Abstract
Background and purpose:
Blood vessel culture is gaining interest for use with transfection-based techniques, but alters the contractile properties of the vessels. The present study tested the effects of culture on the intrinsic tone of rat pulmonary arteries (PAs) and examined the function and expression of K+ channels regulating the resting membrane potential (Em) and tone of pulmonary artery smooth muscle cells (PASMCs).
Experimental approach:
Rat intrapulmonary arteries were isolated and cultured under standard and modified conditions. Contractile responses of fresh and cultured PA were compared using vessel myograph. Electrophysiology experiments on isolated PASMCs used the patch-clamp technique. K+ channel expression was quantified using reverse transcription and real-time PCR.
Key results:
After 4 days in culture vessels contracted to phenylephrine, but relaxation to carbachol was significantly impaired. Contractile responses to 10 mM KCl, 4-aminopyridine and tetraethylammonium increased, and vessels developed an uncharacteristic relaxation response to Ca2+-free solution, nifedipine and levcromakalim. PASMCs from cultured vessels were depolarized and K+ currents reduced, in association with down-regulation of Kv1.5, Kv2.1 and TWIK-related acid-sensitive K+ channel-1 mRNA. These changes were partially reversed by increased oxygenation of the culture medium or removing the endothelium before culture.
Conclusions and implications:
Culture of PA for 3–4 days induced loss of functional K+ channels, depolarization of PASMCs, Ca2+ influx, intrinsic tone and spontaneous constrictions, similar to the effects of chronic hypoxia. This limits the use of cultured vessels for studying excitation–contraction coupling, although oxygenating the culture medium and removing the endothelium can help to retain normal smooth muscle function.
Keywords: pulmonary artery, organ culture, membrane potential, K+ channel
Introduction
The pulmonary vasculature constricts in response to hypoxia, an important physiological response that optimizes gas exchange in the lung by matching blood perfusion to ventilation. In conditions of sustained hypoxia, this adaptation can become chronically established and thus generate pulmonary hypertension, leading to right ventricular hypertrophy, right heart failure and death (McLaughlin and McGoon, 2006;Han et al., 2007). The dynamic properties of vessel tone rely on the contraction of pulmonary artery (PA) smooth muscle cells (PASMC). Contraction is stimulated by a rise in the Ca2+ concentration in the cytosol ([Ca2+]i), which when complexed to calmodulin activates myosin light chain kinase (Hathaway et al., 1991). The rise in [Ca2+]i can be brought about by Ca2+ influx through L-type voltage-gated Ca2+ channels (VGCCs), upon depolarization of the membrane potential (Em). In relaxed rat PASMC, the Em usually is about −50 mV (Suzuki and Twarog, 1982). Background K+ conductances are critical for stabilizing the Em towards the K+ equilibrium potential (EK) and are counterbalanced by opposing leakage conductances (Casteels et al., 1977b; Nelson and Quayle, 1995; Post et al., 1995; Gurney et al., 2002). Loss of PASMC K+ conductance is observed in response to chronic hypoxia in vitro (Platoshyn et al., 2001) and in chronic hypoxia-induced pulmonary hypertension (Osipenko et al., 1998; Michelakis et al., 2002; Pozeg et al., 2003). Reduced K+ conductance and consequent membrane depolarization is therefore thought to play a role in hypoxia-induced PA constriction and pulmonary hypertension.
A number of K+ channels have been proposed to support or regulate the resting Em in PASMCs. The delayed rectifier Kv1.5 and Kv2.1 channels (nomenclature follows Alexander et al., 2008) have been proposed to account for an important component of the oxygen-sensitive K+ conductance in PASMCs (Post et al., 1995; Archer et al., 1998; Archer et al., 2001; Platoshyn et al., 2006), but they can not explain all the properties of the resting conductance in PASMC. Other channels like members of the K2P channel family (Gurney et al., 2003; Gardener et al., 2004; Goldstein et al., 2005; Olschewski et al., 2006), which are growing in importance in the cardiovascular system (Gurney and Manoury, 2009) and KCNQ channels (Joshi et al., 2006; Joshi et al., 2009), have been suggested to play a role.
The quest for the molecular correlates of the resting K+ conductance, however, has suffered from the lack of specific pharmacological tools. The use of RNA interference (RNAi) technology is a promising new approach: the siRNA-induced knock down of the expression of the TWIK-related acid-sensitive K+ channel (TASK)-1 channel gene reduced the oxygen-sensitive current in human PASMC (Olschewski et al., 2006). A similar approach applied to intact vessels indicated a role for the TASK-2 channel (Gonczi et al., 2006), and we recently showed efficient silencing of the TASK-1 (K2P 3.1) protein in intact murine PA using an RNAi-based strategy (Gurney and Hunter, 2005). The application of RNAi techniques to intact tissue requires channel activity to be studied in vessels that are maintained in culture for several days following their removal from the body. The culture of blood vessels has been shown, however, to alter their basic contractile properties. In PAs cultured for 4 days, the histological structure of the vessels was unaltered and the contractile phenotype well maintained (Guibert et al., 2005). Nevertheless the same vessels demonstrated spontaneous rhythmic contractions, increased sensitivity to depolarization by K+ and impaired relaxation to carbamylcholine (carbachol) challenge (Guibert et al., 2005). Interestingly, similar spontaneous contractile activity was described in PA from rats exposed to chronic hypoxia (Bonnet et al., 2001). In the latter study and others, myocytes isolated from these tissues additionally displayed membrane depolarization, basal Ca2+ influx through VGCC and loss of K+ conductance (Osipenko et al., 1998; Bonnet et al., 2001; Platoshyn et al., 2001).
The aim of the present study was to investigate whether, as the above observations suggest, organ culture causes alterations of the resting K+ conductances that regulate Ca2+ influx into the SMC of PAs in a similar manner to hypoxia. We show that rat PAs maintained in culture develop intrinsic tone due to a functional loss of the K+ channels involved in regulating Em, leading to depolarization, enhanced resting [Ca2+]i, raised tone and spontaneous contraction. These changes can be suppressed by removal of the endothelium and by increased oxygenation of the culture medium.
Methods
Tissue preparations and organ culture
All animal care and procedures complied with the UK Scientific Procedures (Animals) Act 1986. Male Sprague Dawley rats (250–300 g) were stunned and then killed by cervical dislocation. The lungs were rapidly removed into physiological salt solution (PSS) containing (in mM): NaCl 122, KCl 5, HEPES 10, NaH2PO4 0.5, KH2PO4 0.5, D-glucose 11, MgCl2 1, CaCl2 1.8, pH adjusted to 7.3 with NaOH. First- and second-order intrapulmonary arteries with external diameters of 200–1000 µm were dissected free of connective tissue and cut into short (1–4 mm) tubular segments. For experiments conducted on freshly isolated tissue, vessels were immediately mounted in a small vessel myograph, or processed for cell and RNA isolation. For organ culture experiments, unpressurized vessels were placed in Dulbecco's modified Eagle's medium (DMEM) with D-glucose (4.5 g·L−1), L-glutamine (4 mM) or glutamax (4 mM) and HEPES (25 mM) (Invitrogen Ltd., Paisley, UK) supplemented with penicillin-streptomycin 1% (PAA Laboratories GmbH, Pasching, Austria), in 96-well tissue culture plates. Tissues were maintained in a humidified incubator at 37°C under 5% CO2 in air. In specified experiments, the culture medium was bubbled with a mixture of 95% O2 and 5% CO2, or a mixture of 21% O2, 74% N2 and 5% CO2. For these ‘bubbling’ experiments the tissue was cultured inside screw-cap microcentrifuge tubes in 0.7 mL of culture medium constantly bubbled with the designated humidified gas mixture, at a rate of one to three bubbles per second. The tubes were maintained in an incubator at a temperature of 35–37°C. In all conditions, medium was renewed on a daily basis. After 1–4 days of culture, vessels with or without endothelium were either mounted on a small vessel myograph, or processed for cell isolation.
