Abstract
Objective
Various lines of evidence suggest that face shape may be a predisposing factor for nonsyndromic cleft lip with or without cleft palate (CL/P). In the present study, 3D surface imaging and statistical shape analysis were used to evaluate face shape differences between the unaffected (non-cleft) parents of individuals with CL/P and unrelated controls.
Methods
Sixteen facial landmarks were collected from 3D captures of 80 unaffected parents and 80 matched controls. Prior to analysis, each unaffected parent was assigned to a subgroup on the basis of prior family history (positive or negative). A geometric morphometric approach was utilized to scale and superimpose the landmark coordinate data (Procrustes analysis), test for omnibus group differences in face shape, and uncover specific modes of shape variation capable of discriminating unaffected parents from controls.
Results
Significant disparity in face shape was observed between unaffected parents and controls (p < 0.01). Notably, these changes were specific to parents with a positive family history of CL/P. Shape changes associated with CL/P predisposition included marked flattening of the facial profile (midface retrusion), reduced upper facial height, increased lower facial height and excess interorbital width. Additionally, a sex-specific pattern of parent-control difference was evident in the transverse dimensions of the nasolabial complex.
Conclusions
The faces of unaffected parents from multiplex cleft families display meaningful shape differences compared with the general population. Quantitative assessment of the facial phenotype in cleft families may enhance efforts to discover the root causes of CL/P.
Keywords: 3D stereophotogrammetry, face shape, geometric morphometrics, nonsyndromic clefting, unaffected parents
Introduction
Nonsyndromic cleft lip with or without cleft palate (CL/P) is the most common craniofacial birth defect, affecting between 1/500–2500 individuals per year depending on population/ethnicity (1, 2). The high incidence of CL/P coupled with the extensive care required for effective treatment underscores the importance of identifying the factors that underlie its etiology. The development of an intact primary palate requires the coordinated growth of the oronasal prominences in a precise temporal-spatial sequence (3, 4). Although still not fully understood, this coordinated growth depends on a tightly orchestrated cascade of molecular signals emanating from the mesenchymal and epithelial cells that comprise the embryonic facial prominences (5–7). Not surprisingly, many of the genes implicated in the formation of the face are also candidate genes for clefting. Altering the function of one or more of these genes may disrupt the normal pattern of facial morphogenesis, e.g., by shifting the rate and/or trajectory of facial prominence growth. Clefting is precipitated when these shifts decrease the likelihood that adjacent oronasal components will meet and subsequently fuse (8). However, even slight shifts in these morphogenetic processes can push an individual toward the threshold for clefting. In such cases, the facial phenotype may be altered in subtle ways, but without any readily visible manifestation of clefting. The phenotypic diversity associated with orofacial clefting is well-described in the clinical literature (9, 10), with cases ranging from complete bilateral clefts of the lip and palate to visible microforms (e.g., bifid uvula). In recent years, it has become increasingly clear that this phenotypic diversity also includes numerous subclinical manifestations, including aspects of craniofacial form (11).
Studies in humans and mice suggest that variation in embryonic face shape contributes to the development of orofacial clefts by mediating the spatial relationship among the rapidly growing tissues that constitute the nascent face. Since the 1960s, numerous studies have documented morphological differences in the embryonic face of mouse strains susceptible to spontaneous clefting (e.g., A/WySn, CL/Fr) compared to non-susceptible strains (12–15). Two findings stand out from among these early comparative studies; cleft-susceptible mice displayed altered orientation of the medial nasal prominences and/or relative underdevelopment of the maxillary prominences. In principle, these morphogenetic shifts lead to an increase in cleft liability by disrupting the normal relationship among facial primordia. More recent studies, drawing on advances in statistical shape analysis, have subsequently confirmed and expanded these findings (16, 17). A handful of studies have also identified craniofacial differences in adults from these same susceptible mouse strains (18, 19), suggesting that altered facial shape persists as a phenotypic marker throughout life.
Investigation of the relationship between face shape and cleft predisposition in humans has focused chiefly on documenting the facial phenotype of unaffected relatives from cleft families as compared to unrelated controls (20–22). The reasoning behind this approach is straightforward: CL/P is a heritable condition and since family members share a large number of genes, relatives of affected individuals are also expected to carry a higher proportion of putative cleft loci than non-relatives with a negative family history. Consequently, systematic differences in facial morphology between “at-risk” relatives and “low-risk” controls can be interpreted as reflecting differences in underlying genetic susceptibility. Evidence for these systematic differences has been steadily accumulating for over four decades. Studies comparing unaffected relatives (parents and sibs) to controls have documented quantitative differences spanning all regions of the craniofacial complex (23–39). Unfortunately, despite positive findings in every study to date, defining the precise nature of these differences has been problematic. Inconsistent and even contradictory findings across studies are ubiquitous, likely reflecting inter-study variation in data acquisition, sample demographics and other methodological factors. Nevertheless, a recent meta-analysis of the cephalometric literature was able to identify a handful of systematic craniofacial differences in the unaffected parents of children with CL/P compared with controls (40); these differences included an increase in nasal cavity width, interorbital width, cranial base length, mandibular protrusion, upper face width and lower face height, along with a reduction in maximum cranial width and upper face height. The primary conclusions of this study were that parent-control differences were generally subtle and in many cases sex-specific and that a great deal of among-study heterogeneity was present.
With current advances in noninvasive 3D surface imaging it is now possible to move beyond the limitations of traditional data capture methods like cephalometry and direct anthropometry. Moreover, the ability to marry the geometric fidelity of 3D imaging data with the capabilities of modern statistical shape analysis offers a potentially powerful strategy for uncovering subtle, yet relevant, aspects of craniofacial dysmorphology (41–43). In a recent study utilizing 3D surface imaging along with a variety of linear distance-based methods, Weinberg et al. (44) demonstrated a number of differences in craniofacial shape between unaffected relatives from multiplex CL/P families and matched healthy controls. Unaffected male relatives displayed a combination of excess upper face and cranial base width, increased lower face height, and reduced upper facial height. Female unaffected relatives also displayed an increase in upper face width, but in contrast to males, did not show major changes in the vertical aspects of the face and exhibited excess nose width and midface retrusion.
