Abstract
Two conditioned suppression experiments with rats investigated the influence on latent inhibition of compounding a Pavlovian conditioned inhibitor with the target cue during preexposure treatment. Results were compared to subjects that received conventional latent inhibition training, no preexposure, or preexposure to the target cue in compound with a neutral stimulus. In Experiment 1, greater attenuation of the latent inhibition effect was observed in subjects that received target preexposure in compound with a Pavlovian conditioned inhibitor relative to subjects that received preexposure with a neutral stimulus or to the target alone. In Experiment 2, this protection from latent inhibition was attenuated if the excitor that was used to train the conditioned inhibitor was extinguished between preexposure and target training. The results are consistent with an account offered by the extended comparator hypothesis.
Keywords: latent inhibition, protection, retrospective revaluation, conditioned inhibition
Latent inhibition is defined as retarded acquisition of stimulus control of behavior following nonreinforced exposure to the to-be conditioned stimulus (CS) before reinforced CS-unconditioned stimulus (US) pairings (Lubow & Moore, 1959). Research has shown that preexposure to the to-be-conditioned stimulus in compound with another stimulus protects the target from latent inhibition. In other words, subjects exhibit faster acquisition of behavioral control by a target CS when it is paired with a US after the subject has been preexposed to the target in compound with another stimulus compared to subjects preexposed to the target CS alone, (hereafter referred to as the Lubow effect, e.g., Ishii, 1999; Lubow, Alek, & Arzy, 1975; Reed, 1991).
The present series of experiments assessed whether compounding a target cue with a conditioned inhibitor during preexposure treatment is capable of providing more protection to the target than does compounding the target cue with a neutral stimulus. One might anticipate more responding to the target cue as a consequence of compounded preexposure with a conditioned inhibitor relative to an associatively neutral stimulus given recent data from Rescorla's laboratory in which he reported that a conditioned inhibitor was more effective at protecting a conditioned excitor from extinction than a neutral stimulus (Rescorla, 2003). Rescorla took this as support for error-correction models such as the Rescorla-Wagner (1972) model, which assume that changes in the associative strength on a given trial depend on the total strength of all cues present on that trial. According to these models, the total amount of associative strength present (i.e., US expectation) during an extinction trial with a conditioned inhibitor present is less than if the inhibitor is replaced with a neutral stimulus. The presence of a conditioned inhibitor should diminish the potential of nonreinforcement to decrease the associative strength of the target, thus protecting it from the normally detrimental effect of nonreinforcement. Because of this mechanism, the model makes an interesting prediction concerning the impact of a conditioned inhibitor on nonreinforced preexposure. The Rescorla-Wagner model predicts that during nonreinforced preexposure, the to-be-conditioned stimulus should gain excitatory strength as a consequence of being compounded with a conditioned inhibitor, which should result in increased responding relative to a stimulus that was preexposed with a neutral stimulus.
The Pearce-Hall model (Pearce & Hall, 1980) offers another mechanism by which a conditioned inhibitor might be more effective in protecting a stimulus from latent inhibition than a neutral stimulus. This model assumes that, as a CS becomes a more consistent predictor of its consequences, it loses associability. Conversely, stimuli that are followed by surprising events will increase in associability. Formally stated, the Pearce-Hall model posits that the associability of a CS is determined by the absolute value of the discrepancy between the perceived magnitude of the US on the last trial that included the target and the total associative strength of all stimuli present on that trial. The total associative strength incorporates both excitatory and inhibitory associative strengths, which are combined in a subtractive manner. Pearce and Hall's model predicts that a target cue preexposed in compound with a conditioned inhibitor should have a higher associability than a target cue preexposed in compound with a neutral stimulus or alone. This prediction is based on the absolute value of total associative strength present during preexposure treatment being positive because the inhibitor predicts nonreinforcement. Thus, accelerated conditioning of the target cue is expected relative to preexposure of the target CS in the presence of a nontarget stimulus.
The extended comparator hypothesis (Denniston, Savastano, & Miller, 2001), an expansion of the original comparator hypothesis (Miller & Matzel, 1988), also predicts elevated responding to a stimulus preexposed in compound with a conditioned inhibitor relative to compound preexposure with a neutral stimulus. This performance-focused model postulates that associations are formed based on contiguity alone, and organisms respond to a target cue based on a comparison of the target-US associative strength with the product of the associative strength between the target cue and other stimuli present during training (first-order comparator stimuli) and the associative strength between these other stimuli and the US. Behavior indicative of inhibition is assumed to reflect relatively strong indirect associations between the target cue and the US (i.e., mediated by US representations activated by comparator stimulus representations), whereas excitatory behavior is assumed to reflect a relatively strong direct target-US association. This model does not postulate inhibitory associations per se, only excitatory associations; behavior indicative of inhibition is merely the consequence of the interaction of these excitatory associations. Furthermore, first-order comparator stimuli are thought to have their own comparator stimuli (i.e., second-order comparator stimuli) that are capable of down modulating the effects of the first-order comparator stimuli, thereby increasing responding to the target cue.
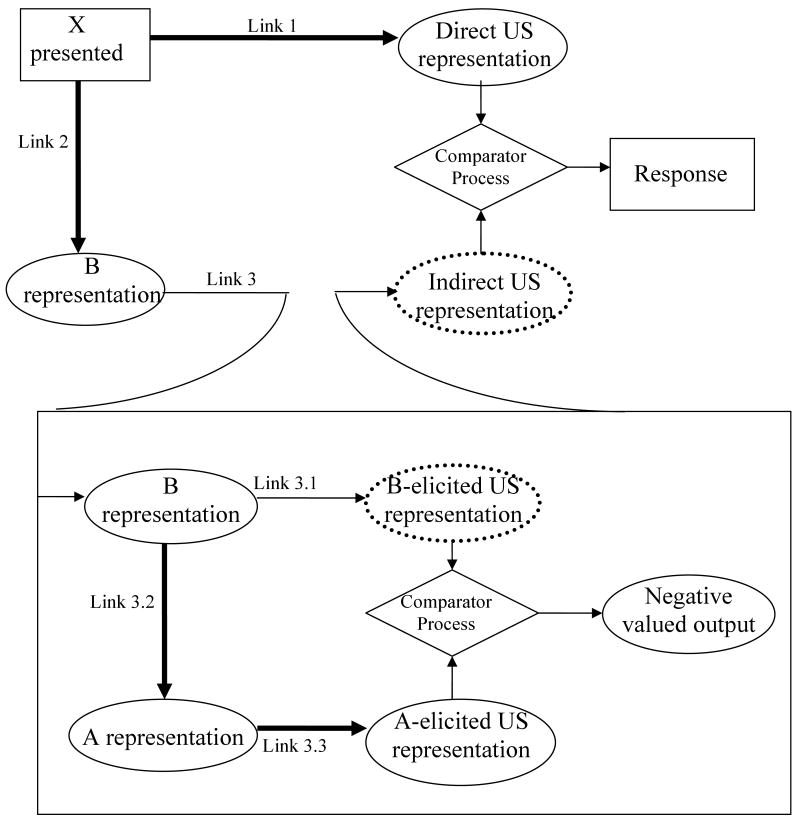
The extended comparator hypothesis predicts that compounding the target cue (X) with a conditioned inhibitor (B) during preexposure will be more effective in protecting X from the retarding effect of latent inhibition than compounding the target stimulus with a neutral stimulus because of B's inhibitory potential. According to the extended comparator hypothesis, both B and the context should act as first-order comparator stimuli to X because they each share a within-compound association with X. As a first-order comparator, the context is responsible for the latent inhibition effect because it is directly associated with both X and the US. The conditioned inhibitor is not directly associated with the US, but it is associated with a conditioned excitor (A) that was used during Pavlovian inhibition conditioning of B (i.e., A+/AB-, where + denotes reinforcement and − denotes nonreinforcement). Thus, X has a strong association with its first-order comparator, B, but B is modulated by a strong association with its own first-order comparator, A, which in turn is strongly associated with the US. This effectively makes A a second-order comparator stimulus for X (Figure 1). A cannot serve as a first-order comparator stimulus to X because there is no direct association between A and X. However, A can act as a first-order comparator stimulus to B and B is a first-order comparator stimulus to X. This makes A exclusively a second-order comparator stimulus with respect to X. The negative output of Link 3 (owing to A being more excitatory than B) enhances behavioral control by the X-US association (Link 1; see Stout & Miller, 2007, which provides a mathematical implementation of the extended comparator hypothesis). Thus, the extended comparator hypothesis also predicts more responding to a target cue that was preexposed with a conditioned inhibitor relative to one preexposed with a neutral stimulus.
