Abstract
Recently identified genetic forms of short QT syndrome (SQTS) are associated with an increased risk of arrhythmia and sudden death. The SQT3 variant is associated with an amino-acid substitution (D172N) in the KCNJ2-encoded Kir2.1 K+ channel. In this study, whole-cell action potential (AP) clamp recording from transiently transfected Chinese Hamster Ovary cells at 37 °C showed marked augmentation of outward Kir2.1 current through D172N channels, associated with right-ward voltage-shifts of peak repolarizing current during both ventricular and atrial AP commands. Peak outward current elicited by ventricular AP commands was inhibited by chloroquine with an IC50 of 2.45 μM for wild-type (WT) Kir2.1, of 3.30 μM for D172N-Kir2.1 alone and of 3.11 μM for co-expressed WT and D172N (P > 0.05 for all). These findings establish chloroquine as an effective inhibitor of SQT3 mutant Kir2.1 channels.
Keywords: Arrhythmia, Antiarrhythmic, Chloroquine, KCNJ2, Kir2.1, QT interval, Short QT syndrome, Sudden death
1. Introduction
The short QT syndrome (SQTS) is linked to abbreviated QT intervals on the electrocardiogram and to an increased incidence of cardiac arrhythmias and of sudden death [1]. The SQT3 variant was identified in an asymptomatic child with an abnormal electrocardiogram and in her father who had a history of presyncopal events and palpitations [2]. Father and daughter had abbreviated rate-corrected QT (QTc) intervals of 320 ms and 315 ms respectively, and programmed electrical stimulation was able to elicit ventricular fibrillation [2]. Genetic analysis [2] revealed that neither individual exhibited mutations in KCNH2 or KCNQ1 (associated with SQT1 and SQT2 variants respectively; [1]), but a single base substitution was identified in KCNJ2, giving rise to an aspartate to asparagine substitution at position 172 (D172N) in the Kir2.1 potassium channel protein. In humans, Kir2.1 is expressed in both atria and ventricles and contributes to channels underlying the inwardly rectifying K+ current IK1 [3]. Patch clamp recordings at ambient temperature showed augmentation of outward but not inward current through D172N-Kir2.1 channels, predicted in cell and tissue simulations to accelerate ventricular repolarization [2].
For the KCNH2-linked SQT1 variant, the action potential voltage-clamp (‘AP clamp’) technique has been used to characterize effects of the SQT1 mutation on ionic current during physiological waveforms [4,5]. In addition, in vitro and in vivo investigations have also identified pharmacological agents that can help restore towards normal the QT intervals of SQT1 patients (e.g. [6–8]). To date, neither of these approaches has been applied to the SQT3 D172N mutation. Accordingly, the present study: (i) provides the first AP clamp information on effects of the Kir2.1 mutation, and does so at physiological temperature; (ii) identifies an effective pharmacological inhibitor of SQT3 D172N mutant Kir2.1.
2. Methods
2.1. Maintenance of cells expressing WT and D172N-Kir2.1 channels
Wild-type (WT) and mutant (D172N) Kir2.1 (in pSVL expression vector) were kindly provided by Professor H Matsuda [9]. Chinese Hamster Ovary (CHO) cells were passaged using a non-enzymatic agent (Enzyme Free, Chemicon®International) and then maintained as described previously [5]. They were transiently transfected with either WT or D172N-Kir2.1 (2 μg of each construct was used) at a ratio of 4:1 with CD8 (in pIRES; courtesy of Dr I Baró and Dr J Barhanin), 24 h after plating cells out, using Lipofectamine™ LTX (Invitrogen), according to the manufacturer's instructions. For co-expression of WT and D172N-Kir2.1 (to mimic the heterozygous condition of the SQT3 proband [2]) cells were transfected with equal amounts of WT and D172N constructs (cf [2]). Cells were plated onto small sterilised collagen-coated glass coverslips 6 h after transfection and recordings were made after at least 24 h incubation at 37 °C. Successfully transfected cells (positive to CD8) were identified using Dynabeads® (Invitrogen).