Cell isolation from rat PAs
Smooth muscle cells were isolated from fresh or cultured vessels by enzymic dissociation in a dissociation medium (DM) of the following composition (in mM): NaCl 110, KCl 5, HEPES 10, KH2PO4 0.5, NaH2PO4 0.5, NaHCO3 10, taurine 10, EDTA 5, D-glucose 10, CaCl2 0.16, MgCl2 2, phenol red 0.03, pH adjusted to 7.0 with NaOH. Vessels were first incubated in DM containing 1.5 mg·mL−1 papain (Sigma Aldrich, Gillingham, UK) for 1 h at 4°C. Then 1 mg dithiothreitol was added to the medium to catalyse papain activity, and vessels were incubated at 37°C for 5–6 min. Vessels were then transferred into a new vial containing 1.4 mg·mL−1 collagenase (type 1A, Sigma Aldrich) and incubated at 37°C for 4–5 min. The vessels were washed two times in fresh DM and gently triturated with a smoothed glass Pasteur pipette. The cells were stored at 4°C and used the same day either for patch-clamp experiments or for RNA isolation.
Isometric tension measurement
The contractile effects of a variety of drugs or ionic solutions on PA segments were measured using a small vessel myograph (Danish Myotechnology, Arhus, Denmark) as reported previously (Joshi et al., 2006). Data acquisition was performed using the Intracept-Chart V4.6.1 software (copyright J Dempster, University of Strathclyde, Glasgow, UK), a National Instruments DAQ Card-6036E and a BNC2110 interface. Vessels with an outer diameter above 800 µm were mounted on pins, smaller vessels were mounted on wire, and a basal tension of 4–5 mN was applied. Throughout the experiment tissues were bathed in PSS at 37°C and continuously aerated. Vessels were allowed to equilibrate for 30–40 min, then a reference constriction to the addition of 50 mM KCl [final concentration of K+ ([K]0): 55 mM] was obtained.
Vessels were washed, and the challenge with 50 mM KCl was repeated until reproducible contractions were produced. After washing and return to basal tension, a test solution was applied for 5–15 min. All drugs were diluted to the final concentration in PSS so that the vehicle volume did not exceed 1% of the final volume. In order to assess the effect of calcium-free solution, vessels were washed once and then incubated with a solution obtained by substituting equimolar MgCl2 for CaCl2 in PSS, adding 1 mM EGTA and readjusting pH to 7.3. Contractile responses were measured as a percentage of the last response to 50 mM KCl in each vessel.
Endothelial function was tested by addition of cumulative doses of carbachol to vessels preconstricted with 1 µM phenylephrine. In some experiments, the endothelium was removed by gently rubbing the lumen of the vessel with a wire, and LG-nitro-L-arginine (L-NAME, 300 µM) and indomethacin (10 µM) were added to the bath. When basal spontaneous constrictions were observed responses to contractile agents were deducted by measuring and averaging the peak force of two to three constrictions after addition of the drug and substracting the averaged peak force during baseline conditions.
Patch-clamp recordings
For electrophysiological experiments, cells were transferred to a recording chamber and superfused with PSS of the following composition (in mM): NaCl 124, KCl 5, HEPES 10, NaH2PO4 0.5, KH2PO4 0.5, D-glucose 10, sucrose 5, CaCl2 1.8, MgCl2 1, pH adjusted to 7.3 with NaOH. The whole-cell patch-clamp technique was used as previously described (Osipenko et al., 1997; Osipenko et al., 1998). Borosilicate glass pipettes were filled with the following solution (in mM): KCl 130, HEPES 10, EGTA 1, MgCl2, pH adjusted to 7.2 with KOH. For K+ current recording under voltage clamp, 10 mM tetraethylammonium (TEA) and 10 µM glibenclamide were added to the bath solution. Delayed rectifier K+ current IK(V) and the non-inactivating K+ current IK(N) were recorded from PASMC as previously described. Briefly, IK(V) was elicited by applying a voltage step to 0 mV for 250 ms from a holding potential of −80 mV. The cells were then clamped at 0 mV for 6–10 min, allowing IK(V) to inactivate, and IK(N) was measured as the non-inactivating current at 0 mV, when the current had stabilized. The current density versus voltage relationship of IK(N) was assessed by applying a 1.2 s voltage ramp from 60 to −100 mV. Resting Em measurements under current clamp (I= 0) were performed in separate experiments where the whole-cell configuration was obtained by perforating the cell membrane using 0.3 mg·mL−1 amphotericin B (Sigma Aldrich) in the pipette solution. This should prevent intracellular medium dialysis, as previously described (Rae et al., 1991). Voltage and current commands were generated with the Whole-Cell Analysis Program V3.6.6 data acquisition software (John Dempster, the University of Strathclyde, Glasgow, UK) through a BNC 2090 interface (National Instruments Corporation, Newbury, UK) and an Axopatch 200A amplifier (Axon CNS, Molecular Devices, Sunnyvale, CA, USA). Em values were corrected by −3 mV to compensate for the liquid junction potential between the external and pipette solutions (Barry and Lynch, 1991).
cDNA synthesis and real-time reverse transcription and PCR (RT-PCR)
Cells isolated from fresh or cultured rat PAs were placed in RNase- and DNase-free collection tubes. PASMC may have been contaminated by endothelial and/or adventitial cells as it was not possible to remove these from the vessels without losing a large amount of smooth muscle. The number of these cells should however have been negligible compared with the bulk of PASMC. After total RNA extraction using a RNeasy Microkit including DNase treatment (Qiagen, Crawley, UK), first-strand cDNA synthesis was performed using SupersciptTM III (Invitrogen Life technology, Paisley, UK), following the manufacturers' protocol but with random hexamers (500 ng, Invitrogen) and RNAse out (Invitrogen). For each sample, at least 250 ng of total RNA was used, and a reaction mix in which SupersciptTM III had been omitted was used as negative control. Real-time PCR was performed using the fluorescent dye, SYBR Green and the ABI Prism 7700 system (Perkin-Elmer, Foster city, CA, USA). Primers pairs to detect TASK-1 and β-actin in PCR reactions were designed using Primer 3 v.0.4.0 software (Whitehead Institute and Howard Hughes Medical Institute, http://primer3.sourceforge.net/). The following primer pairs were used: rat TASK-1 (accession number: NM_033376.1): TTATACCTCCCCTGGGCTCT (forward), CAGAAGGGGAGTGGACACAT (reverse); rat β-actin (accession number: BC063166): TCTGTGTGGATTGGTGGCTCTA (forward), CTGCTTGCTGATCCACATCTG (reverse). Real-time PCR of rat Kv1.5 (accession number: NM_012972) and Kv2.1 (accession number: NM_013186) used Quantitect primer assays (Qiagen, Crawley, UK). First-strand cDNA (1 µL of each) and its respective negative control were used as the template in a PCR reaction with 12.5 µL Sybr Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), forward and reverse primers (MWG Biotech, Ebersberg, Germany, 0.3 µM each) and DNase-free water (10 µL) in a final volume of 25 µL. A first step of 10 min at 95°C was followed by 40 cycles, including denaturation (95°C, 15 s) and annealing-extension (60°C, 60 s) steps. To ensure the specificity of the reaction, a dissociation curve protocol was performed at the end of the reaction, and single peak was obtained. PCR products were sequenced at the DNA sequencing facility, University of Manchester. For each sample, the relative amount of RNA of the gene of interest (GI) was 2-Ct, where Ct stands for ‘cycle threshold’, defined as the cycle number at which the fluorescence signal (i.e. the DNA amount in the reaction) reached an arbitrary intensity threshold. This amount of RNA was normalized by the value of 2-Ct obtained for the PCR reaction targeting the house keeping gene β-actin and always performed simultaneously. Therefore the presented raw data (2-ΔCt) is the ratio of the amount of GI mRNA relative to the amount of β-actin.
