Abstract
Background and purpose:
Chemokines orchestrate neutrophil recruitment to inflammatory foci. In the present study, we evaluated the participation of three chemokines, KC/CXCL1, MIP-2/CXCL2 and LIX/CXCL5, which are ligands for chemokine receptor 2 (CXCR2), in mediating neutrophil recruitment in immune inflammation induced by antigen in immunized mice.
Experimental approach:
Neutrophil recruitment was assessed in immunized mice challenged with methylated bovine serum albumin, KC/CXCL1, LIX/CXCL5 or tumour necrosis factor (TNF)-α. Cytokine and chemokine levels were determined in peritoneal exudates and in supernatants of macrophages and mast cells by elisa. CXCR2 and intercellular adhesion molecule 1 (ICAM-1) expression was determined using immunohistochemistry and confocal microscopy.
Key results:
Antigen challenge induced dose- and time-dependent neutrophil recruitment and production of KC/CXCL1, LIX/CXCL5 and TNF-α, but not MIP-2/CXCL2, in peritoneal exudates. Neutrophil recruitment was inhibited by treatment with reparixin (CXCR1/2 antagonist), anti-KC/CXCL1, anti-LIX/CXCL5 or anti-TNF-α antibodies and in tumour necrosis factor receptor 1-deficient mice. Intraperitoneal injection of KC/CXCL1 and LIX/CXCL5 induced dose- and time-dependent neutrophil recruitment and TNF-α production, which were inhibited by reparixin or anti-TNF-α treatment. Macrophages and mast cells expressed CXCR2 receptors. Increased macrophage numbers enhanced, while cromolyn sodium (mast cell stabilizer) diminished, LIX/CXCL5-induced neutrophil recruitment. Macrophages and mast cells from immunized mice produced TNF-α upon LIX/CXCL5 stimulation. Methylated bovine serum albumin induced expression of ICAM-1 on mesenteric vascular endothelium, which was inhibited by anti-TNF-α or anti-LIX/CXCL5.
Conclusion and implications:
Following antigen challenge, CXCR2 ligands are produced and act on macrophages and mast cells triggering the production of TNF-α, which synergistically contribute to neutrophil recruitment through induction of the expression of ICAM-1.
Keywords: CXCR2, inflammation, chemokines, cytokines, leukocytes, neutrophil recruitment
Introduction
Neutrophil accumulation in tissues is a characteristic of acute inflammatory conditions, including bacterial infection and reperfusion injury. The infiltration of neutrophils is also known to occur and play a role in the acute phase of several chronic inflammatory conditions, such as glomerulonephritis, inflammatory bowel disease, autoimmune vasculitis, dermatitis and rheumatoid arthritis (RA) (Weissmann and Korchak, 1984; Kasama et al., 2005; Larsen et al., 2008; Randis et al., 2008). Experimental models of antigen-induced immune inflammation reproduce some of the features of chronic inflammatory diseases undergoing an acute phase (Trentham et al., 1977; Bacon and Oppenheim, 1998; Canetti et al., 2001).
The subclass of CXC chemokines that possesses the amino acid sequence glutamic acid-leucine-arginine (ELR+) preceding the first cysteine residue of the CXC motif, including KC/CXCL1 (keratinocyte-derived chemokine; CXCL1) and LIX/CXCL5 (lipopolysaccharide-induced CXC chemokine; CXCL5), are a major subclass of chemokines involved in the recruitment and activation of neutrophils (Baggiolini et al., 1989; Rollins, 1997; Bacon and Oppenheim, 1998; Luster, 1998; Smith et al., 2002; Sandler et al., 2007). The major receptor for CXC ELR+ chemokines in mice is the chemokine receptor 2 (CXCR2). Indeed, mice that are CXCR2-deficient or compounds that block CXCR2 function significantly impair neutrophil recruitment in several experimental situations such as pancreatitis, lung injury, autoimmune thyroiditis or arthritis (Chen et al., 2005; Bhatia and Hegde, 2007;Coelho et al., 2008).
Chemokines, such as KC/CXCL1 and LIX/CXCL5, are rapidly released under several experimental situations and play a major role in inducing neutrophil influx. For example, it has been shown that administration of antigen or non-specific irritants, such as carrageenan, induce the release of chemokines that drive neutrophil recruitment in vivo (Garcia-Ramallo et al., 2002; Cunha et al., 2005; Coelho et al., 2008; Grespan et al., 2008). Chemokines are also released and induce neutrophil influx upon injection of cytokines, such as tumour necrosis factor (TNF)-α (Tessier et al., 1997). On the other hand, locally released chemokines may activate resident cells to release cytokines. Indeed, our previous studies have shown that blockade of CXCR2 decreased production of TNF-α in models of gut ischaemia and reperfusion (Souza et al., 2004) and arthritis (Coelho et al., 2008). The present study had three major related objectives: (i) evaluate the ability of KC/CXCL1 and LIX/CXCL5 given exogenously or generated endogenously in an immune peritonitis model to drive neutrophil influx and the local production of TNF-α; (ii) evaluate the role of chemokine-induced TNF-α for neutrophil recruitment; and (iii) evaluate possible cellular sources of TNF-α and mechanisms by which TNF-α could aid in the recruitment of neutrophils in response to chemokine generation.
Methods
Animals
All animal care and experimental procedures were conducted according to the guidelines of the Ethics Committee of the School of Medicine of Ribeirão Preto, University of São Paulo, São Paulo, Brazil. Wild-type Balb/c and C57BL/6 mice, and tumour necrosis factor receptor (TNFR)1-deficient (p55−/−) mice were used in this study. TNFR1−/−mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and maintained in the Faculty of Medicine of Ribeirão Preto (University of Sao Paulo, Sao Paulo, Brazil) as previously described (Canetti et al., 2001). The knockout mice were bred and maintained alongside Balb/c or C57BL/6 wild-type mice. The genetic status of the deficient mice was confirmed by PCR (Canetti et al., 2001). Mice were housed in standard clear plastic cages with free access to food and water, with a 12:12 h light/dark cycle at 21°C.
Immunization procedure
Balb/c, C57BL/6 and TNFR1−/− mice were immunized. Briefly, on day 0 the animals received a single s.c. injection of the protein antigen methylated bovine serum albumin (mBSA; 500 µg) in 0.2 mL of an emulsion containing 0.1 mL of saline and 0.1 mL of complete Freund's adjuvant. Animals received booster injections of mBSA dissolved in incomplete Freund's adjuvant on days 7 and 14. Non-immunized mice were given similar injections but without the antigen (mBSA). Twenty-one days after the initial injection, the immunized and non-immunized mice were challenged with mBSA (30 µg, i.p.), or only saline in immunized mice (control).
Leukocyte recruitment
Neutrophil recruitment was assessed at the indicated times after KC/CXCL1, LIX/CXCL5, TNF-α or mBSA i.p. challenge. The animals were killed, and the cells present in the peritoneal cavity were harvested with 3 mL of phosphate-buffered saline (PBS) containing 1 mM EDTA. Total counts were performed with a cell counter (COULTER® AC T; Coulter Corporation; Miami, FL, USA) and differential cell counts on cytocentrifuge slides (Cytospin® 3; Shandon Lipshaw Inc., Pittsburgh, PA, USA) stained with Rosenfeld. The differential counts were performed with a light microscope and the results presented as the number (means ± SEM) of neutrophils per cavity.
In experiments evaluating the blockade of neutrophil influx by antigen or the chemokines, mice were treated 1 h before the experiment with MK 886 (1 mg·kg−1, p.o.) or reparixin (30 mg·kg−1, s.c.). Antibodies were given 30 min before the experiments: control antibody (30 µL, i.p.), anti-TNF antiserum (30 µL, i.p.), anti-KC/CXCL1 (3 µg, i.p.), anti-MIP-2/CXCL2 (3 µg, i.p.) and anti-LIX/CXCL5 (3 µg, i.p.).