2.2. Electrophysiological recordings
For whole-cell patch-clamp recording cells were continuously superfused (at 37 °C) with an external solution containing (in mM): 140 NaCl, 4 KCl, 2.5 CaCl2, 1 MgCl2, 10 Glucose and 5 HEPES (titrated to pH 7.45 with NaOH). Patch-pipettes (Corning 7052 glass, AM Systems) were pulled and heat polished (Narishige MF83) to 2.5–4 MΩ; pipette dialysate contained (in mM): 130 KCl, 1 MgCl2, 5 EGTA, 5 MgATP and 10 HEPES (titrated to pH 7.2 using KOH). These solutions are similar to those used previously to study the KCNH2 SQT1 mutation [5]. Recordings of Kir2.1 current (IKir2.1) were made using an Axopatch 200 amplifier (Axon Instruments) and a CV201 head-stage. Between 70 and 80% of pipette series resistance was compensated. Voltage-clamp commands were generated using ‘WinWCP’ (John Dempster, Strathclyde University). Human ventricular and atrial AP waveforms were generated using established ventricular [10] and atrial [11] cell models.
2.3. Experimental compounds
Chloroquine-HCl powder (Sigma) was dissolved in Milli-Q water to produce an initial stock solution of 50 mM which was diluted to produce stock solutions ranging down to 300 μM. The Chloroquine-HCl stock solutions were diluted at least 1:1000-fold with Tyrode's solution to achieve concentrations stated in the Results. BaCl2 powder (Sigma) was dissolved in Milli-Q water to produce a stock concentration of 1 M and the test concentration (1 mM) was produced by a 1:1000-fold dilution with Tyrode's solution. External solutions were applied using a home-built, warmed rapid solution exchange device.
2.4. Data analysis
Unless otherwise stated in the text data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using analysis of variance or t-tests as appropriate (Graphpad, Prism v5). P values of less than 0.05 were taken as statistically significant.
3. Results and discussion
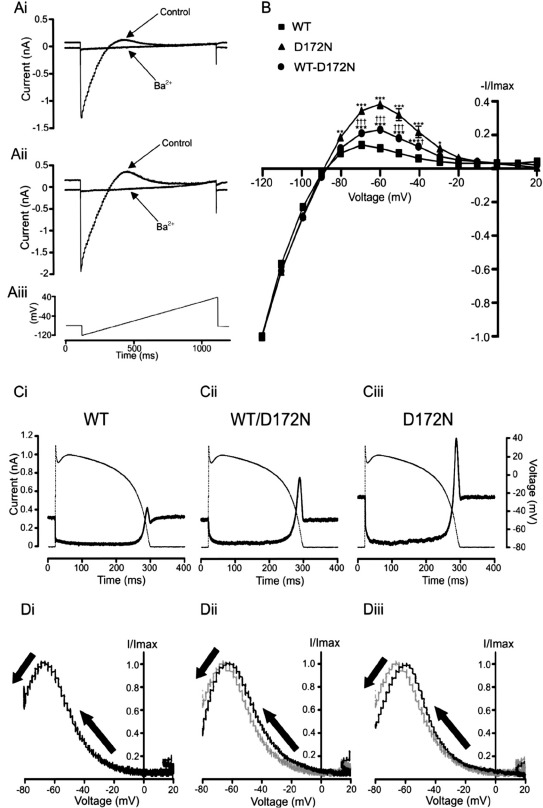
In initial experiments IKir2.1 was elicited by the voltage ramp protocol shown in Fig. 1Aiii, with representative current traces for WT and D172N-Kir2.1 current shown respectively in Figs. 1Ai and Aii, in the absence and presence of 1 mM Ba2+. Fig. 1B shows normalized mean current–voltage (I–V) data for Ba2+-sensitive WT and D172N IKir2.1. The I–V relations for WT and mutant Kir2.1 were similar between − 120 and − 90 mV and the mean reversal potential (Erev) was not significantly different (− 88.3 ± 0.5 mV for WT-Kir2.1 versus − 89.3 ± 1.1 mV for D172N-Kir2.1; P > 0.05). However, outward IKir2.1 differed markedly between the two channels: it was significantly greater for D172N-Kir2.1 between − 80 and − 30 mV and in addition peaked at a more positive membrane