Figure 1.
The extended comparator hypothesis account of responding to the target cue (X) after preexposure to X in the presence of a conditioned inhibitor (B) followed by X-US pairings. Arrows represent the associations between stimuli. The strengths of the directly and indirectly associated outcome representations are compared to determine the strength of responding to the target cue. Ovals depict stimulus representations; rectangles depict physical events; diamonds represent the comparator process. The dotted US representations indicate that B does not have a direct association with the US. Heavy lines indicate strong associations. A is the conditioned excitor used during inhibitory training of B.
The question of whether a conditioned inhibitor provides more protection from latent inhibition than a neutral stimulus is important given that there are competing accounts of what mechanisms might be operating. The first experiment reported here simply attempted to document latent inhibition and the enhanced protection from latent inhibition that might be provided by a conditioned inhibitor relative to a neutral stimulus. The second experiment employed a posttraining manipulation that differentiated between the accounts of Experiment 1.
Experiment 1
In Experiment 1, we preexposed one group of rats to the target cue (X) in compound with a conditioned inhibitor (Group InhibLI). Although conditioned inhibition was not directly assessed in the present series of experiments, the parameters used here were based on many demonstrations of conditioned inhibition in our laboratory using the same preparation (e.g., Friedman, Blaisdell, Escobar, & Miller, 1998). Based on the predictions of several theories of learning (see Introduction), we expected to see strong conditioned suppression to the target cue (i.e., protection from latent inhibition). This behavior was compared to that of a group that received preexposure to X in compound with a neutral stimulus (Group CmpdLI), a group that received preexposure to X elementally (Group LI), and a group given no preexposure treatment (Group Acq). Based on prior research, we anticipated that the subjects in Group CmpdLI would exhibit intermediate suppression (i.e., a mild Lubow effect). Moreover, this group's suppression was expected to be weaker than that in Group InhibLI. Subjects in Group LI were expected to show the least suppression, and subjects in Group Acq were expected to show the highest suppression levels. See Table 1 for the experimental design and predictions. Two different training contexts were used because it was necessary to conduct preexposure treatment in a separate context from that of testing in order to avoid ambiguities in theoretical interpretation. According to the extended comparator hypothesis, latent inhibition is the result of a strong target cue-context association, which presumably is why latent inhibition does not generalize across contexts. A different context was used for inhibition training and testing to avoid testing in a context that might have been made associatively-active as a result of latent inhibition treatment. This insured that it was only the memory of the target training context rather than its presence at testing that would influence responding to the target.
Table 1.
Design of Experiment 1
| Group | Phase 11 Inhibition training | Phase 22 Preexposure | Phase 32 Conditioning | Test X1 Prediction |
|---|---|---|---|---|
| Acq | 48 A+ / 84 AB- | 48 Y- | 3 X+ | CR |
| LI | 48 A+ / 84 AB- | 48 X- | 3 X+ | Cr |
| CmpdLI | 48 C+ / 84 AB- | 48 BX- | 3 X+ | Cr |
| InhibLI | 48 A+ / 84 AB- | 48 BX- | 3 X+ | CR |
Note: A and C were a flashing light and a high frequency tone, counterbalanced; B was a white noise; X and Y were a click train and a low frequency tone, counterbalanced. “+” denotes footshock reinforcement, “-” denotes nonreinforcement. Superscripts indicate different contexts. Predictions are based on the extended comparator hypothesis (Denniston et al., 2001) with uppercase denoting stronger responding than lowercase (i.e., cr < Cr < CR).
Method
Subjects
Subjects were 24 male and 24 female, experimentally naive, Sprague-Dawley descended rats obtained from our own breeding colony. Body-weight ranges were 258-332 g for males and 177-226 g for females. Subjects were randomly assigned to one of four groups (ns = 12), counterbalanced within groups for sex. The animals were individually housed in standard hanging stainless-steel wire-mesh cages in a vivarium maintained on a 16/8-hr light/dark cycle. Experimental manipulations occurred near the middle portion of the light phase. The animals received free access to Purina Lab Chow, whereas water availability was limited to 20 min per day following a progressive deprivation schedule initiated one week prior to the start of the study. From the time of weaning until the start of the study, all animals were handled for 30 s, three times per week.
Apparatus
Six identical copies of each of two different types of experimental chambers were used. Chamber Rectangular (R) was a clear, Plexiglas, rectilinear chamber, measuring 23.0 × 8.5 × 12.5 cm (length × width × height). The floor was constructed of 0.48-cm diameter stainless-steel rods, spaced 1.5 cm apart, center-to-center. The rods were connected by NE-2 neon bulbs that allowed a 0.5-s, 0.7-mA constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Each copy of Chamber R was housed in a separate light- and sound-attenuating environmental isolation chest, which was dimly illuminated by a 2-W (nominal at 120 VAC) incandescent bulb driven at 60 VAC. The houselight was mounted on the upper wall of the environmental chest, approximately 26 cm from the center of the experimental chamber.
Chamber V-shaped (V) was a 22.1-cm long box in a truncated-V shape (25.3 cm height, 21.3 cm wide at the top, 5.1 cm wide at the bottom). The floor and long sides were constructed of stainless steel sheets, and the ends and ceiling were constructed of clear Plexiglas. The floor of each chamber consisted of two parallel metal plates, each 2.0 cm wide, with a 1.1-cm gap between them. Each V-shaped chamber was housed in its own environmental isolation chest, which was dimly illuminated by a 7.5-W (nominal at 120 VAC) incandescent houselight driven at 60 VAC mounted on an inside wall of the environmental chest, approximately 30 cm from the center of the experimental chamber. The light entering the animal chamber was primarily that reflected from the white roof of the environmental chest. The light intensities in the two types of chambers were approximately equal, due to the differences in opaqueness of the walls in Chambers R and V.
