Abstract
Background and purpose:
Hepatic encephalopathy is a neuropsychiatric syndrome caused by liver failure. In view of the effects of cannabinoids in a thioacetamide-induced model of hepatic encephalopathy and liver disease and the beneficial effect of capsaicin (a TRPV1 agonist) in liver disease, we assumed that capsaicin may also affect hepatic encephalopathy.
Experimental approach:
Fulminant hepatic failure was induced in mice by thioacetamide and 24 h later, the animals were injected with one of the following compound(s): 2-arachidonoylglycerol (CB1, CB2 and TRPV1 receptor agonist); HU308 (CB2 receptor agonist), SR141716A (CB1 receptor antagonist); SR141716A+2-arachidonoylglycerol; SR144528 (CB2 receptor antagonist); capsaicin; and capsazepine (TRPV1 receptor agonist and antagonist respectively). Their neurological effects were evaluated on the basis of activity in the open field, cognitive function in an eight-arm maze and a neurological severity score. The mice were killed 3 or 14 days after thioacetamide administration. 2-arachidonoylglycerol and 5-hydroxytryptamine (5-HT) levels were determined by gas chromatography-mass spectrometry and high-performance liquid chromatography with electrochemical detection, respectively.
Results:
Capsaicin had a neuroprotective effect in this animal model as shown by the neurological score, activity and cognitive function. The effect of capsaicin was blocked by capsazepine. Thioacetamide induced astrogliosis in the hippocampus and the cerebellum and raised brain 5-hydroxytryptamine levels, which were decreased by capsaicin, SR141716A and HU-308. Thioacetamide lowered brain 2-arachidonoylglycerol levels, an effect reversed by capsaicin.
Conclusions:
Capsaicin improved both liver and brain dysfunction caused by thioacetamide, suggesting that both the endocannabinoid and the vanilloid systems play important roles in hepatic encephalopathy. Modulation of these systems may have therapeutic value.
Keywords: hepatic encephalopathy, fulminant hepatic failure, capsaicin, cannabinoids, thioacetamide, TRPV1 receptors, astrogliosis, catecholamines, serotonin, cognition
Introduction
Fulminant hepatic failure (FHF), a serious form of liver injury caused by infectious agents, drugs or toxins, may be accompanied by hepatic encephalopathy (HE), a neuropsychiatric syndrome (Lee et al., 2005; Shawcross and Jalan, 2005). The pathogenesis of HE is a complex process involving multiple neurotransmitter systems, astrocyte dysfunction, as well as changes in cerebral perfusion (Ahboucha and Butterworth, 2007; Morgan et al., 2007). Clinical trials of a variety of treatments for HE have been disappointing (Shawcross and Jalan, 2005). Administration of thioacetamide (TAA) to rats induces acute liver failure with histological appearance and biochemical characteristics similar to cirrhosis in man. The hepatotoxicity of TAA is due to the generation of free radicals and oxidative stress (Zimmerman et al., 1989). Liver cirrhosis impairs the conversion of ammonia into urea and high levels of ammonia are toxic to the nervous system and affect gamma-aminobutyric acid (GABA)-ergic neurotransmission and, subsequently, neurological function (Fitz, 2002). Hence, rodents treated with TAA are used as animal models of HE (Zimmerman et al., 1989; Fernández-Martínez et al., 2004; Gabbay et al., 2005; Avraham et al., 2006; 2008a; Dagon et al., 2007)
The endocannabinoids are neuromodulators, conferring a neuroprotective effect in cerebral insults (Panikashvili et al., 2006; Mechoulam and Shohami, 2007). Two cannabinoid receptors, CB1 and CB2, have been characterized and cloned, and CB1 receptors are highly expressed in the brain, whereas CB2 receptors are found mostly in the periphery (Pertwee, 1997; receptor nomenclature follows Alexander et al., 2008). Endocannabinoids mediate via CB1 receptors, the vasodilatation associated with liver cirrhosis (Batkai et al., 2001), leading to fibrogenic effects in the liver (Teixeira-Clerc et al., 2006; 2008;). Activation of the vascular CB1 receptors located on the splanchnic and hepatic vascular endothelium is involved in the vasodilated state associated with cirrhosis (Varga et al., 1998). However, activation of CB2 receptors has an anti-fibrogenic effect (Julien et al., 2005). In some disease states, CB2 receptors are upregulated in the central nervous system (CNS), particularly in glial cells (Benito et al., 2003).
Panikashvili et al. (2001) have shown that following brain injury, levels of the endocannabinoid, 2-arachidonoylglycerol (2-AG) are enhanced significantly, attaining a 10-fold increase after 4 h and declining thereafter. Also, 2-AG alleviated oxidative stress, inhibited NF-kappa B, protected the blood brain barrier, attenuated inflammatory response and ameliorated neurological deficits (Panikashvili et al., 2006; Mechoulam and Shohami, 2007). We have already shown that 2-AG has neuroprotective effects in experimental HE (Avraham et al., 2006).
Anorexia resulting from a loss of appetite, a typical symptom observed in experimental and human cirrhosis, may be associated with altered brain 5-hydroxytryptamine (5-HT) metabolism (Blundell, 1992). Indeed, brain 5-HT levels increase in TAA-treated rats and may play an important role in the pathogenesis of cachexia associated with TAA-induced cirrhosis (Haider et al., 2004).
We have studied the etiology of cerebral dysfunction in a murine model of HE, induced by either bile duct ligation (BDL) or TAA administration. We showed that stimulation of the AMP-activated protein kinase (AMPK) is a compensatory response to liver failure and that it was regulated by endocannabinoids. As CB2 receptor signalling is apparently a cerebral stress response mechanism, its effects on AMPK make it a promising target for the treatment of HE by manipulation of the cannabinoid system (Dagon et al., 2007).
Capsaicin (8-methyl-N-vanillyl-6-nonenamide), the active component of hot peppers (Archer and Jones, 2002), has anti-inflammatory properties (Kim et al., 2003). It causes pro-apoptotic effects in cancer cells (Lee et al., 2004). It also acts on neural cells via vanilloid receptors subtype 1 (TRPV1), a cation channel widely distributed in the CNS. This effect can be blocked by capsazepine (Caterina and Julius, 2001). Di Marzo et al. (1998) raised the possibility of an interaction between the endocannabinoid system and capsaicin-like compounds, on the basis of structural similarities (Di Marzo et al., 1998). Indeed, both anandamide and 2-AG directly activate the TRPV1 receptor (Golech et al., 2004). There are several indications that suggest a possible therapeutic potential of capsaicin in the treatment of hepatic failure (Li et al., 2003).