Coupling of mast cell antibody to magnetic beads
A monoclonal antibody (mAb-AA4) that recognizes two derivatives of the ganglioside GD1b was raised in rats (Guo et al., 1989). The gangliosides are unique to the surface of rodent mast cells (Jamur et al., 2001). mAb-AA4 was conjugated to tosylactivated Dynabeads (Dynal; Lake Success, NY, USA). Briefly, equal volumes of tosylactivated Dynabeads and mAb-AA4 were mixed. The solution was incubated for 24 h at 22°C with slow end-over-end rotation. After incubation, the magnetic beads were collected and washed three times with 5 mL 0.01 M PBS containing 0.1% BSA. The coated beads were resuspended and stored in PBS + 0.1% BSA at a concentration of 30 mg·mL−1.
Mast cell separation
The peritoneal cells were incubated with antibody-coated magnetic beads (see above; three beads per target cell) in PBS containing 2% BSA for 5–10 min at room temperature. After incubation the mast cells were isolated by washing four times with PBS containing 2% BSA and once in PBS (Jamur et al., 1997; Jamur et al., 2005).
In vitro assay for macrophages and mast cells
Macrophage isolation and assay
Naïve mice were treated i.p. with 500 µL of thioglycollate (3%) and 3 days after treatment, peritoneal macrophages were harvested with RPMI 1640 (pH 7.4). Peritoneal macrophages from immunized mice were harvested on day 21 after immunization, without stimulation with thioglycollate, by washing the peritoneum with RPMI 1640 containing 1 mM EDTA (3 mL, pH 7.4). Peritoneal cells from naïve+ thioglycollate-treated or immunized mice were cultured in 48-well culture plates for 1 h at 37°C in an atmosphere of air with 5% CO2. The plates were then washed three times with RPMI to remove the non-adherent cells. The adherent population (95% macrophages ∼5 × 105 cells per well) were incubated for 6 h at 37°C in fresh medium (control) or in medium containing LIX/CXCL5 (100 ng·mL−1). Subsequently, the supernatants were recovered for elisa assay. Cell viability was determined by the Trypan blue test (>95%).
Mast cell assay
Purified mast cells (5 × 105 cells per well) were plated in 48-well culture plates and incubated for 6 h at 37°C in fresh medium (control) or in medium containing LIX/CXCL5 (100 ng·mL−1). Subsequently, the plates were centrifuged and the supernatants recovered for elisa assay.
elisa for KC/CXCL1, MIP-2/CXCL2, LIX/CXCL5 and TNF-α
KC/CXCL1, MIP-2/CXCL2, LIX/CXCL5 and TNF-α levels in the exudates from antigen-challenged immunized mice, or TNF-α levels in the exudates from KC/CXCL1 and LIX/CXCL5-injected mice were detected by elisa (Taktak and Lee, 1991). Briefly, microtiter plates were coated overnight at 40C with an immunoaffinity-purified polyclonal sheep antibody against KC/CXCL1 (1 µg·mL−1), MIP-2/CXCL2 (1 µg·mL−1), LIX/CXCL5 (1 µg·mL−1) or TNF-α (2 µg·mL−1). After blocking the plates, recombinant murine KC/CXCL1, MIP-2/CXCL2, LIX/CXCL5 or TNF-α standards were added at various dilutions, and the samples were then added in duplicate and incubated overnight at 4°C. Rabbit biotinylated immunoaffinity purified polyclonal antibodies (pAbs) anti-KC/CXCL1 (0.1 µg·mL−1), anti-MIP-2/CXCL2 (0.1 µg·mL−1), anti-LIX/CXCL5 (0.1 µg·mL−1) or anti-TNF-α (1:500) were added, followed by incubation at room temperature for 1 h. Finally, 50 µL of avidin-horseradish peroxidase (1:5000 dilution; DAKO A/S, Glostrup, Denmark) were added to each well; after 30 min the plates were washed, and the colour reagent OPD (200 µg per well; Sigma, St. Louis, MO, USA) was added. After 15 min, the reaction was stopped with 1 M H2SO4 and the optical density (OD) measured at 490 nm. The results were expressed as pg·mL−1 of KC/CXCL1, MIP-2/CXCL2, LIX/CXCL5 and TNF-α, based on standard curves.
CXCR2 real-time PCR
Two hours after mBSA injection, the mice were terminally anaesthetized, and the peritoneal cells were purified as described above. The samples were collected, and total RNA was extracted using the SV Total RNA Isolation System (Promega Biosciences Inc., San Luis Obispo, CA, USA). The purity of total RNA was measured with a spectrophotometer, and the wavelength absorption ratio (260/280 nm) was between 1.8 and 2.0 for all preparations. Reverse transcription of total RNA to cDNA was carried out with reverse transcription reaction (Superscript II, Gibco Life Technologies, Grand Island, NY, USA). Real-time PCR quantitative mRNA analysis was performed in an ABI Prism 7500 Sequence Detection System using the SYBR-green fluorescence system (Applied Biosystems, Warrington, UK) for quantification of amplicons. RT-PCR was performed in a 20 µL reaction volume and carried out with heating at 95°C (10 min), and then 40 cycles of 94°C (1 min), 56°C (1 min) and 72°C (2 min). Melting curve analysis was performed (65–95°C) in order to verify that only one product was amplified. Samples with more than one peak were excluded. The data were analysed with the comparative cycle threshold (CT) method. Primer pairs for mouse GAPDH and CXCR2 receptor were as described by Reutershan et al. (2006).
Confocal assay for CXCR2 analysis
Mast cells and macrophages were attached to poly-L-lysine-coated chamber slides (Lab-Tek II, Nunc, Wiesbaden, Germany), fixed with 3.7% paraformaldehyde and permeabilized with 0.1% Triton. After blocking with 5% BSA, samples were incubated with monoclonal anti-mouse CXCR2-phycoerythrin (1:100; R&D Systems, Minneapolis, MN, USA), rat anti-mouse AA4 or rat anti-mouse F4/80 (both 1:100; BD Bioscience, San Jose, CA, USA) antibodies overnight at 4°C. Subsequently, the cells were stained with secondary antibodies for 45 min at room temperature (Alexa594-conjugated anti-rabbit and Alexa488-conjugated anti-rat, both from Molecular Probes, Karlsruhe, Germany). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; 1:500). Controls were performed in the absence of primary antibodies. Images were acquired using a Leica TCS SP5 confocal microscope and a 63× oil objective.
Immunofluorescence assay for intercellular adhesion molecule 1 (ICAM-1)/CD54
An immunofluorescence assay was used to analyse the level of ICAM-1 expression on mouse mesenteric venules, 2 h after mBSA injection. For this, mice were treated for 30 min with anti-LIX/CXCL5 (3 µg, i.p.) or anti-TNF-α subsequently stimulated with saline or mBSA (30 µg, i.p.) for 2 h. Frozen mesenteric tissue sections (5 µm) were fixed with paraformaldehyde (4%) in a wet chamber at room temperature. The slides were incubated with PBS containing 1% bovine serum albumin (PBS-BSA), and then slices were incubated for 1 h with fluorescein isocyanate (FITC)-conjugated anti-mouse CD54/ICAM-1 mAb (1:200; BD Pharmingen, San Jose, CA, USA) in PBS-BSA. The results of qualitative analysis are expressed as fluorescence intensity of stained venules (magnification ×100) present in the fluorescence microscopic field. All images were captured using identical camera settings: time of exposure, brightness, contrast and sharpness, and an appropriate white balance set according to the fluorescence filter and acquired and analysed by Image-Pro Plus 4.0 (Media Cybernetics). The mean fluorescence density was determined from a linear measurement of stained venules fluorescence of at least five randomly chosen fields of each slide, performed in triplicate were analysed.