Each chamber (R and V) could be equipped with a water-filled lick tube that extended 1 cm from the rear of a cylindrical niche, 4.5 cm in diameter, left-right centered in one end wall, with its axis perpendicular to the wall, and positioned with its center 4.25 cm above the floor of the chamber. Each niche had a horizontal infrared photobeam traversing it parallel to the wall on which the niche was mounted, 1 cm in front of the lick tube. In order to drink from the tube, subjects had to insert their heads into the niche, thereby breaking the infrared photobeam. Thus, we could record when subjects had their heads in the niche. Ordinarily, they did this only when they were drinking. Disruption of ongoing drinking by a test stimulus served as our dependent variable.
Each chamber (R and V) was also equipped with three speakers widely separated on the inside walls of the environmental chest. Each speaker could deliver a different auditory stimulus, which consisted of a click train (6 Hz, 6 dB above background), a complex tone composed of two high frequencies (3000 and 3200 Hz, 6 dB above background), or two low frequencies (320 and 300 Hz, 6 dB above background), and a white noise (6 dB above background). A ventilation fan in each chest provided a constant 74-dB (C scale) background noise. A flashing light stimulus (0.25 s on/ 0.25 s off) was provided by a 25-W bulb in Chamber R and by a 100-W bulb in Chamber V, both nominal at 120 VAC, but driven at 60 VAC. The bulbs were mounted on an inside wall of the environmental chest, 30 cm from the center of the experimental chamber. Due to differences in the opaqueness of the walls of Chambers R and V, these two visual stimuli were of similar intensity inside the animal chambers. When presented, the flashing light and the high frequency complex tone served as Stimuli A and C, counterbalanced within groups. The white noise served as Stimulus B. The low frequency complex tone and the clicks served as Stimuli X and Y, counterbalanced within groups. The stimuli were chosen, based on pilot data, to minimize configuring of cues. All stimuli were 10 s in duration except for the 0.5-s US. Two contexts were used in the present experiment. Chambers R and V served as Contexts 1 and 2, counterbalanced within groups. Context 1 was used during Acclimation, Phase 1 (conditioned inhibition training), Reacclimation, and Test. Context 2 was used during Phase 2 (preexposure) and Phase 3 (conditioning).
Procedure
Acclimation
On Day 1, all subjects received one 60-min acclimation session in Context 1 with the lick tubes present. During this session, all cues (A, B, C, X, and Y) were programmed to occur twice for 10 s each in order to reduce possible unconditioned fear to these stimuli. Trial order was light, high tone, white noise, low tone, click, high tone, light, click, low tone, and white noise for all subjects. Stimulus onsets occurred 10, 13, 19, 23, 28, 31, 39, 45, 49, and 52 min into the session. There were no presentations of the US. Subjects had free access to the water-filled lick tubes.
Phase 1
On Days 2-7, all subjects received daily 120-min training sessions in Context 1. Groups InhibLI, LI, and Acq received 8 reinforced presentations of A interspersed with 14 nonreinforced compound presentations of AB each day for a total of 48 A+ trials and 84 AB- trials (+ indicates reinforcement, - indicates nonreinforcement). Group CmpdLI received 8 reinforced presentations of C interspersed with 14 nonreinforced presentations of AB each day for a total of 48 C+ trials and 84 AB- trials. On Days 2, 4, and 6, reinforced trials occurred at 2, 9, 36, 54, 75, 84, 102, and 118 min into the session, and nonreinforced trials occurred at 5, 16, 21, 25, 33, 42, 49, 60, 65, 69, 79, 90, 97, and 109 min into the session. On Days 3, 5, and 7, reinforced trials occurred at 3, 10, 36, 58, 79, 97, 106, and 117 min into the session, and nonreinforced trials occurred at 15, 18, 24, 31, 45, 50, 62, 67, 72, 85, 90, 101, 111, and 115 min into the session. On reinforced trials, the US and CS coterminated. No lick tubes were present during this phase.
Phase 2
On Days 8-13, Groups InhibLI and CmpdLI received 8 daily nonreinforced compound BX presentations each day for a total of 48 BX- trials. Group LI received 8 daily nonreinforced X presentations each day for a total of 48 X- trials. Group Acq received 8 nonreinforced Y presentations each day for a total of 48 Y- trials. All sessions were 24 min in duration and conducted in Context 2. Onsets of the CS occurred at 3, 6, 8 12, 14, 17, 20, and 22 min into the session on Days 8, 10, and 12, and 2, 4, 7, 11, 14, 16, 20, and 23 min into the session on Days 9, 11, and 13. No lick tubes were present during this phase.
Phase 3
On Day 14, all subjects were presented with 3 X-US pairings during a daily 60-min session that took place in Context 2. Onsets of the CS occurred at 6, 12, and 24 min into the session. The US and X coterminated. The lick tubes were not available during this phase.
Reacclimation
On Days 15 and 16, all subjects were reacclimated to Context 1 during a daily 60-min session. Subjects had free access to the water-filled lick tubes, and no nominal stimuli were programmed to occur. The purpose of these sessions was to reestablish a stable rate of drinking behavior (which might have been disrupted by the footshock US), thereby providing similar baseline behavior across the four groups, upon which conditioned lick suppression was to be assessed.
Testing on X
On Day 17, all subjects were tested for conditioned lick suppression to X in Context 1. Upon placement in the test chamber, time spent drinking by each subject was recorded. Immediately after completion of an initial 5 cumulative seconds of licking in the absence of any nominal stimulus, subjects were presented with the target CS X. Thus, all subjects were drinking at the time of CS onset. Time to complete an additional 5 cumulative seconds of licking in the presence of X was recorded. The times recorded during the presentation of X were interpreted as reflecting subjects' expectancy of the US following onset of X. The test session was 16 min in duration, and a ceiling score of 15 min was imposed on the time to complete 5 cumulative seconds of drinking in the presence of X.
All test scores were converted to log10 to better approximate the within-group normal distributions assumed by parametric statistical tests. Following the convention of our laboratory, all animals that took more than 60 s to complete their first 5 cumulative seconds of licking (i.e., prior to CS onset) during the test session were scheduled to be eliminated from the study because such long latencies may be considered indicative of unusually great fear of the test context. In practice, 1 rat in Group Acq was eliminated based on this criterion. Additionally, 13 rats were eliminated from the study due to experimenter error, leaving 34 total subjects (9 in Group InhibLI, 9 in Group CmpdLI, 9 in Group LI, and 7 in Group Acq).