In our previous study we showed that both endocannabinoids and capsaicin are useful in the treatment of FHF in mice. The CB1 receptor antagonist SR141716A, the CB2 receptor agonist HU308 and 2-AG, reduced inflammation and promoted regeneration within a single day after their administration following TAA. The levels of liver enzymes increased after TAA administration and these levels were reversed by administration of SR141716A and 2-AG (either alone or combined), or HU308, but not by administration of SR144528. These observations indicate a CB2 receptor-mediated benefit. However, we noted that in CB2 receptor null mice, 2-AG still modulated liver function (i.e. reversed levels of liver enzymes), indicating that the effect is not solely mediated by CB2 receptors and we showed that this effect is in part mediated by the TRPV1 receptors. Capsaicin improved both liver pathology and function in wild-type mice treated with TAA, whereas capsazepine impaired it. Hence, stimulation of either the CB2 or the TRPV1 receptor may have therapeutic potential in these hepatic injury models (Avraham et al., 2008a).
In the present work we examined the effect of endocannabinoids and capsaicin on HE recovery in order to help understand the disease mechanism and thus suggest new therapeutic modalities. We found that both the endocannabinoid system, as reported previously by us, and the vanilloid system, as reported now, play an important role in HE.
Methods
Mice
All animal care and experimental protocols were approved by the Institutional Committee for the use of animals (number MD-89.52–4). Eight- to 10-week-old female Sabra mice (29–32 g) were randomly assigned into six to seven groups of 10 mice. The food provided was Teklad Certified Global 18% Protein Rodent Diet + Fat (Sterilizable) 2018SC+F (Teklad, Wilmington, DE, USA). Mice were killed by decapitation. Following their rapid removal and dissection, brains were either frozen and kept at −70°C, or were fixed in paraformaldehyde (4%) in phosphate-buffered saline (PBS; pH 7.0) and embedded in paraffin.
Induction of hepatic failure
We adapted the rat model of acute liver failure induced by TAA to mice (Zimmerman et al., 1989; Gabbay et al., 2005; Avraham et al., 2006; 2008a; Dagon et al., 2007; Magen et al., 2008). TAA (Sigma-Aldrich, St. Louis, MO, USA) dissolved in sterile normal saline solution was injected by the intraperitoneal (i.p.) route as a single dose of 200 mg·kg−1. Twenty-four hours later all animals were injected (subcutaneously) with 0.5 mL solution of 0.45% NaCl, 5% dextrose and 0.2% KCl in order to prevent hypovolemia, hypokalemia and hypoglycemia. The mice were intermittently exposed to infrared light in order to prevent hypothermia (Avraham et al., 2006).
Administration of cannabinoid agonists, antagonists and capsaicin
Endocannabinoids and their CB receptor antagonists were dissolved in a vehicle solution composed of ethanol, emulphor and saline in a ratio of 1:1:18, respectively. 2-AG, HU-308 and SR141716A were injected in a dose of 5 mg·kg−1 once only after TAA treatment, based on previous experiments (Avraham et al., 2006; 2008a;). Capsaicin and capsazepine were administered at a dose of 1.25 µg·kg−1 as described by Lu et al. (2005). The solutions were injected i.p. 1 day after TAA administration. Control mice were injected with vehicle. The treatment and sampling schedule is shown in Figure 1.
Figure 1.
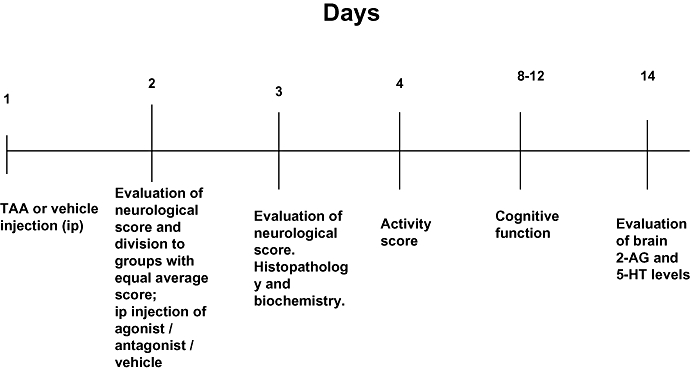
Time course of treatments and of evaluations of different variables. 2-AG, 2-arachidonoylglycerol; i.p., intraperitoneal; TAA, thioacetamide.
Brain tissue
The brain was cut along the midline and separated into two pieces containing brain and cerebellum hemispheres. Both pieces were embedded en bloc in paraffin and 6 µm sagittal sections were adhered on slides. Serial sections were taken in 15 groups of slides (10 slides each, three pairs of sections per slide) at 100 µM intervals. From each group of slides, two were randomly selected and processed for haematoxylin and eosin staining for morphological examination (a total of 30 slides). Adhered slides were used for immunohistochemical detection of glial fibrillary acidic protein (GFAP) (a total of 90 sections), according to standard protocol. Briefly, paraffin sections were deparaffinized, hydrated in xylene and alcohol solutions and rinsed with TBS. Citrate buffer (pH.6) was used for antigen retrieval. The endogenous peroxidase was blocked with H2O2 (0.3% in PBS). Sections were then incubated in blocking buffer for 1 h. A series of reselected sections were then treated with primary antibody against GFAP (DakoCytomation, Glostrup Denmark) and then with goat anti rabbit antibody (Vector, Burligame, CA, USA) as a secondary antibody.
Immunoreactions were visualized with the avidin–biotin complex (Vectastain) and the peroxidase reaction was visualized with diaminobenzidine (DAB) (Vector labs, Burlingame, CA, USA) as chromogen. Sections were finally counterstained with haematoxylin and examined by either light or fluorescent microscopy (Zeiss Axioplan 2, Oberkochen, Baden-Wuerttemberg, Germany). Images were captured with a digital camera (Nikon DS-5Mc-L1, Tokyo, Japan) mounted on a microscope. Astrocytic reaction was evaluated semi-quantitatively in the hippocampus and cerebellum of both hemispheres, with a light microscope. At each area, a total of six randomly selected high power visual fields, per section, were evaluated. A square with 100 square subdivisions, of 930.25 µm2 each, as defined by an ocular morphometric grid adjusted at the prefrontal lens, was centred on each visual field. The scores of the areas studied represented subjective assessments of staining intensity and numbers of labelled cells, and data were expressed as mean surface covered by GFAP positive cells and their processes in µm2. Two independent observers, unaware of sample identities, performed all quantitative assessments. In cases where significant discrepancies were obvious between the two observers, the evaluation was repeated by a third observer.