potential (Fig. 1B). Co-expressed WT-D172N IKir2.1 exhibited a similar I–V profile (Fig. 1B) to WT IKir2.1 between − 120 and − 80 mV (Erev of − 87.6 ± 0.5 mV; P > 0.05 versus WT), with outward current between − 70 and − 30 mV that was intermediate in amplitude between that for individually expressed WT and D172N Kir2.1. These features are qualitatively consistent with those at ambient temperature reported previously [2], although the voltage range over which outward D172N IKir2.1 was significantly greater than WT IKir2.1 was wider in the present study (than a range between − 75 and − 45 mV reported in [2]). The results of AP clamp experiments using an epicardial ventricular AP waveform are shown in Figs. 1C and D. Figs. 1Ci, Cii and Ciii show Ba2+-sensitive IKir2.1 for WT, WT-D172N and D172N Kir2.1 respectively. AP commands were applied from a holding potential of − 80 mV at which there was outwardly directed steady-state IKir2.1. Current markedly decreased following the AP upstroke for each WT and D172N Kir2.1 expression condition; throughout most of the AP plateau phase, a small outward current through WT Kir2.1 was present and this was also the case for co-expressed WT-D172N and for D172N alone. IK1 channels incorporating Kir2.1 would therefore be anticipated to contribute a small amount of repolarizing current throughout much of the AP plateau phase. Towards the latter part of the plateau, outward current increased for each Kir2.1 expression-condition, with a marked outward current during terminal repolarization. The outward current during terminal repolarization of the AP was markedly greater (∼ 4.6-fold, P < 0.01) for D172N-Kir2.1 (1.52 ± 0.47 nA; n = 11) than for WT-Kir2.1 (0.33 ± 0.06 nA; n = 19), with that for co-expressed WT-D172N-Kir2.1 being intermediate between the two (0.72 ± 0.12 nA; n = 18; ∼ 2.2 fold that for WT, P < 0.01 versus WT; P < 0.05 versus D172N). Figs. 1Di–Diii show representative normalized instantaneous I–V relations for IKir2.1 during the repolarizing phase of the AP command. Peak outward current was positively shifted by ∼+ 8 mV for D172N IKir2.1 (from − 68.0 ± 1.0 mV for WT, to − 60.3 ± 2.5 mV for D172N-Kir2.1; P < 0.01 versus WT) and by ∼+ 4 mV for co-expressed WT-D172N-Kir2.1 (to − 63.6 ± 1.6 mV; P < 0.05 versus WT; P > 0.05 versus D172N). The larger and earlier IKir2.1 when D172N Kir2.1 was present would be anticipated to abbreviate AP repolarization in the setting of SQT3. Additional experiments on singly expressed WT or D172N Kir2.1 using endocardial and midmyocardial AP commands (data not shown) showed similar effects of the D172N mutation to those seen with the epicardial ventricular AP waveform.
Fig. 1.