Neutrophil chemotaxis
Bone marrow neutrophils were purified as previously described (Pinho et al., 2007). A modified Boyden chamber assay was performed using a 48-well microchamber (Neuro Probe, Gaithersburg, MD, USA). The stimuli and negative control were added to the lower chambers. A 5 µm pore polycarbonate membrane (Neuro Probe) was placed between the upper and lower chambers, and 5 × 104 cells were added to the top chambers. Cells were allowed to migrate into the membrane for 1 h at 37°C with 5% CO2. Following incubation, the membrane was washed in PBS to remove non-adherent cells before being fixed in methanol and stained using the Diff-Quik system (Dade Behring, Deerfield, IL, USA). Each well-associated membrane area was scored using light microscopy to count the intact cells present in five random fields. The results are expressed as the number of neutrophils per field.
Statistical analysis
The data are reported as means ± SEM, and the number of animals per group was five. The means from different treatments were compared by anova. When statistical significances were identified, individual comparisons were subsequently tested with Bonferroni's t-test for unpaired values. Statistical significance was set at P <0.05. The experiments were repeated two or three times, with similar results
Materials
The pAbs anti-KC/CXCL1, anti-MIP-2/CXCL2 and anti-LIX/CXCL5 for in vivoassays were purchased from Peprotech Inc. Recombinant murine LIX/CXCL5, KC/CXCL1 and MIP-2/CXCL2 or TNF-α, pAbs anti-LIX/CXCL5, anti-KC/CXCL1, anti-MIP-2/CXCL2 or anti-TNF-α and the biotinylated affinity pAbs anti-LIX/CXCL5, anti-KC/CXCL1, anti-MIP-2/CXCL2 or anti-TNF-α for elisa assay were purchased from R&D Systems. The sheep anti-mouse TNF-α and control sera were gifts from Dr S Poole (National Institute for Biological Standards and Control, NIBSC, London, UK). Cromolyn sodium salt, thioglycollate, mBSA, complete Freund's adjuvant and incomplete Freund's adjuvant were purchased from Sigma. MK 886 (inhibitor of FLAP, 5-lipoxygenase activating protein) was obtained from Biomol. Reparixin (CXCR1/2 antagonist) was a kind gift from Dr Riccardo Bertini (Dompè Pharmaceuticals, L'Aquila, Italy).
Results
Dose- and time-dependent neutrophil recruitment
Intraperitoneal challenge of immunized mice with antigen (mBSA) induced a significant dose- and time-dependent neutrophil recruitment when compared with that induced by saline in immunized mice or by antigen in non-immunized mice (Figure 1). Neutrophil recruitment was already significant at 4 h, peaked at 6 h, declining thereafter to reach baseline levels at 24 h after antigen challenge (Figures 1B). For the next series of experiments, immunized mice received an injection of 30 µg of mBSA and neutrophil influx evaluated 6 h after challenge.
Figure 1.
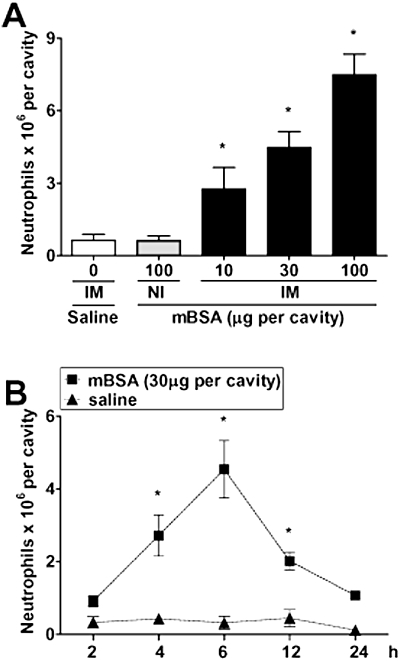
Antigen (methylated bovine serum albumin, mBSA) challenge in immunized (IM) mice induces dose- and time-dependent neutrophil recruitment to the peritoneal cavity. (A) mBSA was injected at the indicated doses into the peritoneal cavity of non-immunized (NI) or IM mice, and neutrophil recruitment was determined 6 h later. Neutrophil recruitment was also evaluated when saline was injected into IM mice (control). (B) Time dependence of the neutrophil recruitment in IM mice challenged with saline or mBSA. Data are means ± SEM (n= 5). *P <0.05 when compared with control mice (anova followed by the Bonferroni test).
KC/CXCL1, LIX/CXCL5 and TNF-α mediate neutrophil recruitment induced by immune peritonitis
Neutrophil recruitment induced by injection of mBSA in immunized mice was inhibited by neutralizing anti-KC/CXCL1 or anti-LIX/CXCL5, but not with anti-MIP-2/CXCL2, antibodies (Figure 2A). Consistent with their functional role, levels of KC/CXCL1 and LIX/CXCL5, but not of MIP-2/CXCL2, were detectable in the peritoneal cavity of antigen-challenged mice. Levels of KC/CXCL1 and LIX/CXCL5 were elevated at 1 h and 2 h after challenge and dropped to baseline at 4 h (Figure 2C).
Figure 2.
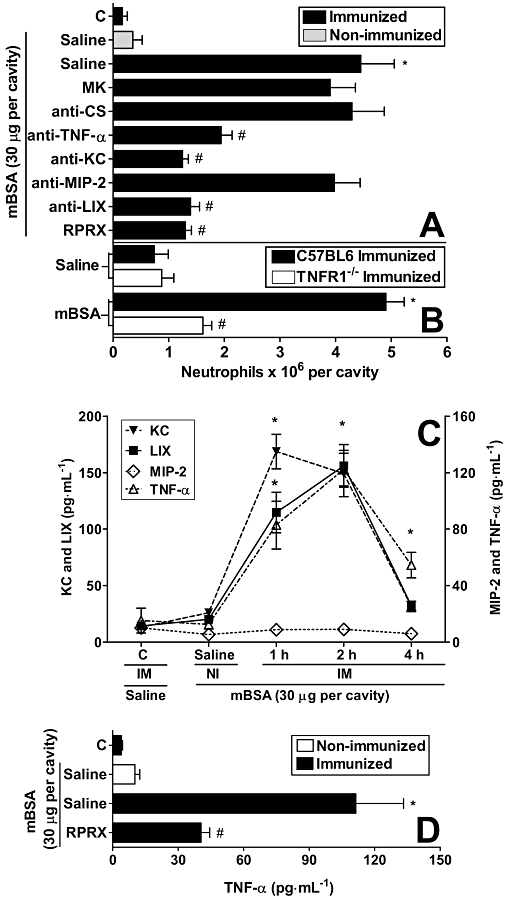
Methylated bovine serum albumin (mBSA) challenge-induced neutrophil recruitment depends on tumour necrosis factor (TNF)-α, KC/CXCL1 and LIX/CXCL5, and time-dependent production of KC/CXCL1, LIX/CXCL5 and TNF-α in the peritoneal exudates. Reparixin reduced TNF-α levels in mBSA-challenged immunized (IM) mice. (A) IM mice were treated, 1 h before challenge, with MK 886 (inhibitor of 5-lipoxygenase activating protein; MK; 1 mg·kg−1, p.o.) or reparixin (CXCR1/2 antagonist; RPRX; 30 mg·kg−1, s.c.), or 30 min before with control antibody (anti-CS; 30 µL, i.p.), anti-TNF antiserum (anti-TNF-α; 30 µL, i.p.), chemokine antibodies, anti-KC/CXCL1 (anti-KC; 3 µg, i.p.), anti-MIP-2/CXCL2 (anti-MIP-2; 3 µg, i.p.) and anti-LIX/CXCL5 (anti-LIX; 3 µg, i.p.) and then challenged with mBSA (30 µg, i.p.). (B) Wild-type or tumour necrosis factor receptor (TNFR)1−/− mice were challenged with mBSA (30 µg, i.p.). Neutrophil recruitment was evaluated 6 h after mBSA or saline i.p. injection. (C) The concentrations of KC/CXCL1, MIP-2/CXCL2, LIX/CXCL5 and TNF-α were determined 1, 2 and 4 h after challenge with mBSA (30 µg, i.p.) in IM and in non-immunized (NI) mice or saline in IM mice (C; control). (D) Animals were treated 1 h before challenge with reparixin (RPRX; 30 mg·kg−1, s.c.) or saline, and the concentrations of TNF-α was determined 2 h after challenge with mBSA (30 µg, i.p.) in IM and NI mice or saline in IM mice (C; control). Data are means ± SEM (n= 5). *P <0.05 when compared with control group; #P <0.05 when compared with IM group after mBSA challenge. (anova followed by the Bonferroni test).