Assessment of neurological function
Neurological function was assessed by a 10-point scale based on reflexes and task performance (Chen et al., 1996): exit from a circle 1 m in diameter in less than 1 min, seeking, walking a straight line, startle reflex, grasping reflex, righting reflex, placing reflex, corneal reflex, maintaining balance on a beam 3, 2 and 1 cm in width, climbing onto a square and a round pole. For each task failed or for an abnormal reflex reaction a score of 1 was assigned. Thus, a higher score indicates poorer neurological function. The neurological score was assessed 1 day after TAA injection (day 2). The mice were then divided between treatment groups so that each group had a similar baseline neurological score after TAA injection. The post-treatment neurological score was assessed 1 day after administration of the agonist or the antagonist or the vehicle (day 3). The following measures were particularly sensitive to TAA: walking a straight line, maintaining balance on a beam 3, 2 and 1 cm in width and climbing onto a square and a round pole.
Assessment of activity
The activity test was performed on day 4. Activity was assessed in the open field (20 × 30 cm field divided into 12 squares of equal size) as described previously (Fride and Mechoulam, 1993). Two mice were observed simultaneously for 5 min. Locomotor activity was recorded by counting the number of crossings by the mice at 1 minute intervals. Results are presented as the mean number of crossings per minute.
Cognitive function
Cognitive function studies were performed 7 days after the induction of hepatic failure (days 8–12) by TAA. The animals were placed in an eight-arm maze, which is a scaled-down version of that developed for rats (Pick and Yanai, 1983). We used water deprivation and a reward of 50µl of water presented at the end of each arm. Water deprivation was achieved by limiting water consumption overnight. Animals were divided between treatment groups so that each group had a similar baseline neurological score after TAA induction. The mice were tested (number of entries) until they made entries into all eight arms or until they completed 24 entries, whichever came first. Hence, the lower the score, the better the cognitive function. Food and water were given at the completion of the test. Maze performance was calculated per each day for five consecutive days. Results were presented as area under the curve (AUC) utilizing the formula: (day 2 + day 3 + day 4 + day 5) − 4*(day 1). The AUC method was used previously (Avraham et al., 1996; 2006;). The validity of the AUC method was also approved by presenting the results as the number of entries, until all eight arms were visited or 24 entries made. The results were the same.
Determination of 2-AG
Determination of 2-AG was performed 14 days after TAA administration as described by Hanušet al. (2003). In brief, immediately after decapitation, whole brains were weighed and placed in glass tubes containing 7 mL chloroform–methanol (2:1) and manually homogenized for 1–2 min. Vacuum filtration was carried out. The solvents were evaporated to dryness. Pre-coated TLC silica gel plates were used to separate 2-AG and mono-arachidin (1-arachidinyl glycerol as an internal standard) from other lipids. Synthetic 2-AG and mono-arachidin were applied alongside the experimental samples, dissolved in chloroform, to help in their identification in the lipid sample.
Gas chromatography-mass spectrometry (GC-MS) analysis
For quantitative analysis the samples were analyzed by GC-MS in a Hewlett-Packard G1800A GCD system (Palo Alto, CA, USA) with a HP-5971 gas chromatograph with electron ionization detector as described (Hanušet al. 2003).
Measurement of 5-HT
Mice were killed 14 days after TAA injection. The whole brain was immediately dissected out and kept at −70°C for all measurements. Whole brains were weighed and placed in glass tubes containing 0.1 M HClO4 and dehydroxybenzylamine (DHBA) as an internal standard and sonicated for 1–2 min and centrifuged, the supernatant was separated and evaluated for 5-HT levels by high-performance liquid chromatography/electrochemical detection (HPLC/ECD) as described (Avraham et al., 1996). Separate groups of mice were used for all analyses.
Data analysis
All data are expressed as mean ± standard error of the mean. Statistical analyses were performed using one-way analysis of variance, following by Tukey's or Bonferroni post hoc test corrected for multiple comparisons.
Materials
Endocannabinoids were synthesized as previously reported (Mechoulam et al., 1995). The antagonists were kindly supplied by the National Institute of Drug Abuse.
Results
Induction of FHF and encephalopathy
We have shown (Avraham et al., 2008a) that 2 days after injecting mice with TAA (200 mg·kg−1), there are clear signs of hepatic failure with 30- to 40-fold increases in plasma levels of the hepatic enzymes, alanine amino transferase (ALT) and aspartate amino transferase (AST), accompanied by less dramatic but still significant increases in plasma total bilirubin (3.7-fold) and ammonia (2.7-fold). In the present study, we assessed development of encephalopathy, at the same time (2 days) after TAA, by measuring the neurological scores (higher score is equivalent to poorer neurological function). As shown in Figure 2, the score was significantly higher in mice injected with TAA, in relation to control mice (P < 0.001) and this increase was reversed following capsaicin (P < 0.01). Although neither SR141716A nor SR144528 significantly modulated the effect of capsaicin, the scores in these groups were also not different from the score after TAA alone. However, the beneficial effect of capsaicin was clearly reversed by the TRPV1 receptor antagonist, capsazepine (P < 0.05). Note that giving capsazepine alone to TAA-treated animals did not affect the raised neurological scores in this group.
Figure 2.
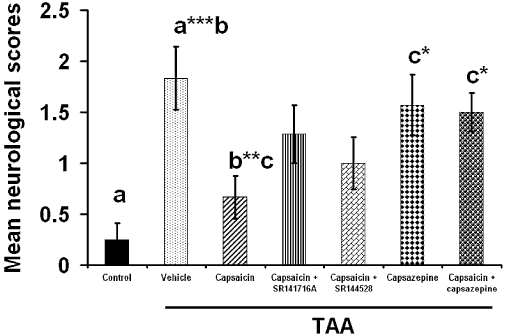
The effect of capsaicin, capsazepine, capsaicin + SR141716A, capsaicin +SR144528 and capsaicin + capsazepine on the neurological score. Sixty mice were given 200 mg·kg−1 thioacetamide (TAA), whereas control mice received saline (n= 10). Neurological score was assessed on day 3 after treatment. Capsaicin caused significant improvement in neurological score (P < 0.01) whereas capsaicin + capsazepine (P < 0.05) impaired the beneficial effect of capsaicin. Capsazepine given alone (P < 0.05) did not produce any effect. The maximum neurological deficit score in an animal is 10. Statistical analysis was performed as described in the Methods section. Data are presented as mean ± standard error of the mean (n= 10 per group). Bars with same letter (a–c) represent group means that are significantly different. *P < 0.05, **P < 0.01, ***P < 0.001.
