Abstract
Improved yields for the syntheses of a variety of spiroisoxazolines were achieved through intramolecular cyclization/methylation reactions of functionalized 5,5-disubstituted isoxazolines in one reaction vessel. Aromatic ring containing nitrile oxides and disubstituted geminal alkenes reacted in a 1,3-dipolar fashion to afford the corresponding 5,5-isoxazoline. A comparison of the relative location of the nucleophile and electrophile on the isoxazoline and two different ester functional groups was performed in order to determine the best isoxazoline system for the intramolecular cyclization/methylation reaction.
Keywords: Intramolecular cyclization, Cycloaddition, Regioselectivity, Spirocycles, Heterocycles
Introduction
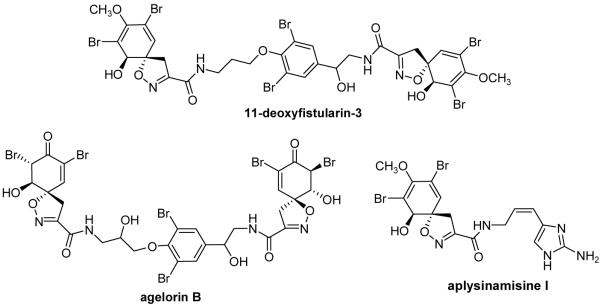
Many synthesized and naturally occurring spiroisoxazolines exhibit biological activity against a variety of disease states, microorganisms, and enzymes. The spiroisoxazolines 11-deoxyfistularin-31 and purealidin Q2 have been shown to be cytotoxic against cancer. Furthermore, other spiroisoxazolines such as aerothionin,3 aplysinamisines I-III,4 and agelorin5 display antifungal, antibiotic, or antimycobacterial activity (Figure 1). Since these and other spiroisoxazoline containing natural products express such a wide array of bioactivities, the synthesis and derivatization of this family of compounds continue to be of interest.6
Figure 1.
Biologically active spiroisoxazoline natural products.
A number of methods exists for the synthesis of functionalized carbocyclic spiroisoxazolines. Some of these methods include the oxidation of an aromatic ring followed by the intramolecular cyclization of a pendant oxime,7,8 the 1,3-dipolar cycloaddition of an exocyclic alkene,9 or other methods.6b,d,e Some oxidative methods for spiroisoxazoline synthesis appear to be limited to aromatic systems, and often require the use of toxic oxidants.7a Furthermore, spiroisoxazoline synthesis via 1,3-dipolar cycloaddition is usually restricted to the use of saturated ring systems with an exocyclic double bond as the dipolarophile.9a,b Herein, we report a facile synthetic methodology for the construction of functionalized unsaturated spiroisoxazolines that involves the intramolecular cyclization/methylation of a 5,5-disubstituted isoxazoline10 in one reaction vessel.
Results and Discussion
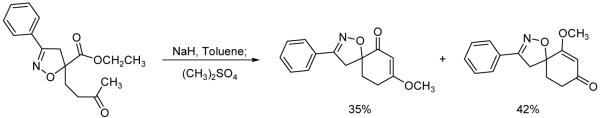
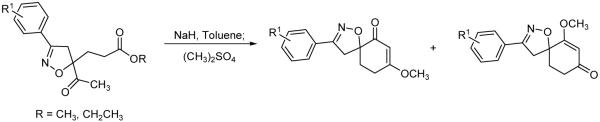
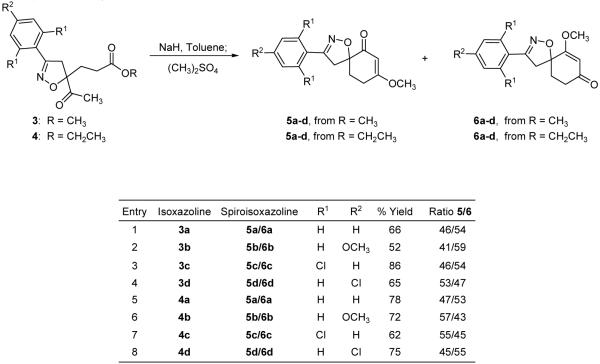
A previous report for the syntheses of spiroisoxazolines through an intramolecular cyclization/methylation methodology used an isoxazoline where the ester functionality was adjacent to the isoxazoline, and the attacking enolate was further away from the isoxazoline11 (Scheme 1). The isolated yield for the intramolecular cyclization was good when the aromatic ring was unsubstituted. However, when other aromatic rings were incorporated onto the isoxazoline, the isolated yields dramatically decreased. Our first attempt to improve the intramolecular cyclization yields was to modify the ester from an ethyl to a methyl ester. Even though ethyl esters are not very bulky, a decrease in ester size could potentially be beneficial. Unfortunately, low yields were also obtained with methyl esters. Other leaving groups were considered, but we decided to relocate the relative positions of the nucleophile and the electrophile for the intramolecular cyclization/methylation reaction as shown in Scheme 2. When the ester was moved away from a position adjacent to the isoxazoline to a more remote location, we believed that the ester carbonyl would be more available for electrophilic attack by the enolate. In order to test this hypothesis, the appropriate isoxazoline was synthesized.
