Abstract
Background and purpose:
A ketogenic diet has been used successfully to treat patients with intractable epilepsy, although the mechanism is unknown. Acetone has been shown to have an anticonvulsive effect in various animal models. The main purpose of this study was to determine whether other ketones, 2-butanone (methyl ethyl ketone: MEK) and 3-pentanone (diethyl ketone: DEK), also show anticonvulsive effects in lithium-pilocarpine (Li-pilocarpine)-induced status epilepticus (SE) in rats.
Experimental approach:
Anticonvulsive effects of MEK and DEK in Li-pilocarpine SE rats were measured by behavioural scoring. Anti-seizure effects of MEK were also evaluated using electroencephalography (EEG). Neuroprotective effect of MEK was investigated by haematoxylin and eosin staining 4 weeks after the treatment with pilocarpine.
Key results:
Acetone, MEK and DEK showed anticonvulsant effects in Li-pilocarpine-induced SE rats. Treatment with MEK twice (8 mmol·kg−1 and 5 mmol·kg−1) almost completely blocked spontaneous recurrent cortical seizure EEG up to 4 weeks after the administration of pilocarpine. MEK also showed strong neuroprotective effects in Li-pilocarpine-treated rats 4 weeks following the administration of pilocarpine. Significant neural cell death occurred in the hippocampus of Li-pilocarpine SE rats, especially in the CA1 and CA3 subfields. In contrast, normal histological characteristics were observed in these regions in the MEK-pretreated rats.
Conclusions and implications:
Both MEK and DEK showed strong anticonvulsive effects in Li-pilocarpine-induced SE rats. They also inhibited continuous recurrent seizure and neural damage in hippocampal region for 4 weeks after the treatment with pilocarpine. These findings appear to be of value in the investigation of epilepsy.
Keywords: lithium-pilocarpine, acetone, methyl ethyl ketone, diethyl ketone, anticonvulsant, seizure, neuroprotection, ketogenic diet
Introduction
A ketogenic diet has been used successfully to treat patients with intractable epilepsy (Schwartzkroin et al., 1997; Jung et al., 2008). Many studies on the anticonvulsant effects of a ketogenic diet have been performed; however, the mechanism remains unknown (Yudkoff et al., 2006; Gasior et al., 2007). Several hypotheses have been proposed to explain the anticonvulsant effects of a ketogenic diet. For example, alterations in energy metabolism in the brain during a ketogenic diet have been observed (Erecinska et al., 1996; Barton et al., 2001; Kaminski et al., 2004; Dahlin et al., 2005). The levels of the ketone bodies, β-hydroxybutylate, acetoacetate and acetone in the plasma of patients receiving a ketogenic diet are significantly increased. Among these three ketone bodies, acetoacetate and acetone easily pass through the blood brain barrier, and partly replace glucose as fuel in the brain (Hartman et al., 2007). During a ketogenic diet, significant alterations in energy metabolism in the brain occur (Gasior et al., 2007; Hartman et al., 2007), and these changes in energy metabolism might be related to the anticonvulsive effect associated with a ketogenic diet in animal models of neurodegenerative diseases.
Several studies have demonstrated that acetone has anti-seizure effects in a variety of animal models of epilepsy. Acetone inhibits seizures induced by pentylenetetrazol (Likhodii and Burnham, 2002), electrical stimulation (Vodickova et al., 1995; Frantic et al., 1996) and audiogenic epilepsy (Frantik et al., 1976). However, to the best of our knowledge, as yet no study has been done on the effect of acetone on epileptic seizures induced by lithium and the non-selective muscarinic agonist pilocarpine (lithium-pilocarpine, Li-pilocarpine), which has been used as a model for temporal lobe epilepsy in humans (Dubéet al., 2001; Szyndler et al., 2005). Hence, in this study we initially investigated the effects of acetone on epileptic seizures induced by Li-pilocarpine in rats.
The second aim of this study was to ascertain whether other ketones, 2-butanone (methyl ethyl ketone: MEK) and 3-pentanone (diethyl ketone: DEK), also show anticonvulsant effects in Li-pilocarpine-induced status epilepticus (SE) rats. MEK and DEK have a similar chemical structure to acetone; however, they are not produced in vivo by ketogenic diet. In addition, neither MEK nor DEK is likely to be used as an energy source in vivo. In this study we found that all three ketone bodies exhibit anti-seizure activity in this Li-pilocarpine model. In addition, we found that MEK induces a neuroprotective effect in this model; MEK completely blocked spontaneous recurrent seizures and protected the hippocampus from neurodegeneration 4 weeks after the treatment with Li-pilocarpine. These new findings will be of value to investigate the mechanism behind the effects of a ketogenic diet.
