Abstract
Summary
Interferon-γ-inducible GTPase (IGTP) expression is upregulated in coxsackievirus B3 (CVB3)-infected murine heart and inhibits CVB3-induced apoptosis through activation of the PI3 kinase/Akt pathway. However, the mechanism of this pathway activation is unknown. In this study, using a doxcycycline-inducible Tet-On HeLa cells that overexpress IGTP, we have demonstrated that focal adhesion kinase (FAK) is phosphorylated in response to IGTP expression and that transfection of the Tet-On HeLa cells with a dominant negative FAK (FRNK) blocks Akt activation. Furthermore, induction of IGTP also promoted the NF-κB activation as evidenced by its enhanced nuclear translocation, binding to transcriptional promoters and increased transcriptional activity. However, FRNK transfection and PI3K inhibitor LY294002 both blocked the IGTP-induced translocation and NF-κB activation. Furthermore, silencing NF-κB with siRNAs significantly inhibited the phosphorylation of FAK and Akt, but not their total expression levels, indicating that NF-κB activation is required for the IGTP-induced activation of FAK and PI3K/Akt. Finally, blocking this survival pathway by transfection of FRNK or silencing of NF-κB reduced CVB3 replication and enhanced cell death during CVB3 infection. Taken together, these results suggest that FAK is a mediator upstream of PI3K/Akt and NF-κB functions as a downstream effector and also positively regulates the activity of upstream kinases.
Introduction
Interferon-γ-inducible GTPase (IGTP), also known as Irgm3, is a recently identified gene in a growing family of 47-kDa IFN-γ-responsive GTPases. Accumulated data has shown that proteins in this family play critical roles in mediating specific resistance to intracellular pathogens including protozoa, bacteria and viruses (Singh et al., 2006; Collazo et al., 2002; Taylor et al., 2000). IGTP localizes predominantly to the endoplasmic reticulum and is therefore assumed to be involved in processing and trafficking of immunologically relevant proteins (Taylor et al., 2004; Taylor et al., 1997). IGTP has been found to be essential for host resistance to acute infection by the protozoans Toxoplasma gondii (Halonen et al., 2001) and Leishmania major (Taylor et al., 2004), but its antimicrobial mechanism is still not clear. Our previous mRNA differential display study demonstrated that IGTP is significantly up-regulated in coxsackievirus B3 (CVB3)-infected mouse hearts, suggesting it plays an important role in viral infection (Yang et al., 1999). Our later in vitro study showed that IGTP expression could confer cell survival during CVB3 infection, and that IGTP inhibited CVB3-induced apoptosis through the activation of the phosphatidylinositol 3-Kinase (PI3K)/Akt pathway and inhibition of viral replication (Zhang et al., 2003).
CVB3 is a common cause of myocarditis, and previous studies by our laboratory and others support the notion that CVB3-induced apoptosis in the early phase of infection is one of the critical determinants of disease severity (Tam, 2006; Yuan et al., 2005; Kawai, 1999; Carthy et al., 1998). Therefore, the findings of IGTP-induced anti-apoptotic pathway shed light on the mechanisms of IGTP-mediated antimicrobial responses in the context of viral myocarditis. However, the exact picture of this IGTP-PI3K/Akt pathway including the mediator upstream of PI3K and downstream effectors still remains unclear.
PI3K is a lipid kinase that synthesizes the second messenger PtdIns(3,4,5)P3, which leads to the recruitment and activation of downstream effectors. Akt, a serine/threonine kinase, is the critical downstream effector of PI3K. Once activated, Akt may protect cells from death by several mechanisms, such as directly phosphorylating and inactivating the proapoptotic protein BAD, or inhibiting pro-apoptotic genes such as BIM (Kane and Weiss, 2003; Vivanco and Sawyers, 2002). Akt can also increase the transcription of survival genes by activating the transcription factor NF-κB (Fresno Vara et al., 2004; Vivanco and Sawyers, 2002). Evidence now suggests that NF-κB is a critical antiapoptotic molecule activating transcription of survival genes such as inhibitor-of-apoptosis proteins (IAPs) (Mattson and Meffert, 2006; Papa et al., 2006). In unstimulated cells, NF-κB complex is localized and inactivated in the cytoplasm by binding to the inhibitory protein IκB. IκB kinase (IKK) can phosphorylate IκB and lead to its degradation. NF-κB is then released to translocate to nucleus and activate transcription. Akt has been reported to be able to activate NF-κB through activating IKK (Chandrasekar et al., 2004; Zou et al., 2004; Tai et al., 2003; Ozes et al., 1999). However, alternative relationships between these two molecules have also been reported in signaling transduction (Schabbauer et al., 2004; Anto et al., 2003; Meng et al., 2002).
Focal adhesion kinase (FAK) is among multiple signaling molecules known capable of activating PI3K (Mitra et al., 2005; Parsons, 2003). FAK is a nonreceptor tyrosine kinase that was originally found to mediate integrin-initiated signal transduction leading to cellular responses such as cell migration and adhesion. FAK is also activated through a variety of cell surface receptors, acting as a point of convergence of a number of signaling pathways (Schaller, 2001; Ilic et al., 1997). There is accumulating evidence that FAK plays an essential role in the regulation of cell survival and protection of cells from apoptosis caused by oxidative stress, etoposide, and ionizing radiation (Kasahara et al., 2002; Sonoda et al., 2000; Sonoda et al., 1999). It has been demonstrated that FAK may inhibit cell apoptosis via activation of PI3K/Akt (Lee et al., 2003; Reif et al., 2003; Yamamoto et al., 2003). Upon stimulation, FAK is phosphorylated at tyrosine397, allowing it to bind SH2 domain of the p85 subunit of PI3K and subsequently activate the PI3K/Akt signal pathway. Activation of FAK-PI3K/Akt pathway, leading to NF-κB–mediated expression of caspase inhibitors of IAPs, has been implicated in protecting human leukemic HL-60 cells from oxidative stress-induced apoptosis (Sonoda et al., 2000). It's plausible that FAK may be involved in the regulation of the IGTP-induced PI3K/Akt pathway.
In this study, using inducible Tet-On HeLa cells overexpressing IGTP, we investigated the signaling mediators of the PI3K/Akt pathway upon IGTP expression, as well as the downstream effectors involved in promoting cell survival during CVB3 infection. We showed that the activation of PI3K/Akt by IGTP is FAK dependent and that the activated FAK-PI3K/Akt pathway in turn activates NF-κB and translocates it to the nucleus. Moreover, the downstream NF-κB also exerts a positive feedback on FAK and Akt activity. Furthermore, inhibition of FAK or NF-κB abrogates the prosurvival effect of IGTP during CVB3 infection and decreases viral replication, which further confirms that IGTP-FAK-PI3K/Akt-NF-κB functions in a signaling cascade to promote cell survival. Finally, the requirement of endogenous IGTP expression for the activation of this pathway was confirmed by using CVB3-infected murine hearts and IFN-γ-treated HL-1 cardiomyocytes.