Levels of TNF-α also increased after antigen challenge of immunized mice. Indeed, significant levels TNF-α were detected at 2 h and were still significant at 4 h after challenge (Figure 2C). Consistent with the detection of TNF-α, neutralization of TNF-α with antibodies (Figure 2A) or experiments in TNFR1-deficient mice (Figure 2B) showed marked decreases in neutrophil influx.
Treatment with the CXCR1/2 receptor blocker, reparixin, greatly reduced neutrophil influx (Figure 2A). Interestingly, treatment with reparixin significantly decreased the production of TNF-α in the peritoneal cavity, suggesting that activation of CXCR1/2 was relevant for the local production of this cytokine (Figure 2D). The 5-lipoxygenase inhibitor, MK 886, failed to modify neutrophil recruitment in response to mBSA challenge of immunized mice (Figure 2A).
Neutrophil recruitment induced by KC/CXCL1 and LIX/CXCL5 depends on the local production of TNF-α
A series of experiments were then conducted to examine further the ability of KC/CXCL1 and LIX/CXCL5 to induce TNF-α. As seen in Figure 3, the i.p. injection of KC/CXCL1 or LIX/CXCL5 induced a dose- and time-dependent influx of neutrophils. Neutrophil influx was optimal at the doses of 3 ng per cavity of KC/CXCL1 or 30 ng per cavity of LIX/CXCL5 and maximal at 4 h after administration of the chemokine (Figure 3).
Figure 3.
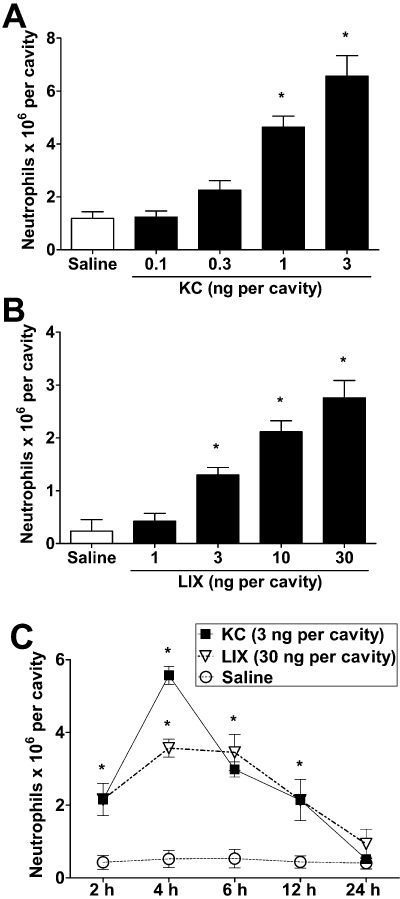
KC/CXCL1 and LIX/CXCL5 induce dose- and time-dependent neutrophil recruitment into peritoneal cavities of mice. (A,B) Saline (control), (A) KC/CXCL1 (KC; 0.1, 0.3, 1 or 3 ng) or (B) LIX/CXCL5 (LIX; 1, 3, 10 or 30 ng) were injected into the peritoneal cavity of mice and 4 h later, neutrophil recruitment was evaluated. (C) Time-dependent neutrophil recruitment after KC/CXCL1 (KC; 3 ng, i.p.) or LIX/CXCL5 (LIX; 30 ng, i.p.) injection. Data are means ± SEM (n= 5). *P <0.05 when compared with control mice (anova followed by the Bonferroni test).
Neutrophil influx induced by KC/CXCL1 or LIX/CXCL5 was greatly reduced by the administration of reparixin (Figure 4A). Moreover, treatment with anti-TNF-α (Figure 4A) or experiments in TNFR1−/− mice (Figure 4B) showed that TNF-α was essential for KC/CXCL1- or LIX/CXCL5-induced neutrophil influx. However, as expected, anti-LIX/CXCL5 and anti-KC/CXCL1 exhibited only marginal effects upon KC/CXCL1- and LIX/CXCL5-induced neutrophil recruitment (Figure 4A). Consistent with the dependence of TNF-α on KC/CXCL1- and LIX/CXCL5-induced neutrophil recruitment, both chemokines induced a rapid production of TNF-α in the peritoneal cavity of mice. TNF-α production peaked at 1 h after administration of the chemokines and decreased markedly thereafter (Figure 4C).
Figure 4.
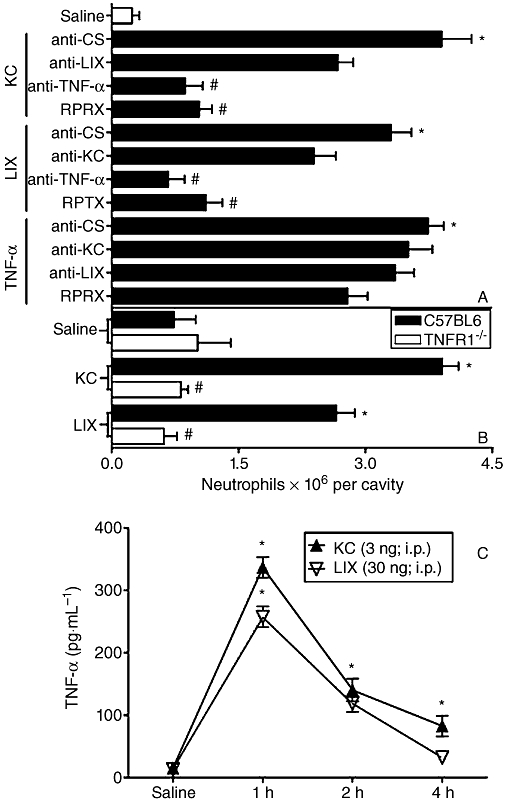
KC/CXCL1 and LIX/CXCL5 induce neutrophil recruitment via tumour necrosis factor (TNF)-α. (A) Mice were treated, 1 h before challenge, with reparixin (RPRX; 30 mg·kg−1, s.c.), or 30 min before challenge, with control antibody (anti-CS; 30 µL, i.p.), anti-TNF antiserum (anti-TNF-α; 30 µL, i.p.), antibody anti-KC/CXCL1 (anti-KC; 3 µg, i.p.), antibody anti-LIX/CXCL5 (anti-LIX; 3 µg, i.p.) and then challenged with KC/CXCL1 (KC; 3 ng), LIX/CXCL5 (LIX; 30 ng) or TNF-α (40 ng) i.p. Neutrophil recruitment was evaluated 4 h after challenge in treated groups. (B) Wild-type or tumour necrosis factor receptor (TNFR)1−/−mice were challenged with KC/CXCL5 (KC; 3 ng, i.p.) or LIX/CXCL5 (LIX; 30 ng, i.p.), and 4 h later the neutrophil recruitment was estimated in mice. Neutrophil recruitment was also evaluated when saline was injected into mice (control). (C) The concentrations of TNF-α were determined 1, 2 and 4 h after challenge i.p. with saline, KC/CXCL1 (KC; 3 ng) or LIX/CXCL5 (LIX; 30 ng) in mice. Data are means ± SEM (n= 5). *P <0.05 when compared with control group; #P <0.05 when compared with group injected with cytokines or chemokines. (anova followed by the Bonferroni test).