Methods
Animals
Male Wistar rats (7–8 weeks old when they were injected with pilocarpine) were purchased from Japan SLC (Shizuoka, Japan). A total of 69 rats were used for this experiment. The rats were housed under a normal 12 h light/dark cycle and allowed free access to food and water. All experiments on the rats were performed with the permission of the Institutional Animal Care and Use Committee, School of Allied Health Sciences, Osaka University.
Surgery
Under sodium pentobarbital anaesthesia (50 mg·kg−1, i.p.), the rat was placed on a stereotaxic frame, and electroencephalography (EEG) electrodes were implanted according to the brain atlas (Paxinos and Watson, 1986). A screw electrode was placed on the skull over the left and right frontal cortex (3.5 mm A, 2.5 mm L) and occipital cortex (−5.0 mm P, 2.5 mm L). Each electrode was then soldered to a connector (SMQ-9SC; Hirose Electric, Tokyo, Japan), which was fixed on the skull with quick self-curing acrylic resin (ADFA, Shofu Inc., Kyoto, Japan). The animals (n= 27) were allowed at least 7 days to recover from the surgery before the start of the study.
Behavioural seizure analysis
Status epilepticus was induced by Li-pilocarpine. Lithium chloride (3 mEq·kg−1, i.p.) was injected 20–24 h prior to the injection of pilocarpine hydrochloride (30 mg·kg−1, i.p., n= 5). After the pilocarpine injection, the animals were isolated in plastic cages, and their behaviour was observed. Control animals received lithium chloride (3 mEq·kg−1, i.p., n= 5) and an equal volume of saline instead of the pilocarpine solution. Acetone (25 mmol·kg−1, i.p., n= 16), MEK (5 mmol·kg−1, i.p., n= 8) or DEK (3.5 mmol·kg−1, i.p., n= 8) were diluted in saline solution (10 mL·kg−1), and the rats were pretreated with one of these three solutions 15 min prior to the administration of the pilocarpine, which was used to induce SE. The presence or absence of any type of behavioural seizure activity was scored according to methods previously described (Racine, 1972): stage 1 seizures consisted of immobility and occasional facial clonus; stage 2 seizures included head nodding; stage 3 seizures had bilateral forelimb clonus; stage 4 included rearing; and stage 5 included rearing and falling.
Electroencepharogram analysis and histopathology
Rats were injected with lithium chloride (3 mEq·kg−1, i.p.) and on the following day, SE was induced by injecting pilocarpine hydrochloride (50 mg·kg−1, i.p.) 1 h after methylscopolamine (1 mg·kg−1, i.p.), which was administered to limit the peripheral effects of the convulsant. MEK (8 mmol·kg−1, i.p.) was given 15 min before the administration of the pilocarpine, and an additional dose of MEK (5 mmol·kg−1, i.p.) was injected 2 h after the pilocarpine injection. Diazepam (5 mg·kg−1, i.p.) was given 3 h after the pilocarpine injection to improve animal survival. SE rats (n= 13) and rats pretreated with MEK (n= 14) underwent EEG for 4 weeks, together with histopathological examination. In control rats, the same protocol was followed except saline was administered instead of MEK.
On the testing day, the rats were placed in the acrylic box for 1 h to allow them to acclimatize to this situation. During the recording period, the rats were unrestricted and could move freely. However, to avoid any artefacts, they were not permitted access to food during EEG recording. EEG signals were recorded from the frontal cortex and occipital cortex through a bioelectric amplifier (AP1532, TEAC Inc., Tokyo, Japan), at a time constant of 0.1 s and a low pass filter setting of 100 Hz. EEG signals were recorded for approximately 1 h at 3 h, 1, 2 and 4 weeks after the pilocarpine treatment. Seven rats out of 13 rats in the control group and eight rats out of 14 rats in the group pretreated with MEK were used for EEG analysis as the electrodes became detached from the head in the other rats. Unusual EEG patterns and behaviours were visually assessed; the spikes, spike-and-slow-wave complexes or seizure discharges for the EEG changes, and twitches or convulsions for behavioural changes. Spectrum analysis of the EEG was performed with spectral analysis software (AP viewer, TEAC Inc.). Consecutive 60 s artefact-free epochs were selected and digitized at a sampling rate of 1 KHz, followed by Fast Fourier Transformation at frequencies from 0.3 to 30 Hz. Absolute power was analysed by the EEG during periods of wakefulness and was plotted as histograms at intervals of 0.49 Hz.