In the present study, a geometric morphometric approach is utilized to assess face shape in unaffected parents with one or more cleft-affected children. Geometric morphometrics describes a suite of statistical tools specifically designed for the analysis of biological shape based on landmark coordinates (45–47). Because of its ability to work directly with 3D landmark data and provide intuitive visualization of shape variation, geometric morphometrics is rapidly replacing more traditional morphometric methods. Based on previous findings, it is predicted that unaffected parents will not only demonstrate statistically significant face shape differences compared to controls, but that these differences will be sex-specific and more pronounced in those with a prior family history of the defect.
Materials and Methods
Sample
After obtaining local ethics committee approval, unaffected parents were identified through index cases (affected probands) served by the Cleft Craniofacial Center at Children’s Hospital of Pittsburgh or the Cleft Palate and Craniofacial Institute at St. Louis Children’s Hospital. For the present study, only Caucasian parents from multiplex CL/P families (i.e., two or more affected individuals) were included. These parents had no visible manifestation of CL/P, including microforms of the lip or soft palate. Parents from families with suspected syndromic cases (screened by a board-certified medical geneticist) or with a history of isolated cleft palate were excluded in an effort to reduce etiological heterogeneity.
The parents in our sample were assigned to one of two groups based on prior family history of CL/P. The first group was limited to unaffected parents with a positive family history of CL/P. In addition to having one or more children with a cleft, eligible parents in this group were required to have at least one other affected biological relative on their side of the family. The second group consisted of parents with a negative family history. The parents in this group have one or more affected children, but no additional affected biological relatives.
A total of 80 unaffected parents were included in this study. For the positive family history parent group, 36 unaffected parents (26 mothers and 10 fathers) met the inclusion criteria. The remaining 44 unaffected parents (21 mothers and 23 fathers) were assigned to the negative family history group. In 26 instances, the unaffected father and mother were the biological parents of the same proband. In the remaining families, only one of the two parents was included. For each parent in each group, an unrelated healthy control was matched on the basis of sex, age (within one year) and ancestry. Each parental group was therefore matched to a separate control group. Controls at each site were ascertained from the same geographic region as the case families, and inclusion was limited to individuals with no personal or family history of a craniofacial birth defect and no personal history of facial plastic or reconstructive surgery.
Data acquisition
Following informed consent, three-dimensional facial surfaces were captured using either a Genex FaceCam 250 (Kensington, MD) or 3dMDface (Atlanta, GA) imaging system. These commercially available imaging systems utilize non-contact digital stereophotogrammetry to capture high-resolution facial surface geometry along with photo-realistic color and texture rendering. These systems are able to acquire very fast captures (< 1 second) and have each been independently validated in terms of measurement precision and accuracy (48–51). Measurements derived from the Genex and 3dMD systems have also been compared directly and found be highly congruent (49).
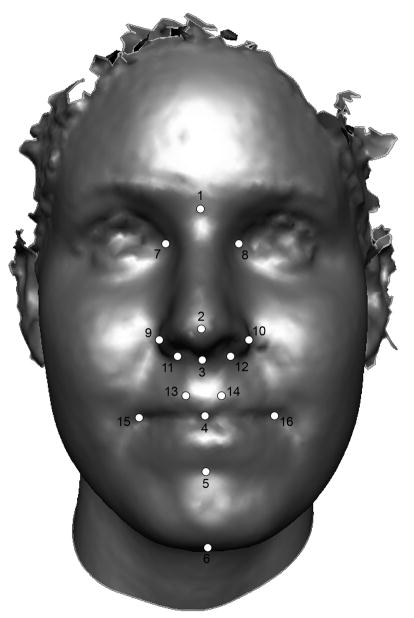
Sixteen standard facial landmarks (52, 53) were collected from each subject’s 3D facial scan (Figure 1) using either the Genex 3D Surgeon or 3dMDpatient software package. These landmarks were chosen for their high reliability and ability to provide adequate facial coverage. Exocanthion (the outer corner of the eye) and tragion (upper margin of the tragus on the ear) were not included in the present study because these landmarks could not be adequately visualized on a large number of subjects. Once the landmarks were digitized on the 3D facial scans, their corresponding x,y,z coordinates were saved for later analysis.
Fig. 1.
Facial landmarks used in the present study: 1 (nasion); 2 (prenasale); 3 (subnasale); 4 (stomion); 5 (sublabiale); 6 (gnathion); 7, 8 (endocanthion); 9, 10 (alare); 11, 12 (subalare); 13, 14 (christa philtri); 15, 16 (chelion).
Statistical approach
Three-dimensional landmark coordinates for each subject were aligned via Procrustes superimposition, which fits the data into a common coordinate system through an iterative least-squares routine to optimally center, scale, and rotate the landmark configurations (54, 55). Procrustes analysis results in a new set of 3D coordinates (Procrustes coordinates) that describe shape. The Procrustes coordinates were then subjected to an omnibus test of group differences in shape (Goodall’s F-test). In the present study, the primary purpose of the F-test is to assess the null hypothesis that mean face shape is equivalent in unaffected parents and controls. Both standard and permutation versions of the F-test for shape difference were carried out using the IMP program Simple3D (56).