WT, WT-D172N and D172N-Kir2.1 during ramp and ventricular AP waveforms. (A) Example traces of WT IKir2.1 (Ai) and D172N IKir2.1 (Aii) elicited by ascending voltage ramp command, shown in (Aiii) (applied at 3 s intervals) in control and following application of 1 mM BaCl2. (B) Mean current–voltage (I–V) relations for Ba2+-sensitive current for WT, WT-D172N and D172N Kir2.1 (n = 18, 24 and 7 cells respectively). For each cell, currents were normalized to the current at − 120 mV to facilitate comparison between the three channel expression conditions. (⁎⁎⁎P < 0.001 versus WT, ⁎⁎P < 0.01 versus WT,⁎P < 0.05 versus WT, †††P < 0.001 versus D172N, †P < 0.05 versus D172N). (C) Profile of 1 mM Ba2+-sensitive WT IKir2.1 (Ci), WT-D172N IKir2.1 (Cii) and D172N IKir2.1 (Ciii) (solid traces) during an epicardial ventricular AP command (dash-dotted trace, 1 Hz). Residual capacitative current transients during the rising phase of the AP command have been blanked for clarity of display. (D) Representative instantaneous I–V relations for WT (Di), WT-D172N (Dii) and D172N (Diii) current during ventricular AP repolarization (direction of repolarization denoted by arrows). For each cell, currents were normalized to the maximal current during repolarization and plotted against the corresponding membrane potential from the AP peak to the return to − 80 mV. To facilitate comparison between the different channel expression conditions the WT I–V relation was superimposed (as a grey trace) on the WT-D172N (Dii) and D172N (Diii) I–V plots (black traces).
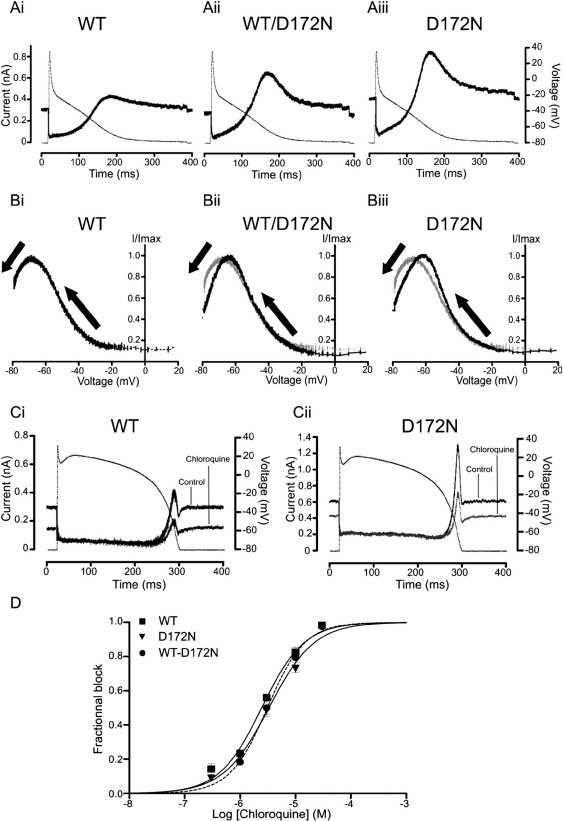
AP clamp experiments were also performed using an atrial AP command waveform. Figs. 2Ai, Aii and Aiii show representative Ba2+-sensitive IKir2.1 for WT, WT-D172N and D172N Kir2.1 respectively. In the absence of a prominent AP plateau phase, the elicited current profiles differed markedly from those seen with a ventricular AP command; however, the WT current configuration is similar to that predicted previously from simulation of IKir2.1 during atrial APs [12], with IKir2.1 contributing repolarizing current throughout much of the AP waveform, after the initial rapid repolarization phase. Peak outward current during repolarization for D172N Kir2.1 (1.22 ± 0.31 nA; n = 10) was ∼ 3-fold that for WT Kir2.1 (0.39 ± 0.07 nA; n = 15; P < 0.01). Peak outward IKir2.1 during AP repolarization for co-expressed WT-D172N (0.77 ± 0.16 nA; n = 12) was ∼ 2-fold that for WT IKir2.1 (P < 0.05). Figs. 2Bi, Bii and Biii show representative instantaneous I–V relations for IKir2.1 during AP repolarization: peak outward current was positively shifted by + 9 mV for D172N IKir2.1 (from − 67.8 ± 1.4 mV for WT, to − 58.8 ± 3.1 mV for D172N; P < 0.01). For WT-D172N co-expression, peak outward IKir2.1 during the atrial AP was shifted by ∼+ 5 mV (to − 63.2 ± 2.0 mV; n = 12; P < 0.05 versus WT; P > 0.05 versus D172N). Thus, our AP clamp findings are consistent with a greater and earlier contribution of IK1 channels containing Kir2.1 subunits to repolarization of both ventricular and atrial APs in SQT3.