Data analysis and statistical analysis
Data handling and statistical analysis were performed with Origin 7.5 software (Originlab corporation, Northampton, MA, USA) and Prism 5.02 (GraphPad Software, Inc., La Jolla, CA, USA). Average data are expressed as the mean ± SEM. For EC50 calculations, dose response curves were fit using the Hill equation. Normality of data distributions was assessed using the Shapiro-Wilk test and parametric or non-parametric statistical tests applied accordingly. Comparisons of two groups employed the Student's t-test or Mann–Whitney U-test. When more than two groups were compared, data were analysed using one-way analysis of variance (anova) followed by a post hoc Dunnett's test (parametric) or a Kruskal-Wallis test (non-parametric) followed by a Dunn post hoc test. Each cultured group was compared with the ‘fresh’ group unless specified. In all cases data samples were considered as significantly different if P < 0.05.
Materials
Phenylephrine, nifedipine, levcromakalim, 4-aminopyridine (4-AP), TEA chloride, L-NAME, indomethacin, glibenclamide, carbachol, dithiothreitol and taurine were purchased from Sigma Aldrich (Gillingham, UK). Phenylephrine, 4-AP, TEA, L-NAME and carbachol were diluted in water. Nifedipine, levcromakalim and glibenclamide were dissolved in DMSO, and indomethacin was dissolved in ethanol. The maximum DMSO or ethanol final concentration in the bath was 0.1% and had no effect on the mechanical properties of the vessels.
Results
Effect of culture on the contractile response to KCl and phenylephrine
Only vessels that constricted to 50 mM KCl (minimum response: 0.14 mN) were analysed. Vessels that fulfilled this criterion were 100% when fresh, 100% at day 1 in culture, 92% at day 2, 85% at day 3 and 77% at day 4 in culture. The average contractile responses to 50 mM KCl at days 1 and 2 of culture were 2.4 ± 0.2 mN and 2.1 ± 0.3 mN, respectively, which were not different from the response observed with fresh vessels (2.5 ± 0.3 mN). The amplitude of contraction diminished significantly by day 3 (1.5 ± 0.1 mN, P < 0.05) and day 4 (0.9 ± 0.2 mN, P < 0.05). At days 3 and 4 in some experiments, spontaneous contractions were observed, mostly in larger vessels dissected from the conduit intrapulmonary artery. The appearance of spontaneous contractions was too variable to be analysed in a meaningful way.
Although the addition of 10 mM KCl (final [K]0= 15 mM) had no or little effect on fresh vessels, it caused significant contraction at culture day 3 (P < 0.001) and day 4 (P < 0.001), amounting to 80% of the reference 50 mM KCl response (Figure 1A,B). The response to 1 µM phenylephrine, measured relative to the contraction induced by 50 mM KCl, appeared slightly increased throughout the culture period, with significance reached at culture day 3 in comparison with fresh vessels (P= 0.04, Figure 1A,C).
Figure 1.
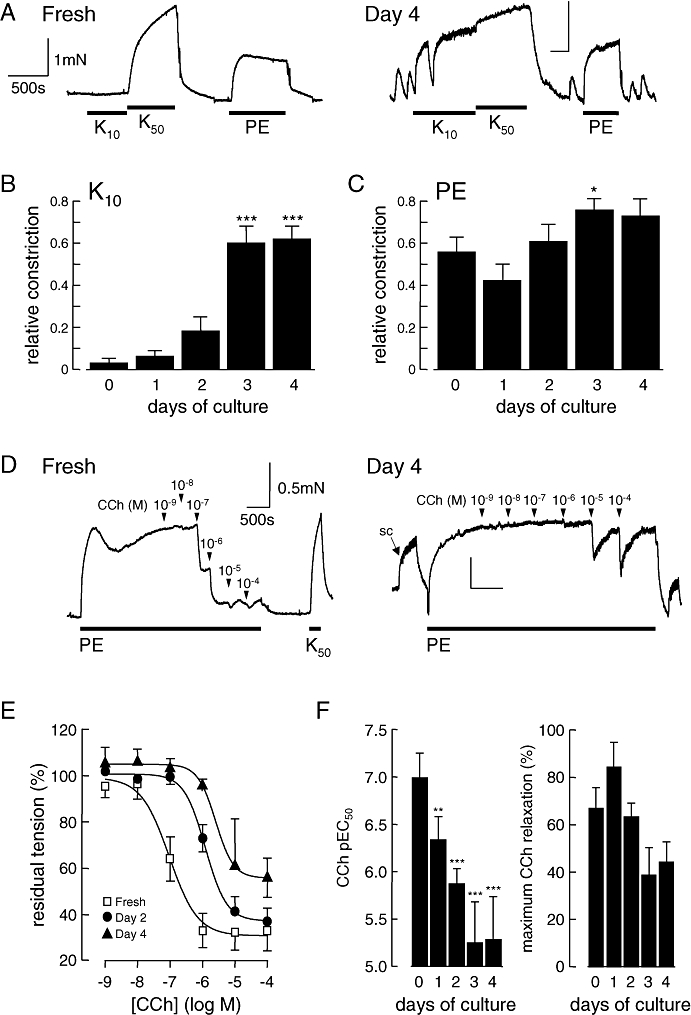
Culture of pulmonary arteries alters their basic pharmaco-mechanical properties. (A) Typical original recordings of tension developed by fresh or 4 day cultured rat intrapulmonary arteries, in basal conditions and after adding 10 mM KCl (K10), 50 mM KCl (K50) and 1 µM phenylephrine (PE). Cultured vessels also demonstrate spontaneous contractions and intense contractile response to 10 mM KCl. Vertical scale: 1 mN. Horizontal scale: 500 s. (B,C) Average responses to 10 mM KCl (B) or 1 µM PE (C) of intrapulmonary arteries segments either freshly isolated or sampled at various culture times. Data are expressed as the average fraction of the reference contractile response to the addition of 50 mM KCl. n= 5–11 animals. *P < 0.05, ***P < 0.001 compared with freshly isolated vessels. (D–F) Endothelium-dependent relaxation of freshly isolated and cultured pulmonary arteries in response to carbachol (CCh). (D) Typical original recordings of relaxation to cumulative doses of CCh (10−9–10−4 M) on freshly isolated (left) or cultured (day 4, right) vessels preconstricted with 1 µM PE. (SC, spontaneous constriction; K50, addition of 50 mM KCl, eliciting constriction of the vessel). Vertical scale: 0.5 mN. Horizontal scale: 500 s. (E) Average responses of freshly isolated and 2 or 4 day cultured pulmonary arteries to increasing CCh concentrations. Isometric tension data are expressed as the fraction of the steady state tension developed by the vessels in response to PE. n= 4–5 animals. (F) pEC50 (left) and maximum response of CCh-induced relaxation (at 100 µM, right) of freshly isolated and cultured pulmonary arteries. n= 4–5 animals. **P < 0.01; ***P < 0.001 compared with fresh vessels.
Effect of culture on endothelium-dependent relaxation
Endothelium-dependent relaxation was assessed by observing the relaxation induced by carbachol when applied to vessels preconstricted with 1 µM phenylephrine. Increasing concentrations of carbachol were added cumulatively to freshly isolated PAs and vessels maintained in culture for 1–4 days (Figure 1D). The potency of carbachol at eliciting relaxation diminished progressively through the culture period in comparison with the response in fresh vessels (Figure 1E). The concentration of carbachol giving a 50% maximal relaxation (EC50) was increased, as indicated by a fall in the pEC50 from 7.0 in fresh tissue to 5.3 after 4 days in culture (Figure 1F, left), reaching significance as soon as culture day 1 (P= 0.01). However, although the maximum response appeared to be smaller after culture, the difference did not reach statistical significance (Figure 1F, right).
Effect of culture on resting tone of PA, role of Ca2+ influx
If the Em of PA smooth muscle becomes depolarized during culture, then it might be expected that Ca2+ influx through VGCC would be activated, leading to an increase in the resting, intrinsic tone of the artery. To test this hypothesis, we first investigated the effect of removing extracellular Ca2+. When exposed to Ca2+-free solution, fresh vessels showed no or little change in tension. In contrast, after 3–4 days in culture, removing extracellular Ca2+ caused significant relaxation, indicating the presence of basal tone due to Ca2+ influx (Figure 2A). Nifedipine (1 µM), a selective inhibitor of VGCC, also caused relaxation in vessels subject to 3–4 days in culture, but not in fresh vessels (Figure 2B). Levcromakalim (10 µM), which activates ATP-sensitive K+ channel (KATP) channels and causes membrane hyperpolarization, also reduced baseline tone in vessels cultured for 3–4 days, but not in fresh vessels (Figure 2C). Responses to Ca2+-free solution, nifedipine and levcromakalim all appeared gradually over 4 days in culture, reaching significance at day 4 with P= 0.01, P= 0.008 and P= 0.03 respectively. In addition, Ca2+ removal, nifedipine and levcromakalim abolished spontaneous constrictions when they were present.