Results
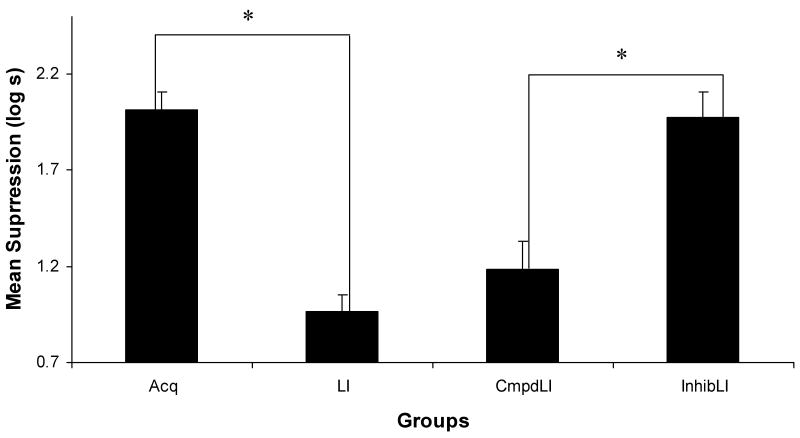
The central results of this experiment were increased responding to the target stimulus X in Group InhibLI relative to Group CmpdLI, suggesting that a conditioned inhibitor provided more protection to the target cue from latent inhibition than did a neutral stimulus (see Figure 2). We also observed increased responding in Group Acq relative to Group LI, indicating that latent inhibition was observed. The following statistics support these conclusions.
Figure 2.
Experiment 1: Mean times (log s) to complete 5 cumulative seconds of licking in the presence of the target cue. All subjects were tested on X alone. * indicates significant differences (ps < .05).
A one-way analysis of variance (ANOVA) conducted on the transformed preCS latencies showed no significant differences between groups in drinking behavior prior to the onset of the test stimulus, p = .65. A similar ANOVA conducted on the transformed CS latencies found an effect of group, F(3, 30) = 19.33, MSE = 0.13, p < .01, Cohen's f = 1.27 (Myers & Wells, 2003). A planned comparison between Groups LI and Acq using the overall error term from the ANOVA detected a basic latent inhibition effect, F(1, 30) = 34.38, p < .01. Although there was a tendency towards demonstrating the Lubow effect, there was not a significant difference between Groups CmpdLI and LI, F(1, 30) = 1.80, p = .19, indicating an absence of an appreciable Lubow effect, which is not surprising given that we selected parameters to minimize this effect (see Discussion below). The critical comparison between Groups InhibLI and CmpdLI indicated that the conditioned inhibitor was more effective in protecting the target cue from latent inhibition than was a neutral stimulus (i.e., Group InhibLI suppressed more than Group CmpdLI, F(1, 30) = 21.94, p < .01).
Discussion
These results suggest that preexposing a target cue in compound with a conditioned inhibitor is more effective in protecting a target CS from latent inhibition than preexposing the target in compound with a neutral stimulus. Notably, in this experiment we did not observe a significant effect of preexposing the target in the presence of a neutral stimulus relative to elemental preexposure of the target (i.e., there was no Lubow effect). Preliminary research in our laboratory had yielded a Lubow effect so strong that Group CmpdLI in those studies suppressed similarly to Group Acq, such that there was not enough sensitivity to detect any further reduction in latent inhibition. Hence, the present Experiment 1 was conducted with parameters selected to minimize the Lubow effect. Specifically, we presented 84 nonreinforced trials during inhibition training rather than the 48 nonreinforced trials that we used in the preliminary experiments. Presenting more nonreinforced trials was intended to reduce the Lubow effect in Group CmpdLI (e.g., Honey & Hall, 1988; Reed, 1991; Reed, 1995; Reed & Tsakanikos, 2002) as well as strengthen the inhibitory properties of B in Group InhibLI. Honey and Hall demonstrated that increased presentations of the companion stimulus prior to compound training attenuates the Lubow effect such that weaker responding is observed at test to the target cue, despite its having been preexposed in compound with another stimulus. In the present experiment, we needed to minimize the Lubow effect so as to have a large latent inhibition effect that might be reduced by preexposure of the target (X) in the presence of a conditioned inhibitor (B). Thus, we increased the number of AB- trials during Phase 1 of conditioned inhibition training. By doing so, we increased the number of exposures to the companion stimulus (B) prior to compound preexposure with X. In accordance with Reed and colleagues and Honey and Hall, this should have weakened B's protection effect. The ECH is unable to offer an explanation for this effect. Honey and Hall suggested that Wagner's (1981) SOP model could account for it. We also lowered the salience of the high frequency complex tone and the white noise used in conditioned inhibition training from 8 dB above background to 6 dB above background. This was intended to reduce the extent to which B overshadowed X during preexposure and to minimize the degree to which A would perceptually mask B during conditioned inhibition training.
Rescorla-Wagner (1972), Pearce-Hall (1980), and the extended comparator hypothesis (Denniston et al., 2001) all account for the protection from latent inhibition observed here. We detail the processes underlying these and other various explanations in the General Discussion. However, there is a less formal interpretation of Experiment 1. Possibly, any associatively active stimulus, regardless of its being inhibitory or excitatory, would increase attention to the neutral target cue when they are presented together without reinforcement. This attentional effect could conceivably attenuate latent inhibition more than the addition of an associatively-neutral cue. If this is the case, then the protection from latent inhibition results from a change in the processing of X that in turn affects the extent to which it later acquires associative strength. Experiment 2 addresses this potential explanation, and informs the more formal interpretations.
Experiment 2
Experiment 1 demonstrated that a conditioned inhibitor provides more protection from latent inhibition than does a neutral stimulus. Multiple models of associative learning employ different mechanisms to predict this effect. It is thus important to differentiate between these competing accounts by testing a unique prediction. The extended comparator hypothesis posits that the protection from latent inhibition observed in Group InhibLI is a performance effect, in which the status of B as a conditioned inhibitor actively promoted responding to X at test. In contrast, the Pearce-Hall model posits that the inhibitory status of B protected the associability of X from decreasing during Phase 2. In this view, the inhibitory associative strength provided by the conditioned inhibitor prevented the associability of X from decreasing as it normally would during elemental preexposure treatment. Moreover, the associability of X should not have been protected during preexposure with a neutral stimulus (Group CmpdLI) because the compound did not have any associative strength and thus the associability of both the non-target cue B and the target cue X would be expected to decrease. The Rescorla-Wagner model suggests that the InhibLI treatment caused B to imbue X with excitatory strength during Phase 2. Thus, if the inhibitory status of B were to be altered after Phase 2, X should still be protected according to both the Pearce-Hall and Rescorla-Wagner models. However, the extended comparator hypothesis indicates that X will no longer be protected if B loses its inhibitory status prior to test.