Scheme 1.
Spiroisoxazoline syntheses from an ethyl ester that is adjacent to the isoxazoline.
Scheme 2.
Spiroisoxazoline syntheses from a methyl ketone that is adjacent to the isoxazoline.
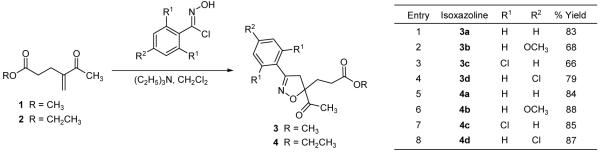
The syntheses of a variety of isoxazolines was achieved through the 1,3-dipolar cycloaddition of disubstituted geminal alkenes, 1 and 2,12 with the requisite nitrile oxide. Compounds 3 a-d and 4 a-d were isolated as a single regioisomer after the respective 1,3-dipolar cycloaddition of 1 and 2 with the corresponding in situ generated nitrile oxidel3 (Scheme 3). Even though an assortment of substituted aromatic rings was incorporated into the isoxazoline, two different ester functionalities were investigated in order to compare the relative efficacy of these two esters during the spiroisoxazoline ring construction through the intramolecular cyclization/methylation strategy.
Scheme 3.
Syntheses of 5,5-disubstituted isoxazolines 3 and 4.
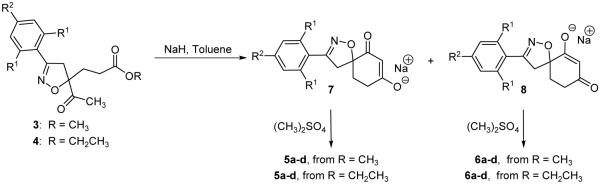
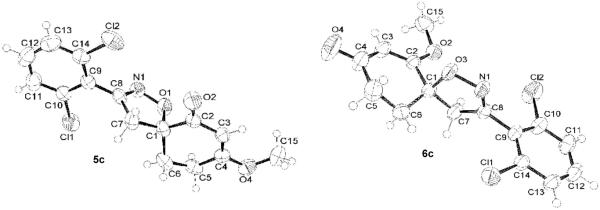
When isoxazolines 3 a-d, which have a methyl ester, were reacted with sodium hydride, intramolecular cyclization insued,14 and the corresponding enolates were methylated with dimethyl sulfate to afford the desired regioisomeric spiroisoxazolines 5 a-d and 6 a-d11 (Scheme 4). The isolation of two spiroisoxazoline regioisomers results from the O-methylation of both spiroisoxazoline intermediate enolates as shown in Scheme 5,11 and the reported ratios between regioisomers 5 and 6 were based upon their respective isolated yields. The spiroisoxazolines arising from the isoxazoline methyl ester were isolated in moderate to good yields, but the ethyl ester containing isoxazoline was examined in order to determine if increased yields of 5 a-d and 6 a-d could be realized. Upon subjecting isoxazolines 4 a-d to the intramolecular cyclization/methylation reaction conditions, the isolated yields of 5 a-d and 6 a-d were examined. In three cases, spiroisoxazolines 5 a-d and 6 a-d were isolated in higher yields when the ethyl ester containing isoxazolines 4 a-d were used as the intramolecular cyclization/methylation substrate. Only spiroisoxazolines 5c and 6c were isolated in higher yields from the methyl ester isoxazoline precursor (Scheme 4). Structural confirmation of the spiroisoxazolines was obtained through NMR studies, and the structures of 5c and 6c were further confirmed through single X-ray crystallographic analysis17 (Figure 2).