Results
IGTP expression promotes phosphorylation of FAK and paxillin
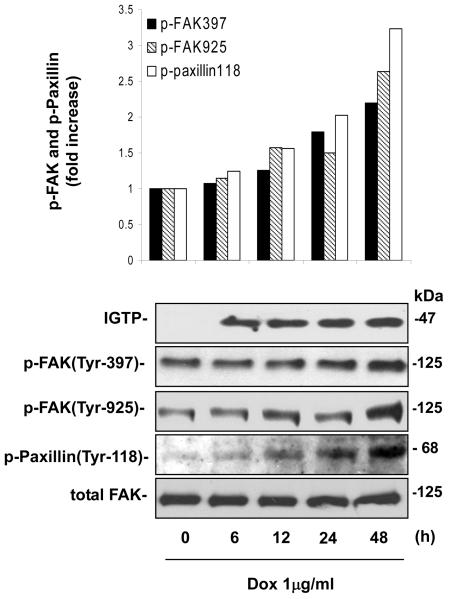
Our previous study demonstrated that IGTP overexpression can activate the PI3K/Akt signaling pathway in the doxycycline(Dox)-inducible Tet-On/IGTP HeLa cell (Zhang et al., 2003). Here we aimed to identify the signaling protein that mediates IGTP-induced activation of the PI3K/Akt pathway, and take FAK as a good candidate since it has been known to be able to direct activate PI3K (Schaller, 2001; Sonoda et al., 1999; Ilic et al., 1997). To this end, we first examined the correlation between IGTP expression and FAK phosphorylation in abovementioned Tet-On/IGTP cell line by Western blotting. During the 48-hour period, following the IGTP expression, the phosphorylation of FAK at both the 397 and 925 sites gradually increased, evident as early as 12h post induction (Fig 1). Tyrosines 397 and 925 are both primary phosphorylation-sites of FAK. In particular, phosphorylation at Tyr397 correlates with increased catalytic activity of FAK, and is critical for activation of other focal-adhesion-associated proteins such as the FAK-binding protein paxillin (Heidkamp et al., 2003; Parsons, 2003). Tyrosine phosphorylation of paxillin occurs in a FAK-dependent manner (Cary and Guan, 1999). As shown in Fig 1, phosphorylation of paxillin at the major Try118 site occurred concomitantly with FAK in a similar linear increase pattern, which further confirmed the activation of FAK. These data suggest that IGTP expression can activate FAK.
Figure 1. Over-expression of IGTP induces up-regulation of p-FAK (Tyr397, Tyr925) and p-Paxillin (Tyr-118).
IGTP expression was induced in Tet-On/IGTP HeLa cells with Dox at 1 μg/ml, and whole cell lysates were prepared at indicated time points and subjected to immunoblot analysis using indicated antibodies. The membrane was then stripped and probed for detection of total FAK, which was used as the equal loading control. Results were quantitated by densitometric analyses of the bands and normalized by the relative ratio of p-FAK or p-Paxillin to total FAK. The relative ratio at time 0 h is arbitrarily set to 1.0. Fold increase is the ratio of the amount of each time point to that of time 0 h. These data are representative of two independent experiments.
IGTP-induced activation of Akt is FAK dependent
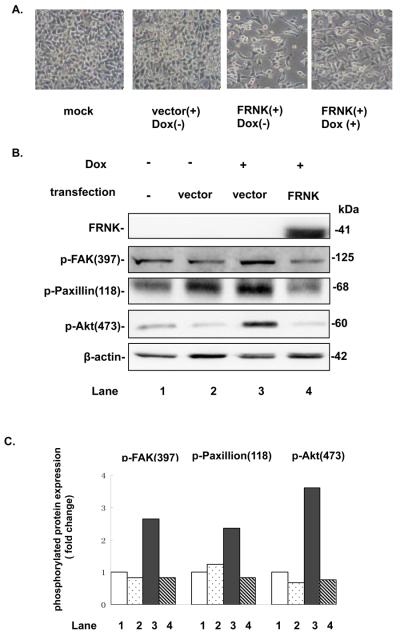
To confirm the involvement of and define the role of FAK in the IGTP-PI3K/Akt survival pathway, we transfected Tet-On/IGTP HeLa cells with FRNK, a dominant negative FAK called FAK-related non-kinase, to examine the regulatory effect of FAK in this pathway. FRNK is a truncated 41-kD protein identical to the C-terminal domain of FAK for targeting. Without catalytical activity, FRNK may act as a competitive inhibitor of FAK in the cells, preventing the localization of FAK to focal adhesions and thereby suppressing its phosphorylation (Schlaepfer et al., 2004; Schaller, 2001). As FRNK affects normal focal adhesion turnover, it may be toxic at high concentrations and cause detrimental cytoskeleton changes and cell death (Schlaepfer et al., 2004; Xia et al., 2004). This prediction is consistent with our observation of the cell rounding and loss after 48 hours of transfection with 2μg/ml FRNK (Fig2A). However, induction of IGTP with doxycycline (Dox) appeared to decrease this effect, suggesting that IGTP expression can partially rescue the FRNK effect, and thereby IGTP and FRNK might converge in the survival pathway. We then transfected Tet-On/IGTP HeLa cells with 1μg/ml HA-tagged FRNK, and examined the signaling change by phospho-specific immunoblot analysis. Phosphorylation of Akt at Ser473 has been used as a marker for PI3K/Akt activation (Vivanco and Sawyers, 2002). Data in Fig2B shows that Dox induction increased the phosphorylation of Akt, agreeing with our previous results (Zhang et al., 2003). Compared to the vector transfected control, FRNK transfection totally blocked the Dox-induced increase of p-FAK (Tyr397) and p-Paxillin (Tyr118). With the inhibition of FAK activity, the upregulation of p-Akt (ser473) after Dox induction was also dramatically reduced. These data strongly suggest that FAK plays a regulatory role for IGTP-induced activation of PI3K/Akt and is the mediator upstream of PI3K/Akt.
Figure 2. FRNK overexpression inhibits IGTP-induced upregulation of p-Akt.
A. Tet-On/IGTP HeLa cells were transfected with 2 μg/ml FRNK-encoding plasmid and then induced with 1 μg/ml Dox 16 h post transfection, Cell morphology was observed under a phase-contrast microscope 48 h pi. B. Tet-On/IGTP HeLa cells at 80% confluence were transfected with 1 μg/ml FRNK using lipofectamine 2000 and then induced with 1 μg/ml Dox 16 h post transfection. Cell lysates were harvested 12 h post induction and subjected to immunoblot analysis with indicated antibodies, where β-actin was used as the loading control. C. Results were quantitated by densitometric analyses and normalized as the relative ratio of p-FAK, p-Paxillin or p-Akt to β-actin. The experiment was repeated twice.
IGTP induces nuclear translocation and activation of NF-κB
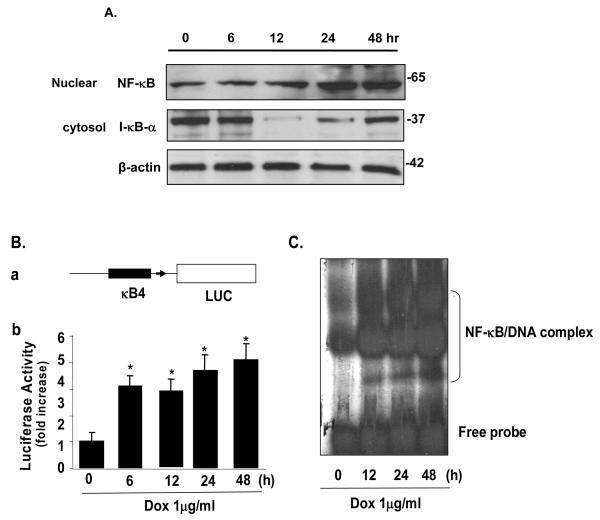
NF-κB is a key anti-apoptotic transcription factor that can be activated by a variety of signals. To determine whether the IGTP-induced PI3K/Akt pathway can lead to activation of NF-κB, we performed Western blot analysis using nuclear and cytosol lysates prepared at time points post Dox induction as indicated in Fig 3. The p65-p50 heterodimer is the most common active NF-κB complex, so we probed p65 in the nuclear extract and also IκBα in cytosol. A significant downregulation of IκBα due to the degradation was observed at 12 h post induction, a time point corresponding the translocation of NF-κB to the nucleus (Fig 3A). Interestingly, when NF-κB is accumulated in nuclear, IκBa begins to recover 24 h post induction, which may be due to re-synthesis under a potential feedback mechanism of NF-κB activation (De Martin et al., 2000). This activation pattern of NF-κB was further confirmed by luciferase assay showing the transcriptional activity of NF-κB (Fig3B), and by gel shift assay demonstrating the association of NF-κB with its DNA binding sequence (Fig3C).