Figure 2.

Unmasking of sensitivity to Ca2+-free solution, nifedipine, and levcromakalim in cultured pulmonary arteries. Typical original recordings of tension developed by freshly isolated (left panels) or cultured (centre panels) rat intrapulmonary arteries and averaged response (right panels) following application of Ca2+-free physiological salt solution (0Ca, A), 1 µM nifedipine (B) and 10 µM levcromakalim (C). Constriction is expressed as the average fraction of the reference contractile response to the addition of 50 mM KCl (K50). n= 5–11 animals for 0Ca and nifedipine; n= 5–6 animals for levcromakalim. *P < 0.05; **P < 0.01, compared with fresh vessels. Horizontal scale: 1000 s.
Effect of culture on the contractile response to K+ channel blockers
At 10 mM, TEA non-specifically blocks large conductance Ca2+-sensitive K+ channel (BKCa) channels (Bolton and Lim, 1989;Clapp and Gurney, 1991;Bolton and Imaizumi, 1996) and some members of the Kv channel family (Coetzee et al., 1999; Smirnov et al., 2002). 4-AP is a non-specific Kv channel blocker and at 1 mM would be expected to block Kv1.5 and Kv2.1 (Osipenko et al., 1997; Archer et al., 1998; Coetzee et al., 1999). Glibenclamide is a selective KATP channel blocker causing close to maximal inhibition at 10 µM (Clapp and Gurney, 1992). In freshly isolated PAs, 4-AP (1 mM) and glibenclamide (10 µM) rarely elicited any response, whereas TEA (10 mM) induced a small contraction, amounting to 10 ± 4% of the reference constriction to 50 mM KCl. During organ culture, PAs became increasingly sensitive to TEA and 4-AP. The contractile response to 1 mM 4-AP was significantly larger (P= 0.04) in cultured vessels at day 4 than in fresh vessels (Figure 3A). TEA-induced constriction of cultured vessels was significantly larger at day 3 (P= 0.05) and day 4 (P= 0.01) (Figure 3B). In contrast, no significant response was observed to glibenclamide in cultured or fresh vessels.
Figure 3.

Contractile response to 4-aminopyridine (4-AP) and tetraethylammonium (TEA) in cultured pulmonary arteries. Average response to 1 mM 4-AP (A) or 10 mM TEA (B) of intrapulmonary arteries segments either freshly isolated (0 day) or taken at various culture times (3 or 4 days). Constriction is expressed as the fraction of the tension developed by the vessels in response to the control 50 mM KCl application. n= 5–6 animals. *P < 0.05; **P < 0.01, compared with freshly isolated vessels.
Effect of endothelium removal on the contractile properties of freshly isolated PAs
The higher reactivity of cultured vessels may be the consequence of the concomitant alteration of endothelial function, which regulates the tone of the smooth muscle layer. In order to test this hypothesis, we investigated the response to adding 10 mM KCl, 1 mM 4-AP, 10 µM levcromakalim or 1 µM nifedipine on freshly isolated PAs from which the endothelium had been functionally removed by rubbing with a hair and incubating with 300 µM L-NAME and 10 µM indomethacin. The functional disruption was confirmed by loss of the relaxing effects of 0.1 and 1 µM carbachol, in vessels preconstricted with 1 µM phenylephrine. In endothelium-free vessels the response to 10 mM KCl was more marked (P= 0.02) in comparison with freshly isolated, intact vessels (Figure 4A,C). However, vessels denuded of endothelium showed little response to nifedipine, levcromakalim or 4-AP, as observed in intact vessels (Figure 4B). So the absence of endothelium per se can partially explain the higher sensitivity of organ-cultured PA to the addition of 10 mM KCl, but not to the other drugs.
Figure 4.

Pharmaco-contractile properties of freshly isolated intrapulmonary arteries segments either intact or having their endothelium removed. To ensure complete endothelium dysfunction in endothelium-free vessels the experiments were carried out in the presence of L-NAME 300 µM and indomethacin 10 µM. (A,B) Typical original recordings of tension developed by intact (E+) or endothelium-disrupted (E−) rat intrapulmonary arteries in response to 10 mM KCl (K10) and 50 mM KCl (K50) (A) and to 1 mM 4-AP, 1 µM nifedipine and 10 µM levcromakalim (B). Vertical scale: 1 mN. Horizontal scale: 2000 s. (C) Average response to 10 mM KCl of intact (E+) or endothelium-disrupted (E−) vessels from n= 5 animals. Data are expressed as the average fraction of the reference contractile response to the addition of 50 mM KCl. *P < 0.05 compared with intact vessel. 4-AP, 4-aminopyridine; CCh, carbachol; L-NAME, LG-nitro-L-arginine; PE, phenylephrine.
Effect of oxygenation of the culture medium on the contractile properties of PAs
The uncharacteristic reactivity of the cultured PAs that we observed resembled the reactivity of PAs exposed to hypoxia (Osipenko et al., 1997; Bonnet et al., 2001). As intact vessels are several layers thick, we hypothesized that there may be barriers to oxygenating the muscle cells during culture, such that they become hypoxic. To test this hypothesis, we increased the O2 gradient through the tissue by bubbling the medium with a gas mixture of 95% O2 and 5% CO2. As a control we bubbled separate vessels from the same animals with a gas mixture containing 21% O2 and 5% CO2, to account for any stirring effect of the gas bubbling in the culture tube. The O2 level, measured at the end of the experiments with an ISO2 oxygen metre (World Precision Instruments, Sarasota, FL, USA), was around 60% in the medium bubbled with the 95% O2 gas mixture, compared with 20% in normal O2 conditions. Figure 5 shows the responses to different drugs and 10 mM K+ applied to vessels cultured for 4 days, in either condition. As observed after culture in standard conditions, vessels bubbled with 21% O2 showed a normal response to 1 µM phenylephrine, constriction in response to 10 mM K+ and relaxation in response to nifedipine and levcromakalim. Bubbling the medium with 95% O2 did not have any effect on the response to 1 µM phenylephrine or 10 mM KCl compared with 21% O2, but it reduced significantly (P < 0.05) the relaxation to nifedipine. Relaxation to levcromakalim also appeared to be reduced, but the difference did not reach statistical significance. As seen in standard culture conditions, the relaxation response to 1 µM carbachol was abolished when vessels were cultured in medium bubbled with 21% O2. In contrast, the same concentration of carbachol elicited a clear relaxation in vessels bubbled with 95% O2, although it was variable in amplitude and the difference between 95% and 21% O2 did not reach statistical significance.
Figure 5.
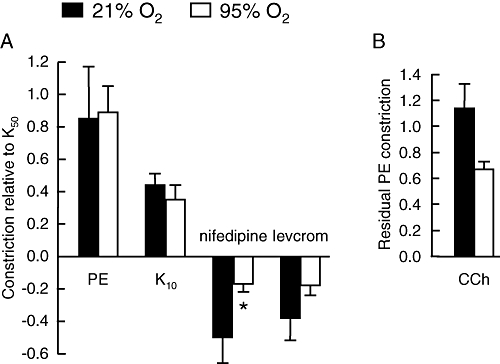
Oxygenation of the culture medium partially inhibits the effects of culture. (A) Mean contractile responses to 1 µM phenylephrine (PE), 10 mM KCl (K10), 1 µM nifedipine and 10 µM levcromakalim (levcrom) measured relative to the response activated by 50 mM KCl (K50). (B) Mean relaxation responses to 1 µM carbachol (CCh) expressed as the residual fraction of PE-induced pre-tone. Intrapulmonary arteries were cultured for 4 days in medium bubbled with a gas mixture containing either 21% O2 or 95% O2. n= 3–4 animals for carbachol, n= 5–14 animals for the other agents. *P < 0.05 comparing culture conditions.