The rats were killed by decapitation after the EEG recording, and the brains were removed 4 weeks after the injection of pilocarpine. The isolated brains were fixed in 10% neutral buffered formalin, processed routinely and embedded in paraffin. Paraffin sections (4 µm) were prepared and stained with haematoxylin and eosin. Based on the previous results of neuronal damage induced by Li-pilocarpine SE, the examination of neuronal degeneration focused on the hippocampus (pyramidal layer of the hippocampus CA1 and CA3) and the hilus of the dentate gyrus, amygdala, thalamus and cortex. For each group, the analysis was performed on the section for each region in four brains per group. Structures were analysed on coronal sections. Analysis was qualitatively performed by a pathologist.
Statistical analysis
Behavioural scores are expressed as the mean ± SD. The significance of differences between values obtained from control (non-pretreated) and pretreated Li-pilocarpine rats was determined by Student's unpaired t-test.
Chemicals
Lithium chloride, acetone, MEK and DEK were obtained from Nacalai Tesque Inc. (Kyoto, Japan). Pilocarpine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO, USA). Diazepam was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other chemicals used were of the highest purity commercially available.
Results
Figure 1 shows the anti-seizure effects of acetone, MEK and DEK 120 min after the administration of pilocarpine into rats pretreated with lithium. In rats not treated with ketones, pilocarpine induced acute seizures with the scores reaching a maximum level (level 5) within 60 min after the injection of pilocarpine. In contrast, all three ketones, acetone, MEK and DEK, showed anticonvulsive effects (Figure 1). The doses of acetone, MEK and DEK required to achieve an anti-seizure effect were 25, 5 and 3.5 mmol·kg−1 respectively.
Figure 1.
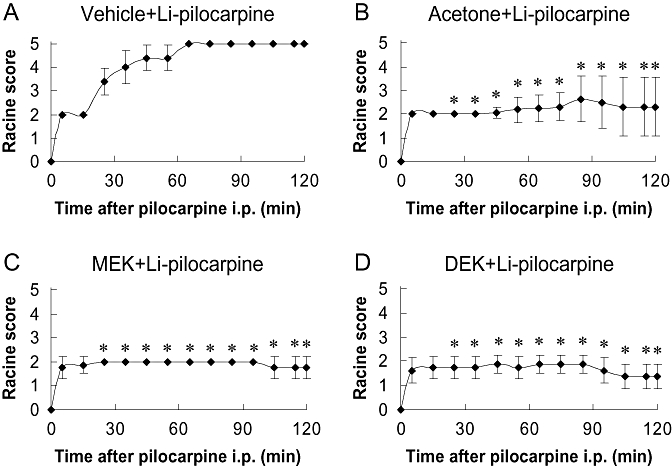
Behavioural scores of seizure according to the Racine scale during the 120 min following the administration of pilocarpine. (A) Rats administered lithium-pilocarpine (Li-pilocarpine) (n= 5), (B) pretreated with 25 mmol·kg−1 acetone (n= 16), (C) pretreated with 8 mmol·kg−1 2-butanone (methyl ethyl ketone: MEK) (n= 8), (D) pretreated with 3.5 mmol·kg−1 3-pentanone (diethyl ketone: DEK) (n= 8). Values are mean ± SD. Significant differences between the ketone-pretreated group and vehicle-treated group (Li-pilocarpine) at each time point is indicated by an asterisk (*P < 0.01).