In the present experiment, we attempted to attenuate the strength of the conditioned inhibitor by extinguishing its training excitor. Changes in behavioral control of a conditioned inhibitor due to posttraining extinction of the training excitor have been reported previously (e.g., Chang, Blaisdell, & Miller, 2003; Hallam, Matzel, Sloat, & Miller, 1990; Kaplan, 1985; Kaplan & Hearst, 1985; Kasprow, Schachtman, & Miller, 1987; Lysle & Fowler, 1985; Miller, Hallam, Hong, & Dufore, 1991). These studies demonstrated that conditioned inhibition is often reduced if the training excitor, the conditioned inhibitor's comparator stimulus, is extinguished (but see Williams, Travis, & Overmier, 1986). In the framework of the extended comparator hypothesis, we expected this treatment would reduce conditioned suppression to the target stimulus because the protection from LI afforded by B is a performance-oriented effect that relies on B's inhibitory potential at the time of test. The model states that the negative output of Link 3 produced by B's strong inhibitory status would be weakened as a result of extinction of A. Because of the existence of a previously masked target-context association, latent inhibition was expected to emerge; that is, less suppression was expected than would be observed in an acquisition control group that received conditioning without any preexposure to the target or a control group that received preexposure to X in compound with a conditioned inhibitor and then had extinguished an excitor other than the one used to produce the conditioned inhibitor.
Experiment 2 tested this prediction by preexposing the target CS with a conditioned inhibitor and then extinguishing the training excitor (Group LI-ExtA). We used a 2 × 2 factorial design in which we manipulated whether subjects received preexposure to the target in the presence of an inhibitor (BX-) or preexposure to the inhibitor alone (B-), and the nature of the treatment after preexposure administered to the excitor used in conditioned inhibition training (Extinction [A-] vs. Control [C-]). We expected that extinction of the excitor used in conditioned inhibition training would selectively affect the group that experienced preexposure in the presence of an inhibitor. All groups other than LI-ExtA were expected to show strong conditioned suppression to X. See Table 2 for the experimental design and predictions.
Table 2.
Design of Experiment 2
| Group | Phase 11 Inhibition training | Phase 22 Preexposure | Phase 31 Extinction | Phase 42 Conditioning | Test X1 Prediction |
|---|---|---|---|---|---|
| LI-ExtA | 48 A+ / 84 AB- / 48 C+ | 48 BX- | 200 A- | 3 X+ | cr |
| LI-ExtC | 48 A+ / 84 AB- / 48 C+ | 48 BX- | 200 C- | 3 X+ | CR |
| NoLI-ExtA | 48 A+ / 84 AB- / 48 C+ | 48 B- | 200 A- | 3 X+ | CR |
| NoLI-ExtC | 48 A+ / 84 AB- / 48 C+ | 48 B- | 200 C- | 3 X+ | CR |
Note: A and C were a flashing light and a high frequency tone, counterbalanced; B was a white noise; X was a click train. “+” denotes footshock reinforcement, “-” denotes nonreinforcement. Superscripts indicate different contexts. Predictions are based on the extended comparator hypothesis (Denniston et al., 2001) with uppercase denoting stronger responding than lowercase (i.e., cr < Cr < CR).
Method
Subjects
Subjects were 48 male and 48 female, experimentally naive, Sprague-Dawley descended rats obtained from our own breeding colony. Body-weight ranges were 205-400 g for males and 160-262 g for females. Except where noted, all procedures were the same as in Experiment 1.
Apparatus
This experiment used the click train, the high frequency complex tone, the white noise, and the flashing light. The flashing light and the high frequency complex tone served as CSs A and C, counterbalanced within groups. The white noise served as B, and the clicks served as X. All stimuli were 10 s in duration except for the 0.5-s, 0.7-mA footshock US.
Procedure
Acclimation
On Day 1, all subjects received one 60-min acclimation session in Context 1. During this session, all cues (A, B, C, and X) were programmed to occur twice for 10 seconds each. Trial order was light, tone, white noise, click, tone, light, click, and white noise for all subjects. Stimulus onsets occurred 10, 15, 24, 30, 37, 42, 49, and 52 min into the session. The US was not programmed to occur.
Phase 1
On Days 2-7, all groups received daily 120-min training sessions in Context 1 consisting of 8 reinforced A trials, 14 nonreinforced compound AB trials, and 8 reinforced C trials for a total of 48 A+ presentations, 84 AB- presentations, and 48 C+ presentations. The mean intertrial interval was 4.0 min (range: 2-6). On Days 2, 4, and 6 reinforced A trials occurred 4, 27, 48, 64, 71, 81, 98, and 110 min into the session. Reinforced C trials occurred 9, 17, 44, 67, 92, 107, 117, and 119 min into the session. Nonreinforced trials occurred at 14, 23, 32, 36, 39, 51, 55, 59, 76, 84, 88, 95, 102, and 113 min into the session. On Days 3, 5, and 7 reinforced A trials occurred 15, 36, 39, 59, 72, 79, 99, and 118 min into the session. Reinforced C trials occurred 3, 20, 28, 56, 76, 88, 104, and 114 min into the session. Nonreinforced trials occurred at 7, 12, 24, 31, 43, 48, 52, 64, 69, 83, 91, 95, 107, and 111 min into the session. On reinforced trials, the US and CS coterminated.
Phase 2
On Days 8-13, all groups received daily 24-min sessions conducted in Context 2. Groups LI-ExtC and LI-ExtA received eight daily nonreinforced compound BX presentations for a total of 48 preexposure trials. Groups NoLI-ExtC and NoLI-ExtA received eight daily nonreinforced B alone presentations for a total of 48 trials. The schedule of presentations was the same as in Experiment 1.
Phase 3
On Days 14 and 15, all groups received daily 60-min sessions of extinction trials conducted in Context 1. Subjects in groups LI-ExtC and NoLI-ExtC received 100 daily extinction trials of C for a total of 200 C- trials. Subjects in groups LI-ExtA and NoLI-ExtA received 100 daily extinction trials of A for a total of 200 A- trials. On both days, the first CS onset occurred 3 min into the session, and the last CS onset occurred 57 min into the session. Mean ITI was 0.6 min (range: 0.25-1.75 from CS onset to CS onset).
Phase 4
On Day 16, all subjects were presented with three X-US pairings during a 30-min session in Context 2 just like in Phase 3 of Experiment 1.
Reacclimation and testing
Reacclimation (Days 17 and 18) and testing (Day 19) occurred as in Experiment 1.
Results
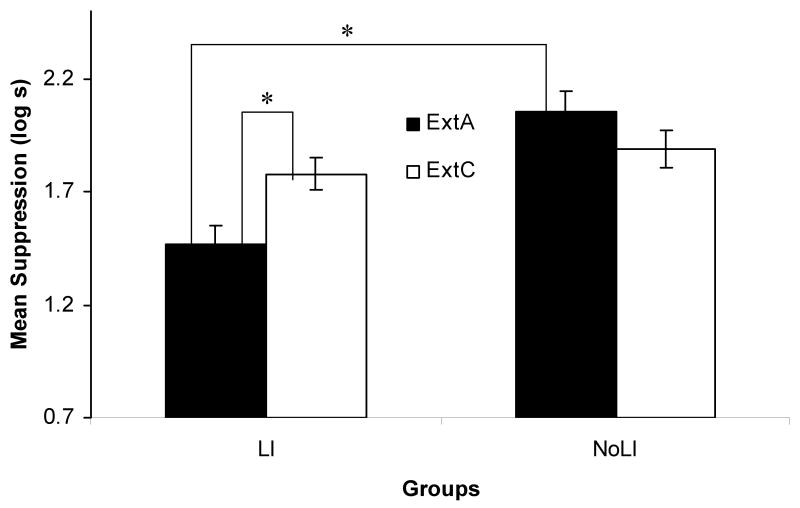
The results of Experiment 2 are depicted in Figure 3. Replicating Experiment 1, protection from latent inhibition provided by a conditioned inhibitor was observed in the groups that controlled for the posttraining extinction manipulation (LI-ExtC and NoLI-ExtC). As anticipated by the extended comparator hypothesis, extinguishing the excitor used in conditioned inhibition training restored latent inhibition in that Group LI-ExtA suppressed less than all other groups. Moreover, a comparison between Group LI-ExtA and LI-ExtC suggests that the effect of the extinction manipulation was specific to the excitor A, given that extinction of an excitatory stimulus that was not part of inhibition training (C) did not restore latent inhibition. The present observations were confirmed by the following statistical analyses.