To determine the nature of the shape variation both within and across groups, principal components analysis (PCA) and canonical variates analysis (CVA) were applied to the Procrustes coordinate data (46). In shape analysis, PCA reduces the dimensionality of the Procrustes coordinate data into a more manageable number of uncorrelated summary variables or “components”, each capturing a distinct aspect of shape variation. By plotting the component scores for each subject along a set of orthogonal axes, PCA allows for the identification of those modes of shape variation capable of separating pre-existing groups within a dataset (e.g., parents and controls). CVA is also a multivariate data reduction method, but in contrast to PCA, groups are specified a priori and the variance parameters are optimized to maximally discriminate between groups. Each canonical variate (CV) is a linear combination of variables (i.e., shape coordinates), weighted to reflect a distinct mode of shape variation. The ultimate goal in CVA is to discover the aspects of shape variation that best distinguish among existing groups in a dataset.
Because the geometric morphometric approach preserves the intrinsic geometry present in landmark coordinate data, shape variation along a given PC or canonical discriminant axis can be visualized as a displacement of points in 3D space (57). In the context of the current study, modes of shape variation that discriminate unaffected parents from controls can be displayed graphically as shifts in the relative position of facial landmarks, providing an intuitive approach to visualize group differences in shape. PCA of shape coordinates was carried out using the program Morphologika v2.5 (58), while CVA was performed in MorphoJ v1.0 (59). All other statistical tests were performed in SPSS v15 (Chicago, IL).
Results
Parents with positive family history
For parents with a positive family history of CL/P, the omnibus shape test revealed a significant difference in mean face shape compared to demographically matched controls (Procrustes distance = 0.023; Goodall’s F = 1.576; p = 0.01). A permutation version of the above F-test (400 resamples) yielded similar results (p < 0.01). Despite the apparent difference in mean shape, the level of within-group variance in shape was found to be equivalent between unaffected parents and controls (p > 0.05).
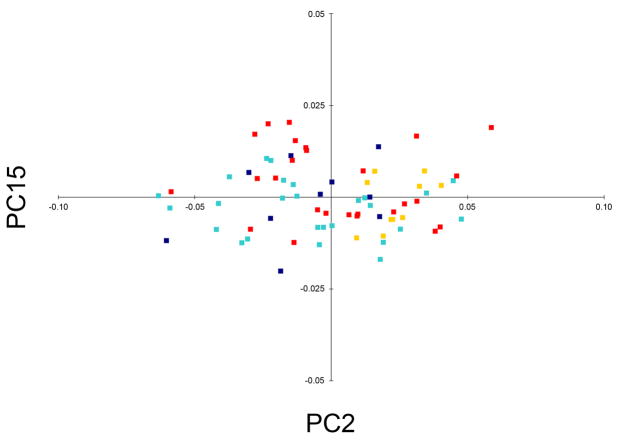
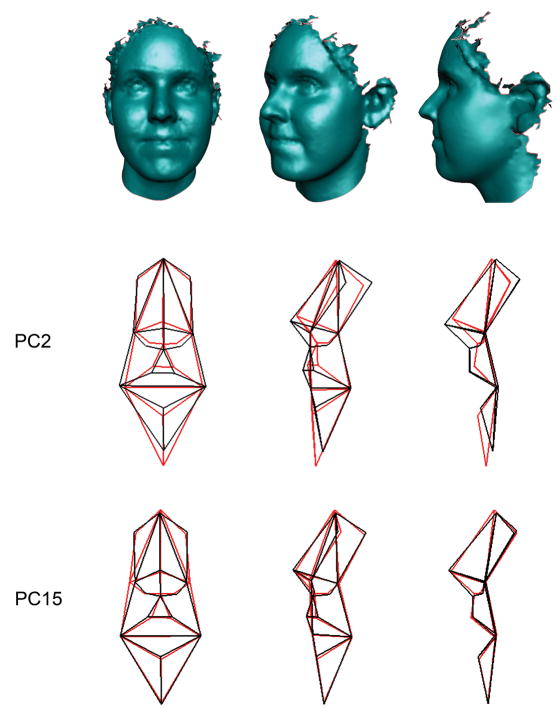
PCA of Procrustes coordinates showed evidence of group discrimination along the second and fifteenth principle components (PC2 and PC15), which together accounted for 14.5% of the total shape variance. Separation of unaffected parents from controls along PC2 was limited to males (Figure 2). This finding was confirmed by results showing that mean PC scores on the second component differed significantly between unaffected fathers and male controls (p = 0.001). The shape changes associated with PC2 (shifting from male controls to unaffected fathers along the second principal axis of variation) primarily involved an inferior and slightly anterior shift in the position of the mandible, and a concomitant superior, posterior and medial shift of coordinates comprising the nasolabial complex (Figure 3). There was also a slight inferior and lateral shift of the endocanthion points. Thus, compared with male controls, unaffected fathers with a positive family history appear to possess a vertically shorter and more retrusive midface coupled with vertical elongation of the lower face and a reduction in width of the mouth and lower nose. In contrast to PC2, PC15 showed evidence of significant discrimination between unaffected mothers and female controls (p = 0.006; Figure 2). The shape changes associated with PC15 (shifting from female controls to unaffected mothers) were subtle and primarily involved the lateral displacement of the alare points, resulting in a broadening of the nasal base (Figure 3). Unaffected mothers also displayed some evidence of reduced upper face height and decreased philtrum width. No other PC showed evidence of group discrimination in either sex.
Fig. 2.
Plot of PC scores for unaffected parents with a positive history of CL/P and matched controls on the second and fifteenth shape components. In this figure, the two axes represent different principal components, each of which describe a distinct mode of face shape variation. Every subject in the sample receives a score on each component, represented by a single discrete point on the PC plot. By color-coding the points according to group status, components of shape variation related to group discrimination are revealed visually by the clustering of points along one or more axes. Male controls (dark yellow); unaffected fathers (violet); female controls (red); unaffected mothers (light blue).
Fig. 3.
Face shape variation associated with PC2 and PC15. The top row of faces shows the orientation of the wireframe models below. The wireframe models represent the extreme ends of the shape variation associated with each component axis. The black wireframe represents the hypothetical control extreme, whereas the superimposed red wireframe represents the hypothetical unaffected parent extreme.