Brain immunohistochemistry
Astrogliosis was evaluated at the area of cerebellum and hippocampus in control and TAA-treated animals on day 3 (Figures 3 and 4). The TAA treatment induced intensive GFAP staining and increased process complexity (i.e. more processes, increased branching). These changes were minimal in samples from TAA-treated mice subsequently given capsaicin, or SR141716A (CB1 receptor antagonist) or HU308 (CB2 receptor agonist) (summary data in Figure 5; P < 0.001), whereas treatment with the CB2 receptor antagonist (SR144528) or the CB1 receptor agonist noladin (data not shown) did not alter either the number or the morphology of activated astroglia, compared with those in mice injected with TAA only (Figure 5).
Figure 3.
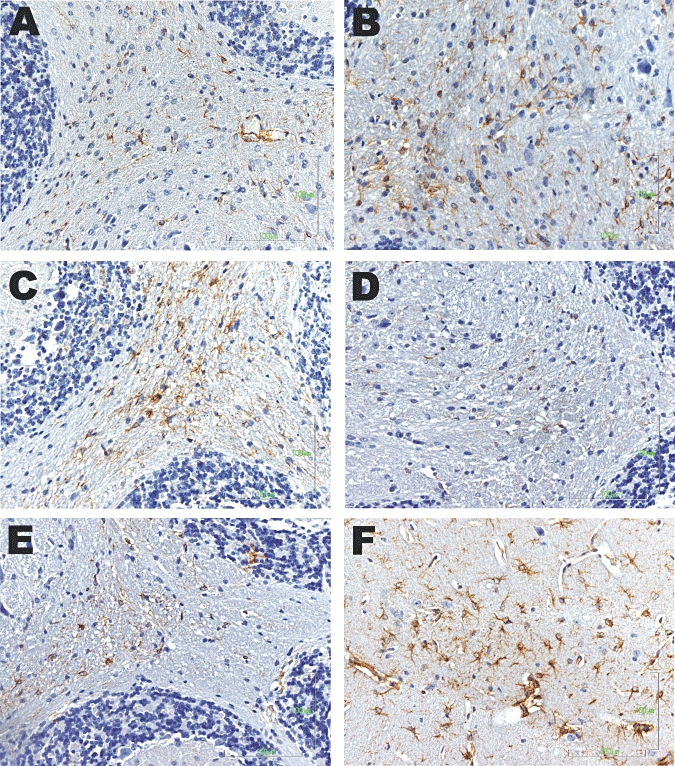
Astrogliosis in the cerebellum in saline-treated animals (A) and following thioacetamide (TAA) administration (B–F). Samples from mice with hepatic encephalopathy induced by TAA showed intensive glial fibrillary acidic protein (GFAP) staining and increased process complexity (i.e. more processes, increased branching). These changes were minimal in mice given TAA followed by capsaicin (C) or the CB1 receptor antagonist (SR141716A) (D) or the CB2 receptor agonist (HU308) (E). Note that administration of the CB2 receptor antagonist (SR144528) (F) to mice treated with TAA, did not alter either the number or the morphology of activated astroglia compared with TAA-treated animals (B).
Figure 4.
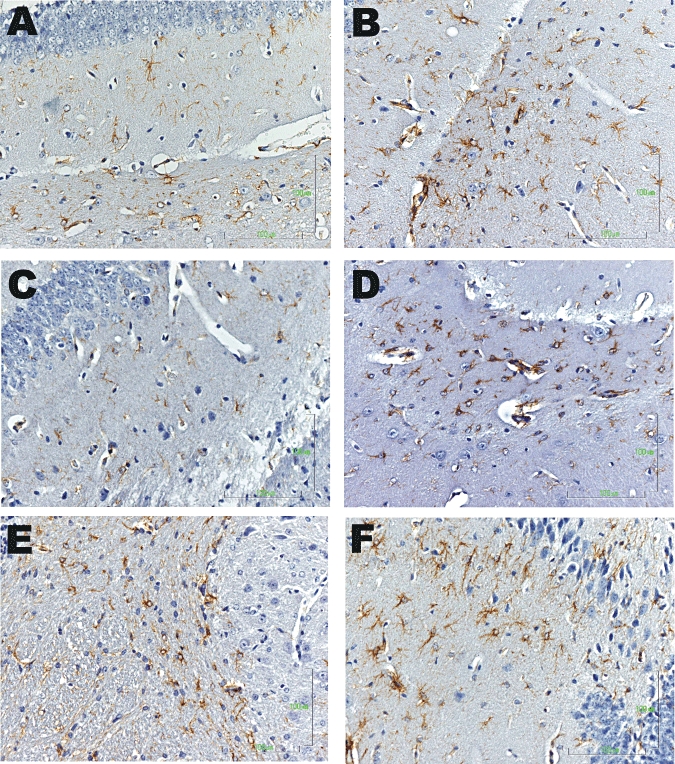
Astrogliosis in the hippocampus in saline-treated animals (A) and following thioacetamide (TAA) administration (B–F). Samples from mice with HE induced by TAA showed intensive GFAP staining and increased process complexity (i.e. more processes, increased branching). These changes were minimal in mice given TAA followed by capsaicin (C); or the CB1 receptor antagonist (D) or the CB2 receptor agonist (E) treatment. Note that administration of the CB2 receptor antagonist (F) did not alter either the number or the morphology of activated astroglia compared with TAA-treated animals (B).
Figure 5.
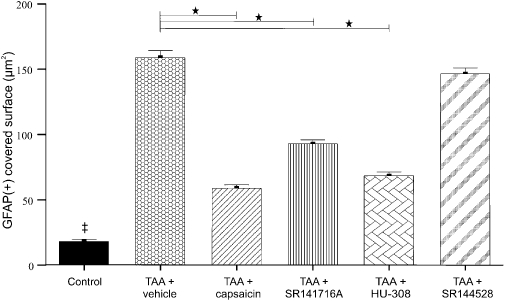
Quantitative evaluation of astrocytic activation in the brain samples illustrated in Figures 3 and 4. Activation of astrocytes was assessed by the area of glial fibrillary acidic protein (GFAP) staining and data represent the mean (with standard error mean) area covered by astrocytes per visual field in the hippocampus and cerebellum. The statistical test performed was the two-sided Fisher's exact test. ‡Significant difference (P < 0.001) between saline-treated controls and all other groups: *Significant difference (P < 0.001) between the group given thioacetamide (TAA) only and those given treatment after TAA (capsaicin or SR141716A or HU 308 or SR2144528).