Scheme 4.
Syntheses of spiroisoxazolines 5 and 6.
Scheme 5.
Intramolecular cyclization of 3 and 4 to afford regioisomeric enolates 7 and 8.
Figure 2.
Thermal ellipsoid plots for the structures of 5c and 6c.
In summary, starting from a disubstituted geminal alkene, spiroisoxazolines were synthesized in two steps. After the regioselective synthesis of the desired 5,5-disubstituted isoxazoline through nitrile oxide mediated 1,3-dipolar cycloaddition with a disubstituted geminal alkene, regioisomeric spiroisoxazoline were constructed through an intramolecular cyclization/methylation synthetic sequence. Structural confirmation of some of the spiroisoxazolines was realized through X-ray crystallographic analysis.
Acknowledgements
We thank the National Institutes of Health SCORE and RCMI programs (3S06 GM 0080407-34S1 and G12RR13459 (NMR and Analytical CORE facilities)), the MRFN program, and the National Science Foundation grant DMR05-20415. EJV gratefully acknowledges the support of the National Science Foundation grant MRI 0618148 and the W. M. Keck Foundation for crystallographic resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Compagnone RS, Avila R, Suarez AI, Abrams OV, Rangel HR, Arvelo F, Pina IC, Merentes E. J. Nat. Prod. 1999;62:1443. doi: 10.1021/np9901938. [DOI] [PubMed] [Google Scholar]
- 2.Tabudravu JN, Jaspars M. J. Nat. Prod. 2002;65:1798. doi: 10.1021/np020275n. [DOI] [PubMed] [Google Scholar]
- 3.(a) Moody K, Thomson RH, Fattorusso E, Minale L, Sodano G. J. Chem. Soc., Perkin Trans. 1. 1972:18. doi: 10.1039/p19720000016. [DOI] [PubMed] [Google Scholar]; (b) El Sayed KA, Bartyzel P, Shen X, Perry TL, Zjawiony JK, Hamann MT. Tetrahedron. 2000;56:949. [Google Scholar]; (c) Encarnacion-Dimayuga R, Ramirez MR, Luna-Herrera J. Pharm. Biol. 2003;41:384. [Google Scholar]
- 4.Rodriguez AD, Pina IC. J. Nat. Prod. 1993;56:907. doi: 10.1021/np50096a014. [DOI] [PubMed] [Google Scholar]
- 5.Koenig GM, Wright AD. Heterocycles. 1993;36:1351. [Google Scholar]
- 6.(a) Goldenstein K, Fendert T, Proksch P, Winterfeldt E. Tetrahedron. 2000;56:4173. [Google Scholar]; (b) Adamo MFA, Chimichi S, De Sio F, Donati D, Sarti-Fantoni P. Tetrahedron Lett. 2002;43:4157. [Google Scholar]; (c) Harburn JJ, Rath NP, Spilling CD. J. Org. Chem. 2005;70:6398. doi: 10.1021/jo050846r. [DOI] [PubMed] [Google Scholar]; (d) Adamo MFA, Donati D, Duffy EF, Sarti-Fantoni P. J. Org. Chem. 2005;70:8395. doi: 10.1021/jo051181w. [DOI] [PubMed] [Google Scholar]; (e) Marsini MA, Huang Y, Van De Water R, Pettus TRR. Org. Lett. 2007;9:3229. doi: 10.1021/ol0710257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Nishiyama S, Yamamura S. Tetrahedron Lett. 1983;24:3351. [Google Scholar]; (b) Nishiyama S, Yamamura S. Bull, Chem. Soc. Jpn. 1985;58:3453. [Google Scholar]; (c) Murakata M, Tamura M, Hoshino O. J. Org. Chem. 1997;62:4428. doi: 10.1021/jo970082i. [DOI] [PubMed] [Google Scholar]; (d) Wasserman HH, Wang J. J. Org. Chem. 1998;63:5581. [Google Scholar]; (e) Boehlow TR, Harburn JJ, Spilling CD. J. Org. Chem. 2001;66:3111. doi: 10.1021/jo010015v. [DOI] [PubMed] [Google Scholar]