Effect of organ culture on the passive electrical properties of PASMCs
Using the ‘perforated’ configuration of the patch-clamp technique in current clamp mode (current clamped at 0 mA), the Em of PASMCs isolated from fresh PAs or arteries maintained in culture for 4 days was recorded. Cells from vessels cultured under standard conditions were slightly although significantly depolarized by 4 mV (P < 0.05) in comparison with cells from freshly isolated PAs (Figure 6A). In contrast, the Em of cells isolated from PAs cultured in an O2-enriched medium was not significantly different from fresh cells. In our patch-clamp experiments, the mean input resistances of the cells in different conditions were as follows: fresh: 6 ± 2 GΩ, n= 20; standard culture: 6 ± 1 GΩ, n= 17; culture bubbled with O2: 7 ± 2 GΩ, n= 21. None of these values differed significantly. The mean cell capacitance values were 12 ± 1 pF (fresh, n= 20), 12 ± 1 pF (cultured in control medium, n= 18) and 13 ± 1 pF (cultured in oxygenated medium, n= 21) with no significant difference among the three groups.
Figure 6.

Culture of pulmonary arteries depolarizes the membrane potential and decreases the K+ currents IK(V) and IK(N) in smooth muscle cells. (A) Mean membrane potential ± SEM values in freshly isolated and 4 day cultured in either control (Standard culture) or O2-enriched medium (Bubbled O2) conditions are represented. n= 11–12 cells, from 3–5 animals per groups. *P < 0.05 compared with fresh vessels. (B,C) K+ current recording from isolated pulmonary artery smooth muscle cell using the whole-cell configuration of the patch-clamp technique. (B) Left: IK(V) recorded from myocytes isolated from either freshly isolated (Fresh) or 4 day cultured pulmonary artery (PA) (Cultured), using the described voltage protocol (upper panel). Each trace figures one representative current normalized against the cell capacitance. Right: Mean current density ± SEM of IK(V) at 0 mV (maximal value of outward current during the 0 mV step) from myocytes isolated from freshly isolated (Fresh, n= 8) and 4 day cultured PA in either standard conditions (Std culture, n= 6) or in O2-enriched medium (Bubbled O2, n= 8). ***P < 0.001 compared with Fresh. (C) Left: IK(N) recorded from myocytes isolated from either freshly isolated (Fresh) or 4 day cultured PA (Cultured) using the described voltage ramp stimulation (inset). Each trace figures one representative current normalized against the cell capacitance. Right: mean current density of IK(N)± SEM, measured as the non-inactivating current at 0 mV, from myocytes isolated from freshly isolated (Fresh, n= 8) and 4 day cultured PA in either standard conditions (Std culture, n= 6) or in O2-enriched medium (Bubbled O2, n= 9). **P < 0.01 compared with Fresh.
Effect of organ culture on the K+ currents IK(V) and IK(N)
The observed depolarization of PASMC isolated from cultured PA may be caused by a functional decrease in the background K+ currents responsible for driving the Em towards EK. In order to test this hypothesis, we measured the amplitudes of the delayed rectifier K+ current IK(V) and the non-inactivating K+ current IK(N), normalized against cell capacitance, in PASMCs from either freshly isolated or 4 day cultured PA. The amplitude of IK(V) at 0 mV was measured as the maximal value of the outward current, recorded during a step to 0 mV from the −80 mV holding potential. Figure 6B shows that IK(V) in PASMCs from vessels cultured in standard conditions or in O2-enriched conditions were significantly reduced (P < 0.001) compared with IK(V) in cells from freshly isolated vessels. The amplitude of IK(N) was also significantly (P < 0.01) smaller in PASMC from vessels cultured in standard conditions or O2-enriched conditions compared with cells from freshly isolated vessels (Figure 6C). K+ currents recorded from PASMC isolated from vessels cultured in medium bubbled with air displayed similar reductions in amplitude (data not shown).
Effect of organ culture on the mRNA levels of TASK-1, Kv1.5 and Kv2.1 in PA
The TASK-1, Kv1.5 and Kv2.1 genes code for K+ channels that have been proposed to regulate the resting Em in PASMCs (Archer et al., 2001; Gurney et al., 2003; Archer et al., 2004; Olschewski et al., 2006). The mRNAs corresponding to the TASK-1, Kv1.5 and Kv2.1 genes were quantified in 2 day cultured PA and compared with their corresponding levels in vessels freshly isolated from the same animals using RT-PCR (Figure 7). The relative amounts of TASK-1, Kv1.5 and Kv2.1 mRNAs in PA cultured in standard conditions were reduced by 75%, 84% and 74% compared with the respective amount in freshly isolated vessels, and these reductions were statistically significant for all three channels (P < 0.001 for TASK-1 and Kv1.5; P < 0.05 for Kv2.1). Oxygenation did not prevent the loss of expression, with respective reductions of 64%, 89% and 93% respectively (P < 0.001 for TASK-1 and Kv1.5, P < 0.01 for Kv2.1).
Figure 7.

Culture of pulmonary arteries decreases the mRNA expression of K+ channels. The expressions of TWIK-related acid-sensitive K+ channel (TASK)-1 (n= 5–6), Kv1.5 (n= 5–6) and Kv2.1 (n= 4–5) genes in pulmonary arteries (PAs) from freshly isolated (Fresh) and 2 day cultured PA in either standard conditions (Std culture) or in O2-enriched medium (Bubbled O2) were investigated by RT-PCR. The relative amount of each K+ channel subunit mRNA was normalized to β-actin mRNA level. Data are expressed as the average ratio [2-Ct (channel subunit)/2-Ct (β-actin)]± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 compared with freshly isolated vessels.
Effect of endothelium removal on the contractile properties of cultured isolated PAs
The small depolarization observed in cultured and isolated PASMC may not explain all the effects of culture on contractility. As it is clear that culture alters endothelial function, perhaps a constrictor mediator released by the endothelium contributes to increased intrinsic tone and reactivity of the vessels. This hypothesis was tested by investigating the response to nifedipine in arteries that had their endothelium gently removed either immediately before the culture period or at the end of the culture period just before mounting the vessels for myography. Endothelium disruption was confirmed by the lack of response to 10 µM carbachol (Figure 8A,B). The vessels that had their endothelium removed after the culture clearly relaxed to nifedipine by over 30% of the response to KCl (Figure 8B,C). Vessels from the same animals, but cultured without the endothelium, did not display this sensitivity to nifedipine (Figure 8A,C). The response to nifedipine was significantly larger in vessels cultured with an intact endothelium (P= 0.02). Thus the presence of the endothelium is necessary for intrinsic tone to develop during culture, but not necessary for the intrinsic tone to be maintained, for it was persistent during the myography study following culture in the absence of endothelium (Figure 8B).
Figure 8.

Removal of endothelium of pulmonary artery (PA) before organ culture prevents the sensitivity to nifedipine from appearing. (A,B) Original recordings of tension developed by rat PA in which endothelium was removed either before (A) or after (B) 4 days of organ culture, in response to 1 µM phenylephrine (PE), carbachol (10−7–10−5 M) and 1 µM nifedipine. Both traces represent recordings from vessels isolated from the same animal, and average responses of vessels from n= 3 animals are presented in (C). Data are expressed relative to the reference contractile response to addition of 50 mM KCl. *P < 0.05.