Figure 3. Expression of IGTP promotes translocation and activity of nuclear transcription factor NF-κB (p65).
A. Tet-On/IGTP HeLa cells were induced with 1 μg/ml Dox, whole cell lysates and nuclear lysates were prepared at indicated time points. NF-κB and I-κBα were detected by Western blot. B. Luciferase assay of IGTP-induced NF-κB activity. (a) Schematic structure of the pNF-κB-Luc plasmid. LUC: firefly luciferase gene; κB4: four copies of the NF-κB consensus enhancer sequence fused to a TATA-like promoter. (b) Luciferase activity assay. Tet-On/IGTP HeLa cells were cotransfected with pNF-κB-luc and pCMV-β-gal control vector, induced with Dox and harvested at indicated time points. Control cells were not induced but cultured for 48 h. Assay reaction and data normalization were performed as described in Experimental Procedures. Data from three independent experiments are presented as a fold increase. Values are means ± SE. *p<0.05. C. Expression of IGTP increases sequence-specific DNA-binding activity of NF-κB. Tet-On/IGTP HeLa cells were induced with Dox and the nuclear proteins were prepared at indicated time points. A gel mobility shift assay was performed using nuclear proteins and 32P-end-labeled oligonucleotides containing a consensus NF-κB binding site.
Inhibition of FAK and Akt by FRNK and LY294002 blocks the IGTP-induced activation of NF-κB
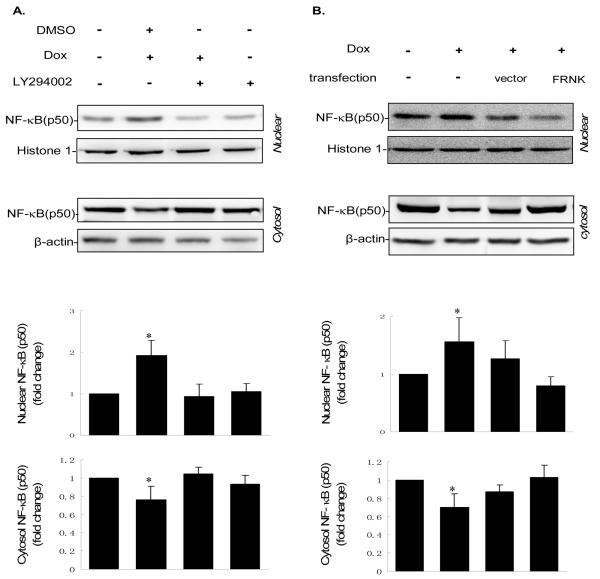
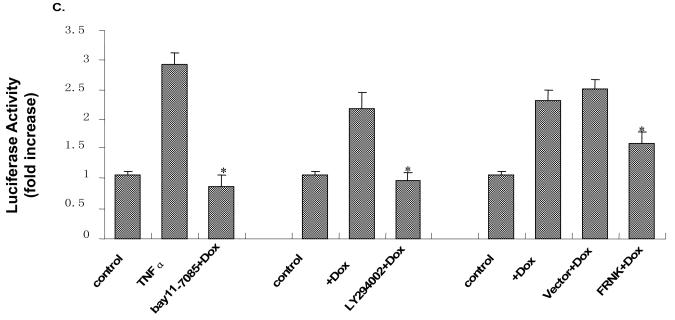
To determine the position of NF-κB in the IGTP-induced PI3K/Akt survival cascade, we further examined the IGTP-induced activity of NF-κB under the suppression of FAK and Akt. We added LY294002, a specific inhibitor of PI3K that competitively inhibits its catalytic activity, to examine the effect of PI3K/Akt suppression on NF-κB nuclear translocation. After inhibitor treatment and dox induction, Fractionated cell lysates were collected, and NF-κB (p50) was probed by Western blotting. Here, histone-1 was used as a nuclear loading control to further validate the fractionation technique. The amount of NF-κB in cytosolic and nuclear extracts exhibited a complementary pattern in all groups, clearly showing the translocation from cytosol to nucleus in the Dox-treated samples (lane 2 from the left, Fig4A). Compared to DMSO control, LY294002 specifically abrogated this IGTP-induced translocation of NF-κB. The quantification of NF-κB was performed by densitometric analyses (lower panel). To suppress FAK activity, we transfected FRNK into the cells using the vector-transfected cell as a control. Again, the FRNK transfection almost completely blocked the IGTP-induced increase of NF-κB translocation (lane 4, Fig4B). The vector control shows slightly inhibited translocation, as was the cases in later experiments, probably due to the detrimental impact of the transfection reagent itself. However, the change was negligible compared to the effect of FRNK. We further examined the NF-κB transcriptional activation by luciferase reporter assay, using the same treatments as Western blotting. TNF-α, a well recognized stimulator of NF-κB, and NF-κB inhibitor Bay11-7085 were used as the positive and negative control, respectively. The results correlate well with the change of NF-κB translocation. Both FRNK transfection and LY294002 treatment substantially blocked IGTP-induced increase of NF-κB transcriptional activity, although to a lesser extent by FRNK than by LY294002 in this assay. Taken together, inhibition of FAK and Akt both block the IGTP-induced activation of NF-κB.
Figure 4. FRNK and PI3K inhibitor LY294002 block NF-κB translocation and activity induced by IGTP overexpression.
A. Tet-On/IGTP HeLa cells at 70% confluence were treated with 10 μM LY294002 or DMSO and induced with 1 μg/ml Dox 1 h post treatment. Nuclear lysates and cytosol lysates were prepared 10 h post induction and subjected to immunoblot analysis with the indicated antibodies, among which the β-actin was used as the cytosol loading control and the histone-H1 as the nuclear protein loading control. Results were quantitated by densitometric analyses and normalized as the relative ratio of nuclear p50 to histone-H1 and cytosol p50 to β-actin, respectively. The relative ratio from untreated cell lysates is arbitrarily set to 1.0. Fold increase is presented as described in Fig 1. The values are representative of three independent experiments.*p<0.05. B. Tet-On/IGTP HeLa cells at 70% confluence were transfected with 1 μg/ml FRNK using lipofectamine 2000 and induced with 1 μg/ml Dox 24 h post transfection. Nuclear lysates and cytosol lysates were harvested and separated 10 h post induction and then subjected to immunoblot analysis with the indicated antibodies. Loading control and densitometric analyses are as described above. *p<0.05. C. Tet-On/IGTP HeLa cells were cotransfected with pNF-κB-luc and pCMV-β-gal control plasmid, and treated with PI3K inhibitor or transfected with FRNK as described above. The cells were then induced with Dox and harvested at 10 h post induction. Control cells were not Dox-induced but cultured for 48 h. TNF-α and Bay 11-7085 were applied as positive and negative controls, respectively. Luciferase assay reaction and data normalization were performed as described above in Fig. 3. Data represents values obtained from three independent experiments. Values are means ± SE. *p<0.05.