Fig. 2.
WT, WT-D172N and D172N-Kir2.1: atrial AP clamp and sensitivity to chloroquine. (A) Profile of 1 mM Ba2+-sensitive WT IKir2.1 (Ai), WT-D172N (Aii) and D172N IKir2.1 (Aiii) (solid traces) during an atrial AP command (dash-dotted trace, 1 Hz). Residual capacitative current transients during the rising phase of the AP command have been blanked for clarity of display. (B) Instantaneous I–V relations for WT (Bi), WT-D172N (Bii) and D172N (Biii) current during atrial repolarization. For each cell, currents were normalized as for Fig. 1D. Direction of repolarization is denoted by arrows. To facilitate comparison between the different channel expression conditions, the WT I–V curve was superimposed (as a grey trace) on the WT-D172N (Bii) and D172N (Biii) IV curves. (C) Representative traces showing the effect of 3 μM chloroquine application on WT IKir2.1 (Ci) and D172N IKir2.1 (Cii), elicited by ventricular AP command (superimposed). (D) Concentration–response relations for inhibition by chloroquine of maximal outward peak Kir2.1 current elicited during AP clamp. Data for WT (squares), WT-D172N (circles) and D172N (triangles) IKir2 are shown overlaid (for each expression condition n = 4–8 cells per concentration). For IC50 values see ‘Results and discussion’ text.
The D172 residue is located in a highly conserved region of the transmembrane pore of the Kir2.1 channel [2] and its mutation has been reported to influence channel block by Mg2+ ions and polyamines [9,13]. The quinolone agent chloroquine blocks potently native IK1 [14] and has recently been shown to inhibit Kir2.1 by interacting at a site in the cytoplasmic domain (i.e. distinct from the transmembrane pore region) of the channel, with residues E224, D259 and E299 being strong binding determinants, F254 also acting as a (weaker) determinant, whilst the alanine mutant D172A produced a comparatively small attenuation of chloroquine potency [15]. We reasoned, therefore, that chloroquine might be an effective inhibitor of D172N-Kir2.1 channels. To test this proposition, a range of chloroquine concentrations was tested against WT, D172N-Kir2.1 and co-expressed WT-D172N Kir2.1. Inhibition of peak outward current during ventricular AP repolarization was assessed [cf 15], using an identical AP waveform for each channel/expression condition to enable direct comparison of observed blocking potency. Figs. 2Ci and Cii show representative traces of WT and D172N IKir2.1 before and during exposure to 3 μM chloroquine. Whole-cell current through the two channels was attenuated by chloroquine to a similar extent. Fig. 2D contains concentration–response relations for effects of chloroquine on singly expressed WT and D172N and on co-expressed WT-D172N IKir2.1, showing these to be similar between the WT and differing D172N Kir2.1 expression conditions. The half-maximal inhibitory concentration (IC50) value for WT IKir2.1 was 2.45 μM (95% confidence interval (C.I.) 2.14 to 2.79 μM; Hill coefficient 1.13 ± 0.07) in fair agreement with [15]. The observed IC50 value for inhibition of D172N IKir2.1 was 3.30 μM (95% C.I. from 2.90 to 3.75 μM; Hill coefficient 1.05 ± 0.06; P > 0.05 versus WT). Chloroquine inhibited co-expressed WT and D172N IKir2.1 with an IC50 value of 3.11 μM (95% C.I. 2.72 to 3.55 μM; Hill coefficient 1.28 ± 0.10; P > 0.05 versus both WT and D172N alone). As the voltage at which peak IKir2.1 during AP repolarization occurred was positively shifted for D172N compared to the WT channel, an additional comparison of blocking potency was