Figure 3.
Experiment 2: Mean times (log s) to complete 5 cumulative seconds of licking in the presence of the target cue. All subjects were tested on X alone. * indicates significant differences (ps < .05).
A 2 (Preexposure stimulus: BX or B) × 2 (Extinguished stimulus: C or A) ANOVA conducted on the transformed preCS latencies showed no significant differences between groups in drinking behavior prior to the onset of the test stimulus, p = .43. A similar ANOVA conducted on the transformed CS latencies detected an interaction, F(1, 92) = 8.33, MSE = 0.16, p < .01, Cohen's f = 0.28. This suggests that posttraining extinction of A attenuated suppression when X had been paired with B in Phase 2 (Condition LI), but had little effect otherwise (Condition NoLI). A planned comparison between Groups LI-ExtC and LI-ExtA indicated that subjects that received extinction of the training excitor A experienced a loss of protection against the latent inhibition effect compared to subjects that received nonreinforced exposure to an irrelevant excitor stimulus, F(1, 92) = 7.14, p < .01. A second planned comparison between Groups LI-ExtA and NoLI-ExtA provided evidence of restored latent inhibition only when X was preexposed in the presence of B, F(1, 92) = 25.30, p < .01. Lastly, a planned comparison between Groups LI-ExtC and NoLI-ExtC indicated a robust protection from latent inhibition, F(1, 92) = 0.90, p > .05; there was not a difference between Group LI-ExtC, which was given preexposure with a conditioned inhibitor and extinguished on an irrelevant excitor, and Group NoLI-ExtC, which was not given preexposure to the target, p > .05.
Discussion
In this experiment, we observed protection from latent inhibition (Group LI-ExtC) and demonstrated that extinction of the excitor used to train the conditioned inhibitor (that was subsequently preexposed in compound with the target CS) resulted in at least partial emergence of the latent inhibition effect (Group LI-ExtA). This latter effect is explained within the framework of the extended comparator hypothesis (Denniston et al., 2001; see Figure 1) by assuming that extinction of A weakens the A-US association (Link 3.3) and possibly the B-A association (Link 3.2), which attenuates A's potential to support a negative output from Link 3. This presumably caused conditioned suppression to X to decrease because B, as an effectively neutral first-order comparator stimulus, should not be able to enhance responding to X to the extent that it did when B was an effective conditioned inhibitor. The two NoLI groups showed high suppression because X was essentially a novel cue when it was reinforced, excluding the two preexposures during acclimation. Clearly, extinction of excitor A, relative to extinction of neutral stimulus C, did not attenuate suppression to X when X had not been preexposed in the presence of B. Strong suppression was observed in Groups LI-ExtC because Link 3 had a negative output that summated with Link 1 rather than down modulating it. The Pearce and Hall (1980) and Rescorla-Wagner (1972) models both successfully explained the results of Experiment 1, but they fail to anticipate the reemergence of latent inhibition observed in Experiment 2. Similarly, the simple attentional account of Experiment 1 also fails to explain the results of Experiment 2.
General Discussion
This series of experiments demonstrated attenuation of latent inhibition if the target cue was preexposed in compound with a conditioned inhibitor. Experiment 1 found that preexposure to the target cue in compound with a conditioned inhibitor attenuated latent inhibition more than preexposure of the target in the presence of a neutral stimulus. Experiment 2 determined that, following preexposure to the target in compound with the conditioned inhibitor, extinction of the conditioned excitor used in inhibition training (A) reduced the protection from latent inhibition that otherwise occurred. Although we did not test the putative inhibitor B for conditioned inhibition, we based our parameters on those of a previous study (Friedman et al., 1998) that explicitly confirmed that these procedures produced a conditioned inhibitor as demonstrated by negative summation and retardation tests. Thus, it appears reasonable to assume our inhibition training also produced a conditioned inhibitor. Moreover, the control conditions confirm the specificity of the present effects to preexposure in the presence of an inhibitor. We now consider how various models of acquired behavior fare in explaining these observations.
The Pearce-Hall model (1980) postulates that the associability of a stimulus increases when it is presented on a trial with a surprising outcome and decreases on a trial with an expected outcome. Latent inhibition is expected because during preexposure the target becomes a good predictor of nonreinforcement. This causes it to lose associability, which results in retarded acquisition of excitation when it is subsequently reinforced. The Pearce-Hall model also predicts that preexposure in the presence of a conditioned inhibitor will increase the associability of the target cue which should result in faster acquisition of excitation relative to elemental preexposure or preexposure in compound with a neutral stimulus. This is expected because during preexposure the absolute value of the total associative strength for target cue and inhibitor predicting nonreinforcement is positive. This results in a positive increment in the associability of the target stimulus; consequently it should condition quickly. Thus, the Pearce-Hall model can predict all of the effects observed in Experiment 1. However, the Pearce and Hall model cannot account for the results of Experiment 2 in which the excitor used in inhibition training was extinguished and an emergence of the latent inhibition effect was observed. This failure arises because the Pearce-Hall model does not anticipate a change in the associability of the target cue as a result of extinguishing the excitor used in inhibition training in the absence of the target cue.
Conditioned attention theory (CAT) (Lubow, 1973; Lubow et al., 1975) can explain latent inhibition as a conditioned decrement in attention to the target stimulus, and the Lubow effect as overshadowing of this conditioned decrement. However, CAT cannot account for the enhanced protection effect. According to this theory, any mechanism that enhances normal conditioning of attention (or excitation) to a CS should also enhance conditioning of inattention to a CS that is not reinforced. There have been many reports that reinforcing a stimulus in the presence of a conditioned inhibitor results in very strong responding to that CS, that is, superconditioning (e.g., Rescorla, 1971, 2004; Urusihara, Wheeler, Pineño, & Miller, 2005). In the framework of CAT, preexposing X in the presence of a conditioned inhibitor should augment conditioning of inattention to the target, which would result in even weaker responding to X at test, not stronger.