Discussion
Membrane depolarization underpins intrinsic tone and spontaneous activity in organ culture
This study has established that organ culture of small segments of rat PA causes significant depolarization of the smooth muscle cells, resulting in the development of intrinsic tone due to Ca2+ influx through VGCC. The appearance of intrinsic tone was demonstrated by the relaxant effect, in cultured but not freshly isolated PA, of conditions blocking Ca2+ entry through VGCCs. Both nifedipine, which directly blocks VGCC, and levcromakalim, which blocks VGCCs by opening KATP channels and hyperpolarizing the Em, caused vasodilation. These observations imply that a basal Ca2+ influx through VGCCs exists in cultured PA in the absence of any stimulus. The intrinsic tone seen after 3–4 day culture could be due in part to reduced activity of the K+ channels mediating the resting Em. The K+ currents IK(N) and IK(V), which drive Em towards EK and act as a brake to prevent membrane depolarization (Evans et al., 1996; Gurney et al., 2002; Archer et al., 2004), were smaller in PASMCs from cultured vessels compared with fresh vessels. Moreover, the culture process led to the down-regulation of mRNAs encoding the putative molecular correlates of these conductances, namely the K2P channel, TASK-1 (Gurney et al., 2003) and the delayed rectifiers Kv1.5 and Kv2.1 (Archer et al., 2004), all of which have been proposed to maintain the Em of PASMCs (Osipenko et al., 1997; Archer et al., 1998; Archer et al., 2001; Gurney et al., 2003; Archer et al., 2004; Olschewski et al., 2006).
Patch-clamp recordings of Em confirmed the depolarization of PASMC isolated from cultured vessels. Recordings were made using the perforated-patch configuration, in order to prevent dialysis of the intracellular medium and to match conditions to the intact artery as closely as possible. The range of Em values measured (−43 to −38 mV) is similar to previous reports in similar conditions (Reeve et al., 1995). The depolarization at culture day 4 was only about 4–5 mV. This is sufficient, however, to increase significantly the opening probability of L-type channels, which rises exponentially from −50 mV (Nelson et al., 1990). Casteels et al. (1977b) demonstrated a steep relationship between the Em and contraction over the range −40 to −35 mV, with the contraction threshold at around −40 mV. The authors noted that an initial depolarization of 4 mV was sufficient to evoke contraction. Thus the small depolarization caused by culture would be sufficient for the excitation–contraction threshold to be reached, inducing steady leak of Ca2+ into the cells and intrinsic tone.
In addition to raised intrinsic tone, vessels cultured for 4 days often displayed spontaneous rhythmic contractions. In accordance with the study of Guibert et al. (2005), these spontaneous contractions were abolished by Ca2+-free solution, nifedipine and levcromakalim. Therefore the spontaneous contractions may also be a consequence of the depolarized Em, suggesting that the raised intrinsic tone and spontaneous activity in cultured vessels may be linked through the loss of K+ conductance and membrane depolarization. We cannot rule out that additional Ca2+ entry pathways contribute to the increased basal vessel tone and spontaneous activity that develop in culture. Although not studied here, increased expression or altered voltage sensitivity of VGCCs might also contribute. On the other hand, L-type currents were reduced after organ culture of cerebral arteries, whereas non-specific, store-operated cationic conductances were shown to be increased by 4 days of organ culture in rat tail and cerebral arteries (Dreja et al., 2001; Bergdahl et al., 2005). Thus abnormal calcium handling in cultured PAs may be another cause for alteration of the basal tone.
Does endothelial dysfunction account for the increased vasoreactivity after organ culture?
In freshly isolated PA, the smooth muscle depolarization induced by the addition of 10 mM KCl, which evokes about 7 mV depolarization, was not sufficient to launch measurable constriction: the threshold for a significant response is usually around 20 mM (Casteels et al., 1977a; Guibert et al., 2005). However, in 3 and 4 day cultured vessels, 10 mM KCl was sufficient to evoke contraction. This is consistent with the depolarization we recorded in PASMC, which makes the threshold potential easier to be reached. Nevertheless, a significant contractile response to 10 mM KCl was also observed in fresh vessels when the endothelium was removed. Endothelial dysfunction can therefore explain the culture-induced sensitivity to 10 mM KCl, because over the same period in culture there was a marked decrease in the ability of carbachol to produce endothelium-dependent dilation. In freshly isolated PA, basal release of NO (Fleming and Busse, 1999) or endothelium-derived hyperpolarizing factor (Busse et al., 2002) from the endothelium may provide a tonic dilator influence, which antagonizes the constrictor effects of high [K]0 and is lost in culture. Consistent with our data, Guibert et al. (2005) found that 4 days of culture induced an increased sensitivity to K+ and complete loss of response to carbachol, without a change in the endothelium-dependent response to the Ca2+ ionophore, A23187, or loss of endothelial cell structure. Therefore the response to moderate K+ in cultured PAs may be explained by a loss of the pathway linking muscarinic receptors in endothelial cells to vasodilators combined with PASMC depolarization.
Interestingly, we also showed that removal of the endothelium just before culture could abolish the increased sensitivity to nifedipine. This last observation implies that altered activity of an endothelium-derived mediator, which remains to be identified, is responsible for depolarizing the smooth muscle cells and causing the development of the intrinsic tone during culture. Removal of the endothelium just after organ culture could not prevent the development of an intrinsic tone in PA from the same animals. Two conclusions can be drawn from these data: (i) altered activity in the muscle layer of cultured artery is sufficient to explain the nifedipine-sensitive tone, which may reflect the small depolarizations measured in isolated PASMC; and (ii) the altered properties of the SMC layer in cultured vessels are the consequence of long-lasting and/or ‘genomic’ effects caused by the influence of an ‘abnormal’ endothelium. Those irreversible effects would possibly include down-regulation of the expression of K+ channels, which we observed in the PASMC from the cultured vessels.
Together the data suggest that during culture, the endothelium undergoes specific alterations that switch off the normal ‘relaxing’ acetylcholine/NO pathway, and modify the properties of the smooth muscle layer through the altered activity of an unknown factor. Endothelin-1 (ET-1), which is known to produce a long-lasting depolarization of arterial smooth muscle (Van Renterghem et al., 1988), is an endothelium-derived agent that could potentially mediate such an effect. There is evidence that ET-1 can reduce K+ channel expression in PASMC (Whitman et al., 2008), although it is not clear how hypoxia affects ET-1 secretion. Although 24 h exposure to hypoxia was reported to enhance ET-1 secretion from bovine coronary artery endothelial cells (Hieda and Gomez-Sanchez, 1990), it reduced the release of ET-1 from pulmonary endothelial cells (Markewitz et al., 1995) while stimulating the release of contracting factors distinct from ET-1 (Gaine et al., 1998). On the other hand, short-term organ culture was reported to increase sensitivity to ETB receptor agonists and increase the expression of ETB receptors in several vascular beds (Adner et al., 1998; Johnsson et al., 2008), which would support an increased action of ET-1. Although increased release of a constrictor, such as endothelin, would provide a simple explanation for the development of intrinsic tone in culture, we cannot rule out the involvement of other substances, the release of which changes during culture.
Organ culture mimics chronic hypoxia-induced vasoconstriction
Membrane depolarization is consistently observed in PASMCs from rats exposed to chronic hypoxia (Smirnov et al., 1994; Osipenko et al., 1998; Bonnet et al., 2001) and in PASMC cultured in a hypoxic environment (Wang et al., 1997; Platoshyn et al., 2001). Hypoxic vessels also show relaxation responses to nifedipine (Rodman, 1992; Bonnet et al., 2001) and levcromakalim (Bonnet et al., 2001), spontaneous contractile activity and increased PASMC [Ca2+]i (Bonnet et al., 2001). Moreover, chronic in vivo hypoxia induces down-regulation of Kv1.5 protein, as well as the K+ currents IK(V) and IK(N) (Smirnov et al., 1994; Osipenko et al., 1998; Hong et al., 2004). Hypoxia also down-regulates Kv channels in cultured rat PASMCs (Wang et al., 1997; Platoshyn et al., 2001). Although the consequences of chronic hypoxia on TASK-1 expression are not known, channel activity is inhibited by acute hypoxia in some cell types (Buckler et al., 2000; Olschewski et al., 2006). Interestingly, the pattern of effects induced by hypoxia is reminiscent of those in organ culture, where PASMC Em is depolarized, IK(N) and IK(V) are suppressed, Kv1.5, Kv2.1 and TASK-1 channel subunits are down-regulated, and there is increased sensitivity to Ca2+-free solution, nifedipine and levcromakalim.