The i.p. injection of TNF-α also induced a marked influx of neutrophils. TNF-α-induced neutrophil influx was independent of the local production of KC/CXCL1 or LIX/CXCL5, as seen by the lack of effect of neutralizing antibody against these chemokines (Figure 4A). Moreover, treatment of mice with reparixin failed to alter TNF-α-induced neutrophil influx (Figure 4A). The treatments of the mice with neutralizing antibodies against MIP-2/CXCL2 also did not affect LIX/CXCL5- or TNF-α-induced neutrophil migration (data not shown in figures).
Macrophages and mast cells express CXCR2 and release TNF-α upon LIX/CXCL5 activation
Next, we investigated whether resident macrophages and mast cells could respond to LIX/CXCL5 and influence neutrophil recruitment. We did not test KC/CXCL1 in this set of experiment because the previous experiments demonstrated that it acts by mechanism similar to LIX/CXCL5. Initial experiments using confocal microscopy showed that both macrophages and mast cells from the peritoneal cavity of mice expressed CXCR2, 92% ± 5 and 69% ± 3 respectively. Indeed, both F4/80+ macrophages and AA4+ mast cells harvested from the peritoneal cavity of mice co-expressed CXCR2 (Figure 5A,B). Both mast cells and macrophages in culture also produced TNF-α when stimulated in vitro with LIX/CXCL5 (Figure 5D).
Figure 5.
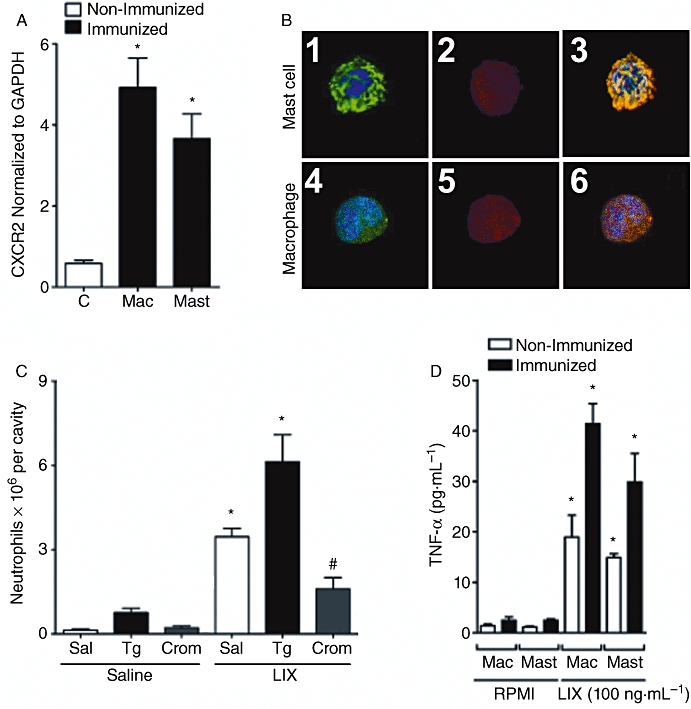
LIX/CXCL5 induced neutrophil recruitment by acting on CXCR2 in macrophages and mast cells, stimulating production of tumour necrosis factor (TNF)-α. (A) Real-time PCR analysis of CXCR2 expression of purified macrophages (Mac) or mast cells (Mast) from peritoneal cavity of immunized and non-immunized mice 2 h after methylated bovine serum albumin (mBSA) (30 µg, i.p.) challenge. (B) Confocal analysis of mast cells and macrophages from immunized mice expressing CXCR2. (B1) 4,6-diamidino-2-phenylindole (DAPI) and anti-murine AA4 conjugated with Alexa488. (B2,B5) mAb anti-mouse CXCR2-phycoerythrin. (B4) DAPI and anti-murine F4/80 conjugated with Alexa488. (B3,B6) Double staining with Alexa488 and phycoerythrin was performed to discriminate the cell type and CXCR2 receptor respectively. Nuclei were counterstained with DAPI. (C) Thioglycollate 3% (Tg; 200 µL, i.p.) injected i.p. induces an increase in mononuclear cells (15.3 ± 1.8 × 106; data not shown), and after 4 days the mice were further challenged with saline or LIX/CXCL5 (30 ng, i.p.). Mice were treated, 1 h before challenge, with cromolyn sodium (Crom; 10 mg·kg−1, i.p.) that induced stabilization of mast cells in the peritoneal cavity. Under these conditions, saline or LIX/CXCL5 (LIX; 30 ng) was injected i.p., and neutrophil recruitment was determined 4 h after stimulus injection. (D) Concentrations of TNF-α determined 6 h after saline (C; control) or LIX/CXCL5 (LIX; 100 ng·mL−1) in vitro stimulation of macrophages (Mac) or mast cells (Mast) from immunized mice. Data are means ± SEM (n= 5). *P <0.05 when compared with control group; #P <0.05 when compared with immunized group after mBSA challenge. (anova followed by the Bonferroni test).
To examine the relevance of these resident cells for TNF-α production in vivo, we used pharmacological approaches (Ribeiro et al., 1991). The number of macrophages in the peritoneal cavity was increased by 2.3-fold by the treatment with thioglycollate (data not shown). Injection of LIX/CXCL5 in the peritoneal cavity of mice, which previously received thioglycollate, induced neutrophil migration that was twice that observed in the peritoneal cavity of control mice (Figure 5C). On the other hand, the previous injection of thioglycollate did not alter neutrophil recruitment induced by fMLP (data not shown). To examine the role of mast cells, animals were treated with the mast cell-stabilizing agent, cromolyn sodium. This treatment greatly reduced LIX/CXCL5-induced neutrophil recruitment (Figure 5C).
TNF-α controls ICAM-1 expression in immune peritonitis
Next, we examined whether expression of cell adhesion molecules could account for the ability of TNF-α to facilitate neutrophil influx induced by chemokines. As seen in Figure 6, the injection of antigen in immunized mice induced a significant up-regulation of ICAM-1 expression on the endothelium of mesenteric vessels when compared with i.p. injection of saline. Pretreatment of mice with anti-TNF-α significantly decreased the enhanced expression of ICAM-1 on the vascular endothelium (Figure 6). In agreement with the ability of LIX/CXCL5 to induce TNF-α production (see Figure 4C) and LIX/CXCL5 receptor antagonist to reduce mBSA-induced TNF-α production (Figure 2D), administration of anti-LIX/CXCL5 reduced the expression of ICAM-1 on the vascular endothelium induced by mBSA (Figure 6). We also examined whether, besides inducing expression of adhesion molecules, TNF-α also induces neutrophil chemotaxis. We found that TNF-α, over a range of concentrations (10−8−10−4 M), induced similar increase (∼3.3-fold) in neutrophil locomotion, compared with RPMI (RPMI = 10 ± 4; TNF-α; 10−4M = 38 ± 4 neutrophils per microscopy field, n= 8), which was not reduced by addition of TNF-α into both microchamber compartments (upper and lower). As control, we observed that, under the same experimental conditions, KC/CXCL1 (10−6 M) induced neutrophil chemotaxis (65 ± 4 neutrophils per microscopy field, n= 8), which was completely inhibited by simultaneous addition of KC/CXCL1 into both upper and lower chamber compartments. These findings suggest that the observed increase in the neutrophil locomotion induced by TNF-α is not due to chemotaxis, but due to chemokinesis
Figure 6.