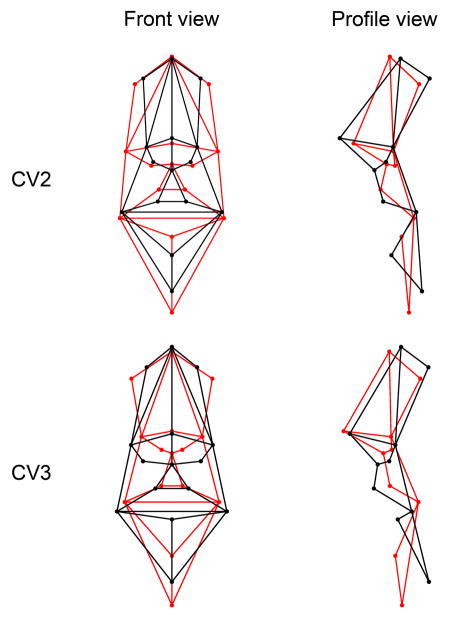
CVA of shape coordinates resulted in the extraction of three CVs, all associated with meaningful, yet distinct, modes of group discrimination. CV1 was associated with general sexual dimorphism. The second and third CVs were both related to parent-control discrimination; CV2 separated unaffected mothers from female controls (Mahalanobis distance = 2.151, p < 0.001), whereas CV3 was associated with the separation of unaffected fathers and male controls (Mahalanobis distance = 3.059, p = 0.002). Figure 4 shows a plot of subjects’ scores along the second and third canonical discrimination axes. The shape changes associated with CV2 (moving from female controls to unaffected mothers) included the inferior, anterior and lateral displacement of the endocanthion points, the lateral excursion of points relating to the alar cartilage, the superior and posterior movement of the nasolabial complex, the anterior displacement of nasion, and the inferior and anterior projection of the mandible (Figure 5). Thus, the face of unaffected mothers was characterized by an increased interorbital and nasal width, a reduction in upper face height, a vertical lengthening of the lower face, and a loss of facial convexity due to a combination of midface retrusion, mandibular protrusion and forward projection of the superior nasal bridge. The shape changes associated with CV3 were similar in many respects to those observed in CV2; compared to male controls, unaffected fathers demonstrated a marked flattening of the facial profile, increased hypertelorism, a reduction in the height of the upper face and a lengthening of the lower face (Figure 5). However, in contrast to unaffected mothers, unaffected fathers displayed a medial displacement of landmarks comprising the nasolabial complex, resulting in a prominent narrowing of the nose, philtrum and oral fissure.
Fig. 4.
Plot of scores on the second and third canonical discrimination axes. Four groups are represented: unaffected mothers with a positive family history of CL/P (light blue), female controls (red), unaffected fathers with a positive family history of CL/P (violet), and males controls (dark yellow).
Fig. 5.
Face shape variation associated with CV2 and CV3. The wireframe models represent the extreme ends of the shape variation associated with each canonical axis. The black wireframe represents the hypothetical control extreme, whereas the superimposed red wireframe represents the hypothetical unaffected parent extreme. The magnitude of the shape change is exaggerated for the purposes of visualization.
Parents with negative family history
For parents with a negative family history of CL/P, the omnibus shape test revealed no difference in mean face shape compared to matched controls (Procrustes distance = 0.016; Goodall’s F = 0.859; p = 0.72). Accordingly, both PCA and CVA of shape coordinates showed no statistical evidence of group separation for either sex. In addition, there was no overall shape difference between parents with a positive family history and parents with a negative family history (Procrustes distance = 0.018; Goodall’s F = 1.114; p = 0.29).
Discussion
For over four decades, studies have sought to establish a link between facial morphology and orofacial cleft predisposition. The primary strategy for investigating this relationship has been to describe the facial features of the unaffected biological relatives of affected individuals. Systematic facial differences between these “at-risk” individuals and controls drawn from the general population are hypothesized to indicate a subclinical manifestation of CL/P. Such differences are likely to be subtle in nature, requiring a rigorous quantitative approach capable of dealing with the complex 3D geometry of the human face. The present study represents the first attempt to combine 3D surface imaging with geometric morphometrics to investigate face shape in unaffected parents from CL/P families. This is also the first study of its kind to divide parents explicitly on the basis of their family history. As predicted, significant face shape differences were present in unaffected parents compared with controls. Moreover, these differences were statistically significant only in parents with a positive family history of CL/P and were manifested, to a limited extent, in a sex-specific manner.
The facial characteristics associated with CL/P predisposition (regardless of sex) included retrusion of nasolabial structures coupled with mandibular protrusion and forward projection of the orbital-nasal bridge. This combination of morphological changes resulted in a pronounced flattening, or loss of convexity, of the entire facial profile. Furthermore, in the vertical dimension, the proportional relationship between the upper and lower parts of the face was altered in unaffected parents; middle and upper portions of the face were reduced in height, while the lower face was simultaneously elongated. In addition, unaffected fathers, and mothers to a lesser extent, showed evidence of increased interorbital distance. There were also some sex-specific aspects of shape variation associated with discriminating unaffected parents from controls. These primarily involved oronasal structures. As evidenced in PC2 and CV3, unaffected fathers displayed a reduction in the width of the nose, philtrum and mouth, giving the midface a more pinched appearance. In contrast, unaffected mothers demonstrated minimal changes in the width of the philtrum and mouth as well as a dramatic broadening of the nasal ala.