The converging effects of our culture model with in vivo and in vitro models of chronic hypoxia raised the hypothesis that the smooth muscle cells in cultured vessels might be inadequately oxygenated, despite incubation in 20% O2. In contrast to the situation with cell culture, the thickness of the tissue might impair gas exchange and the O2 supply to the inner muscle layer, causing cells to undergo changes similar to those in chronic hypoxia. This hypothesis was tested by raising the O2 level in the culture medium to 60%. In these conditions we observed partial protection from the effects of culture on PA, including the absence of PASMC depolarization, partial recovery of the endothelium-dependent carbachol response and return of the sensitivity to levcromakalim and nifedipine towards normal. As the protection was not seen when the culture medium was bubbled with a gas mixture containing 21% O2, it seems that the protective effects were due to the raised level of O2 in the culture medium per se. Although the exact mechanisms are still unclear, these data provide interesting insights in the quest to optimize the conditions for organ culture of blood vessels. While elevating O2 might be a useful adjunct to culturing PA, consideration should be given to its potential adverse effects.
As seen here with organ culture, chronic in vivo hypoxia enhanced the pulmonary vasoconstrictor response to 4-AP (Osipenko et al., 1998). As hypoxia did this without any change in the sensitivity of K+ currents to 4-AP (around 43% inhibition of IK(V) and only 5–9% of IK(N) at 1 mM), the simplest explanation was that down-regulation of the resting K+ conductance, IK(N), led to depolarization, where the open probability of 4-AP-sensitive, Kv channels was higher (Osipenko et al., 1998). As a consequence, 4-AP-sensitive channels contributed more to the resting potential in hypoxic vessels, resulting in a larger effect of 4-AP on Em, greater activation of VGCC and constriction. The same mechanism could operate in cultured vessels. The increased contractile response of cultured vessels to 10 mM TEA implies that there was also greater activity of TEA-sensitive channels at the resting potential. These channels include BKCa, Kv2.1 but not Kv1.5, (reviewed in Coetzee et al., 1999; Smirnov et al., 2002). This finding is consistent with depolarization and Ca2+ influx triggering a higher [Ca2+]i, which would in turn activate BKCa channels (Bolton and Imaizumi, 1996; Bolton, 2006). The increased activity of Kv and BKCa channels would help to counteract depolarization and could prevent excessive depolarization in culture conditions. Glibenclamide had no significant effect on cultured PA, consistent with previous findings that KATP channel activity is not involved in setting the Em and tone in resting conditions (Clapp and Gurney, 1991). This finding also implies that KATP channels remain closed in culture, indicating that ATP levels in the PASMCs were well maintained.
Although oxygenation prevented significant depolarization of Em in PASMC, it did not seem to prevent the loss of IK(V) or IK(N). This raises doubt over the involvement of these channels in setting the resting potential. A possible explanation is that K+ current recordings employed a different patch-clamp method (perforated vs. whole-cell) and it is possible that intracellular dialysis altered pathways that regulate channel activity. The electrophysiological data are however supported by TASK-1, Kv1.5 and Kv2.1 mRNA data, which show that the expression of all three subunits was significantly down-regulated both in standard and oxygen-enriched culture. Other ionic conductances must therefore play an important role in the oxygenation-induced ‘protection’ of Em. These could include K+ channels that would have been blocked in the presence of TEA and glibenclamide, used to isolate IK(N), or cation conductances, which may be up-regulated in culture (Dreja et al., 2001; Bergdahl et al., 2005).
Comparison with other vascular beds
The effects of culture on the contractile properties described here are not restricted to the pulmonary vascular bed. For instance, increases in spontaneous tone after culture were described in rat renal artery (De Mey et al., 1989). Impaired Ca2+ handling after organ culture has been reported in many different systemic vascular beds, including cerebral artery (Bergdahl et al., 2005), tail, basilar arteries (Dreja et al., 2001) and mesenteric (Tai et al., 2009) artery. Our results therefore address technical limitations to organ culture that affect all types of cultured vessels. The effects of organ culture on PA converge with the effects of chronic hypoxia on PA previously described by our group and others (Osipenko et al., 1998; Bonnet et al., 2001; Platoshyn et al., 2001). Despite striking similarities between the two conditions and the fact that a raised O2 level in organ culture is protective, it remains unclear whether a hypoxic environment existed in our culture model. Thus it may be misleading to consider organ culture as a potential model of chronic hypoxia without knowledge of the micro-environment of the smooth muscle cells.
In conclusion, we present here the first study that establishes a link between increased resting tone, voltage-dependent Ca2+ influx, depolarization of PASMC Em and loss of K+ channels induced by blood vessel organ culture. Moreover we provide encouraging insight into limiting some of those changes by increasing the oxygenation of the tissue, and by gently removing the endothelium before culture. Compared with classical cell culture systems, organ culture offers a more integrated physio-pathological model to study blood vessel responses to challenges, such as hypoxia (Murata et al., 2001) or stretch injury (Bergdahl et al., 2005). Cultured vessels are also valuable as an integrated model for pharmacogenomical studies, such as RNAi targeting of a gene (Gurney and Hunter, 2005; Corteling et al., 2007) or expression of recombinant proteins (Chen et al., 2006). The ability to maintain blood vessels in culture without alteration in their properties is therefore an important goal. Conventional approaches use serum-free medium, which preserves contractility and does not stimulate cell proliferation (Lindqvist et al., 1999). Here we provide a time scale for the effects of organ culture on the contractile phenotype of PAs, showing altered ionic conductances and pharmaco-contractile properties of the vessels over a 2–4 day period. There was little change in these pharmaco-mechanical properties during the first 2 days of culture, but down-regulation of mRNA encoding Kv1.5, Kv2.1 and TASK-1 channels was detected as soon as day 2. The progressive down-regulation of these channels is one mechanism to explain the depolarization of PASMCs and voltage-dependent Ca2+ influx, which gave rise to the appearance of intrinsic tone and spontaneous contractile activity between days 3 and 4. These changes may limit the use of organ culture for some studies. Our data support the conclusions of Guibert et al. (2005) that cultured vessels are suitable for pharmacological studies using agonists, as the contractile phenotype and response to agonist is well maintained. The alterations that we describe would, however, limit investigations of the links between ion channels, Em and the tone of PAs. Raising the O2 level in the medium can help to counteract changes in Em, as well as partially restoring the pharmaco-mechanical properties of PAs, but does not reverse the loss of expression of some K+ channels. Full maintenance of vascular function during culture of intact arteries will require further, as yet unidentified, interventions. Importantly, this study draws attention to the necessity of describing fully the behaviour of vessels for any study involving organ culture.
Acknowledgments
The authors acknowledge the Biotechnology and Biological Sciences Research Council (BBSRC) and the British Heart Foundation for their financial support.
Glossary
Abbreviations:
- BKCa
large conductance Ca2+-sensitive K+ channel
- [Ca2+]i
intracytosolic Ca2+ concentration
- EK
K+ equilibrium potential
- Em
membrane potential
- ET-1
endothelin-1
- [K]0
concentration of K+ in bath solution
- KATP
ATP-sensitive K+ channel
- Kv, delayed rectifier
voltage-gated K+ channel
- PA
pulmonary artery
- PASMC
pulmonary artery smooth muscle cell
- RT-PCR
reverse transcription and PCR
- TASK
TWIK-related acid-sensitive K+ channel
- VGCC
voltage-gated Ca2+ channel
Conflicts of interest
The authors declare no conflict of interest.
