Abstract
We report the first use of a photoinduced 1,3-dipolar cycloaddition reaction in “stapling” peptide side chains to reinforce a model peptide helical structure with moderate to excellent yields. The resulting pyrazoline “staplers” exhibit unique fluorescence useful in a cell permeability study.
Peptide helices are frequent mediators of key protein-protein interactions that regulate many important biological processes such as stress response and apoptosis.1 However, when peptide helices are taken out of protein context and placed into aqueous buffer in isolation, they usually adopt random coil conformations, leading to a drastic reduction in biological activity and thus diminished therapeutic potential. Among numerous strategies that aim to stabilize or mimic peptide helices,2 the most straightforward, yet effective, strategy is sidechain cross-linking (“peptide stapling”).3-6 In addition to structural reinforcement, peptide stapling usually leads to increased metabolic stability, improved membrane permeability, and potentially enhanced binding affinity to protein targets due to pre-organization.
Since peptide stapling necessitates macrocyclization, an entropically unfavorable process,7 very few reactions are known to date that give rise to good yields along with the reinforced structures. These include disulfide bond formation,3 lactam formation,4 ruthenium-catalyzed ring closing metathesis,5 and copper-catalyzed azide-acetylene cycloaddition.6 While these reactions have enabled the synthesis of stapled peptide helices, the development of additional stapling reactions with high yields and predictable structural effect is still highly desirable. Herein, we report the first synthesis of stapled peptide helices using a photoinduced nitrile imine-mediated intramolecular 1,3-dipolar cycloaddion reaction, and the subsequent structural, photophysical, and preliminary cellular uptake studies of the stapled peptides.
We recently reported a photoactivated nitrile imine-mediated 1,3-dipolar cycloaddition as a new bioorthogonal reaction for protein labeling both in vitro and in vivo.8 During these studies, we observed that the nitrile imine species, while reactive toward suitable alkenes, was exceedingly stable in the aqueous medium. To probe whether this unique reactivity profile can be harnessed to “staple” peptides, we appended an alkene and a tetrazole moiety, respectively, to peptide sidechains located at the i and i + 4 positions of Balaram’s 310-helix9 (scheme in Table 1). We chose this peptide helix model because it has been studied previously by Grubbs and co-workers5a in demonstrating ruthenium-catalyzed ring closing metathesis chemistry for peptide stapling. We envisioned that upon photoirradiation, tetrazole would undergo the cycloreversion reaction to generate the nitrile imine dipole in situ, which would then react with proximal alkene dipolarophile to form a fluorescent pyrazoline cross-linker. To examine conformational effect on the reaction efficiency, we prepared a series of linear peptide precursors (1-8) by attaching various alkene and tetrazole moieties, respectively, at the sidechains of either lysines or ornithines located at the 2- and 6-positions (Table 1). To effect macrocycloaddition, the linear peptides (150 μM in acetonitrile) were photoirradiated with a 302-nm handheld UV lamp (UVM, 0.16 AMPS) for 2 hours and the resulting stapled peptides were purified by reverse-phase HPLC. Interestingly, we found: 1) the lysine sidechains gave higher yields than the ornithine sidechains (compare peptides 3-8 to 1-2), suggesting that larger rings cause less strains and therefore are more conducive to the macrocycloaddition reactions; 2) N-(4-methoxy)- and N-(4-dimethylamino)-phenyl tetrazoles gave higher yields than simple N-phenyl tetrazole (compare 6 and 8 to 3), which can be attributed to higher reactivities of the corresponding nitrile imines;10 3) linear peptide 8 carrying methacrylic and N-(4-dimethylamino) phenyl tetrazole sidechains afforded the highest yield (94%), suggesting that both the tetrazole reactivity and the alkene conformational rigidity (methacrylic vs. acrylic) are important for the macrocycloaddition reaction; and 4) the stapling reaction involving peptide 8 was found to be tolerant of protic solvents such as EtOH and iPrOH as well as EtOH/H2O (1:1) mixture, affording similar yields (see Table S1 in ESI). Additionally, a preliminary study with a p53 analog, Ac-LTFαHYWAQLβS-NH2 where α and β represent methacrylic and N-(4-methoxy) phenyl-tetrazole modified lysine, respectively, showed that the cyclized product was isolated in 74% yield, indicating that the stapling is compatible with the polar side chains.11
Table 1.