- 8.(a) Forrester AR, Thomson RH, Woo S-O. Liebigs Ann. Chem. 1978:66. [Google Scholar]; (b) Murakata M, Yamada K, Hoshino O. J. Chem. Soc. Chem. Commun. 1994:443. [Google Scholar]; (c) Masatoshi M, Yamada K, Hoshino O. Tetrahedron. 1996:14713. [Google Scholar]; (d) Murakata M, Yamada K, Hoshino O. Heterocycles. 1998;47:921. [Google Scholar]; (e) Ogamino T, Nishiyama S. Tetrahedron. 2003;59:9419. [Google Scholar]; (f) Adamo MFA, Donati D, Duffy EF, Sarti-Fantoni P. J. Org. Chem. 2005;70:8395. doi: 10.1021/jo051181w. [DOI] [PubMed] [Google Scholar]
- 9.(a) Kumar HM, Anjaneyulu S, Yadav JS. Synth. Commun. 1999;29:877. [Google Scholar]; (b) Reddy DB, Reddy AD, Padmaja A. Synth. Commun. 1999;29:4433. [Google Scholar]; (c) Manikandan S, Jayashankaran J, Raghunathan R. Synth. Commun. 2003;33:4063. [Google Scholar]; (d) Bardhan S, Schmitt DC, Porco JA., Jr. Org. Lett. 2006;8:927. doi: 10.1021/ol053115m. [DOI] [PubMed] [Google Scholar]
- 10.(a) Hamme AT, II, Xu J, Wang J, Cook T, Ellis E. Heterocycles. 2005;65:2885. [Google Scholar]; (b) Yamauchi M. J. Heterocyclic Chem. 2002;39:1013. [Google Scholar]
- 11.Xu J, Wang J, Ellis ED, Hamme AT., II Synthesis. 2006:3815. [Google Scholar]
- 12.Kalaus G, Juhasz I, Greiner I, Kajtar-Peredy M, Brlik J, Szabo L, Szantay C. J. Org. Chem. 1997;62:9188–9191. [Google Scholar]
- 13.Zamponi GW, Stotz SC, Staples RJ, Andro TM, Nelson JK, Hulubei V, Blumenfeld A, Natale NR. J. Med. Chem. 2003;46:87. doi: 10.1021/jm020354w. [DOI] [PubMed] [Google Scholar]
- 14.Boers RB, Gast P, Hoff AJ, De Groot HJM, Lugtenburg J. Eur. J. Org. Chem. 2002:189. [Google Scholar]
- 15.General Procedure for 1,3-Dipolar Cycloaddition: A solution of the alkene (3.0 mmol) and the hydroximoyl chloride (3.0 mmol) in 4 mL of dichloromethane was heated to 50 °C for 10 minutes. Triethylamine (0.46 mL, 3.3 mmol) was then added dropwise, and the resulting reaction mixture was heated for an additional 5 minutes at 50 °C. The reaction mixture was stirred at rt until the disappearance of the starting materials, as evidenced by TLC. After the reaction was complete, the reaction mixture was washed with water (3 × 4 mL) and brine (4 mL). The organic layer was dried over anhydrous sodim sulfate, filtered, and the solvent was evaporated under reduced pressure. Based upon TLC and NMR, no purification was necessary, and the crude products were used in the subsequent step.
- 16.General Procedure for Intramolecular Cyclization/Methylation: To a stirred solution of the isoxazoline (0.87-1.00 mmol) and 5 mL of anhydrous toluene was added a 60% dispersion of sodium hydride in mineral oil. The reaction mixture was then heated to 50 °C and stirred overnight. After the disappearance of starting material, as evidenced by TLC, 1 mmol of dimethyl sulfate was added to the enolate reaction mixture. The reaction mixture was then heated to 50 °C, and the mixture was stirred overnight. After the reaction was complete, NH4OH (1 mL) was added, and the mixture was stirred for 30 minutes to 3 h. The reaction mixture was washed with water (3 × 5 mL), and the organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The crude product was purified via column chromatography over silica gel using a 2:1 hexanes-ethyl acetate ratio as an eluant system.
- 17.Structural information for 5c and 6c has been deposited with the CCDC as 738659 and 739766, respectively, available free of charge from www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033).