The Rescorla-Wagner (1972) model assumes that excitation and inhibition reflect a common mechanism with the two phenomena simply being indicative of positive and negative values of a single associative variable. One prediction that follows from this model is that a neutral stimulus presented in compound with a conditioned inhibitor on nonreinforced trials should gain excitatory value (Rescorla, 1971). That is, according to the Rescorla-Wagner model, on a given nonreinforced trial the inhibitor creates a negative expectation of the US, and, because the US is not presented on that trial, the inhibitor loses inhibitory strength, resulting in a net loss of its previously-established inhibitory strength. Additionally, the neutral cue that is present during this trial shares gains in excitatory strength. In the framework of this experiment, this model would predict stronger conditioned suppression to the target cue following preexposure with a conditioned inhibitor because the target was made excitatory to some degree during preexposure (notably, the prediction of the Rescorla-Wagner model that a stimulus should acquire excitatory properties as a result of being presented nonreinforced in compound with a conditioned inhibitor has repeatedly failed to be confirmed [e.g., Baker, 1974; Rescorla, 1976]. Thus, the theoretical mechanism behind the model's prediction appears to be incorrect; nevertheless, the model's empirical prediction of enhanced protection from latent inhibition is correct). This leads to a prediction of enhanced suppression to the target cue relative to a group not given preexposure to the target, a group given elemental preexposure to the target (note that the Rescorla-Wagner model does not predict the basic latent inhibition effect), and a group given preexposure to the target compounded with a neutral stimulus. These three latter groups should have performed equally and shown less suppression than a group preexposed with an inhibitor. In fact, more suppression was observed relative to an elemental preexposure group and a group preexposed with a neutral stimulus, but there was not more suppression observed relative to a group not preexposed to the target. Of note, because conventional statistics cannot offer support based on a null result, one cannot categorically falsify the prediction. However, suppression was not at ceiling levels, so at least we cannot attribute this lack of difference to a ceiling effect. Additionally, the control groups did not show equal suppression; the subjects that received elemental preexposure clearly showed less conditioned suppression than the subjects that were not preexposed (i.e., the basic latent inhibition effect). Notably the Rescorla-Wagner model cannot account for the basic latent inhibition effect, which makes its account of protection from latent inhibition unsatisfying. A revision of the Rescorla-Wagner model was proposed by Van Hamme and Wasserman (1994) that allows the associability of the target cue (α) to take on a negative value when it is absent provided an associate of the target is present. This modification allows the Rescorla-Wagner model to account for retrospective revaluation, such as the attenuation of conditioned inhibition as a result of extinction of the excitor used in inhibition training. However, the revised version is no better than the original Rescorla-Wagner model in explaining the results of either of the current experiments. It too fails to account for the basic latent inhibition observed in Experiment 1. Moreover, in Experiment 2 CSs A and X were never paired; hence, the revised Rescorla-Wagner model predicts no effect of extinguishing A on responding to X.
Wagner's SOP (1981) model and its revision by Dickinson and Burke (1996; modified SOP [MSOP]) are capable of accounting for the Lubow effect by assuming that, during preexposure, the target cue competes with the other stimulus present for activation in working memory, which attenuates the target's potential association with the context (it is this association that produces latent inhibition). However, these models cannot account for the enhanced suppression observed in these experiments to a target that was preexposed with a conditioned inhibitor relative to a target that was preexposed in compound with a neutral stimulus. According to these models, these two types of preexposure should have resulted in equal suppression because they bring into play the same processes; they do not predict more suppression to the target after preexposure with an inhibitor as was observed. Moreover, extinction of A is not expected to influence suppression to X even in MSOP because the non-target stimulus which was manipulated (A in this case) should have had no direct association with the target cue.
The extended comparator hypothesis is capable of accounting for all of the effects observed in this series of experiments (see Table 3). It predicts more protection from latent inhibition (i.e., greater suppression) when the target cue is preexposed in compound with a conditioned inhibitor relative to its being preexposed with a neutral stimulus. Further, the extended comparator hypothesis is the only contemporary model that predicts that extinguishing the training excitor used in inhibition training should reverse the protection effect and result in less suppression to the target. Theoretically, these effects are attributed to higher-order comparator processes that involve second-order comparators (i.e., A) that modulate the effectiveness of first-order comparators (B in this instance). The explanation offered by the extended comparator hypothesis depends on the assumption that there are two opposing comparator processes; the context serves to reduce responding as a first-order comparator, whereas B serves to increase responding as a first-order comparator through its association with A. However, B is expected to be more influential in determining the response potential of X because it is a punctate, and therefore more salient, stimulus compared to the diffuse context. These assumptions follow from the model because X, B, and the context share within-compound associations. A cannot serve as a first-order comparator stimulus to X because there is no direct association between these two stimuli; however, A can serve as a second-order comparator to X through its relation to B. Finally, X cannot serve as a higher-order comparator to itself. During BX preexposure, X presumably formed an association with B and the context. As a first-order comparator, the context down modulates responding to X, which ordinarily produces latent inhibition. If B is a conditioned inhibitor, it has its own comparator stimulus, A, which in turn is associated with the US. Because B's indirect association with the US is stronger than its direct association (which does not exist because it was never directly paired with shock) Link 3 should have a negative output (see Figure 1), thereby enhancing suppression to X. This is essentially the same way that the extended comparator hypothesis accounts for superconditioning (e.g., Urushihara, Wheeler, Pineño, & Miller, 2005). However, if A's association with the US is extinguished, A should then have less of an effect on B, which in turn should eliminate the negative output of Link 3. That is, with extinction of A, Group LI-ExtA of Experiment 2 should perform like Group CmpdLI of Experiment 1, which is what was observed.
Table 3.
| Model | Latent inhibition | Protection by a neutral stimulus | Enhanced Protection by an inhibitor | Attenuated protection by extinction of the inhibitor's excitor |
|---|---|---|---|---|
| Pearce-Hall | Yes | No | Yes | No |
| Conditioned Attention Theory | Yes | Yes | No | No |
| Rescorla-Wagner and Revised Rescorla-Wagner | No | No | Yes | No |
| SOP and Modified SOP | Yes | Yes | No | No |
| Extended Comparator Hypothesis | Yes | Yes | Yes | Yes |
Summary of the phenomena observed in Experiments 1 and 2 accounted for by various models. Only the extended comparator hypothesis is capable of explaining all of the effects. See text for details.
It is important to note that the extended comparator hypothesis also anticipates a Lubow effect in Experiment 1. According to the model, this can occur because the neutral B stimulus acts as a second-order comparator for X. When X is tested, it activates a representation of the context based on the strength of Link 2, but this activation can be modulated by any other stimulus (in this case B) that has a strong association with the context and X. The protection afforded by a neutral stimulus is less than that afforded by an inhibitor because the inhibitor also works as a first-order comparator to potentiate responding to the target cue (as described above). Thus, the extended comparator hypothesis would anticipate moderate responding in Group CmpdLI relative to Groups LI and InhibLI. This did not occur for a couple potential reasons. First, there was a nonsignificant tendency toward a Lubow effect, and it can be very difficult to observe three levels of responding in the lick-suppression preparation. It is possible that the paradigm was simply not statistically sensitive enough to simultaneously detect a Lubow effect and protection from latent inhibition. Second, we intentionally selected parameters (such as salience and number of trials) that would produce a small Lubow effect (see Discussion of Experiment 1; as mentioned previously, the ECH is unable to account for these parameter changes reducing the Lubow effect, but they proved to be effective in reducing the Lubow effect, thereby giving us the sensitivity we needed to observe an enhanced protection from latent inhibition effect). Consequently, retarded acquisition of conditioned suppression is anticipated when X is paired with the US. Collectively, these experiments lend support to the position of the extended comparator hypothesis, and as previously suggested by Grahame, Barnet, Gunther, and Miller (1994), that protection from latent inhibition is due to a performance deficit rather than an acquisition deficit.