Is the beneficial effect of capsaicin on neurological score, activity and cognitive function mediated by the CB1, CB2 or vanilloid receptors?
TAA-treated mice had a higher neurological severity (NSS) score, indicating neurological impairment (P < 0.001), diminished motor activity (P < 0.05) and impaired cognitive function (P < 0.001) compared with the control mice. Treatment with capsaicin reversed neurological function (P < 0.01), motor activity (P < 0.01) and cognitive function (P < 0.05) to control values (Figures 2, 6, 7), whereas capsazepine co-administered with capsaicin abolished the effect of capsaicin. Caspazepine alone had no effect on the impairment produced by TAA.
Figure 6.
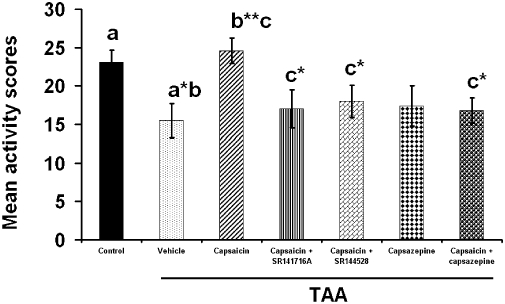
The effect of capsaicin, capsazepine, capsaicin + SR141716A, capsaicin + SR144528 capsaicin + capsazepine on the activity score. Sixty mice were given 200 mg·kg−1 thioacetamide (TAA), whereas control mice received saline (n= 10). Activity score was assessed on day 4 by the open-field test. TAA caused significant decrease in activity score (P < 0.05) whereas capsaicin reversed it significantly (P < 0.01) Blocking the CB1 or the CB2 receptors reversed the beneficial effect of capsaicin (P < 0.05). Statistical analysis was performed as described in the methods. Data are presented as mean ± standard error of the mean (n= 10 per group). Bars with same letter (a–c) represent group means that are significantly different. *P < 0.05, **P < 0.01.
Figure 7.
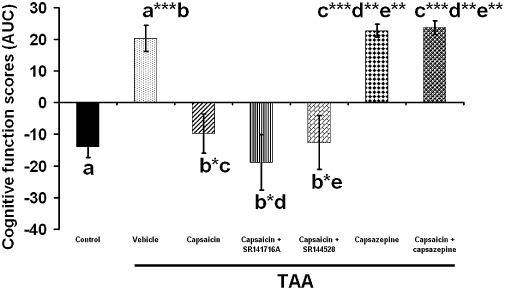
The effect of capsaicin, capsazepine, capsaicin + SR141716A, capsaicin + SR144528 capsaicin + capsazepine on the cognitive function. Sixty mice were given 200 mg·kg−1 thioacetamide (TAA), whereas control mice received saline (n= 10). The cognitive function was assessed by the eight arm maze beginning on day 8. The score was calculated as the area under the curve (AUC) averaged for the performance in the maze for five consecutive days. TAA induction caused significant deterioration in performance (P < 0.001). Capsaicin (P < 0.05) capsaicin + SR141716A (P < 0.05), capsaicin + SR144528 (P < 0.05) significantly improved cognitive function whereas capsazepine (P < 0.001) and capsaicin + capsazepine (P < 0.001) impaired it. Statistical analysis was performed as described in the methods. Data are presented as mean ± standard error of the mean (n= 10 per group). Bars with same letter (a–e) represent group means that are significantly different. *P < 0.05, **P < 0.01, ***P < 0.001.
Following TAA treatment, the beneficial effect of capsaicin on neurological function (Figure 2) and activity (Figure 6), but not on cognitive function (Figure 7), was impaired by both SR141716A and SR144528 (P < 0.05). Capsazepine administration impaired the beneficial effect of capsaicin not only on the neurological score and activity (P < 0.05), but also on cognitive function (P < 0.001) (Figures 2, 6, 7). Thus, the effect of capsaicin on neurological score, activity and cognitive function was mediated by both TRPV1 and CB receptors. Treatment with SR141716A (Avraham et al., 2006) alone resulted in significantly improved neurological score, activity and cognitive function whereas treatment with SR144528 alone was not effective (not shown).
Do capsaicin and endocannabinoids affect brain 5-HT levels?
TAA increased brain 5-HT levels significantly (P < 0.001). SR141716A (P < 0.05), capsaicin (P < 0.05), 2-AG and HU308 (P < 0.001) returned these increased 5-HT levels to control values. Blocking the CB1 receptor did not change the effect of the capsaicin treatment (Figure 8).
Figure 8.
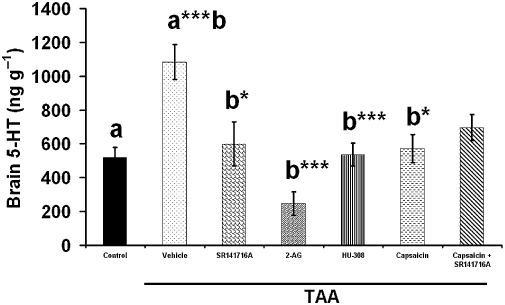
The effect of SR141716A, 2-arachidonoylglycerol (2-AG), HU308, capsaicin, SR141716A + capsaicin on brain 5-HT levels. Sixty mice were given 200 mg·kg−1 thioacetamide (TAA), whereas control mice received saline (n= 10). Fourteen days after TAA administration mice were killed and 5-HT levels were evaluated using high-performance liquid chromatography/electrochemical detection. TAA administration increased significantly brain 5-HT levels (P < 0.001). SR141716A (P < 0.05), 2-AG (P < 0.001), HU308 (P < 0.001), capsaicin (P < 0.05) and SR141716A + capsaicin reversed brain 5-HT to control levels. Statistical analysis was performed as described in the methods. Data are presented as mean ± standard error of the mean (n= 10 per group). Bars with same letter (a,b) represent group means that are significantly different. *P < 0.05, **P < 0.01, ***P < 0.001.
Effects on brain 2-AG levels
2-AG levels in whole brain were decreased 14 days after TAA administration (P < 0.001) and reversed by capsaicin treatment almost to control levels (P < 0.05). Blocking the CB1 or the CB2 receptors had no significant effect on the beneficial effect of capsaicin (Figure 9), although there seemed to be a trend towards reversal of capsaicin.
Figure 9.