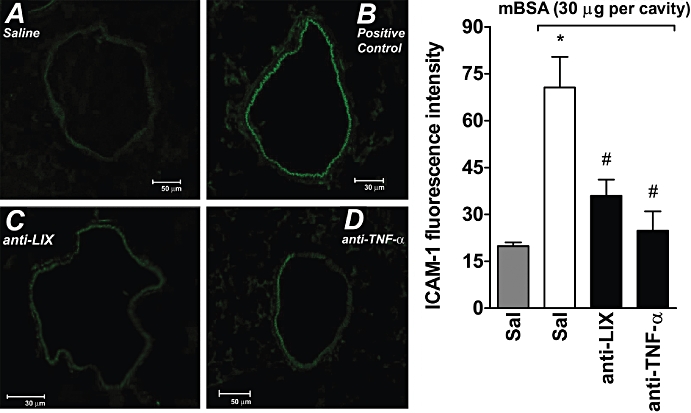
Effects of anti-LIX/CXCL5 and anti-TNF-α on mBSA-induced ICAM-1 expression in the mesenteric microcirculation. ICAM-1 expression in the microcirculation in mesenteric tissues was determined by fluorescent immunohistochemical staining using anti-ICAM-1-specific mAb conjugated with FITC (A–D). (A) Immunofluorescence staining for ICAM-1 in PBS i.p. injected animals. (B) Mice treated with PBS (0.2 mL, s.c.) and then injected with mBSA (30 µg per cavity). (C) Mice treated with anti-LIX/CXCL5 (anti-LIX; 3 µg, i.p., 30 min before) and then injected with mBSA. (D) Mice treated with anti-TNF-α (4 µg, i.p., 30 min before) and then injected with mBSA. Panels show images representative of at least three independent experiments. In addition, the endothelial fluorescence intensity was quantified. Data represent mean ± SD from three independent experiments. *P <0.05 when compared with control group; #P <0.05 when compared with immunized group after mBSA challenge. (anova followed by the Bonferroni test). ICAM-1, intercellular adhesion molecule 1; mBSA, methylated bovine serum albumin; PBS, phosphate-buffered saline; TNF, tumour necrosis factor.
Discussion and conclusions
The influx of neutrophils in tissues is thought to be relevant to the pathophysiology of several acute and chronic inflammatory diseases, including reperfusion injury, arthritis and inflammatory bowel disease. In the present study we have investigated the ability of chemokines to induce TNF-α production and the role of TNF-α for chemokine-induced neutrophil influx in vivo. Our major findings can be summarized as follows: (i) the chemokines KC/CXCL1 and LIX/CXCL5 and the chemokine receptor CXCR1/2 were crucial for neutrophil recruitment after the i.p. injection of antigen in immunized mice; (ii) the local production of KC/CXCL1 and LIX/CXCL5 in response to antigen preceded TNF-α production, and injection of exogenous KC/CXCL1 and LIX/CXCL5 induced TNF-α production; (iii) blockade of CXCR1/2 during antigen challenge or anti-KC/CXCL1 or anti-LIX/CXCL5 treatments prevented neutrophil influx and local TNF-α production; (iv) peritoneal resident mast cells and macrophages expressed CXCR2 and responded to LIX/CXCL5 by producing TNF-α; and (v) the expression of ICAM-1 was enhanced after antigen challenge, an effect that was prevented by anti-TNF-α or anti-LIX/CXCL5 antibodies.
In our present experiments, we used a model of delayed-type hypersensitivity (DTH) with mBSA as antigen. DTH type I is an inflammatory reaction mediated by effector memory T lymphocytes that infiltrate the site of injection of an antigen against, which the immune system has been primed (Hadden, 1994). We observed that, 21 days after the first immunization, challenge with mBSA induced a dose- and time-dependent neutrophil recruitment that was inhibited by treatment with reparixin, anti-TNF-α, anti-KC/CXCL1 or anti-LIX/CXCL5, showing the importance of CXCR1/2 and TNF-α in this inflammatory process.
Angiogenesis, the growth of new blood vessels, is vital to the ingress of inflammatory leukocytes in RA synovial tissue and to the growth and proliferation of RA pannus, and ENA-78/CXCL5 is an important contributor to the angiogenic activity found in the inflamed RA joint (Koch et al., 2001). In addition our group has demonstrated high levels of GRO-α/CXCL1 and ENA-78/CXCL5 in joint synovial fluid of RA patients (Grespan et al., 2008). Another study demonstrated that smokers with stable chronic obstructive pulmonary disease have a chronic inflammation with a further increase of neutrophils and various inflammatory mediators including ENA-78/CXCL5 and its receptor CXCR2 (Papi et al., 2006). Other relevant studies showed that GRO-α/CXCL1 and ENA-78/CXCL5, acting through CXCR2, are involved in the inflammatory process of many diseases such acute coronary syndrome, prostate and pancreatic cancer, idiopathic pulmonary fibrosis, colorectal adenoma and carcinoma, type 2 diabetic nephropathy and RA (Begley et al., 2008; Frick et al., 2008; Higurashi et al., 2008; Rubie et al., 2008; Smith et al., 2008). Our report shows that KC/CXCL1 and LIX/CXCL5, but not MIP-2/CXCL2, are relevant and work via CXCR2 to induce inflammatory responses. The blockade of CXCR1/2 by reparixin evidenced the importance of CXCR2. CXCR1 has been described recently in mice and is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8 (Fu et al., 2005; Moepps et al., 2006). CXCR1 does not bind to nor is activated by murine or human GRO-α/CXCL1 and human GRO-β-γ/CXCL2-3, ENA-78/CXCL5 and NAP-2/CXCL7 (Fan et al., 2007). Altogether, these data reinforce that CXCR2 is the crucial receptor to trigger responses by KC/CXCL1 and LIX/CXCL5 in mice.
One of the most important cytokines in inflammatory processes is TNF-α, which is an important target for the treatment of patients with RA. We observed that the blockade of CXCR2 by reparixin was able to reduce TNF-α production by resident cells. In another set of experiments, we observed that anti-TNF-α was capable of inhibiting KC/CXCL1- or LIX/CXCL5-induced neutrophil recruitment, and KC/CXCL1 and LIX/CXCL5 greatly induced production of TNF-α. On the other hand, reparixin, anti-KC/CXCL1 or anti-LIX/CXCL5 antibody failed to reduce neutrophil recruitment induced by TNF-α. These results show that KC/CXCL1 and LIX/CXCL5 drive TNF-α production in the system and that TNF-α release plays a relevant role in driving neutrophil influx.
Mast cells and macrophages are resident tissue cells thought to play an important role in the development of inflammatory reactions. Indeed, several studies highlight the importance of macrophages and mast cell-derived TNF-α in driving tissue inflammation (Sandler et al., 2007; Reuter et al., 2008). CXCR2 may be expressed on the surface of mast cells and macrophages and the triggering of this receptor induced the release of TNF-α (Mercer-Jones et al., 1999; Biedermann et al., 2000; Inamura et al., 2002; Oliveira et al., 2008). Indeed, there is evidence that LIX/CXCL5, acting on CXCR2, induces the activation of nuclear factor kappa B, which is related to the further induction of cytokine production (Chandrasekar et al., 2003). In our experiments, peritoneal macrophages and mast cells expressed CXCR2 and when stimulated with LIX/CXCL5 released TNF-α. To investigate the relevance of these cell types for neutrophil recruitment, we used pharmacological strategies to increase macrophage numbers and reduce mast cell function (Ribeiro et al., 1997; Ginsburg and Baldwin, 2004). Our results showed that an increase of macrophage numbers in the peritoneal cavity increased neutrophil recruitment induced by LIX/CXCL5. On the other hand, stabilization of mast cells reduced the ability of LIX/CXCL5 to induce neutrophil recruitment. Altogether, these results argue for an important role of resident peritoneal mast cells and macrophages in driving LIX/CXCL5-induced TNF-α production and neutrophil recruitment.