We have provided further evidence here that even after preexposure, a stimulus is capable of eliciting strong behavioral control given that the appropriate manipulations are employed. Additionally, latent inhibition reemerged when further treatment was given to effectively undo the manipulation that allowed the target stimulus to control behavior. This strongly suggests that a preexposed stimulus acquires an association with the US but is prevented from displaying this behavioral control unless further treatments are administered. Additionally, these data support the view that animals encode more than simple CS-US association in cue interaction situations. They are consistent with recent data from our laboratory that suggested an important role for higher-order associative chains in determining the response potential of a CS (e.g., Urushihara et al., 2005; Witnauer, Urcelay, & Miller, 2008). More generally, the present observations suggest that the prevailing emphasis on information processing at the time of acquisition to the exclusion of subsequent information processing, particularly at the time of testing, is misdirected. That is, among contemporary models of learning, only the extended comparator hypothesis is able to account for the results of Experiment 2 because it assumes that the response potential of stimuli are influenced by not only the associative status of the associates of the target, but by associates of associates of the target (i.e., second-order comparator stimuli).
References
- Baker AG. Conditioned inhibition is not the symmetrical opposite of conditioned excitation: A test of the Rescorla-Wagner model. Learning and Motivation. 1974;5:369–379. [Google Scholar]
- Chang RC, Blaisdell AP, Miller RR. Backward conditioning: Mediation by the context. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:171–183. doi: 10.1037/0097-7403.29.3.171. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding by relative strength. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgments. Quarterly Journal of Experimental Psychology. 1996;49B:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Friedman BX, Blaisdell AP, Escobar M, Miller RR. Comparator mechanisms and conditioned inhibition: Conditioned stimulus preexposure disrupts Pavlovian conditioned inhibition but not explicitly unpaired inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:453–466. [PubMed] [Google Scholar]
- Grahame NJ, Barnet RC, Gunther LM, Miller RR. Latent inhibition as a performance deficit results from CS-context associations. Animal Learning & Behavior. 1994;22:395–408. [Google Scholar]
- Hallam SC, Matzel LD, Sloat JS, Miller RR. Excitation and inhibition as a function of posttraining extinction of the excitatory cue used in Pavlovian inhibition training. Learning and Motivation. 1990;21:59–84. [Google Scholar]
- Honey RC, Hall G. Overshadowing and blocking procedures in latent inhibition. The Quarterly Journal of Experimental Psychology. 1988;42B:163–180. [PubMed] [Google Scholar]
- Ishii I. Attenuation of latent inhibition after compound conditioning. Japanese Psychological Research. 1999;41:102–111. [Google Scholar]
- Kaplan PS. Explaining the effects of relative time in trace conditioning: A preliminary test of a comparator hypothesis. Animal Learning & Behavior. 1985;13:233–238. [Google Scholar]
- Kaplan PS, Hearst E. Contextual control and excitatory versus inhibitory learning: Studies of extinction, reinstatement, and interference. In: Balsam PD, Tomie A, editors. Context and Learning. Hillsdale, NJ: Erlbaum; 1985. pp. 195–224. [Google Scholar]
- Kasprow WJ, Schachtman TR, Miller RR. The comparator hypothesis of conditioned response generation: Manifest conditioned excitation and inhibition as function of relative excitatory strengths of CS and conditioning context at the time of testing. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:395–406. [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition. Psychological Bulletin. 1973;79:398–407. doi: 10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Alek M, Arzy J. Behavioral decrement following stimulus preexposure: Effects of number of preexposures, presence of a second stimulus, and interstimulus interval in children and adults. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:178–188. doi: 10.1037//0097-7403.1.2.178. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced preexposure of the conditioned stimulus. Journal of Comparative and Physiological Psychology. 1959;52:416–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Fowler H. Inhibition as a “slave” process: Deactivation of conditioned inhibition through extinction of conditioned excitation. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:71–94. doi: 10.1037//0097-7403.11.1.71. [DOI] [PubMed] [Google Scholar]
- Miller RR, Hallam SC, Hong JY, Dufore DS. Associative structure of differential inhibition: Implications for models of conditioned inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:141–150. doi: 10.1037//0097-7403.17.2.141. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. 51 pp.92 pp. [Google Scholar]
- Myers JM, Wells AD. Research design and statistical analysis. 2nd. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Reed P. Blocking of latent inhibition. Bulleting of Psychonomic Society. 1991;29:292–294. [Google Scholar]
- Reed P. Enhanced latent inhibition following compound pre-exposure. Quarterly Journal of Experimental Psychology. 1995;48:32–45. [PubMed] [Google Scholar]
- Reed P, Tsakanikos E. The influence of a distractor during compound preexposure on latent inhibition. Animal Learning & Behavior. 2002;30:121–131. doi: 10.3758/bf03192914. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Variation in the effectiveness of reinforcement and nonreinforcement following prior inhibitory conditioning. Learning and Motivation. 1971;2:113–123. [Google Scholar]
- Rescorla RA. Second-order conditioning of Pavlovian conditioned inhibition. Learning and Motivation. 1976;7:161–172. [Google Scholar]
- Rescorla RA. Protection from extinction. Learning & Behavior. 2003;31:124–132. doi: 10.3758/bf03195975. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Superconditioning from a reduced reinforcer. The Quarterly Journal of Experimental Psychology. 2004;57B:133–152. doi: 10.1080/02724990344000051. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Stout SC, Miller RR. Sometimes-competing retrieval (SOCR): A formalization of the comparator hypothesis. Psychological Review. 2007;114:759–783. doi: 10.1037/0033-295X.114.3.759. [DOI] [PubMed] [Google Scholar]
- Urcelay GP, Miller RR. A comparator view of Pavlovian and differential inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:271–283. doi: 10.1037/0097-7403.32.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Wheeler DS, Pineño O, Miller RR. An extended comparator hypothesis account of superconditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:184–198. doi: 10.1037/0097-7403.31.2.184. [DOI] [PubMed] [Google Scholar]
- Van Hamme LJ, Wasserman EA. Cue competition in causality judgments: The role of nonrepresentation of compound stimulus elements. Learning and Motivation. 1994;25:127–151. [Google Scholar]
- Wagner AR. SOP: A model of automatic processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Williams DA, Travis GM, Overmier JB. Within-compound associations modulate the relative effectiveness of differential and Pavlovian conditioned inhibition procedures. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:351–362. [Google Scholar]
- Witnauer JE, Urcelay GP, Miller RR. Reduced blocking as a result of increasing the number of blocking cues. Psychonomic Bulletin & Review. 2008;15:651–655. doi: 10.3758/pbr.15.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]