Synthesis of stapled peptides based on the Karle and Balaram’s heptapeptidic 310 helixa
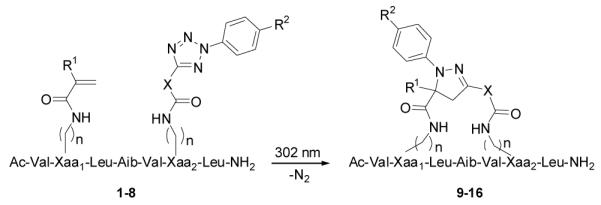 | ||||||
|---|---|---|---|---|---|---|
| linear peptide |
n | X | R1 | R2 | stapled peptide |
yield (%) |
| 1 | 3 | - | Me | H | 9 | 15 |
| 2 | 3 | -Ph- | Me | H | 10 | 15 |
| 3 | 4 | - | Me | H | 11 | 41 |
| 4 | 4 | -Ph- | Me | H | 12 | 38 |
| 5 | 4 | - | H | OMe | 13 | 64 |
| 6 | 4 | - | Me | OMe | 14 | 53 |
| 7 | 4 | - | H | NMe2 | 15 | 63 |
| 8 | 4 | - | Me | NMe2 | 16 | 94 |
The reaction was performed by irradiating linear peptides (150 μM in acetonitrile) in a quartz round-bottom flask with a handheld UV lamp at 302 nm. Isolated yields were reported. Aib = aminoisobutyric acid.
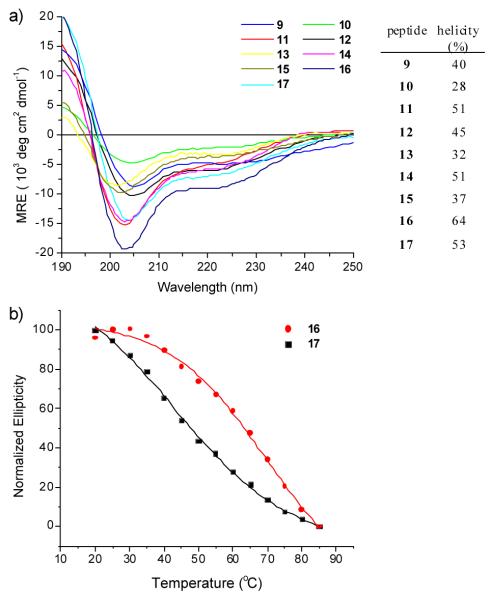
To examine the stapling effect on peptide secondary structure, we measured the far-UV circular dichroism (CD) spectra of the stapled peptides in trifluoroethanol (TFE) at 25 °C. Because the tetrazoles in the linear peptides are labile to the CD scanning light (short wavelength UV light), we prepared a photo stable control peptide, Ac-Val-Lys(Ac)-Leu-Aib-Val-Lys(Ac)-Leu-NH2 (17; Ac = acetyl; Aib = amino isobutyric acid) as a linear surrogate. Peptide 17 showed typical CD spectrum of a right-handed 310-helix with a strong negative band around 208 nm (π→π*) and a weak negative band around 222 nm (n→π*) (Figure 1a).12 The percent helicity of 17 was determined to be 53% on the basis of mean residue ellipticity (MRE) at 208 nm.13 By comparison, stapled peptides 11 and 14 showed similar percent helicity (51% for both) while 16 exhibited slightly higher percent helicity (64%) (Fig. 1a), indicating that the methyl group attached to the pyrazoline rings reinforces the helical structures. To further characterize the stability derived from the stapling, we measured thermal melting point (Tm) of the most helical peptide 16 by following its ellipticity over a wide temperature range (20 - 85 °C),14 and compared it to that of the linear photo stable peptide 17 (Fig. 1b). We found that stapled peptide 16 exhibited higher melting temperature (Tm = 63 °C) than linear peptide 17 (Tm = 48 °C); the 15 °C-increase is greater than 10 °C-increase observed for a double cysteine alkylation cross-linker spanning the i and i + 11 positions of an α-helical peptide,3b suggesting that helical reinforcement afforded by the pyrazoline cross-linker is robust.