Many of the facial differences observed in the present sample of unaffected relatives have been previously reported in the literature. Prior studies have documented prominent flattening of the facial profile (23–27), decreased upper facial height (25, 27, 29, 31, 35, 37, 38), increased lower facial height (28–30, 60) and increased interorbital width (26, 27, 29, 34, 39, 60, 61) in unaffected parents. These findings are further supported by a recent meta-analysis of the parent-control cephalometric literature (40). Although contrary evidence exists for each of these findings, such agreement is noteworthy given the sizable disparity in research methodology between the present study and earlier reports. For instance, the vast majority of previous studies utilize 2D cephalometry and/or direct anthropometry, make little or no attempt to minimize sources of heterogeneity in their study sample, and employ statistical methods inadequate for describing shape. In a corresponding study, Weinberg et al. (44) applied Euclidean distance matrix analysis (EDMA), an alternative method of statistical shape analysis, to 3D facial landmark data from a partially overlapping sample of unaffected relatives (sibs and parents) from multiplex CL/P families and demographically-matched controls. In agreement with the present study, they found that unaffected relatives displayed excess lower facial height, reduced upper facial height and greater soft tissue nasal breadth (in females). Taken together, these results suggest that a more definitive picture of the facial phenotype associated with CL/P predisposition is emerging.
Many of the facial characteristics observed in unaffected parents are plausible, from a developmental perspective, as risk markers for CL/P. Studies describing the embryonic face of cleft-susceptible mouse strains consistently report changes in the size, shape and orientation of the facial prominences (12–17). The loss of facial convexity in unaffected parents, due in part to excess midface retrusion, may relate back to a localized reduction in early nasomaxillary prominence growth. Relative underdevelopment of the maxillary prominences has been reported in susceptible mice (15–17), and there is evidence that midface reductions may persist into adulthood (18). A similar phenomenon of deficient maxillary growth may explain the pattern of reduced middle and upper facial height in “at-risk” parents. Facial prominence growth is mediated by a number of genes including Msx1, Bmp4, Shh and Fgf8 (6, 7, 62), all considered important candidates for CL/P.
Unaffected parents were also characterized by a conspicuous increase in interorbital distance. This trait has been observed in individuals affected with CL/P (63, 64) and animal models with experimentally-induced cleft lip (65). In the embryo, variation in spacing between the orbits is most intimately related to frontonasal prominence growth, which is mediated in part by local Shh expression (65, 66). During later phases of development, hypertelorism may be linked to a broader set of changes relating to the horizontal proportions of the upper face and cranial base. Excessive upper facial breadth is one of the most consistently reported features in unaffected relatives (24, 29, 34, 36, 39, 61) and was recently reported in adult cleft-susceptible mice (19). Mechanistically, both excessive facial width and relative underdevelopment of the maxillary prominences will alter normal spatial relationships during the critical period of primary palate formation, ultimately decreasing the probability of successful contact and fusion.
Some of the shape differences between unaffected parents and controls were manifested in a sex-specific manner. CVA revealed two completely sex-specific modes of facial shape variation (CV2 and CV3) separating unaffected parents and controls. Specifically, excessive nasal breadth was observed in female parents, whereas male parents displayed significant narrowing of the entire nasolabial complex. The finding of increased soft-tissue nose width in females, but not males, has been noted previously (44). In general, studies using traditional morphometric methods to compare the faces of unaffected mothers and fathers independently to sex-matched controls report sex-specific differences (26–28, 32, 33, 37, 39, 40, 44). However, McIntyre and Mossey (36) failed to find any sex-related shape changes in their cephalometric analysis of unaffected parents and controls. In terms of magnitude, there was evidence in the present study to suggest that the parent-control facial differences were more pronounced in males. While there is some agreement with this in the literature (27, 28), many other studies report either no systematic change in the magnitude of parent-control facial differences across the sexes (33, 34, 36, 39), or the opposite pattern, that these differences are in fact more pronounced in females (32). Thus, the sex-specific nature of the face shape changes associated with CL/P predisposition remains unclear. It is perhaps noteworthy that other subclinical cleft traits (e.g., subepithelial lip defects) have also been shown to be more frequent in male relatives (67), particularly given the roughly 2:1 male bias observed in CL/P.
The discovery of reliable phenotypic markers associated with elevated CL/P risk may offer a number of benefits. These markers may facilitate the detection of clinically unaffected but genetically informative individuals; these are individuals who may be carrying putative susceptibility alleles, but due to reduced penetrance, do not display any visible manifestation of an overt cleft. At a practical level, the identification of “at-risk” individuals within CL/P families can improve the accuracy of recurrence risk estimation, ultimately leading to improvements in genetic counseling. For researchers seeking to uncover the genetic and environmental factors that lead to CL/P, the sub-phenotyping approach will likely enhance the power of epidemiological and statistical mapping methods (68). Consequently, efforts are currently underway to incorporate sub-phenotype data into formal genetic analyses of CL/P.
Significant face shape changes in the present study were detected only in unaffected parents with a positive family history of the defect (i.e., they had at least one addition biological relative with CL/P). Nevertheless, our results may also have important implications for parents without a prior family history, particularly those in simplex families. It is likely that clefting has a genetic basis in some portion of these families. Since simplex families make up the vast majority of nonsyndromic cleft cases, identifying additional susceptible family members in this population is a priority. The critical issue is whether we can identify simplex families that “look more genetic,” since doing so would substantially increase the number of families eligible for inclusion in genetic analyses. The sub-phenotyping approach utilized here may facilitate the identification of simplex families with a higher risk of recurrence. However, because any collection of simplex families is likely to include a heterogeneous mix of clefting forms, approaches like hierarchical cluster analysis will be required to sort out families that naturally fall into different etiological categories (30). Moreover, the facial changes are likely to be more subtle in unaffected family members from simplex families, requiring more sensitive methods for quantifying face shape based on whole surfaces (41) and much larger samples.