Suppression of NF-κB expression blocks the IGTP-induced activation of both FAK and Akt
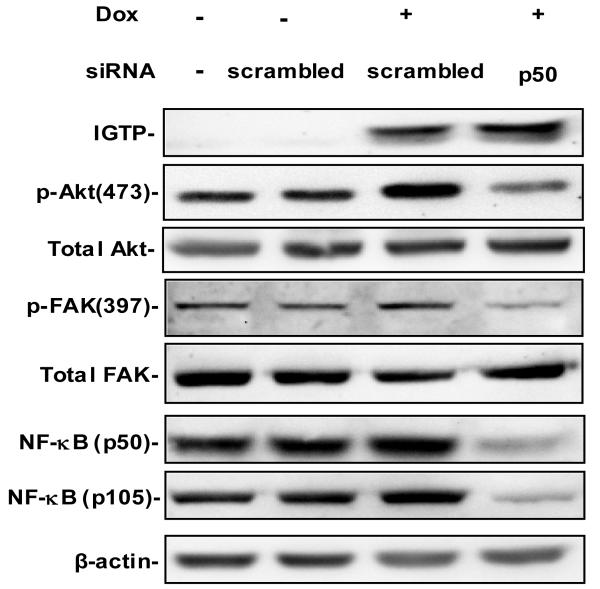
The regulatory network for NF-κB is complex, and multiple feedback mechanisms have been suggested to be associated with NF-κB (Liu and Malik, 2006). In order to further elucidate the role of NF-κB in this pathway, we examined whether NF-κB has influence on FAK and Akt activity. We measured the phosphorylation of FAK and Akt after silencing NF-κB using validated siRNAs specifically targeting NF-κB1 p50/p105. P105 serves as the precursor of p50, and is constitutively processed to p50 in cell. 70 nM NF-κB1 siRNA effectively silenced the expression of both p105 and p50 (lane 4 from the left, Fig 5). Specificity of this silencing can be confirmed by the fact that no inhibition was observed with the scrambled control siRNA (lane 2 and 3, Fig 5) and that expression of other proteins, such as β-actin, was not significantly affected. With the suppression of NF-κB, the IGTP-induced upregulation of p-FAK and p-Akt was dramatically reduced, compared with the cells induced with Dox but transfected with scrambled siRNA (lane 3 and 4, Fig 5). However, the expression levels of total FAK and Akt were unaltered by silencing NF-κB expression. These data suggest that NF-κB is required for the IGTP-induced activation of FAK and Akt.
Figure 5. NF-κB p50 siRNA inhibits the IGTP-induced upregulation of p-FAK and p-Akt.
Tet-On/IGTP HeLa cells at 60% confluence were transfected with NF-κB p50 siRNA at a final concentration of 70 nM using Oligofectimine. Scrambled siRNAs at the same concentration were transfected as the control, and 1 μg/ml Dox was added 6 h post transfection. Cell lysates were harvested 30 h later and subjected to immunoblot anaylsis with the indicated antibodies. β-actin was used as the loading control. The experiments were repeated twice.
Blockage of FAK or NF-κB attenuates IGTP-induced cell survival and decreases virus replication
We have shown previously the prosurvival effect of the IGTP-PI3K/Akt pathway during the CVB3 infection (Zhang et al., 2003). Moreover, we have revealed that both FAK and NF-κB are mediators of this pro-survival cascade. Thus, we next tested their involvement in mediating the pro-survival effect of IGTP during CVB3 infection.
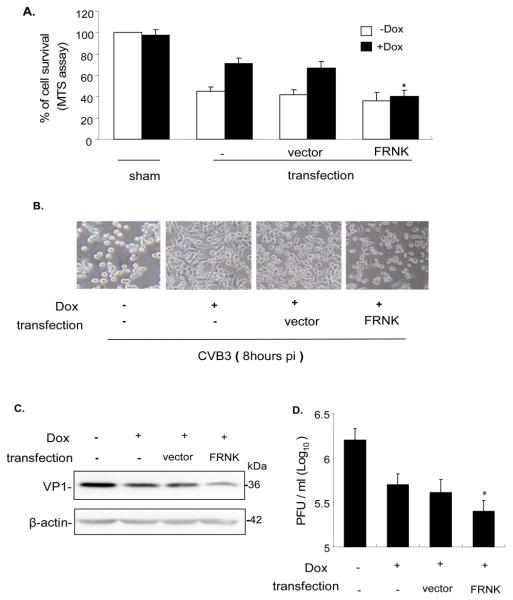
To check the effect of FAK, Tet-On/IGTP HeLa cells were transfected with FRNK or vector control, and induced with Dox before CVB3 infection. Cell viability was determined by the MTS assay 9 h post-CVB3 infection. Compared to non-induced cells, Dox-induced cells exhibit a much higher survival rate (Fig 6A). However, compared to vector-transfected cells, FRNK transfection significantly attenuated the protective effect of the IGTP-induced survival pathway, decreasing cell viability almost to levels of non-Dox-induced cells. This observation was further corroborated by a morphological assessment of the cultured cells (Fig 6B), which shows a nearly similar degree of cell loss and detachment in both non-induced/non-transfected cells and Dox-induced/FRNK-transfected cells. To examine the corresponding effect of FRNK on viral replication and viral protein synthesis, we performed viral plaque assay to measure viral titer, and Western blotting to measure CVB3 capsid protein VP1. In both experiments, FRNK transfection further reduced viral protein synthesis and viral replication (Fig 6C and D).
Figure 6. FRNK blocks the IGTP-induced cell survival, and decreases viral protein synthesis and viral release.
Tet-On/IGTP HeLa cells at 70% confluence were transfected with 1 μg/ml FRNK 24 h prior to infection, and induced with 1 μg/ml Dox 3 h prior to infection. The cells were then infected with CVB3 at 10 MOI. A. Cell viability was measured by MTS assay 9 h post infection. Absorbance values were corrected by substracting background reading. B. Cell morphology was observed under a phase-contrast microscope 8 h post infection. C. Cell lysates were collected 9 h post infection and Western blotting was performed to probe the viral capsid protein VP1. β-actin was used as the loading control. D. Supernatants from cell cultures were collected 9 h post infection, and the pfu/ml was determined by plaque assay on HeLa cell monolayers. Data represents values obtained from three independent experiments, values are means ± SE. *p<0.05.
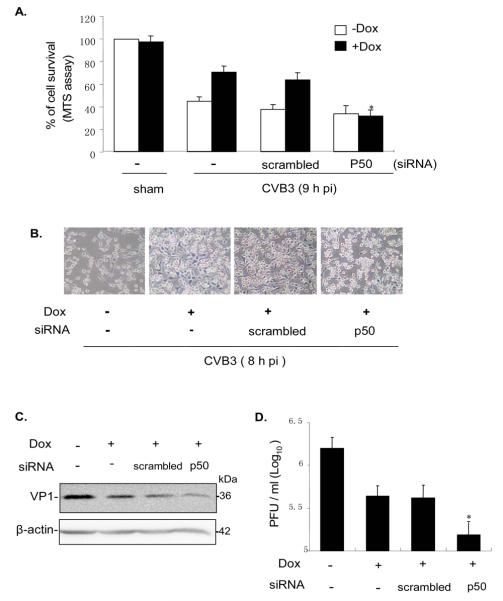
To check the effect of NF-κB during CVB3 infection, NF-κB siRNA or scrambled siRNA was transfected into Tet-On/IGTP HeLa cells, and followed by Dox induction and then CVB3 infection. The same set of assays was performed to examine the cell viability and viral replication as described above. As shown in Fig 7, the suppression of NF-κB also substantially reduced the IGTP-induced pro-survival effect and inhibited viral replication. The reduction of virus titer by NF-κB siRNAs was greater in magnitude than that of FRNK. All these data further confirmed that both FAK and NF-κB are essential for the pro-survival effect of the IGTP-induced PI3K/Akt pathway during CVB3 infection.