The typical EEG patterns in control and MEK-pretreated rats measured 3 h, 1 and 4 weeks after the administration of pilocarpine are shown in Figure 2. In the vehicle-pretreated group, spontaneous recurrent seizures or spike and slow wave complexes were observed for 4 weeks after the injection of pilocarpine (Figure 2). On the other hand, almost normal EEG patterns were seen at all time points measured in the MEK-pretreated rats (Figure 2). Power spectral analysis indicated that EEG power from the prefrontal cortex 3 h after the administration of pilocarpine in Li-pilocarpine rats was increased at all the frequency bands compared with the pre-dosing EEGs, whereas those in the MEK-treated rats was decreased (Figure 3). Four weeks after the administration of pilocarpine, the power spectra exhibited a peak frequency at 2 Hz; this noticeable peak was not observed 3 h after the administration of pilocarpine (Figure 4). This change seemed to reflect an increase in the spike and slow wave complexes. Pretreatment with MEK improved the power spectra from the frontal cortex so that the level was similar to the normal baseline in Li-pilocarpine SE rats. Power spectra in frontal cortex were similar to those of the occipital cortex (data not shown).
Figure 2.
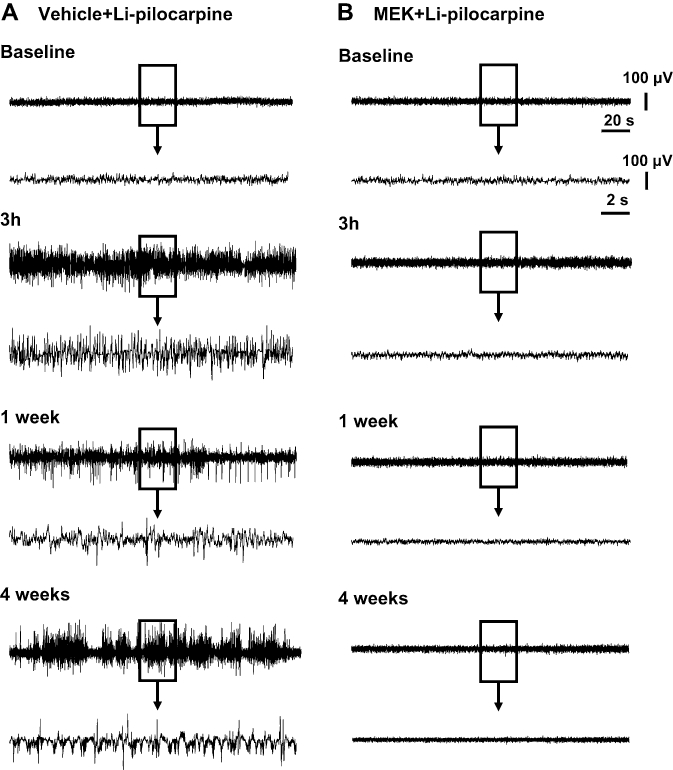
Typical electroencephalography (EEG) patterns at 3 h, 1 and 4 weeks following the administration of pilocarpine in lithium-pilocarpine (Li-pilocarpine) status epilepticus rats treated with vehicle (n= 7) and 2-butanone (methyl ethyl ketone: MEK) (n= 8). MEK (8 mmol·kg−1, i.p.) was given 15 min before the administration of the pilocarpine, and an additional dose of MEK (5 mmol·kg−1, i.p.) was injected 2 h after the pilocarpine injection. Li-pilocarpine induced spike or seizure discharge in the frontal cortex, which continued for 4 weeks. However, slow wave components in the recurrent seizures at 4 weeks were increased compared with those occurring 3 h after the administration of pilocarpine. EEGs were recorded for 4 weeks after the administration of pilocarpine.
Figure 3.