Because TNF-α was essential for endogenous or exogenous LIX/CXCL5-induced neutrophil influx, a series of experiments were carried out to understand how TNF-α would participate in the cascade of events leading to neutrophil influx. In agreement with earlier studies, (Smith et al., 1991; Drost and MacNee, 2002;Onnheim et al., 2008), we found that TNF-α did not induce neutrophil chemotaxis in vitro. However, as seen in Figure 6, TNF-α was crucial for the enhanced expression of ICAM-1 induced by antigen challenge of immunized mice. ICAM-1 (CD54) mediates vascular endothelium/neutrophil adhesion, a crucial step that precedes neutrophil extravasation (Engelhardt and Wolburg, 2004). Although ICAM-1 may be constitutively expressed on endothelial cells, its expression can be enhanced by cytokines and at sites of inflammation (Anderson et al., 1996; Ho et al., 2008). The fact that TNF-α does not induce neutrophil chemotaxis is in apparent contradiction with the demonstration that i.p. administration of TNF-α induces neutrophil recruitment (Canetti et al., 2001). A possible explanation for this contradiction is that exogenous TNF-α induces adhesion molecule expression, as described in the present study, and, the release of other mediators that are able to induce the neutrophil locomotion. For example, it has been shown that TNF-α induces LTB4 and PAF production, and these eicosanoids are involved in neutrophil recruitment (Canetti et al., 2001; Belanger et al., 2008; Gountopoulou et al., 2008).
Together the results of the present study suggest that LIX/CXCL5 appears to have at least two major functions in the neutrophil influx after antigen challenge of immunized mice (Figure 7): (i) KC/CXCL1 and LIX/CXCL5 stimulate TNF-α production by CXCR2-expressing tissue resident cells, including macrophages and mast cells, and the TNF-α production drives ICAM-1 expression on endothelial cells; and (ii) KC/CXCL1 and LIX/CXCL5 may also bind to CXCR2 on neutrophils and mediate their consequent adhesion and recruitment into tissues. These two actions of KC/CXCL1 and LIX/CXCL5 cooperate to promote neutrophil recruitment. Therefore, targeting KC/CXCL1 or LIX/CXCL5 or its CXCR1/2 receptors could be an alternative pharmacological approach for the treatment of inflammatory diseases displaying enhanced TNF-α and neutrophil recruitment.
Figure 7.
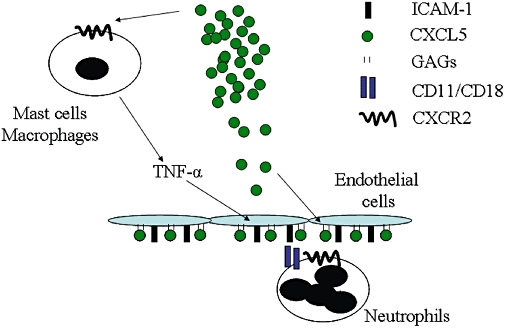
Schematic representation of the actions of LIX/CXCL5 and cooperation with tumour necrosis factor (TNF)-α-induced intercellular adhesion molecule 1 (ICAM-1) to drive neutrophil influx in vivo.
Acknowledgments
We thank Giuliana B Francisco, Ieda RS Schivo, Fabiola Leslie A Mestriner, Sérgio R Rosa, Ana K dos Santos and Jose Wilson dos Santos Meirelles for technical assistance. This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (Brazil), Fundação de Amparo à Pesquisa do Estado do Amazonas – FAPEAM (Brazil), Conselho Nacional de Pesquisa (Brazil) and Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (Brazil). SM Vieira is a recipient of a fellowship from FAPEAM.
Glossary
Abbreviations:
- CXCL
α-chemokine ligand
- CXCR
α-chemokine receptor
- DTH
delayed-type hypersensitivity
- ICAM-1
intercellular adhesion molecule 1
- mBSA
methylated bovine serum albumin
- TNF
tumour necrosis factor
- TNFR
tumour necrosis factor receptor
Conflict of interest
The authors declare no conflict of interest.
References
- Anderson JA, Lentsch AB, Hadjiminas DJ, Miller FN, Martin AW, Nakagawa K, et al. The role of cytokines, adhesion molecules, and chemokines in interleukin-2-induced lymphocytic infiltration in C57BL/6 mice. J Clin Invest. 1996;97:1952–1959. doi: 10.1172/JCI118627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon KB, Oppenheim JJ. Chemokines in disease models and pathogenesis. Cytokine Growth Factor Rev. 1998;9:167–173. doi: 10.1016/s1359-6101(98)00005-7. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley LA, Kasina S, Mehra R, Adsule S, Admon AJ, Lonigro RJ, et al. CXCL5 promotes prostate cancer progression. Neoplasia. 2008;10:244–254. doi: 10.1593/neo.07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger C, Elimam H, Lefebvre J, Borgeat P, Marleau S. Involvement of endogenous leukotriene B4 and platelet-activating factor in polymorphonuclear leucocyte recruitment to dermal inflammatory sites in rats. Immunology. 2008;124:295–303. doi: 10.1111/j.1365-2567.2007.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Hegde A. Treatment with antileukinate, a CXCR2 chemokine receptor antagonist, protects mice against acute pancreatitis and associated lung injury. Regul Pept. 2007;138:40–48. doi: 10.1016/j.regpep.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetti C, Silva JS, Ferreira SH, Cunha FQ. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Br J Pharmacol. 2001;134:1619–1628. doi: 10.1038/sj.bjp.0704403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, Melby PC, Sarau HM, Raveendran M, Perla RP, Marelli-Berg FM, et al. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway. J Biol Chem. 2003;278:4675–4686. doi: 10.1074/jbc.M207006200. [DOI] [PubMed] [Google Scholar]
- Chen K, Wei Y, Alter A, Sharp GC, Braley-Mullen H. Chemokine expression during development of fibrosis versus resolution in a murine model of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol. 2005;78:716–724. doi: 10.1189/jlb.0205102. [DOI] [PubMed] [Google Scholar]
- Coelho FM, Pinho V, Amaral FA, Sachs D, Costa VV, Rodrigues DH, et al. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheum. 2008;58:2329–2337. doi: 10.1002/art.23622. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost EM, MacNee W. Potential role of IL-8, platelet-activating factor and TNF-alpha in the sequestration of neutrophils in the lung: effects on neutrophil deformability, adhesion receptor expression, and chemotaxis. Eur J Immunol. 2002;32:393–403. doi: 10.1002/1521-4141(200202)32:2<393::AID-IMMU393>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Wolburg H. Mini-review: transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, et al. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- Frick VO, Rubie C, Wagner M, Graeber S, Grimm H, Kopp B, et al. Enhanced ENA-78 and IL-8 expression in patients with malignant pancreatic diseases. Pancreatology. 2008;8:488–497. doi: 10.1159/000151776. [DOI] [PubMed] [Google Scholar]
- Fu W, Zhang Y, Zhang J, Chen WF. Cloning and characterization of mouse homolog of the CXC chemokine receptor CXCR1. Cytokine. 2005;31:9–17. doi: 10.1016/j.cyto.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramallo E, Marques T, Prats N, Beleta J, Kunkel SL, Godessart N. Resident cell chemokine expression serves as the major mechanism for leukocyte recruitment during local inflammation. J Immunol. 2002;169:6467–6473. doi: 10.4049/jimmunol.169.11.6467. [DOI] [PubMed] [Google Scholar]
- Ginsburg MI, Baldwin AL. Disodium cromoglycate stabilizes mast cell degranulation while reducing the number of hemoglobin-induced microvascular leaks in rat mesentery. Am J Physiol Heart Circ Physiol. 2004;286:H1750–H1756. doi: 10.1152/ajpheart.00605.2003. [DOI] [PubMed] [Google Scholar]