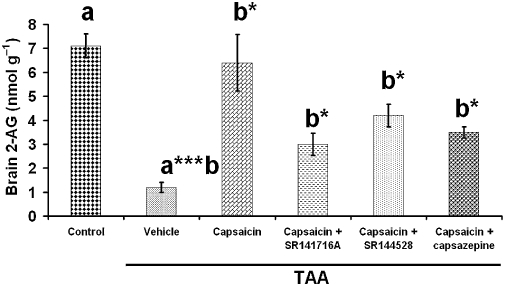
The effect of capsaicin, capsaicin + SR141716A, capsaicin + SR144528 or capsazepine on brain 2-arachidonoylglycerol (2-AG) levels. Fifty mice were given 200 mg·kg−1 thioacetamide (TAA), whereas the control mice received saline (n= 10). 2-AG levels were measured 14 days after TAA administration. 2-AG levels decreased 14 days after TAA administration. Administration of capsaicin (P < 0.05) reversed the lowered 2-AG levels to control values, whereas capsaicin + SR141716A (P < 0.05), capsaicin + SR144528 (P < 0.05) and capsazepine produced intermediate effects. Statistical analysis was performed as described in the methods. Data are presented as mean ± standard error of the mean (n= 10 per group). Bars with same letter (a,b) represent group means that are significantly different. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Capsaicin, a TRPV1 agonist, has a neuroprotective role in HE
Administration of TAA to mice induced acute liver failure, which led to CNS changes related to those seen in HE (Zimmerman et al., 1989; Gabbay et al., 2005; Avraham et al., 2006; 2008a; Dagon et al., 2007; Magen et al., 2008; Izzo and Camilleri, 2008). As agonists of the vanilloid and the CB2 receptor systems were found to ameliorate liver damage in FHF (Avraham et al., 2008a), we investigated their possible potential in treating HE. The principal finding in the present study is that capsaicin, a TRPV1 agonist, has a neuroprotective role in HE. This was supported by the following observations. First, TAA administration caused impaired neurological score, decreased activity and diminished cognitive function. Low dose capsaicin reversed these effects to almost to control levels, whereas capsazepine, a TRPV1 antagonist, reduced the beneficial effect of capsaicin indicating that the effects observed were vanilloid receptor-mediated. Second, TAA administration induced astrogliosis in both hippocampus and cerebellum. Capsaicin, SR141716A (a CB1 receptor antagonist) and HU308 (a CB2 receptor agonist), ameliorated astrogliosis, whereas SR144528 (a CB2 receptor antagonist) and noladin (a CB1 agonist) did not have any effect. Third, TAA increased brain 5-HT levels and capsaicin, SR141716A, HU308 and 2-AG reversed this effect of TAA on brain 5-HT. Fourth, brain levels of 2-AG decreased 14 days after TAA administration and capsaicin reversed this effect. Lastly, the effect of capsaicin on the neurological score, activity and 5-HT levels was impaired by SR141716A – which probably competes with capsaicin for binding to the vanilloid receptor and by SR144528, which probably blocked the beneficial effect of endogenous 2-AG via the CB2 receptors. Cognitive function was not impaired either by SR141716A or by SR144528, presumably indicating that a maximal effect was attained by capsaicin. Capsazepine alone did not affect either the neurological score or cognitive function, but it did reduce the activity score (P < 0.05) (not shown).
Antagonism of CB1 receptors and agonism at CB2 receptors have a neuroprotective role in HE, and capsaicin administration normalizes brain 2-AG levels
In a previous study of ours, the effects of endocannabinoids on activity, as well as on neurological and cognitive functions in HE, were studied. Encephalopathic mice treated with SR141716A or 2-AG or both, showed improved neurological function, activity and cognitive function compared with untreated controls. SR141716A showed a dose-response improvement of neurological function. HU308 treatment improved neurological score via the CB2 receptors. CNS levels of endogenous 2-AG measured 2 days after TAA administration were found to be elevated when compared with healthy controls (Avraham et al., 2006). However, in the current study, brain levels of 2-AG were found to be markedly decreased 14 days after TAA (Figure 9). Capsaicin administration normalized brain 2-AG levels mostly through the TRPV1 receptor, apparently in part through the CB1 and CB2 receptors (Figure 9).
In a previous report, using a model of brain injury, levels of 2-AG increased significantly, up to tenfold, 4 h after the injury, and declined thereafter. Administration of 2-AG alleviated oxidative stress, inhibited NF-kappa B, protected the blood-brain-barrier, attenuated neuroinflammatory response and ameliorated neurological deficits (Panikashvili et al. 2001; Panikashvili et al., 2006; Mechoulam and Shohami, 2007). Furthermore, our previous studies have shown neuroprotective effects of 2-AG in experimental HE (Avraham et al., 2006). We have shown also that 2-AG probably plays a role in the pathophysiological changes in the liver, heart, lung and kidney that follow BDL, an animal model of chronic liver disease (Avraham et al., 2008b).
Modulation of increased 5-HT levels following TAA administration
Anorexia, one of the most typical symptoms observed in experimental and human cirrhosis, is assumed to be associated with altered brain 5-HT metabolism. Hence, increased brain 5-HT concentration in TAA treated rats, may be relevant to the pathogenesis of anorexia associated with TAA-induced cirrhosis (Haider et al., 2004). We now show that increased brain 5-HT levels were reversed by SR141716A, 2-AG, HU308 and capsaicin. Decreased 5-HT levels may therefore be associated with increased appetite.
The complex therapeutic mechanism involves both the cannabinoid and the vanilloid systems
The amelioration of astrogliosis following treatment with either SR141716A, HU308 or capsaicin, as well as the modulation of brain 5-HT levels by the same agents, as well as 2-AG, clearly indicate the complexity of the mechanism(s) involved. Previous findings have also shown that both endocannabinoids and capsaicin improve liver function and histopathology (Avraham et al., 2008a). These results may be related to those in a recent publication by Fioravanti et al. (2008), who showed that constitutive activity at the CB1 receptor is required to maintain the TRPV1 receptor in a ‘sensitized’ state responsive to chemical stimuli.
We have confirmed now that a very low dose of capsaicin improves neurological function, activity and cognitive function, whereas its antagonist capsazepine reverses the capsaicin improvement. It seems that part of the effect of capsaicin is mediated also via the CB1 and CB2 receptors and that SR141716A also competes with capsaicin for the vanilloid receptors.
Thus, the simultaneous blockade of CB1 receptors and stimulation of both the CB2 and vanilloid receptors has the best therapeutic effect on the liver. The treatment was very effective, as it was given 1 day after TAA administration and showed therapeutic effects on brain histopathology and function, liver enzyme levels as well as on liver pathology, on day 3.