made at a single standardized membrane potential value (− 68 mV; the mean value for peak WT outward current in Fig. 1D). This comparison (concentration–response relations not shown) also yielded little variation in blocking potency between WT and mutant channels, with IC50 values of: 2.91 μM for WT IKir2.1 (95% C.I. 2.46 to 3.45 μM, and not significantly different from 2.45 μM; Hill coefficient 1.08 ± 0.08); 3.68 μM for D172N IKir2.1 (95% C.I. from 3.24 to 4.18 μM; Hill coefficient 1.09 ± 0.07; P > 0.05 versus WT) and 3.25 μM for co-expressed WT-D172N IKir2.1 (95% C.I. 2.77 to 3.80 μM; Hill coefficient 1.1 ± 0.10; P > 0.05 versus both WT and D172N alone). Thus, chloroquine produced similar inhibition of IKir2.1 for WT, D172N and co-expressed WT and D172N Kir2.1.
Chloroquine has been shown previously to block preferentially outward over inward current for both IK1 and IKir2.1 [14,15] and this would appear well-suited to reducing consequences of a gain-of-function mutation that selectively augments outward IKir2.1. Chloroquine can be anticipated also to inhibit KCNH2-encoded hERG channel subunits (which underlie native IKr) [e.g. 16] and in additional experiments (not shown) we found chloroquine to inhibit WT-hERG channels at overlapping concentrations (IC50 of 2.18 μM; 95% C.I. 1.98 to 2.39 μM; though, in contrast to Kir2.1, blocking potency of the SQT1-linked N588K-hERG mutation was reduced ∼ 8.5-fold in our hands; data not shown). After oral administration, plasma levels of chloroquine range from ∼ 1–5 μM (depending on the dose administered) and these levels are accompanied by some rate-corrected QT (QTC) interval prolongation [17]. As IC50 values for chloroquine inhibition of both Kir2.1 and hERG seen under our conditions (and cf [15,16]) fall within this clinical range, it seems likely that inhibition of both these channel types may contribute to observed QTC interval prolongation. Our data show that the chloroquine sensitivity of WT and D172N Kir2.1 is similar to one another when tested under identical conditions. As the QTC interval and, by extension, ventricular action potential waveform duration in SQT patients differ from those in normal individuals, it cannot be assumed that the precise level of Kir2.1 (or hERG) block would be identical in both settings. However, due to the significant overlap between the clinical concentration range and the concentration range over which these channels are sensitive to chloroquine, it seems reasonable to propose that both IK1 and IKr in SQT3 patients would be susceptible to chloroquine inhibition, effects that would be anticipated to be synergistic in reducing net repolarizing current. It should be noted that chloroquine is associated with a range of adverse effects, particularly gastrointestinal problems (including nausea, vomiting, and diarrhea), lightheadedness and dizziness [17]. Therefore, chloroquine may not itself be ideal for long-term administration to normalize QT intervals in SQT3. On the other hand, chloroquine might represent a template chemical structure from which more clinically useful Kir2.1 inhibitors could be developed.
Thus, considered collectively, our findings provide evidence that compounds with chemical structures related to chloroquine may be able to offset abbreviated cardiac AP repolarization caused by augmented Kir2.1 function. Further work may be warranted to determine the potency of chloroquine against other Kir2.x isoforms and also whether or not it is effective against other gain-of-function KCNJ2 mutations.