- Gountopoulou A, Leondaritis G, Galanopoulou D, Mavri-Vavayanni M. TNFalpha is a potent inducer of platelet-activating factor synthesis in adipocytes but not in preadipocytes. Differential regulation by PI3K. Cytokine. 2008;41:174–181. doi: 10.1016/j.cyto.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Grespan R, Fukada SY, Lemos HP, Vieira SM, Napimoga MH, Teixeira MM, et al. CXCR2-specific chemokines mediate leukotriene B(4)-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 2008;58:2030–2040. doi: 10.1002/art.23597. [DOI] [PubMed] [Google Scholar]
- Guo NH, Her GR, Reinhold VN, Brennan MJ, Siraganian RP, Ginsburg V. Monoclonal antibody AA4, which inhibits binding of IgE to high affinity receptors on rat basophilic leukemia cells, binds to novel alpha-galactosyl derivatives of ganglioside GD1b. J Biol Chem. 1989;264:13267–13272. [PubMed] [Google Scholar]
- Hadden JW. T-cell adjuvants. Int J Immunopharmacol. 1994;16:703–710. doi: 10.1016/0192-0561(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Higurashi M, Ohya Y, Joh K, Muraguchi M, Nishimura M, Terawaki H, et al. Increased urinary levels of CXCL5, CXCL8 and CXCL9 in patients with Type 2 diabetic nephropathy. J Diabetes Complications. 2008;23:178–184. doi: 10.1016/j.jdiacomp.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ho AW, Wong CK, Lam CW. Tumor necrosis factor-alpha up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology. 2008;213:533–544. doi: 10.1016/j.imbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Inamura H, Kurosawa M, Okano A, Kayaba H, Majima M. Expression of the interleukin-8 receptors CXCR1 and CXCR2 on cord-blood-derived cultured human mast cells. Int Arch Allergy Immunol. 2002;128:142–150. doi: 10.1159/000059405. [DOI] [PubMed] [Google Scholar]
- Jamur MC, Grodzki AC, Moreno AN, Swaim WD, Siraganian RP, Oliver C. Immunomagnetic isolation of rat bone marrow-derived and peritoneal mast cells. J Histochem Cytochem. 1997;45:1715–1722. doi: 10.1177/002215549704501215. [DOI] [PubMed] [Google Scholar]
- Jamur MC, Grodzki AC, Moreno AN, de Mello Lde F, Pastor MV, Berenstein EH, et al. Identification and isolation of rat bone marrow-derived mast cells using the mast cell-specific monoclonal antibody AA4. J Histochem Cytochem. 2001;49:219–228. doi: 10.1177/002215540104900209. [DOI] [PubMed] [Google Scholar]
- Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, Oliver C. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood. 2005;105:4282–4289. doi: 10.1182/blood-2004-02-0756. [DOI] [PubMed] [Google Scholar]
- Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- Koch AE, Volin MV, Woods JM, Kunkel SL, Connors MA, Harlow LA, et al. Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil activating peptide 78 in the rheumatoid joint. Arthritis Rheum. 2001;44:31–40. doi: 10.1002/1529-0131(200101)44:1<31::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Larsen JM, Bonefeld CM, Poulsen SS, Geisler C, Skov L. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J Allergy Clin Immunol. 2008;123:486–492. doi: 10.1016/j.jaci.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Mercer-Jones MA, Shrotri MS, Heinzelmann M, Peyton JC, Cheadle WG. Regulation of early peritoneal neutrophil migration by macrophage inflammatory protein-2 and mast cells in experimental peritonitis. J Leukoc Biol. 1999;65:249–255. doi: 10.1002/jlb.65.2.249. [DOI] [PubMed] [Google Scholar]
- Moepps B, Nuesseler E, Braun M, Gierschik P. A homolog of the human chemokine receptor CXCR1 is expressed in the mouse. Mol Immunol. 2006;43:897–914. doi: 10.1016/j.molimm.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Oliveira SH, Canetti C, Ribeiro RA, Cunha FQ. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation. 2008;31:36–46. doi: 10.1007/s10753-007-9047-x. [DOI] [PubMed] [Google Scholar]
- Onnheim K, Bylund J, Boulay F, Dahlgren C, Forsman H. Tumour necrosis factor (TNF)-alpha primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization. Immunology. 2008;125:591–600. doi: 10.1111/j.1365-2567.2008.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:245–251. doi: 10.1513/pats.200512-125SF. [DOI] [PubMed] [Google Scholar]
- Pinho V, de Castro Russo R, Amaral FA, de Sousa LP, Barsante MM, de Souza DG, et al. Tissue- and stimulus-dependent role of phosphatidylinositol 3-kinase isoforms for neutrophil recruitment induced by chemoattractants in vivo. J Immunol. 2007;179:7891–7898. doi: 10.4049/jimmunol.179.11.7891. [DOI] [PubMed] [Google Scholar]
- Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. Eur J Immunol. 2008;38:1215–1224. doi: 10.1002/eji.200838266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand EW, Stassen M, et al. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008;31:773–782. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RA, Flores CA, Cunha FQ, Ferreira SH. IL-8 causes in vivo neutrophil migration by a cell-dependent mechanism. Immunology. 1991;73:472–477. [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RA, Souza-Filho MV, Souza MH, Oliveira SH, Costa CH, Cunha FQ, et al. Role of resident mast cells and macrophages in the neutrophil migration induced by LTB4, fMLP and C5a des arg. Int Arch Allergy Immunol. 1997;112:27–35. doi: 10.1159/000237427. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Rubie C, Frick VO, Wagner M, Schuld J, Graber S, Brittner B, et al. ELR+ CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. doi: 10.1186/1471-2407-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler C, Lindstedt KA, Joutsiniemi S, Lappalainen J, Juutilainen T, Kolah J, et al. Selective activation of mast cells in rheumatoid synovial tissue results in production of TNF-alpha, IL-1beta and IL-1Ra. Inflamm Res. 2007;56:230–239. doi: 10.1007/s00011-007-6135-1. [DOI] [PubMed] [Google Scholar]
- Smith E, McGettrick HM, Stone MA, Shaw JS, Middleton J, Nash GB, et al. Duffy antigen receptor for chemokines and CXCL5 are essential for the recruitment of neutrophils in a multicellular model of rheumatoid arthritis synovium. Arthritis Rheum. 2008;58:1968–1973. doi: 10.1002/art.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Wadleigh DJ, Xia YR, Mar RA, Herschman HR, Lusis AJ. Cloning and genomic localization of the murine LPS-induced CXC chemokine (LIX) gene, Scyb5. Immunogenetics. 2002;54:599–603. doi: 10.1007/s00251-002-0501-5. [DOI] [PubMed] [Google Scholar]
- Smith WB, Gamble JR, Clark-Lewis I, Vadas MA. Interleukin-8 induces neutrophil transendothelial migration. Immunology. 1991;72:65–72. [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–142. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taktak YS, Lee M. A solid phase enzyme immunoassay for serum amyloid A (SAA) protein. Clinical evaluation. J Immunol Methods. 1991;136:11–16. doi: 10.1016/0022-1759(91)90243-9. [DOI] [PubMed] [Google Scholar]
- Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G, Korchak H. Rheumatoid arthritis. The role of neutrophil activation. Inflammation. 1984;8:S3–S14. doi: 10.1007/BF00915708. [DOI] [PubMed] [Google Scholar]