Fig. 1.
(a) CD spectra of the stapled peptides 9-16 and the linear peptide 17 at 25 °C. Peptides were dissolved in TFE to derive 100 μM solutions. The calculated percent helicity values were listed in the table. (b) Thermal melting curves of peptides 16 and 17. 100 μM peptide solutions in 20% TFE/H2O were used in the CD scans.
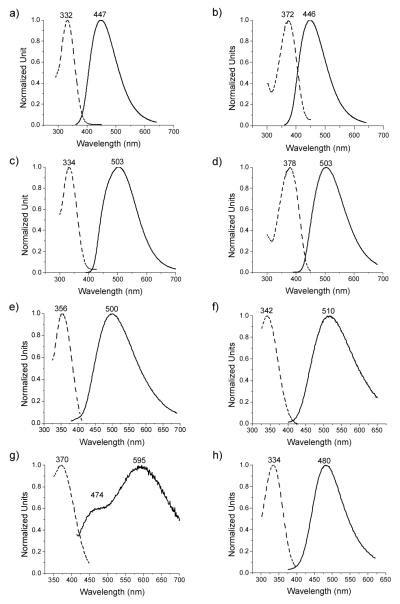
Since pyrazoline crosslinkers are fluorescent, we measured the UV and fluorescence spectra of the eight stapled peptides (Fig. 2). As expected, large Stokes shifts (74 ~ 169 nm) were observed, in excellent agreement with our previous observation.8a In general, it appears that the strained, stapled peptides with lower percent helicity (see Fig. 1a) showed consistently smaller Stokes shifts compared to their relaxed counterparts (compare 10 to 9, 12 to 11, and 13 to 14) (Fig. 2).15 Since Stokes shift reflects the electronic displacement in potential surfaces between the ground and excited states of the chromophore, the decreases in Stokes shift observed in 10, 12, and 13 can be attributed to the rigidified ground states and thus increased potential surfaces—the result of macrocyclic ring strains.16
Fig. 2.
UV-Vis and fluorescence spectra of the stapled peptides: (a) 9; (b) 10; (c) 11; (d) 12; (e) 13; (f) 14; (g) 15; (h) 16. Dashed lines represent the UV absorbance spectra while solid lines represent the fluorescence emission spectra. The absorption and emission maxima were marked on top of the spectra.
To assess whether the stapled peptides are capable of penetrating cell membrane, we took advantage of the intrinsic fluorescence of the pyrazoline cross-linkers8 and monitored the stapled peptide cellular uptake by fluorescent microscopy. Because stapled peptide 13 showed maximum absorption at 356 nm and a broad emission band at 400-700 nm (Fig. 2e), matching closely to commercial DAPI filter settings (ex 365 nm, em 445 ± 25 nm), we decided to use peptide 13 in our cellular uptake assay.17 After incubating HeLa cells with 100 μM of peptide 13 for 4 hours in a 37 °C CO2 incubator, the cells were washed twice with PBS before fixing with 4% paraformaldehyde and the subcellular distribution of peptide 13 was examined by fluorescent microscopy. Interestingly, punctuated fluorescence was observed in discrete cytoplasmic regions within HeLa cells (Fig. 3a), resembling closely to the intracellular distribution pattern of the hydrocarbon-stapled BH3 helix,18 which in turn suggests that the pyrazoline stapled peptides penetrates cell membrane via a similar pinocytotic pathway. In a control experiment, treatment of HeLa cells with a linear analog 18 (Ac-Val-Lys(Pyr)-Leu-Aib-Val-Lys(Ac)-Leu-NH2; Pyr = pyrazoline fluorophore with the same structure as that of peptide 13) did not yield cellular fluorescent pattern under the identical conditions (Fig. 3b), suggesting that the membrane permeation is indeed endowed by the sidechain stapling.