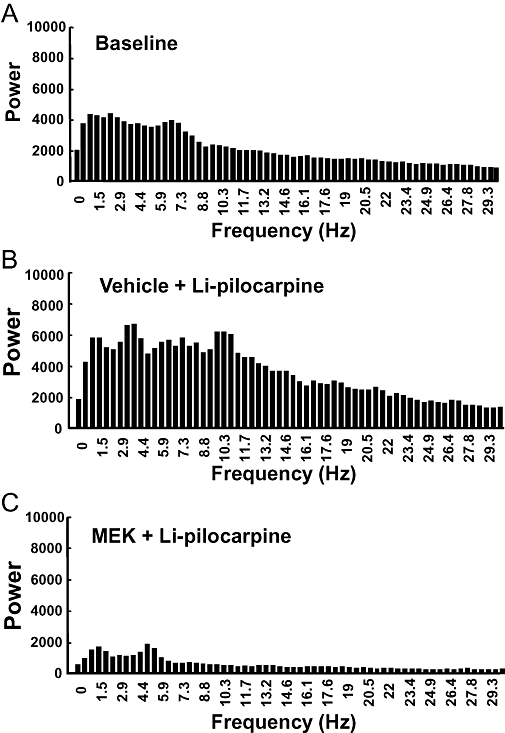
Power spectra indicating cortical electroencephalography (EEG) activity during an acute seizure discharge in rats 3 h following the administration of pilocarpine. (A) Baseline (n= 8), (B) lithium-pilocarpine (Li-pilocarpine) (n= 7), (C) pretreated with 2-butanone (methyl ethyl ketone: MEK) (n= 8). Average power from the prefrontal cortex 3 h after the administration of pilocarpine in Li-pilocarpine status epilepticus rats was increased at the all frequency bands compared with the pre-dosing EEG, whereas those of the MEK-pretreated rats was decreased. Mean results shown are from seven to eight animals.
Figure 4.
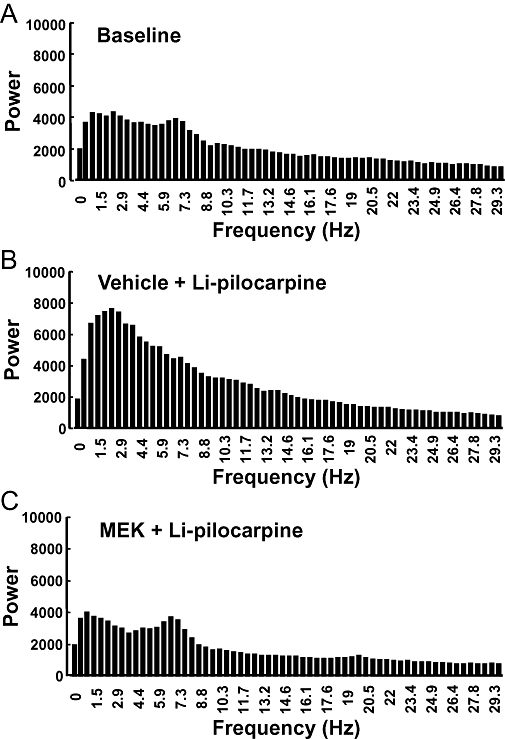
Power spectra indicating cortical electroencephalography activity during a partial seizure (spike and wave) 4 weeks after the administration of pilocarpine to rats. (A) Baseline (n= 8), (B) lithium-pilocarpine (Li-pilocarpine) (n= 7), (C) pretreated with 2-butanone (methyl ethyl ketone: MEK) (n= 8). Four weeks after the administration of pilocarpine, the power spectra with a peak frequency at 2 Hz was improved in the Li-pilocarpine status epilepticus rats that had been pretreated with MEK. Mean results shown are from seven to eight animals.
The histopathological findings in brain slices including the hippocampus formation are shown in Figure 5. In all Li-pilocarpine rats (n= 4), significant neural cell death was observed in the CA1 and CA3 areas of the hippocampus. Neural damage in the CA2 area was also seen in one of the four rats. In addition, gliosis and calcium deposits were observed in the thalamus (data not shown). These pathological changes in the thalamus might indicate the severe damage induced by epilepsy. In contrast, a normal histological profile with no neural cell death was observed in the brains from MEK-pretreated rats (n= 4).
Figure 5.