Conclusions
The results of this study indicate that significant face shape differences were present in the unaffected parents of individuals with CL/P compared to matched controls. The major predisposing features included increased flattening of the facial profile resulting from a combination of excess midface retrusion and mandibular protrusion, decreased middle and upper facial height, increased lower facial height, increased interorbital width, and altered breadth of nasolabial structures. These shape differences were limited to parents with a prior family history of the defect, were manifested in a partly sex-specific manner, and were biologically plausible as risk factors for clefting. Our results suggest that certain facial features should be considered part of the phenotypic spectrum of clefting and highlight the potential importance of subclinical phenotypic assessment for both recurrence estimation and the study of CL/P etiology. Based on the present findings, a strategy involving detailed evaluation of the craniofacial phenotype coupled with large samples and sophisticated multivariate analysis methods is recommended for future family studies of CL/P.
Acknowledgments
The authors would like to thank all the families that participated in this research. We are indebted to Lance Kennelty, James Gallagher and Dan Govier for their valuable technical support and assistance and to Dr. Alex Kane for his assistance in facilitating recruitment of families at the St. Louis site. The study was funded by the following grants from the National Institute for Dental and Craniofacial Research: R01-DE016148 and P50-DE016215. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health.
References
- 1.Schutte BC, Murray JC. The many faces and factors of orofacial clefts. Hum Mol Genet. 1999;8:1853–9. doi: 10.1093/hmg/8.10.1853. [DOI] [PubMed] [Google Scholar]
- 2.Marazita ML, Mooney MP. Current concepts in the embryology and genetics of cleft lip and cleft palate. Clin Plast Surg. 2004;31:125–40. doi: 10.1016/S0094-1298(03)00138-X. [DOI] [PubMed] [Google Scholar]
- 3.Diewert VM, Lozanoff S. Growth and morphogenesis of the human embryonic midface during primary palate formation analyzed in frontal sections. J Craniofac Genet Dev Biol. 1993;13:162–83. [PubMed] [Google Scholar]
- 4.Johnston MC, Bronsky PT. Craniofacial embryogenesis: abnormal developmental mechanisms. In: Mooney MP, Siegel MI, editors. Understanding Craniofacial Anomalies: The Etiopathogenesis of Craniosynostoses and Facial Clefting. New York: Wiley-Liss, Inc; 2002. pp. 61–124. [Google Scholar]
- 5.Chong SS, Cheah FSH, Jabs EW. Genes implicated in lip and palate development. In: Wyszynski DF, editor. Cleft Lip and Palate: From Origin to Treatment. Oxford: Oxford University Press; 2002. pp. 25–39. [Google Scholar]
- 6.Francis-West PH, Robson L, Evans DJ. Craniofacial development: the tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol. 2003;169:1–138. doi: 10.1007/978-3-642-55570-1. [DOI] [PubMed] [Google Scholar]
- 7.Jiang R, Bush JO, Lidral AC. Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn. 2006;235:1152–66. doi: 10.1002/dvdy.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trasler DG, Fraser FC. Time-position relationships with particular reference to cleft lip and cleft palate. In: Wilson JG, Fraser FC, editors. Handbook of Teratology, Vol 2: Mechanisms and Pathogenesis. New York: Plenum Press; 1977. pp. 271–92. [Google Scholar]
- 9.Saal HM. Classification and description of nonsyndromic clefts. In: Wyszynski DF, editor. Cleft Lip and Palate: From Origin to Treatment. Oxford: Oxford University Press; 2002. pp. 47–52. [Google Scholar]
- 10.Eppley BL, van Aalst JA, Robrey A, Havlik RJ, Sadove AM. The spectrum of orofacial clefting. Plast Reconstr Surg. 2005;115:101e–14e. doi: 10.1097/01.prs.0000164494.45986.91. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg SM, Neiswanger K, Martin RA, Mooney MP, Kane AA, Wenger SL, et al. The Pittsburgh Oral-Facial Cleft Study: expanding the cleft phenotype. Background and justification. Cleft Palate Craniofac J. 2006;43:7–20. doi: 10.1597/04-122r1.1. [DOI] [PubMed] [Google Scholar]
- 12.Trasler DG. Pathogenesis of cleft lip and its relation to embryonic face shape in A/J and C57BL mice. Teratology. 1968;1:33–50. doi: 10.1002/tera.1420010106. [DOI] [PubMed] [Google Scholar]
- 13.Juriloff DM, Trasler DG. Test of the hypothesis that embryonic face shape is a causal factor in genetic predisposition to cleft lip in mice. Teratology. 1976;14:35–41. doi: 10.1002/tera.1420140106. [DOI] [PubMed] [Google Scholar]
- 14.Millicovsky G, Ambrose LJH, Johnston MC. Developmental alterations associated with spontaneous cleft lip and palate in CL/Fr mice. Am J Anat. 1982;164:29–44. doi: 10.1002/aja.1001640104. [DOI] [PubMed] [Google Scholar]
- 15.Wang KY, Diewert VM. A morphometric analysis of craniofacial growth in cleft lip and noncleft mice. J Craniofac Genet Dev Biol. 1992;12:141–54. [PubMed] [Google Scholar]
- 16.Young NM, Wat S, Diewert VM, Browder LW, Hallgrimsson B. Comparative morphometrics of embryonic facial morphogenesis: implications for cleft-lip etiology. Anat Rec. 2007;290:123–39. doi: 10.1002/ar.20415. [DOI] [PubMed] [Google Scholar]
- 17.Parsons TE, Kristensen E, Hornung L, Diewert VM, Boyd SK, German RZ, et al. Phenotypic variability and craniofacial dysmorphology: increased shape variance in a mouse model for cleft lip. J Anat. 2008;212:135–43. doi: 10.1111/j.1469-7580.2007.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trasler DG, Machado M. Newborn and adult face shapes related to mouse cleft lip predisposition. Teratology. 1979;19:197–206. doi: 10.1002/tera.1420190210. [DOI] [PubMed] [Google Scholar]