Figure 7. NF-kB siRNA abrogates the IGTP-induced cell survival, and decreases viral protein synthesis and viral release.
Tet-On IGTP HeLa cells at 60% confluence were transfected with NF-κB p50 siRNA at a final concentration of 70 nM using Oligofectimine. Scrambled siRNAs at the same concentration were transfected as the control. Cells were induced with 1 μg/ml Dox 3 h prior to infection and then the cells were infected by CVB3 at 10 MOI 24 h post transfection. A. cell viability was measured by MTS assay 9 h post infection. Absorbance values were corrected with the reading of sham-transfected control. B. Cell morphology was observed under a phase-contrast microscope 8 h post infection. C. Cell lysates were collected 9 h post infection and Western blotting was performed to probe the viral capsid protein VP1, and β-actin was used as the loading control. D. Supernatants from cell cultures were collected 9 h post infection, and the pfu/ml was determined by plaque assay on HeLa cell monolayers. Data represents values obtained from three independent experiments, values are means ± SE. *p<0.05.
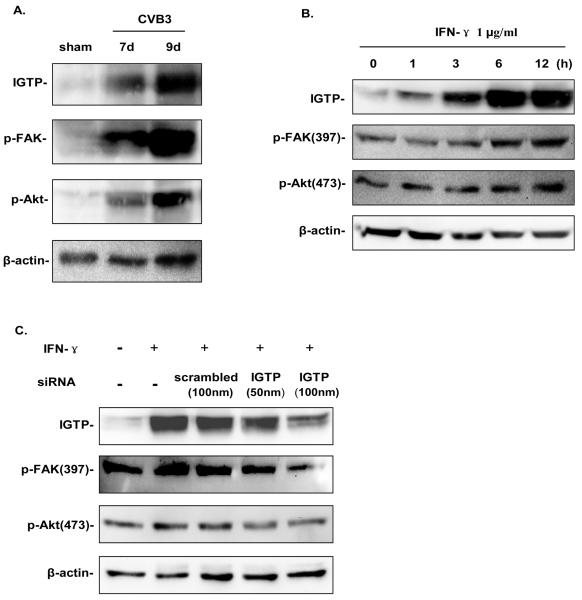
Expression of endogenous IGTP is required for activation of FAK and Akt in murine cardiomyocytes
To demonstrate the endogenous activation of FAK and Akt by IGTP during CVB3 infection, we performed in vivo CVB3 infection on A/J mice. Heart lysates were prepared at days 7 and 9 post infection (pi), when inflammatory T-cells have infiltrated into the myocardium and secreted a large amount of cytokines including IFN-γ. As shown in Fig 8A, the IGTP expression is gradually and significantly upregulated after CVB3 infection, and such up-regulation of endogenous IGTP was remarkably correlated with phosphorylation of both FAK and Akt. We further examined whether such endogenous IGTP expression occurs in cardiomyocytes and is required for FAK and Akt activation by treating murine HL-1 cardiomyocytes with recombinant mouse interferon-γ. The data shown in Fig 8B demonstrates that upregulation of endogenous IGTP levels, with corresponding increased phosphorylation of FAK and Akt after IFN-γ treatment, resembling the expression pattern obtained from the above in vivo experiment using CVB3-infected murine hearts. This data was further confirmed by applying specific siRNAs to knockdown IGTP expression. As shown in Fig 8C, increasing concentrations of siRNAs targeting IGTP (especially at 100 nM) significantly suppressed endogenous IGTP expression compared to the scrambled control. The change is paralleled by the suppression of p-FAK and p-Akt production after IFN-γ treatment.
Figure 8. Expression of endogenous IGTP is required for activation of FAK and Akt in murine cardiomyocytes.
A. Endogenous IGTP was increased in concert with activation of FAK and Akt in CVB3 infected murine hearts. A/J mice were injected intraperitoneally with 105 PFU of CVB3 or PBS, and sacrificed at days 7 and 9 pi. Hearts were collected, and cell lysates were subjected to immunoblot anaylsis using the indicated antibodies. β-actin was used as the loading control. B. HL-1 cardiomyocytes at 80% confluence were treated with IFN-γ at a final concentration of 1μg/ml for an indicated period of time. Cells were then harvested for Western blot analysis using indicated antibodies. C. HL-1 cells at 60% confluence were transfected with IGTP siRNA at a final concentration of 50 nM and 100 nM, respectively. Scrambled siRNAs at 100 nM of concentration were transfected as a control, and 1 μg/ml IFN-γ was added 24 h post transfection. Cell lysates were harvested 10 h post induction and subjected to immunoblot anaylsis with indicated antibodies. The data are representative of two independent experiments.
Discussion
Since the discovery of this p47 GTPases (also known as Immunity-related GTPases, IRGs), data accumulated in the past decade have clearly defined this protein family as a key regulator of cell autonomous resistance to intracellular pathogens (Taylor, 2007). Remarkably, each confers gene-specific and pathogen-specific resistance (Taylor et al., 2004). The critical but differential roles for the p47 proteins in host resistance against bacteria, protozoa or viruses have been illustrated in many recent studies, including our previous report on IGTP in CVB3 infections (Zhang et al., 2003; Yang et al., 1999). The study also revealed that besides limiting viral replication in vitro, this p47 GTPase also functions as a “survival factor” for the host cell, activating the PI3K/Akt survival pathway. However, many aspects of this signaling pathway remain unclear, including the potential mediators and the downstream effectors. In the present study, using a Tet-On/IGTP HeLa cell line and cardiomyocytes, we found that FAK mediates the activation of the PI3K/Akt pathway by IGTP, and that NF-κB serves as a downstream effector. Moreover, our data found that NF-κB can provide positive feedback for the activation of this FAK-PI3K/Akt pathway.
Based on our negative results on the co-immunoprecipitation of the IGTP and PI3K (data not shown), we hypothesize that IGTP may activate PI3K/Akt indirectly through certain mediator(s). Growing evidence has shown that after autophosphorylation, activated FAK can form a complex with the p85 subunit of PI3K (Schaller, 2001; Sonoda et al., 1999; Ilic et al., 1997). The FAK-PI3K/Akt survival pathway has been emerging as an important pathway conferring protection against apoptosis induced by various stimuli such as integrin (Xia et al., 2004) and growth factor (Casamassima and Rozengurt, 1998). However, it has also been suggested that integrin signaling activates the PI3K and FAK pathways in parallel (Velling et al., 2004), and that in the study of IGF-I pro-survival effect, PI3K even regulates FAK activity indirectly, casting into doubt about the order and directionality of molecules within the pathway.
In the present study, we examined FAK and found it was significantly phosphorylated following IGTP expression. The sustained phosphorylation at both primary sites of FAK and at the site of its binding partner paxillin verified the full activation of FAK. IGTP expression protected the cell from apoptosis resulting from disruption of FAK signaling. Expression of exogenous dominant negative FAK completely blocked the activation of Akt by IGTP overexpression. Therefore, our data suggests that FAK is not only involved in this IGTP signaling, but actually positioned upstream of PI3K/Akt in this pathway. To further validate the role of endogenous IGTP in activating this pathway, we examined CVB3-infected murine hearts as well as IFN-γ-treated murine HL-1 cardiomyocytes. Results from both model systems support the notion that endogenous IGTP expression is responsible for the activation of FAK and Akt.
