI] [PubMed] [Google Scholar]
- Wang P, Kim RB, Chowdhury JR, Wolkoff AW. The human organic anion transport protein SLC21A6 is not sufficient for bilirubin transport. J Biol Chem. 2003;278:20695–20699. doi: 10.1074/jbc.M301100200. [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology. 2002;36:164–172. doi: 10.1053/jhep.2002.34133. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Toliat MR, Schirmer M, Meineke I, Nürnberg P, Brockmöller J. The polymorphisms Asn130Asp and Val174Ala in OATP1B1 and the CYP2C9 allele *3 independently affect torsemide pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;83:815–817. doi: 10.1038/sj.clpt.6100404. [DOI] [PubMed] [Google Scholar]
- Xiang X, Jada SR, Li HH, Fan L, Tham LS, Wong CI, et al. Pharmacogenetics of SLCO1B1 gene and the impact of *1b and *15 haplotypes on irinotecan disposition in Asian cancer patients. Pharmacogenet Genomics. 2006;16:683–691. doi: 10.1097/01.fpc.0000230420.05221.71. [DOI] [PubMed] [Google Scholar]
- Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, et al. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007;35:2166–2176. doi: 10.1124/dmd.107.017459. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Kobayashi M, Okada M, Takeuchi T, Unno M, Abe T, et al. Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett. 2008;260:163–169. doi: 10.1016/j.canlet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34:1247–1254. doi: 10.1124/dmd.105.008938. [DOI] [PubMed] [Google Scholar]
- Zhang W, He YJ, Han CT, Liu ZQ, Li Q, Fan L, et al. Effect of SLCO1B1 genetic polymorphism on the pharmacokinetics of nateglinide. Br J Clin Pharmacol. 2006;62:567–572. doi: 10.1111/j.1365-2125.2006.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