- 19.Hallgrimsson B, Dorval CJ, Zelditch ML, German RZ. Craniofacial variability and morphological integration in mice susceptible to cleft lip and palate. J Anat. 2004;205:501–17. doi: 10.1111/j.0021-8782.2004.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntyre GT, Mossey PA. The craniofacial morphology of the parents of children with orofacial clefting: a systematic review of cephalometric studies. J Orthod. 2002;29:23–9. doi: 10.1093/ortho/29.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Ward RE, Moore ES, Hartsfield JK. Morphometric characteristics of subjects with oral facial clefts and their relatives. In: Wyszynski DF, editor. Cleft Lip and Palate: From Origin to Treatment. Oxford: Oxford University Press; 2002. pp. 66–86. [Google Scholar]
- 22.Maulina I, Urtane I, Jakobsone G. The craniofacial morphology of the parents of children with cleft lip and/or palate: a review of cephalometric studies. Stomatologija. 2006;8:16–20. [PubMed] [Google Scholar]
- 23.Dixon DA. Abnormalities of the teeth and supporting structures in children with clefts of lip and palate. In: Drillien CM, Ingram TTS, Wilkinson EM, editors. The Causes and Natural History of Cleft Lip and Palate. Edinburgh: E & S Livingstone; 1966. pp. 178–205. [Google Scholar]
- 24.Fraser FC, Pashayan H. Relation of face shape to susceptibility to congenital cleft lip. J Med Genet. 1970;7:112–7. doi: 10.1136/jmg.7.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coccaro PJ, D’Amico R, Chavoor A. Craniofacial morphology of parents with and without cleft lip and palate children. Cleft Palate J. 1972;9:28–38. [PubMed] [Google Scholar]
- 26.Figalova P, mahel Z. Cephalometric study of families with clefts. Acta Chir Plast. 1974;16:247–55. [PubMed] [Google Scholar]
- 27.Kurisu K, Niswander JD, Johnston MC, Mazaheri M. Facial morphology as an indicator of genetic predisposition to cleft lip and palate. Am J Hum Genet. 1974;26:702–14. [PMC free article] [PubMed] [Google Scholar]
- 28.Shibasaki Y, Ohtsuka S, Hattori M, Nukatsuka S. A cephalometric study of craniofacial morphology of parents of children with cleft lip and palate [in Japanese] J Jpn Cleft Palate Assoc. 1978;3:31–43. [Google Scholar]
- 29.Nakasima A, Ichinose M. Characteristics of craniofacial structures of parents of children with cleft lip and/or palate. Am J Orthod. 1983;84:140–6. doi: 10.1016/0002-9416(83)90178-1. [DOI] [PubMed] [Google Scholar]
- 30.Ward RE, Bixler D, Raywood ER. A study of cephalometric features in cleft lip-cleft palate families I: phenotypic heterogeneity and genetic predisposition in parents of sporadic cases. Cleft Palate J. 1989;26:318–25. [PubMed] [Google Scholar]
- 31.Raghavan R, Sidhu SS, Kharbanda OP. Craniofacial pattern of parents of children having cleft lip and/or palate anomaly. Angle Orthodont. 1994;64:137–44. doi: 10.1043/0003-3219(1994)064<0137:CPOPOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Mossey PA, McColl J, O’Hara M. Cephalometric features in the parents of children with orofacial clefting. Br J Oral Maxillofac Surg. 1998;36:202–12. doi: 10.1016/s0266-4356(98)90498-3. [DOI] [PubMed] [Google Scholar]
- 33.AlEmran SES, Fatani E, Hassanain JE. Craniofacial variability in parents of children with cleft lip and cleft palate. J Clin Pediatr Dent. 1999;23:337–41. [PubMed] [Google Scholar]
- 34.Suzuki A, Takenoshita Y, Honda Y, Matsuura C. Dentocraniofacial morphology in parents of children with cleft lip and/or palate. Cleft Palate Craniofac J. 1999;36:131–8. doi: 10.1597/1545-1569_1999_036_0131_dmipoc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre GT, Mossey PA. Posteroanterior cephalometric analysis of the parental craniofacial morphology in orofacial clefting. Cleft Palate Craniofac J. 2003;40:416–25. doi: 10.1597/1545-1569_2003_040_0416_pcaotp_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 36.McIntyre GT, Mossey PA. Parental craniofacial morphology in orofacial clefting. Eur J Orthod. 2004;26:375–84. doi: 10.1093/ejo/26.4.375. [DOI] [PubMed] [Google Scholar]
- 37.Perkiomaki MR, Yoon KJ, Tallents RH, Barillas I, Herrera-Guido R, Moss ME, et al. Association of distinct craniofacial features in nonsyndromic cleft lip and palate family members. Cleft Palate Craniofac J. 2003;40:397–402. doi: 10.1597/1545-1569_2003_040_0397_aodcfi_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 38.Chatzistavrou E, Ross RB, Tompson BD, Johnston MC. Predisposing factors to formation of cleft lip and palate: inherited craniofacial skeletal morphology. Cleft Palate Craniofac J. 2004;41:613–21. doi: 10.1597/03-090.1. [DOI] [PubMed] [Google Scholar]
- 39.Yoon KJ, Perkiomaki MR, Tallents RH, Barillas I, Herrera-Guido R, Fong CT, et al. Transverse craniofacial features and their genetic predisposition in families with nonsyndromic unilateral cleft lip and palate. Cleft Palate Craniofac J. 2004;41:256–61. doi: 10.1597/02-134.1. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg SM, Maher BS, Marazita ML. Parental craniofacial morphology in cleft lip with or without cleft palate as determined by cephalometry: a meta-analysis. Orthod Craniofacial Res. 2006;9:18–30. doi: 10.1111/j.1601-6343.2006.00339.x. [DOI] [PubMed] [Google Scholar]