NF-κB is a central player in the complex signal transduction network and acts by regulating expression of immune, stress response, and inflammation related genes (Liu and Malik, 2006; Mattson and Meffert, 2006; Papa et al., 2006). In the majority of systems, NF-κB activation provides a survival-promoting signal (Perkins and Gilmore, 2006; Madrid et al., 2000). PI3K/Akt signaling stimulated by TNF, PDGF, interleukin-1 and interferons has been reported to suppress apoptosis by activating NF-κB (Mansell et al., 2001; Yang et al., 2001; Sizemore et al., 1999). Activation of NF-κB was also observed in transgenic mice that express constitutively activated Akt (Jones et al., 2000). The mechanisms by which PI3K/Akt signaling activates NF-κB appear to be pleiotropic and cell type-specific (Pasparakis et al., 2006; Gustin et al., 2004). Contrary to the above studies, inhibition of PI3K was found to enhance LPS activation of NF-κB in monocytic cells (Guha and Mackman, 2002). Moreover, results from two recent studies place Akt as a downstream target of NF-κB in the TNF and EGF transducing pathways (Anto et al., 2003; Meng et al., 2002), which add to the complexity of the relationship between Akt and NF-κB. Additionally, studies on anti-apoptotic role of FAK provide evidence of a link between FAK and NF-κB. FAK overexpression enhanced NF-κB activity and inhibited apoptosis in an NF-κB dependent manner (Schlaepfer et al., 2004; Sonoda et al., 2000). All these led us to examine whether NF-κB is involved, and its putative position in this pathway. Our data have shown that IGTP expression activates NF-κB by downregulating IκB, allowing its translocation to the nucleus, which is consistent with the mechanism whereby Akt leads to IκB degradation via IKK (Zou et al., 2004; Tai et al., 2003; Ozes et al., 1999). However, both IGTP-induced nuclear translocation and increased activity of NF-κB were totally blocked with the transfection of dominant negative FAK or treatment of the specific PI3K inhibitor. These data suggest that activation of NF-κB by IGTP overexpression is regulated by both FAK and PI3K, and that NF-κB functions as a downstream effector in this IGTP survival pathway.
To further define the role of NF-κB in this pathway, we examined the effects of NF-κB knockdown on the activation of FAK and Akt. Interestingly, we found that with the inhibition of NF-κB expression by siRNAs, the phosphorylation of FAK and Akt following IGTP expression was almost completely blocked, without alternating their overall expression levels. This result demonstrates that NF-κB is required for IGTP-induced activation of FAK and Akt, thereby suggesting that a positive feedback loop exists here in this FAK-PI3K/Akt-NF-κB pathway. Positive and negative feedback mechanisms regulating NF-κB activity within the NF-κB and IκB family have been intensively studied, and exemplified respectively by RelA positive feedback for high-affinity NF-κB complexes (Yang et al., 2003), and upregulation of IκB (Ito et al., 2004; Karin and Ben-Neriah, 2000). Remarkably, new evidence has emerged, suggesting that, in addition to autocrine loop for TNF (Coward et al., 2002), NF-κB activated targets can also stimulate its upstream kinases, and thus form positive feedback loops (Anrather et al., 2006; Hosokawa et al., 2005; Kim et al., 2004) or negative feedback mechanisms (Liu and Malik, 2006). In tumor cell lines, a positive feedback loop was revealed whereby Akt activation of NF-κB further stimulates Akt via down-regulation of the PI3K inhibitor PTEN. These findings fit in line with our observation of the inhibition of NF-κB reciprocally reducing phosphorylation of FAK and Akt. Therefore, although the promoter region of the human FAK gene was indicated to contain NF-κB binding sites, our data suggest that NF-κB may positively regulate the upstream FAK and Akt activity indirectly through some intermediate, in the same way that PTEN regulates Akt (Kim et al., 2004). As for FAK, some NF-κB-regulated genes may be prerequisites for FAK activation in focal adhesion (Cohen-Hillel et al., 2006; Ishibe et al., 2004). Moreover, a peculiarity of NF-κB is the rapid and transient nature of its activity, as we observed an obvious NF-κB increase observed as early as 6 h post induction in our luciferase assay. A significant fraction of genes can be upregulated rapidly following NF-κB activation in a stimulus-specific fashion, acting only for a limited period of time before shutting down, which could influence and contribute to later prolonged activation of NF-κB (Perkins, 2007; De Martin et al., 2000). Therefore, it is conceivable that early initial activation of NF-κB through upregulation of IGTP could regulate some intermediates that are essential for the full activation of FAK (and Akt), and the activated FAK-PI3K/Akt pathway further stimulates the sustained activation of NF-κB, thereby amplifying the pro-survival signaling to a necessary level.
Our experiments also showed that inhibition of FAK and NF-κB significantly suppresses the host cell viability during CVB3 infection, which is consistent with our previous observation of the inhibition of Akt (Zhang et al., 2003), highlighting the pro-survival role of this IGTP-FAK-PI3K/Akt-NF-κB signaling pathway. During the early phase of virus infection, it would be advantageous for the virus to preserve the cell viability, thereby allowing for efficient production of viral progeny. Therefore, as previous studies have shown (Esfandiarei, 2007; Zhang et al., 2003), inhibition of the survival pathway in our study on FAK and NF-κB, led to inhibition of CVB3 viral progeny production and release. It is noteworthy that two recent studies suggested the central role of NF-κB activation in CVB3 infection. On one hand, virus may manipulate NF-κB to augment its replication (Esfandiarei, 2007). On the other hand, the host may detect IκBα cleavage during CVB3 infection and mount a counteracting response (Zaragoza et al., 2006). The interactions of virus and host defense response converging at NF-κB further underscore the significance of this survival cascade from IGTP to NF-κB.
In summary, in the present study, we further investigated the IGTP-induced cell survival signaling pathway in the context of CVB3 infection. We identified FAK, functioning upstream of PI3K/Akt, as the mediator for IGTP-induced activation of the PI3K/Akt pathway, and NF-κB as both a downstream effector and a positive regulator of FAK and PI3K/Akt activity. These findings may add insights into the role of this p47 GTPase in response to CVB3 infection. Further studies are required to dissect and fully understand this survival pathway and viral resistance mechanism of IGTP.
Experimental procedures
Cells, animals and virus
CVB3 was routinely propagated in HeLa cells (ATCC). The virus supernatant was obtained by three cycles of freeze-thaw and centrifugation to remove cell debris and stored at −80 °C. Virus titers were determined by plaque assay prior to infection. A previously established double-stable Tet-On/IGTP HeLa cell line was used in this study. Tet-On/IGTP HeLa cells were grown and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 μg/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, 1 mM HEPES, and 10% Clontech approved fetal bovine serum specially prepared for the Tet-On system in the presence of both 100 μg/ml G418 and 100μg/ml hygromycin. To induce the expression of IGTP, cells were cultured in the above medium containing 1 μg/ml Dox. Cell cultures prepared for detection of cell signals were serum-starved overnight before performing the experiments. HL-1 cells were grown in Claycomb medium (JRH Biosciences) containing 10% FBS, 0.1 mmol/L norepinephrine (Sigma-Aldrich), and 2 mM L-glutamine. Five week old male A/J mice (Jackson Laboratories) were injected intraperitoneally with 105 plague-forming unit (PFU) of CVB3 or phosphate-buffered saline (PBS), and were sacrificed at days 7 or 9 pi (n=5 per time point per group). Heart tissue was homogenized, and cell lysates were collected.