Our studies are in agreement with those of Teixeira-Clerc et al. (2006; 2008;) and Lotersztajn et al. (2008), who identified the CB1 receptor as a molecular target for the treatment of liver fibrosis. Our data suggest that TRPV1 receptors might also be involved, although in our experiments an anti-fibrotic effect could not be confirmed morphologically, as the animals were killed a short time after TAA administration. Moreover, our findings raise the possibility of using TRPV1 agonists to treat HE.
Lotersztajn's group (2008) showed that cirrhosis was associated with increased number of fibrogenic cells regulated by cannabinoid receptors (Teixeira-Clerc et al., 2006). CB2 receptor-mediated suppression of fibrosis is in agreement with our findings and the beneficial effect of HU308 on HE (Julien et al., 2005; Lotersztajn et al., 2008). Earlier studies by Kunos' group showed that endocannabinoids acting at CB1 receptors in the hepatic vasculature may mediate the vasodilated state and hypotension that accompany advanced liver cirrhosis (Batkai et al., 2001). Thus, CB1 receptor blockade may improve the prognosis of cirrhotic individuals not only by delaying the fibrotic process, but also by improving the associated haemodynamic abnormalities (Varga et al., 1998; Batkai et al., 2001; Kunos et al., 2006).
The therapeutic effect on brain is either independent or due to liver recovery
Decreased inflammatory cell infiltration was noticed following HU308, SR141716A and capsaicin treatment (Figures 3–5). However, administration of a CB2 receptor antagonist did not result in an anti-inflammatory effect. These findings may indicate that the anti-inflammatory effect of these reduced compounds may, at least partly, be related to the improved function of the liver and are CB2 receptor-mediated.
The regenerative capacity of liver was increased following all but capsaicin treatment, a finding that may be correlated with the reduced hepatic cell injury noticed in this group of animals. Histological findings indicate that the CB2 receptor-mediated anti-inflammatory effect may be related to the improved functional outcome of the remaining intact and regenerating hepatic cells following TAA administration. However, in the case of capsaicin, an additional factor might be the protection of the hepatic cells. Whether this effect is related to the concomitant anti-inflammatory effect of this compound and/or to a direct protective mechanism could not be clarified in the present study. The beneficial effect of capsaicin on reducing ammonia levels in our previous article (Avraham et al., 2008a) might clarify a possible mechanism.
In a previous study of ours (Dagon et al., 2007), a Δ9-tetrahydrocannabinol (THC)-related, AMPK-mediated improvement of cerebral function (but not of hepatic function) was reported. However, our current results indicate that low dose of capsaicin affects both liver and brain function shortly after TAA administration, without side effects.
Astrogliosis following TAA administration and the effect of capsaicin, HU308 or SR141716A
Astrogliosis is a phenomenon that develops in various CNS disorders. Reactive astrocytes are involved in neuronal survival and regeneration. Major reactive changes of astrocytes in vivo are the upregulation of the intermediate filaments, GFAP and vimentin, with accompanying cellular hypertrophy and/or hyperplasia (Eng and Ghirnikar, 1994; Röhl et al., 2007). The cellular and molecular mechanisms leading to astrogliosis are still not completely understood. In general, microglia, which are normally in a quiescent status, become activated by different stimuli associated with neuropathological changes of the CNS. This activation precedes or accompanies astrogliosis (Herber et al., 2006). Glutamine is synthesized in astrocytes from ammonia and glutamate and causes brain swelling that correlates with neuropsychological deterioration. As a result, alterations of astrocytic–neuronal cross-talk occur, affecting brain function (Shawcross and Jalan, 2005; Ahboucha and Butterworth, 2007).
At the molecular level, cytokines predominantly secreted by microglia seem to play an important role as triggers and modulators of astrogliosis and within the CNS. The effect of single cytokines on the induction of astrogliosis has been examined in several studies. Prominent representatives of proinflammatory cytokines such as IL-1 and TNFα have been shown to induce, enhance or accompany astrogliosis in vivo (Balasingam et al., 1994). In the present work activated astrocytes at the areas of hippocampus and cerebellum, in particular, following TAA administration, were detected. However, capsaicin, HU308 or SR141716A treatment resulted in the amelioration of astrogliosis. 2-AG levels increase within 3 days following TAA administration and decrease thereafter below the control levels over 14 days post-HE induction. Likewise, brain 5-HT levels increase significantly following TAA administration, which parallels the observations of lack of appetite and decrease in weight in humans with HE.
In conclusion, the effects of capsaicin, HU308 or SR141716A, were due to both cerebral and hepatic actions. As capsaicin, SR141716A and HU308 have the advantage of access through the blood–brain barrier; the brain itself may be a direct therapeutic target. However, an indirect benefit for the brain via liver recovery, cannot be excluded.
Acknowledgments
We would like to thank the Israel Science Foundation for the support of the study.
Glossary
Abbreviations:
- 2-AG
2-arachidonoylglycerol
- 5-HT
5-hydroxytryptamine
- ALT
alanine amino transferase
- AMPK
AMP-activated protein kinase
- AST
aspartate amino transferase
- FHF
fulminant hepatic failure
- GFAP
glial fibrillary acidic protein
- HE
hepatic encephalopathy
- TAA
thioacetamide
- THC
Δ9-tetrahydrocannabinol
Conflict of interest
There are no conflicts of interest.