Fig. 3.
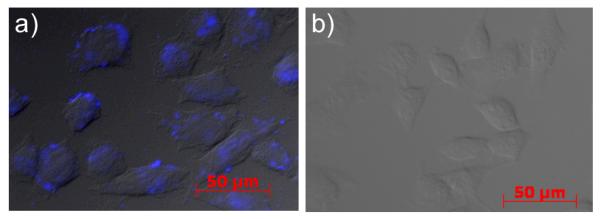
Fluorescent images of fixed HeLa cells overlaid on the DIC images after treatment of 100 μM of (a) peptide 13 or (b) linear control peptide 18.
In summary, we have demonstrated a facile synthesis of stapled peptide helices using a photoinduced, nitrile imine-mediated, intramolecular 1,3-dipolar cycloaddition reaction. When appropriate alkenes and tetrazoles were employed, high stapling yields were obtained along with the reinforced helical structures. Moreover, one stapled peptide was found to be capable of permeating the HeLa cell membrane. With this new orthogonal stapling reaction, it might be possible to combine several orthogonal reactions to design novel, multiply stapled peptide structures. By taking advantage of the spatiotemporal resolution of light activation, it is also possible to “switch-on” the biologically active form of a peptide in specific cell types.
Supplementary Material
Acknowledgments
This work is supported by New York State Center of Excellence in Bioinformatics and Life Sciences.
Footnotes
Electronic supplemental information (ESI) available: Full experimental details and compound characterization data. See DOI: 10.1039/b000000x
Notes and references
- 1 (a).Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]; (b) Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Science. 1997;275:983. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 2 (a).Balaram P. Cur. Opin. Struct. Biol. 1992;2:845. [Google Scholar]; (b) Kemp DS, Allen TJ, Oslick SL, Boyd JG. J. Am. Chem. Soc. 1996;118:4240. [Google Scholar]; (c) Orner BP, Ernst JT, Hamilton AD. J. Am. Chem. Soc. 2001;123:5382. doi: 10.1021/ja0025548. [DOI] [PubMed] [Google Scholar]; (d) Chin JW, Schepartz A. Angew. Chem., Int. Ed. 2001;40:3806. [PubMed] [Google Scholar]; (e) Chapman RN, Dimartino G, Arora PS. J. Am. Chem. Soc. 2004;126:12252. doi: 10.1021/ja0466659. [DOI] [PubMed] [Google Scholar]; (f) Horne WS, Boersma MD, Windsor MA, Gellman SH. Angew. Chem., Int. Ed. 2008;47:2853. doi: 10.1002/anie.200705315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 (a).Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. J. Am. Chem. Soc. 1991;113:9391. [Google Scholar]; (b) Zhang F, Sadovski O, Xin SJ, Woolley GA. J. Am. Chem. Soc. 2007;129:14154. doi: 10.1021/ja075829t. [DOI] [PubMed] [Google Scholar]
- 4 (a).Bracken C, Gulyas J, Taylor JW, Baum J. J. Am. Chem. Soc. 1994;116:6431. [Google Scholar]; (b) Phelan CJ, Skelton NJ, Braisted AC, McDowell RS. J. Am. Chem. Soc. 1997;119:455. [Google Scholar]; (c) Schievano E, Bisello A, Chorev M, Bisol A, Mammi S, Peggion E. J. Am. Chem. Soc. 2001;123:2743. doi: 10.1021/ja0027261. [DOI] [PubMed] [Google Scholar]; (d) Fujimoto K, Oimoto N, Katsuno K, Inouye M. Chem. Commun. 2004:1280. doi: 10.1039/b403615h. [DOI] [PubMed] [Google Scholar]; (e) Fujimoto K, Kajino M, Inouye M. Chem. Eur. J. 2008;14:857. doi: 10.1002/chem.200700843. [DOI] [PubMed] [Google Scholar]; (f) Ousaka N, Sato T, Kuroda R. J. Am. Chem. Soc. 2008;130:463. doi: 10.1021/ja077857h. [DOI] [PubMed] [Google Scholar]