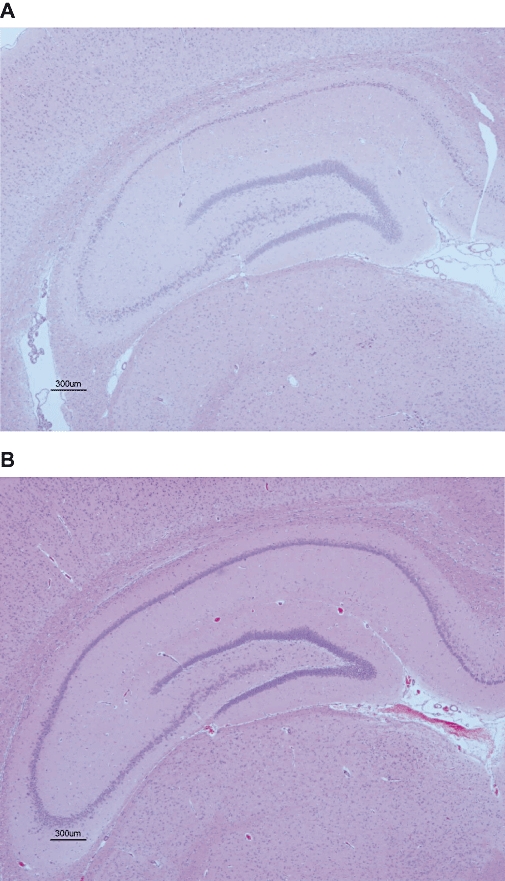
Photomicrographs of haematoxylin and eosin stained sections from the brains at the level of the hippocampus of lithium-pilocarpine status epilepticus rats treated with vehicle (A) or 2-butanone (methyl ethyl ketone: MEK) (B). Significant neuronal cell death was observed in CA1 and CA3 areas of the hippocampus in status epilepticus rats (A), whereas almost no neuronal cell death was seen in MEK-treated rats (B). Four rats in each group were used for histopathology.
Discussion
Acetone has been reported to have anticonvulsive effects in several animal models of epilepsy. For example, in the pentylentetrazol-induced seizure model, acetone showed anti-seizure effects in 20% and 60% of the mice with doses of 1 mmol·kg−1 and 10 mmol·kg−1 respectively (Likhodii and Burnham, 2002). In the present study, 25 mmol·kg−1 of acetone demonstrated anti-seizure effects in 70% of the rats treated with Li-pilocarpine. As far as we know, this is the first time that acetone has been shown to have an anticonvulsive effect in the Li-pilocarpine SE rat model, which has been well characterized as a model for human temporal lobe epilepsy. In addition, both MEK and DEK also showed anticonvulsive effects in the Li-pilocarpine SE model. The doses of MEK and DEK required to achieve anticonvulsive effects were 5 and 3.5 mmol·kg−1, respectively, which were about one-fifth and one-seventh of the effective dose of acetone on its own. The reason for the smaller amounts of MEK or DEK being sufficient to achieve anticonvulsive effects seemed to be related to the lipophylicities of these ketones due to differences in the length of their carbon chain. The fact that not only acetone, but also other ketones, MEK and DEK, showed anticonvulsive effects would appear to be a very important finding in the investigation of the mechanism underlying the anticonvulsive effects of a ketogenic diet.
It has been demonstrated that acetone is converted to acetyl-CoA by acetoacethyl-CoA-thiolase in the brain, enters the Krebs cycle and produces ATP (Hartman et al., 2007). It has been proposed that a significant alteration in brain energy metabolism occurs during a ketogenic diet, as the main ketone bodies produced by a ketogenic diet, β-hydroxybutyrate, acetoacetate and acetone partly replace glucose as fuel (Hartman et al., 2007). However, it is unlikely that either MEK or DEK is converted to acetyl-CoA in the brain (DiVincenzo et al., 1976); therefore, the anticonvulsive effects of these three ketones (acetone, MEK and DEK) might be due to a different mechanism, as was previously believed to be the case in acetone.
The most important findings of this study are the following: firstly, MEK almost completely blocked spontaneous recurrent seizures after Li-pilocarpine SE (as shown in Figure 2). Secondly, MEK also showed a strong neuroprotective effect in Li-pilocarpine SE rats at 4 weeks after the administration of pilocarpine (as shown in Figure 5).
In untreated Li-pilocarpine SE rats, significant neural cell death was observed in the hippocampal formation. On the other hand, almost normal histology was observed in the MEK-pretreated group. Zhao et al. (2004) found that a ketogenic diet reduced the number of spontaneous seizures after Li-pilocarpine SE. However, no differences were seen in the pathology scores of Li-pilocarpine SE rats between a group fed a ketogenic diet and the group on a regular diet (Zhao et al., 2004). Therefore, MEK would appear to have much stronger anticonvulsive and neuroprotective effects in Li-pilocarpine rats as compared with a ketogenic diet on its own.
The mechanism behind the anticonvulsive and neuroprotective effects of MEK is unknown, and it certainly is an important subject meriting future study. One possible hypothesis is that MEK directly acts on the cell membrane or microenvironments in the extracellular space. Binder et al. (2004) demonstrated that the diffusion rate of macromolecules in the extracellular space is much slower during a convulsion. It would be of interest to ascertain whether MEK alters the diffusion rate of macromolecules in the extracellular space.