- 41.Hammond P, Hutton TJ, Allanson JE, Campbell LE, Hennekam RCM, Holden S, et al. 3D analysis of facial morphology. Am J Med Genet Part A. 2004;126A:339–48. doi: 10.1002/ajmg.a.20665. [DOI] [PubMed] [Google Scholar]
- 42.Hennessy RJ, Baldwin PA, Browne DJ, Kinsella A, Waddington JL. Three-dimensional laser surface imaging and geometric morphometrics resolve frontonasal dysmorphology in schizophrenia. Biol Psychiatry. 2007;61:1187–94. doi: 10.1016/j.biopsych.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 43.Mutsvangwa T, Douglas TS. Morphometric analysis of facial landmark data to characterize the facial phenotype associated with fetal alcohol syndrome. J Anat. 2007;210:209–20. doi: 10.1111/j.1469-7580.2006.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberg SM, Neiswanger K, Richtsmeier JT, Maher BS, Mooney MP, Siegel MI, et al. Three-dimensional morphometric analysis of craniofacial shape in the unaffected relatives of individuals with nonsyndromic orofacial clefts: a possible marker for genetic susceptibility. Am J Med Genet Part A. 2008;146A:409–20. doi: 10.1002/ajmg.a.32177. [DOI] [PubMed] [Google Scholar]
- 45.Dryden IL, Mardia KV. Statistical Shape Analysis. Chichester: Wiley; 1998. [Google Scholar]
- 46.Zelditch ML, Swiderski DL, Sheets HD, Fink WL. Geometric Morphometrics for Biologists: A Primer. Amsterdam: Elsevier Academic Press; 2004. [Google Scholar]
- 47.Slice D. Modern Morphometrics in Physical Anthropology. New York: Kluwer Academic; 2005. [Google Scholar]
- 48.Weinberg SM, Scott NM, Neiswanger K, Brandon CA, Marazita ML. Digital three-dimensional photogrammetry: evaluation of anthropometric precision and accuracy using a Genex 3D camera system. Cleft Palate Craniofac J. 2004;41:507–18. doi: 10.1597/03-066.1. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg SM, Naidoo S, Govier DP, Martin RA, Kane AA, Marazita ML. Anthropometric precision and accuracy of digital three-dimensional photogrammetry: comparing the Genex and 3dMD imaging systems to one another and to direct anthropometry. J Craniofac Surg. 2006;17:477–83. doi: 10.1097/00001665-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Aldridge K, Boyadjiev SA, Capone GT, DeLeon VB, Richtsmeier JT. Precision and error of three-dimensional phenotypic measures acquired from 3dMD photogrammetric images. Am J Med Genet Part A. 2005;138A:247–53. doi: 10.1002/ajmg.a.30959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong JY, Oh AK, Ohta E, Hunt AT, Rogers GF, Mulliken JB, et al. Validity and reliability of craniofacial anthropometric measurement of 3D digital photogrammetric images. Cleft Palate Craniofac J. 2008;45:232–9. doi: 10.1597/06-175. [DOI] [PubMed] [Google Scholar]
- 52.Farkas LG. Anthropometry of the Head and Face. 2. New York: Raven Press; 1994. [Google Scholar]
- 53.Kolar JC, Salter EM. Craniofacial Anthropometry: Practical Measurement of the Head and Face for Clinical, Surgical and Research Use. Springfield: Charles C. Thomas; 1997. [Google Scholar]
- 54.Rohlf F, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- 55.Goodall CR. Procrustes methods in the statistical analysis of shapes. J R Stat Soc B. 1991;53:285–339. [Google Scholar]
- 56.Sheets HD. Simple3D: Integrated Morphometrics Package (IMP) Software Series. Canisius College; Buffalo, NY: 2004. [Google Scholar]
- 57.O’Higgins P, Jones N. Facial growth in Cercocebus torquantus: an application of three-dimensional geometric morphometric techniques to the study of morphological variation. J Anat. 1998;193:251–72. doi: 10.1046/j.1469-7580.1998.19320251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Higgins P, Jones N. Morphologika: Tools for Statistical Shape Analysis. Hull York Medical School; 2006. [Google Scholar]
- 59.Klingenberg CP. Morpho J. University of Manchester; UK: 2008. [Google Scholar]
- 60.Sato T. Craniofacial morphology of parents with cleft lip and palate children [in Japanese] Shikwa Gakuho. 1989;89:1479–506. [PubMed] [Google Scholar]
- 61.Blanco R, Cifuentes L, Maldonado MJ, Rameau X, Munoz MA. Cleft lip and cleft palate: cephalometric characteristics of affected individuals, their relatives and a control population [in Spanish] Rev Med Chil. 1992;120:13–9. [PubMed] [Google Scholar]
- 62.Thomason HA, Dixon MJ, Dixon J. Facial clefting in Tp63 deficient mice results from altered Bmp4, Fgf8 and Shh signaling. Dev Biol. 2008;321:273–82. doi: 10.1016/j.ydbio.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 63.Moss ML. Hypertelorism and cleft palate deformity. Acta Anat (Basel) 1965;61:547–57. doi: 10.1159/000142714. [DOI] [PubMed] [Google Scholar]
- 64.Aduss H, Pruzansky S, Miller M. Interorbital distance in cleft lip and palate. Teratology. 1974;4:171–82. [Google Scholar]
- 65.Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–84. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- 66.Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–67. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- 67.Neiswanger K, Weinberg SM, Rogers CR, Brandon CA, Cooper ME, Bardi KM, et al. Orbicularis oris muscle discontinuities as an expanded phenotypic feature in nonsyndromic cleft lip with or without cleft palate. Am J Med Genet Part A. 2007;143A:1143–9. doi: 10.1002/ajmg.a.31760. [DOI] [PubMed] [Google Scholar]
- 68.Marazita ML. Subclinical features in non-syndromic cleft lip with or without cleft palate (CL/P): review of the evidence that subepithelial orbicularis oris muscle defects are part of an expanded phenotype for CL/P. Orthod Craniofacial Res. 2007;10:82–7. doi: 10.1111/j.1601-6343.2007.00386.x. [DOI] [PubMed] [Google Scholar]