Antibodies, plasmids and reagents
Antibodies against phosphorylated Akt (p-Akt), total Akt, and phosphorylated ERK (p-ERK) as well as the PI3K inhibitor LY294002 were purchased from Cell Signaling Technologies. Antibodies against phosphorylated FAK (pFAK) at Tyr397, pFAK at Tyr925, total FAK (tFAK), and NF-κB p65 and p50 subunits were purchased from Santa Cruz Biotechnology. Goat antibodies against mouse IgG and rabbit IgG conjugated to horseradish peroxidase were obtained from BD Biosciences and Santa Cruz Biotechnology, respectively. Rabbit polyclonal antibody against the HA-tag was purchased from Covance Research Product. The pHK-FRNK (FAK-related non-kinase) construct containing a HA-His tag at the 3′ end of its open reading frame was obtained from Dr. Junlin Guo, University of Michigan. NF-κB siRNA p50 was purchased from Ambion.
Western blot analysis
Cells either untreated or treated with different experimental reagents were washed twice with ice-cold PBS containing 5% phosphatase inhibitor (Active Motiff Company) and Cell lysates were prepared as described previously (Zhang et al., 2003). The protein concentration was determined by the Bradford assay (Bio-Rad). Twenty to 80 micrograms of extracted proteins were fractionated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech), and blocked with PBS containing 0.1% Tween 20 and 5% nonfat dry milk for 40 min. Afterward, the membrane was incubated with the specific primary antibody overnight at 4°C, followed by the secondary antibody for 1 h at room temperature. The immunoblots were visualized with an enhanced chemiluminescence detection system according to the protocol of the manufacturer (Pierce). Densitometry analysis was performed by using the Genetools software from Syngene. Density values for proteins were normalized to the level of control groups (arbitrarily set to 1.0-fold).
Luciferase assay
The NF-κB-dependent luciferase reporter gene construct containing the synthetic sequence with four copies of connective NF-κB-binding elements (p-NF-κB-luc) and the pCMV-β-galactosidase control plasmid were obtained from Clontech and used for transfection. The cells were collected at 30 h after transfection with Lipofectamine 2000 (Invitrogen Life Technologies), and the values of luciferase activity from individual transfection were measured using the Luciferase Assay System from Clonetech. The experiments were conducted in triplicate and the data was normalized by division with corresponding β-galactosidase activity.
Preparation of nuclear protein and electrophoretic mobility Shift Assay (EMSA)
Nuclear proteins were prepared with a nuclear extraction kit (Pierce) following the manufacturer's instructions, and the protein contents were measured using the Bradford method. The double-stranded oligonucleotides containing a consensus NF-κB binding site (QIAGEN) were end labeled with [γ-32P]ATP using T4 polynucleotide kinase (Promega). For gel mobility shift assay, 32P-labeled oligonucleotides (30,000 cpm) and 10 μg of nuclear proteins were incubated in a total volume of 20 μl in the presence of 10 mM Tris·HCl (pH7.5), 50 mM NaCl, 1 mM EDTA, 1 mM DTT, 50% glycerol, and 1 μl of poly(dI-dC). The binding reactions were allowed to proceed at room temperature for 20 min. Two μl of bromophenol blue (0.1% in water) were added, and protein-DNA complexes were resolved by performing electrophoresis on nondenaturing 5% polyacrylamide gels and visualized by autoradiography.
Cell viability assay
Cell viability following virus infection was measured using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphen yl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) assay reagents, according to the manufacturer's instructions (Promega). Briefly, cells were incubated with MTS solution for 2 h and absorbance was measured at 492 nm using an enzyme-linked immunosorbent assay plate reader (Tecan, spectra fluro plus). The absorbance of sham-infected cells was defined as a value of 100% survival and the remaining data were converted to the ratio of the sham-infected sample. Morphological changes of cells following CVB3 infection were evaluated by phase contrast microscopy.
Viral plaque assay
Cells either untreated or treated with different reagents were infected with CVB3 at multiplicity of infection (MOI) of 10 for 1 h. Cells were washed and replenished with serum-free DMEM for 9 h. The supernatants were collected to determine viral titer on HeLa cell monolayers in triplicate following the standard procedures described previously (Zhang et al., 2003). Agar overlays were fixed and Cells were stained to visualize plaques at day 3 after infection. The virus titer was calculated as PFU per milliliter.
Statistical analysis
Two-way analysis of variance with multiple comparisons and paired Student's t tests were performed. Values shown are the mean ± standard deviation. A P value of <0.05 was considered statistically significant.
Acknowledgements
We thank Dr. Junlin Guo, University of Michigan, for providing the pHK-FRNK plasmid. This work was supported by grants from the Canadian Institutes of Health Research and Heart and Stroke Foundation of BC and Yukon. Zhen Liu is a recipient of the Doctoral Research Award of the Heart and Stroke Foundation of Canada. Dr. Alhousseynou Sall is supported in part by an IMPACT postdoctoral fellowship, Ji Yuan is a recipient of the Doctoral Research Award from the Canadian Institutes of Health Research and Michael Smith Foundation of Health.
References
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Anto RJ, Venkatraman M, Karunagaran D. Inhibition of NF-kappaB sensitizes A431 cells to epidermal growth factor-induced apoptosis, whereas its activation by ectopic expression of RelA confers resistance. J Biol Chem. 2003;278:25490–25498. doi: 10.1074/jbc.M301790200. [DOI] [PubMed] [Google Scholar]
- Carthy CM, Granville DJ, Watson KA, Anderson DR, Wilson JE, Yang D, et al. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J Virol. 1998;72:7669–7675. doi: 10.1128/jvi.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- Casamassima A, Rozengurt E. Insulin-like growth factor I stimulates tyrosine phosphorylation of p130(Cas), focal adhesion kinase, and paxillin. Role of phosphatidylinositol 3′-kinase and formation of a p130(Cas).Crk complex. J Biol Chem. 1998;273:26149–26156. doi: 10.1074/jbc.273.40.26149. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Bysani S, Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem. 2004;279:3188–3196. doi: 10.1074/jbc.M311660200. [DOI] [PubMed] [Google Scholar]
- Cohen-Hillel E, Yron I, Meshel T, Soria G, Attal H, Ben-Baruch A. CXCL8-induced FAK phosphorylation via CXCR1 and CXCR2: cytoskeleton- and integrin-related mechanisms converge with FAK regulatory pathways in a receptor-specific manner. Cytokine. 2006;33:1–16. doi: 10.1016/j.cyto.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Collazo CM, Yap GS, Hieny S, Caspar P, Feng CG, Taylor GA, Sher A. The function of gamma interferon-inducible GTP-binding protein IGTP in host resistance to Toxoplasma gondii is Stat1 dependent and requires expression in both hematopoietic and nonhematopoietic cellular compartments. Infect Immun. 2002;70:6933–6939. doi: 10.1128/IAI.70.12.6933-6939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, Church MK. NF-kappa B and TNF-alpha: a positive autocrine loop in human lung mast cells? J Immunol. 2002;169:5287–5293. doi: 10.4049/jimmunol.169.9.5287. [DOI] [PubMed] [Google Scholar]
- De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20:E83–88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- Esfandiarei M, Boroomand , Suarez , Si N, Rahmani , McManus B. Cell Microbiol. 2007;9:2358–2371. doi: 10.1111/j.1462-5822.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Gustin JA, Ozes ON, Akca H, Pincheira R, Mayo LD, Li Q, et al. Cell type-specific expression of the IkappaB kinases determines the significance of phosphatidylinositol 3-kinase/Akt signaling to NF-kappa B activation. J Biol Chem. 2004;279:1615–1620. doi: 10.1074/jbc.M306976200. [DOI] [PubMed] [Google Scholar]