Acetone, MEK and DEK easily pass through the blood brain barrier and seem to rapidly disappear from the brain. In fact, Gerasimov et al. (2005) established the rapid kinetics of [11C]-acetone in the rat brain. This result together with the finding that only two doses of MEK were needed to block spontaneous recurrent seizures as well as provide neuroprotection indicate that suppression of the initial convulsions is very important to protect against epileptic progression. Another important target for future study would be to clarify whether MEK and the other ketones used here show anticonvulsive and neuroprotective effects in other animal models of epilepsy.
Our findings strongly suggest that MEK and DEK showed much stronger anticonvulsive effects in the Li-pilocarpine SE model than acetone itself; MEK almost completely blocked spontaneous recurrent seizer as well as protecting the brain against neural cell death. Although the mechanism of this anticonvulsant effect is still unknown, taken together, these findings would appear to be of value in the investigation of epilepsy.
Glossary
Abbreviations:
- DEK
3-pentanone (diethyl ketone)
- Li
lithium
- MEK
2-butanone (methyl ethyl ketone)
- SE
status epilepticus
Conflict of interest
The authors state no conflict of interest.
References
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci. 2004;37:8049–8056. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–125. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Divincenzo GD, Kaplan CJ, Dedinas J. Characterization of the metabolites of methyl n-butyl ketone, methyl iso-butyl ketone, and methyl ethyl ketone in guinea pig serum and their clearance. Toxicol Appl Pharmacol. 1976;36:511–522. doi: 10.1016/0041-008x(76)90230-1. [DOI] [PubMed] [Google Scholar]
- Dubé C, Boyet S, Marescaux C, Nehlig A. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2001;167:227–241. doi: 10.1006/exnr.2000.7561. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem. 1996;67:2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- Frantic E, Vodickova L, Hornychova M, Nosek M. Pattern of inhalation exposure: blood levels and acute subnarcotic effects of toluene and acetone in rats. Cent Eur J Public Health. 1996;4:226–232. [PubMed] [Google Scholar]
- Frantik F, Horvath M, Vodickova I. Effects of solvent mixtures on behavior and seizure characteristics at the utmost additive. Act Nerv Super (Praha) 1976;30:260–264. [PubMed] [Google Scholar]
- Gasior M, French A, Joy MT, Tang RS, Hartman AL, Rogawski MA. The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia. 2007;48:793–800. doi: 10.1111/j.1528-1167.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Ferrieri RA, Pareto D, Logan J, Alexoff D, Ding YS. Synthesis and evaluation of inhaled [11C]butane and intravenously injected [11C]acetone as potential radiotracers for studying inhalant abuse. Nucl Med Biol. 2005;32:201–208. doi: 10.1016/j.nucmedbio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung da E, Kang HC, Kim HD. Long-term outcome of the ketogenic diet for intractable childhood epilepsy with focal malformation of cortical development. Pediatrics. 2008;122:e330–e333. doi: 10.1542/peds.2008-0012. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Likhodii SS, Burnham WM. Ketogenic diet: does acetone stop seizures? Med Sci Monit. 2002;8:HY19–HY24. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 2nd ed. Sydney: Academic Press; 1986. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:295–299. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin TD, Vining EP, Freeman JM. The ketogenic diet. Adv Pediatr. 1997;44:297–329. [PubMed] [Google Scholar]
- Szyndler J, Wierzba-Bobrowicz T, Skórzewska A, Maciejak P, Walkowiak J, Lechowicz W, et al. Behavioral, biochemical and histological studies in a model of pilocarpine-induced spontaneous recurrent seizures. Pharmacol Biochem Behav. 2005;81:15–23. doi: 10.1016/j.pbb.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Vodickova L, Frantik E, Vodickova A. Neutrotropic effects and blood levels of solvents at combined exposures: binary mixtures of toluene, o-xylene and acetone in rats and mice. Cent Eur J Public Health. 1995;3:57–64. [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A, et al. Short-term fasting, seizure control and brain amino acid metabolism. Neurochem Int. 2006;48:650–656. doi: 10.1016/j.neuint.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]