References
- Ahboucha S, Butterworth RF. The neurosteroid system: an emerging therapeutic target for hepatic encephalopathy. Metab Brain Dis. 2007;22:291–308. doi: 10.1007/s11011-007-9065-2. [DOI] [PubMed] [Google Scholar]
- Archer VE, Jones DW. Capsaicin pepper, cancer and ethnicity. Med Hypotheses. 2002;59:450–457. doi: 10.1016/s0306-9877(02)00152-4. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham Y, Bonne O, Berry EM. Behavioral and neurochemical alterations caused by diet restriction – the effect of tyrosine administration. Brain Res. 1996;732:133–144. doi: 10.1016/0006-8993(96)00514-8. [DOI] [PubMed] [Google Scholar]
- Avraham Y, Israeli E, Gabbay E, Okun A, Zolotarev O, Silberman I, et al. Endocannabinoids affect neurological and cognitive function in thioacetamide-induced hepatic encephalopathy in mice. Neurobiol Dis. 2006;21:237–245. doi: 10.1016/j.nbd.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Avraham Y, Zolotarev O, Grigoriadis N, Pautahidis T, Magen I Vorobiav L, et al. Cannabinoids and capsaicin improve liver function following thioacetamide-induced acute injury in mice. Am J Gastroenterol. 2008a;103:3047–3056. doi: 10.1111/j.1572-0241.2008.02155.x. [DOI] [PubMed] [Google Scholar]
- Avraham Y, Magen I, Zolotarev O, Vorobiav L, Nachmias A, Pappo O, et al. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist, in various rat tissues during the evolution of experimental cholestatic liver disease. Prostaglandins Leukot Essent Fatty Acids. 2008b;79:35–40. doi: 10.1016/j.plefa.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong VW. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14:846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rábano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell JE. Serotonin and the biology of feeding. Am J Clin Nutr. 1992;55:155–159. doi: 10.1093/ajcn/55.1.155s. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology and cognitive deficits. J Neurotrauma. 1996;13:557–568. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Ilan Y, Mechoulam R, Berry EM. Cannabinoids ameliorate cerebral dysfunction in experimental hepatic encephalopathy via AMP-activated protein kinase. FASEB J. 2007;21:2431–2441. doi: 10.1096/fj.06-7705com. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, et al. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Fitz GJ. Hepatic encephalopathy, hepatopulmonary syndromes, hepatorenal syndrome, coagulopathy and endocrine complications of liver diseases. In: Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 7th edn. London: W.B. Saunders Press; 2002. pp. 1543–1565. [Google Scholar]
- Fernández-Martínez A, Callejas NA, Casado M, Boscá L, Martín-Sanz P. Thioacetamide-induced liver regeneration involves the expression of cyclooxygenase 2 and nitric oxide synthase 2 in hepatocytes. J Hepatol. 2004;40:963–970. doi: 10.1016/j.jhep.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Fioravanti B, De Felice M, Stucky CL, Medler KA, Luo M-C, Gardell LR, et al. Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J Neurosci. 2008;28:11593–11602. doi: 10.1523/JNEUROSCI.3322-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 1993;231:313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Gabbay E, Avraham Y, Ilan Y, Israeli E, Berry EM. Endocannabinoids and liver disease – review. Liver Int. 2005;25:921–926. doi: 10.1111/j.1478-3231.2005.01180.x. [DOI] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Haider S, Saleem S, Shameem S, Ahmed SP, Parveen T, Haleem DJ. Is anorexia in thioacetamide-induced cirrhosis related to an altered brain serotonin concentration? Pol J Pharmacol. 2004;56:73–78. [PubMed] [Google Scholar]
- Hanuš L, Avraham Y, Ben-Shushan D, Zolotarev O, Berry EM, Mechoulam R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res. 2003;983:144–151. doi: 10.1016/s0006-8993(03)03046-4. [DOI] [PubMed] [Google Scholar]
- Herber DL, Maloney JL, Roth LM, Freeman MJ, Morgan D, Gordon MN. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia. 2006;53:382–391. doi: 10.1002/glia.20272. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, et al. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Kim CS, Kawada T, Kim BS, Han IS, Choe SY, Kurata T, et al. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15:299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D, Bátkai S, Gao B. Cannabinoids hurt, heal in cirrhosis. Nat Med. 2006;12:608–610. doi: 10.1038/nm0606-608. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chang JS, Lee JY, Kim JA. Capsaicin-induced apoptosis and reduced release of reactive oxygen species in MBT-2 murine bladder tumor cells. Arch Pharm Res. 2004;27:11457–11453. doi: 10.1007/BF02975121. [DOI] [PubMed] [Google Scholar]
- Lee WS, McKiernan P, Kelly DA. Etiology, outcome and prognostic indicators of childhood fulminant hepatic failure in the United Kingdom. J Pediatr Gastroenterol Nutr. 2005;40:575–581. doi: 10.1097/01.mpg.0000158524.30294.e2. [DOI] [PubMed] [Google Scholar]
- Li Y, Song D, Zhang Y, Lee SS. Effect of neonatal capsaicin treatment on haemodynamics and renal function in cirrhotic rats. Gut. 2003;52:293–299. doi: 10.1136/gut.52.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotersztajn S, Teixeira-Clerc F, Julien B, Deveaux V, Ichigotani Y, Manin S, et al. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol. 2008;153:286–289. doi: 10.1038/sj.bjp.0707511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu IJ, Lee KZ, Lin JT, Hwang JC. Capsaicin administration inhibits the abducent branch but excites the thyroarytenoid branch of the recurrent laryngeal nerves in the rat. J Appl Physiol. 2005;98:1646–1652. doi: 10.1152/japplphysiol.01133.2004. [DOI] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Berry EM, Mechoulam R. Endocannabinoids in liver disease and hepatic encephalophathy. Curr Pharm Des. 2008;14(23):2362–2369. doi: 10.2174/138161208785740063. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Shohami E. Endocannabinoids and traumatic brain injury. Mol Neurobiol. 2007;36:68–74. doi: 10.1007/s12035-007-8008-6. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharm. 1995;50:83–100. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Morgan MY, Blei A, Grüngreiff K, Jalan R, Kircheis G, Marchesini G, et al. The treatment of hepatic encephalopathy. Metab Brain Dis. 2007;22:389–405. doi: 10.1007/s11011-007-9060-7. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanuš L, Breuer E, Mechoulam R, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shein NA, Mechoulam R, Trembovler V, Kohen R, Alexandrovich A, et al. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurob Dis. 2006;22:257–264. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pick CG, Yanai J. Eight arm maze for mice. Int J Neurosci. 1983;21:63–66. doi: 10.3109/00207458308986121. [DOI] [PubMed] [Google Scholar]
- Röhl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129:43–52. doi: 10.1016/j.brainres.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Shawcross D, Jalan R. Dispelling myths in the treatment of hepatic encephalopathy. Lancet. 2005;365:431–433. doi: 10.1016/S0140-6736(05)17832-5. [DOI] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Julien B, Grenard P, Van Nhieu T, Deveaux J, Li V, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- Teixeira-Clerc F, Julien B, Grenard P, Van Nhieu T, Deveaux J, Hezode V, et al. The endocannabinoid system as a novel target for the treatment of liver fibrosis. Pathol Biol (Paris) 2008;56:36–38. doi: 10.1016/j.patbio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet and macrophage derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Zimmerman P, Ferenci C, Pifl C, Yurdaydin J, Ebner H, Lassman E, et al. Hepatic encephalopathy in thioacetamide induced acute liver failure in rats: characterization of an improved animal model and study of amino acid-ergic neurotransmission. Hepatology. 1989;9:594–601. doi: 10.1002/hep.1840090414. [DOI] [PubMed] [Google Scholar]