References
- Adner M, Uddman E, Cardell LO, Edvinsson L. Regional variation in appearance of vascular contractile endothelin-B receptors following organ culture. Cardiovasc Res. 1998;37:254–262. doi: 10.1016/s0008-6363(97)00206-x. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1, S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, et al. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, London B, Hampl V, Wu X, Nsair A, Puttagunta L, et al. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J. 2001;15:1801–1803. doi: 10.1096/fj.00-0649fje. [DOI] [PubMed] [Google Scholar]
- Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, et al. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, et al. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005;288:C872–C880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- Bolton TB. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J Physiol. 2006;570:5–11. doi: 10.1113/jphysiol.2005.095604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/s0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Lim SP. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, Hyvelin JM, Bonnet P, Marthan R, Savineau JP. Chronic hypoxia-induced spontaneous and rhythmic contractions in the rat main pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2001;281:L183–L192. doi: 10.1152/ajplung.2001.281.1.L183. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(1):135–142. doi: 10.1111/j.1469-7793.2000.00135.x. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Casteels R, Kitamura K, Kuriyama H, Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977a;271:63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R, Kitamura K, Kuriyama H, Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977b;271:41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res. 2006;99:53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- Clapp LH, Gurney AM. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991;76:677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Clapp LH, Gurney AM. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992;262:H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Corteling RL, Brett SE, Yin H, Zheng XL, Walsh MP, Welsh DG. The functional consequence of RhoA knockdown by RNA interference in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;293:H440–H447. doi: 10.1152/ajpheart.01374.2006. [DOI] [PubMed] [Google Scholar]
- De Mey JG, Uitendaal MP, Boonen HC, Vrijdag MJ, Daemen MJ, Struyker-Boudier HA. Acute and long-term effects of tissue culture on contractile reactivity in renal arteries of the rat. Circ Res. 1989;65:1125–1135. doi: 10.1161/01.res.65.4.1125. [DOI] [PubMed] [Google Scholar]
- Dreja K, Bergdahl A, Hellstrand P. Increased store-operated Ca2+ entry into contractile vascular smooth muscle following organ culture. J Vasc Res. 2001;38:324–331. doi: 10.1159/000051063. [DOI] [PubMed] [Google Scholar]
- Evans AM, Osipenko ON, Gurney AM. Properties of a novel K+ current that is active at resting potential in rabbit pulmonary artery smooth muscle cells. J Physiol. 1996;496(2):407–420. doi: 10.1113/jphysiol.1996.sp021694. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol. 1999;31:5–14. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- Gaine SP, Hales MA, Flavahan NA. Hypoxic pulmonary endothelial cells release a diffusible contractile factor distinct from endothelin. Am J Physiol. 1998;274:L657–L664. doi: 10.1152/ajplung.1998.274.4.L657. [DOI] [PubMed] [Google Scholar]
- Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol. 2004;142:192–202. doi: 10.1038/sj.bjp.0705691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- Gonczi M, Szentandrassy N, Johnson IT, Heagerty AM, Weston AH. Investigation of the role of TASK-2 channels in rat pulmonary arteries; pharmacological and functional studies following RNA interference procedures. Br J Pharmacol. 2006;147:496–505. doi: 10.1038/sj.bjp.0706649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert C, Savineau JP, Crevel H, Marthan R, Rousseau E. Effect of short-term organoid culture on the pharmaco-mechanical properties of rat extra- and intrapulmonary arteries. Br J Pharmacol. 2005;146:692–701. doi: 10.1038/sj.bjp.0706379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- Gurney AM, Hunter E. The use of small interfering RNA to elucidate the activity and function of ion channel genes in an intact tissue. J Pharmacol Toxicol Methods. 2005;51:253–262. doi: 10.1016/j.vascn.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Gurney AM, Osipenko ON, MacMillan D, Kempsill FE. Potassium channels underlying the resting potential of pulmonary artery smooth muscle cells. Clin Exp Pharmacol Physiol. 2002;29:330–333. doi: 10.1046/j.1440-1681.2002.03653.x. [DOI] [PubMed] [Google Scholar]
- Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FE. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- Hathaway DR, March KL, Lash JA, Adam LP, Wilensky RL. Vascular smooth muscle. A review of the molecular basis of contractility. Circulation. 1991;83:382–390. doi: 10.1161/01.cir.83.2.382. [DOI] [PubMed] [Google Scholar]
- Hieda HS, Gomez-Sanchez CE. Hypoxia increases endothelin release in bovine endothelial cells in culture, but epinephrine, norepinephrine, serotonin, histamine and angiotensin II do not. Life Sci. 1990;47:247–251. doi: 10.1016/0024-3205(90)90327-n. [DOI] [PubMed] [Google Scholar]
- Hong Z, Weir EK, Nelson DP, Olschewski A. Subacute hypoxia decreases voltage-activated potassium channel expression and function in pulmonary artery myocytes. Am J Respir Cell Mol Biol. 2004;31:337–343. doi: 10.1165/rcmb.2003-0386OC. [DOI] [PubMed] [Google Scholar]
- Johnsson E, Maddahi A, Wackenfors A, Edvinsson L. Enhanced expression of contractile endothelin ET(B) receptors in rat coronary artery after organ culture. Eur J Pharmacol. 2008;582:94–101. doi: 10.1016/j.ejphar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res. 2006;7:31. doi: 10.1186/1465-9921-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther. 2009;329:368–376. doi: 10.1124/jpet.108.147785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Nordstrom I, Malmqvist U, Nordenfelt P, Hellstrand P. Long-term effects of Ca(2+) on structure and contractility of vascular smooth muscle. Am J Physiol. 1999;277:C64–C73. doi: 10.1152/ajpcell.1999.277.1.C64. [DOI] [PubMed] [Google Scholar]
- McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- Markewitz BA, Kohan DE, Michael JR. Hypoxia decreases endothelin-1 synthesis by rat lung endothelial cells. Am J Physiol. 1995;269:L215–L220. doi: 10.1152/ajplung.1995.269.2.L215. [DOI] [PubMed] [Google Scholar]
- Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- Murata T, Yamawaki H, Hori M, Sato K, Ozaki H, Karaki H. Hypoxia impairs endothelium-dependent relaxation in organ cultured pulmonary artery. Eur J Pharmacol. 2001;421:45–53. doi: 10.1016/s0014-2999(01)01015-9. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Olschewski A, Li Y, Tang B, Hanze J, Eul B, Bohle RM, et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res. 2006;98:1072–1080. doi: 10.1161/01.RES.0000219677.12988.e9. [DOI] [PubMed] [Google Scholar]
- Osipenko ON, Evans AM, Gurney AM. Regulation of the resting potential of rabbit pulmonary artery myocytes by a low threshold, O2-sensing potassium current. Br J Pharmacol. 1997;120:1461–1470. doi: 10.1038/sj.bjp.0701075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipenko ON, Alexander D, MacLean MR, Gurney AM. Influence of chronic hypoxia on the contributions of non-inactivating and delayed rectifier K currents to the resting potential and tone of rat pulmonary artery smooth muscle. Br J Pharmacol. 1998;124:1335–1337. doi: 10.1038/sj.bjp.0702006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, et al. Chronic hypoxia decreases K(V) channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L801–L812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- Platoshyn O, Brevnova EE, Burg ED, Yu Y, Remillard CV, Yuan JX. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C907–C916. doi: 10.1152/ajpcell.00028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post JM, Gelband CH, Hume JR. Ca2+ i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Reeve HL, Weir EK, Nelson DP, Peterson DA, Archer SL. Opposing effects of oxidants and antioxidants on K+ channel activity and tone in rat vascular tissue. Exp Physiol. 1995;80:825–834. doi: 10.1113/expphysiol.1995.sp003890. [DOI] [PubMed] [Google Scholar]
- Rodman DM. Chronic hypoxia selectively augments rat pulmonary artery Ca2+ and K+ channel-mediated relaxation. Am J Physiol. 1992;263:L88–L94. doi: 10.1152/ajplung.1992.263.1.L88. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol. 1994;266:H365–H370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. J Physiol. 2002;538:867–878. doi: 10.1113/jphysiol.2001.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Twarog BM. Membrane properties of smooth muscle cells in pulmonary arteries of the rat. Am J Physiol. 1982;242:H900–H906. doi: 10.1152/ajpheart.1982.242.5.H900. [DOI] [PubMed] [Google Scholar]
- Tai K, Vandenberg G, Hamaide MC, Wibo M, Morel N. Effect of organ culture on noradrenaline-evoked contraction, calcium signalling and TRPC expression in rat mesenteric artery. J Vasc Res. 2009;46:353–364. doi: 10.1159/000189796. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C, Vigne P, Barhanin J, Schmid-Alliana A, Frelin C, Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988;157:977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, et al. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2008;294:L309–L318. doi: 10.1152/ajplung.00091.2007. [DOI] [PubMed] [Google Scholar]