- 5 (a).Blackwell HE, Grubbs RH. Angew. Chem., Int. Ed. 1998;37:3281. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; (b) Schafmeister CE, Po J, Verdine GL. J. Am. Chem. Soc. 2000;122:5891. [Google Scholar]; (c) Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. Mol. Cell. 2006;24:199. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]; (e) Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. J. Am. Chem. Soc. 2007;129:2456. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantel S, Isaad ALC, Scrima M, Levy JJ, DiMarchi RD, Rovero P, Halperin JA, D’Ursi A,M, Papini AM, Chorev M. J. Org. Chem. 2008;73:5663. doi: 10.1021/jo800142s. [DOI] [PubMed] [Google Scholar]
- 7.Illuminati G, Mandolini L. Acc. Chem. Res. 1981;14:95. [Google Scholar]
- 8 (a).Song W, Wang Y, Qu J, Madden MM, Lin Q. Angew. Chem., Int. Ed. 2008;47:2832. doi: 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]; (b) Song W, Wang Y, Qu J, Lin Q. J. Am. Chem. Soc. 2008;130:9654. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]
- 9 (a).Karle IL, Flippen-Anderson JL, Uma K, Balaram P. Proteins. 1990;7:62. doi: 10.1002/prot.340070107. [DOI] [PubMed] [Google Scholar]; (b) Karle IL, Flippen-Anderson JL, Uma K, Balaram P. Biopolymers. 1993;33:827. doi: 10.1002/bip.360330511. [DOI] [PubMed] [Google Scholar]
- 10.The HOMO energies of C-amido nitrile imines were calculated to be −6.9618 eV for (4-dimethylamino)phenyl substituent, −7.4201 eV for (4-methoxyamino) phenyl substituent, and −7.7458 eV for simple phenyl substituent. The higher the HOMO energies, the more reactive the nitrile imines, See:Wang Y, Song W, Hu WJ, Lin Q. Angew. Chem. Int. Ed. 2009;48:5330. doi: 10.1002/anie.200901220.
- 11.Madden MM, Lin Q. unpublished results.
- 12.Formaggio F, Crisma M, Kamphuis J. J. Am. Chem. Soc. 1996;118:2744. [Google Scholar]
- 13.The equation of % helicity = 100 × (4000 − [θ]208MRE)/29000 was used in the calculations.
- 14.Flint DG, Kumita JR, Smart OS, Woolley GA. Chem. Biol. 2002;9:391. doi: 10.1016/s1074-5521(02)00109-6. [DOI] [PubMed] [Google Scholar]
- 15.The valid comparison between 15 and 16 could not be made because peptide 15 exhibited two emission maxima, presumably due to the presence of two charged states arose from the protonation of dimethylamino group in aqueous buffer.
- 16.Similar strain-dependent variable Stokes shifts were also observed for spirocyclic benzofuranone compounds, see:D’Souza DM, Rominger F, Müller TJ. Angew. Chem., Int. Ed. 2005;44:153. doi: 10.1002/anie.200461489.
- 17.We attempted to monitor the cellular uptake of peptide 16, the most helical peptide identified in this study, with no success because its fluorophore could not be adequately excited (λex = 334 nm) using the commercial DAPI filter set.
- 18.Bird GH, Bernal F, Pitter K, Walensky LD. Methods Enzymol. 2008;446:369. doi: 10.1016/S0076-6879(08)01622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.