Acknowledgments
We thank the British Heart Foundation for funding (PG/06/147) and Mrs Lesley Arberry for technical assistance
References
- 1.Schimpf R., Borggrefe M., Wolpert C. Clinical and molecular genetics of the short QT syndrome. Curr. Opin. Cardiol. 2008;23:192–198. doi: 10.1097/HCO.0b013e3282fbf756. [DOI] [PubMed] [Google Scholar]
- 2.Priori S.G., Pandit S.V., Rivolta I., Berenfeld O., Ronchetti E., Dhamoon A. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ. Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 3.Gaborit N., Le Bouter S., Szuts V., Varro A., Escande D., Nattel S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordeiro J.M., Brugada R., Wu Y.S., Hong K., Dumaine R. Modulation of IKr inactivation by mutation in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc. Res. 2005;67:498–509. doi: 10.1016/j.cardiores.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 5.McPate M.J., Duncan R.S., Milnes J.T., Witchel H.J., Hancox J.C. The N588K-HERG K+ channel mutation in the ‘short QT syndrome’: mechanism of gain-in-function determined at 37 °C. Biochem. Biophys. Res. Commun. 2005;334:441–449. doi: 10.1016/j.bbrc.2005.06.112. [DOI] [PubMed] [Google Scholar]
- 6.Wolpert C., Schimpf R., Giustetto C., Antzelevitch C., Cordeiro J., Dumaine R. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J. Cardiovasc. Electrophysiol. 2005;16:54–58. doi: 10.1046/j.1540-8167.2005.04470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPate M.J., Duncan R.S., Witchel H.J., Hancox J.C. Disopyramide is an effective inhibitor of mutant HERG K+ channels involved in variant 1 short QT syndrome. J. Mol. Cell. Cardiol. 2006;41:563–566. doi: 10.1016/j.yjmcc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Schimpf R., Veltmann C., Giustetto C., Gaita F., Borggrefe M., Wolpert C. In vivo effects of mutant HERG K+ channel inhibition by disopyramide in patients with a short QT-1 syndrome: a pilot study. J. Cardiovasc. Electrophysiol. 2007;18:1157–1160. doi: 10.1111/j.1540-8167.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda H., Oishi K., Omori K. Voltage-dependent gating and block by internal spermine of the murine inwardly rectifying K+ channel, Kir2.1. J. Physiol. 2003;548:361–371. doi: 10.1113/jphysiol.2003.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ten Tusscher K.H., Noble D., Noble P.J., Panfilov A.V. A model for human ventricular tissue. Am. J. Physiol, Heart Circ. Physiol. 2004;286:H1573–H1589. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 11.Nygren A., Fiset C., Firek L., Clark J.W., Lindblad D.S., Clark R.B. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ. Res. 1998;82:63–81. doi: 10.1161/01.res.82.1.63. [DOI] [PubMed] [Google Scholar]
- 12.Dhamoon A.S., Pandit S.V., Sarmast F., Parisian K.R., Guha P., Li Y. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ. Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 13.Abrams C.J., Davies N.W., Shelton P.A., Stanfield P.R. The role of a single aspartate residue in ionic selectivity and block of a murine inward rectifier K+ channel Kir2.1. J. Physiol. 1996;493:643–649. doi: 10.1113/jphysiol.1996.sp021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez-Chapula J.A., Salinas-Stefanon E., Torres-Jácome J., Benavides-Haro D.E., Navarro-Polanco R.A. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J. Pharmacol. Exp. Ther. 2001;297:437–445. [PubMed] [Google Scholar]
- 15.Rodríguez-Menchaca A.A., Navarro-Polanco R.A., Ferrer-Villada T., Rupp J., Sachse F.B., Tristani-Firouzi M. The molecular basis of chloroquine block of the inward rectifier Kir2.1 channel. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1364–1368. doi: 10.1073/pnas.0708153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traebert M., Dumotier B., Meister L., Hoffmann P., Dominguez-Estevez M., Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur. J. Pharmacol. 2004;484:41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Mzayek F., Deng H., Mather F.J., Wasilevich E.C., Liu H., Hadi C.M. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin. Trials. 2007;2(1):e6. doi: 10.1371/journal.pctr.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]