- Halonen SK, Taylor GA, Weiss LM. Gamma interferon-induced inhibition of Toxoplasma gondii in astrocytes is mediated by IGTP. Infect Immun. 2001;69:5573–5576. doi: 10.1128/IAI.69.9.5573-5576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Scully BT, Eble DM, Samarel AM. Activation of focal adhesion kinase by protein kinase C epsilon in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H1684–1696. doi: 10.1152/ajpheart.00016.2003. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Suzuki H, Nakagawa M, Lee TH, Seto M. API2-MALT1 fusion protein induces transcriptional activation of the API2 gene through NF-kappaB binding elements: evidence for a positive feed-back loop pathway resulting in unremitting NF-kappaB activation. Biochem Biophys Res Commun. 2005;334:51–60. doi: 10.1016/j.bbrc.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Ilic D, Damsky CH, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110(Pt 4):401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell. 2004;16:257–267. doi: 10.1016/j.molcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ito T, Morimatsu M, Oonuma T, Shiina T, Kitamura H, Syuto B. Transcriptional regulation of the MAIL gene in LPS-stimulated RAW264 mouse macrophages. Gene. 2004;342:137–143. doi: 10.1016/j.gene.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Koguchi E, Funakoshi M, Aizu-Yokota E, Sonoda Y. Antiapoptotic action of focal adhesion kinase (FAK) against ionizing radiation. Antioxid Redox Signal. 2002;4:491–499. doi: 10.1089/15230860260196290. [DOI] [PubMed] [Google Scholar]
- Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–1100. doi: 10.1161/01.cir.99.8.1091. [DOI] [PubMed] [Google Scholar]
- Kim S, Domon-Dell C, Kang J, Chung DH, Freund JN, Evers BM. Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-alpha/nuclear factor-kappaB (NF-kappaB)-inducing kinase/NF-kappaB pathway is linked to a default IkappaB-alpha autoregulatory loop. J Biol Chem. 2004;279:4285–4291. doi: 10.1074/jbc.M308383200. [DOI] [PubMed] [Google Scholar]
- Lee YC, Tang YC, Chen YH, Wong CM, Tsou AP. Selenite-induced survival of HuH7 hepatoma cells involves activation of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt pathway and Rac1. J Biol Chem. 2003;278:39615–39624. doi: 10.1074/jbc.M304095200. [DOI] [PubMed] [Google Scholar]
- Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr., Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell A, Khelef N, Cossart P, O'Neill LA. Internalin B activates nuclear factor-kappa B via Ras, phosphoinositide 3-kinase, and Akt. J Biol Chem. 2001;276:43597–43603. doi: 10.1074/jbc.M105202200. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Meng F, Liu L, Chin PC, D'Mello SR. Akt is a downstream target of NF-kappa B. J Biol Chem. 2002;277:29674–29680. doi: 10.1074/jbc.M112464200. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, et al. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Luedde T, Schmidt-Supprian M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13:861–872. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, et al. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278:8083–8090. doi: 10.1074/jbc.M212927200. [DOI] [PubMed] [Google Scholar]
- Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Watanabe S, Matsumoto Y, Aizu-Yokota E, Kasahara T. FAK is the upstream signal protein of the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen peroxide-induced apoptosis of a human glioblastoma cell line. J Biol Chem. 1999;274:10566–10570. doi: 10.1074/jbc.274.15.10566. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Matsumoto Y, Funakoshi M, Yamamoto D, Hanks SK, Kasahara T. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J Biol Chem. 2000;275:16309–16315. doi: 10.1074/jbc.275.21.16309. [DOI] [PubMed] [Google Scholar]
- Tai YT, Podar K, Mitsiades N, Lin B, Mitsiades C, Gupta D, et al. CD40 induces human multiple myeloma cell migration via phosphatidylinositol 3-kinase/AKT/NF-kappa B signaling. Blood. 2003;101:2762–2769. doi: 10.1182/blood-2002-09-2813. [DOI] [PubMed] [Google Scholar]
- Tam PE. Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol. 2006;19:133–146. doi: 10.1089/vim.2006.19.133. [DOI] [PubMed] [Google Scholar]
- Taylor GA. IRG proteins: key mediators of interferon-regulated host resistance to intracellular pathogens. Cell Microbiol. 2007;9:1099–1107. doi: 10.1111/j.1462-5822.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Feng CG, Sher A. p47 GTPases: regulators of immunity to intracellular pathogens. Nat Rev Immunol. 2004;4:100–109. doi: 10.1038/nri1270. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Stauber R, Rulong S, Hudson E, Pei V, Pavlakis GN, et al. The inducibly expressed GTPase localizes to the endoplasmic reticulum, independently of GTP binding. J Biol Chem. 1997;272:10639–10645. doi: 10.1074/jbc.272.16.10639. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, et al. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc Natl Acad Sci U S A. 2000;97:751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velling T, Nilsson S, Stefansson A, Johansson S. beta1-Integrins induce phosphorylation of Akt on serine 473 independently of focal adhesion kinase and Src family kinases. EMBO Rep. 2004;5:901–905. doi: 10.1038/sj.embor.7400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Sonoda Y, Hasegawa M, Funakoshi-Tago M, Aizu-Yokota E, Kasahara T. FAK overexpression upregulates cyclin D3 and enhances cell proliferation via the PKC and PI3-kinase-Akt pathways. Cell Signal. 2003;15:575–583. doi: 10.1016/s0898-6568(02)00142-0. [DOI] [PubMed] [Google Scholar]
- Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM. Interferon alpha /beta promotes cell survival by activating nuclear factor kappa B through phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2001;276:13756–13761. doi: 10.1074/jbc.M011006200. [DOI] [PubMed] [Google Scholar]
- Yang D, Yu J, Luo Z, Carthy CM, Wilson JE, Liu Z, McManus BM. Viral myocarditis: identification of five differentially expressed genes in coxsackievirus B3-infected mouse heart. Circ Res. 1999;84:704–712. doi: 10.1161/01.res.84.6.704. [DOI] [PubMed] [Google Scholar]
- Yang L, Ross K, Qwarnstrom EE. RelA control of IkappaBalpha phosphorylation: a positive feedback loop for high affinity NF-kappaB complexes. J Biol Chem. 2003;278:30881–30888. doi: 10.1074/jbc.M212216200. [DOI] [PubMed] [Google Scholar]
- Yuan J, Zhang J, Wong BW, Si X, Wong J, Yang D, Luo H. Inhibition of glycogen synthase kinase 3beta suppresses coxsackievirus-induced cytopathic effect and apoptosis via stabilization of beta-catenin. Cell Death Differ. 2005;12:1097–1106. doi: 10.1038/sj.cdd.4401652. [DOI] [PubMed] [Google Scholar]
- Zaragoza C, Saura M, Padalko EY, Lopez-Rivera E, Lizarbe TR, Lamas S, Lowenstein CJ. Viral protease cleavage of inhibitor of kappaBalpha triggers host cell apoptosis. Proc Natl Acad Sci U S A. 2006;103:19051–19056. doi: 10.1073/pnas.0606019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Yuan J, Cheung P, Luo H, Yanagawa B, Chau D, et al. Overexpression of interferon-gamma-inducible GTPase inhibits coxsackievirus B3-induced apoptosis through the activation of the phosphatidylinositol 3-kinase/Akt pathway and inhibition of viral replication. J Biol Chem. 2003;278:33011–33019. doi: 10.1074/jbc.M305352200. [DOI] [PubMed] [Google Scholar]
- Zou T, Rao JN, Guo X, Liu L, Zhang HM, Strauch ED, et al. NF-kappaB-mediated IAP expression induces resistance of intestinal epithelial cells to apoptosis after polyamine depletion. Am J Physiol Cell Physiol. 2004;286:C1009–1018. doi: 10.1152/ajpcell.00480.2003. [DOI] [PubMed] [Google Scholar]