Abstract
Manganese is an essential nutrient, and a healthy human with good liver and kidney function can easily excrete excess dietary manganese. Inhaled manganese is a greater concern, because it bypasses the body’s normal homeostatic mechanisms and can accumulate in the brain. Prolonged exposure to high manganese concentrations (>1 mg/m3) in air leads to a Parkinsonian syndrome known as “manganism.” Of greatest concern are recent studies which indicate that neurological and neurobehavioral deficits can occur when workers are exposed to much lower levels (<0.2 mg/m3) of inhaled manganese in welding fumes. Consequently, researchers at NIOSH are conducting a risk assessment for inhaled manganese. Novel components of this risk assessment include an attempt to quantify the range of inter-individual differences using data generated by the Human Genome Project and experimental work to identify genetically based biomarkers of exposure, disease and susceptibility. The difficulties involved in moving from epidemiological and in vivo data to health-based quantitative risk assessment and ultimately enforceable government standards are discussed.
Keywords: Manganese, Occupational health, Epidemiology, Genetic susceptibility, DNA, Policy
1. Introduction
Manganese (Mn) is an essential nutrient that can be neurotoxic when inhaled (Aschner et al., 2007; Dobson et al., 2004; Erikson and Aschner, 2003), when manganese homeostasis is disrupted by liver damage (Krieger et al., 1995) or when delivered via parenteral nutrition (Fell et al., 1996). Infants consuming high levels of Mn in drinking water or infant formulas are also at risk (Ljung and Vahter, 2007) due to differential pharmacokinetics. Airborne manganese exposure is common in the workplace with an estimated 376,000 workers employed in welding and thousands of others exposed in the mining and refining industries (BLS, 2006). Environmental exposures occur in neighborhoods near manganese industries or more generally through the use of manganese-based anti-knock agents in gasoline (Cooper, 1984; Davis et al., 1998; Kaiser, 2003; Loranger and Zayed, 1997). Inhaled manganese can reach the brain after entering the circulation in the lungs, or to a lesser degree, via olfactory neurons (Leavens et al., 2007).
Occupational exposure to high concentrations of manganese (>1 mg/m3) through mining, metal processing or pesticide exposure has long been associated with an increased risk of neurological disorders and the development of a form of Parkinsonism known as manganism (Feldman, 1999; Kirkey et al., 2001; Montgomery, 1995; Olanow, 2004). More recently, it was demonstrated that chronic exposure to lower levels (<0.5 mg/m3) of manganese leads to more subtle effects on learning, memory and behavior (Aschner et al., 2007; Bowler et al., 2007a,b; Fitsanakis et al., 2006; Klos et al., 2006). Effects include tremors, weakness, reduced hand–eye coordination and psychological impairments. In a preliminary risk assessment, Park et al. (2006) reported that exposure to Mn below 100 μg/m3 for 2 years results in a statistically significant doubling in the prevalence of neurological impairment. Clewell et al. (2003) recommended an occupational exposure limit between 0.1 and 0.3 mg/m3 based on benchmark dose modeling. In contrast, Santamaria et al. (2007) and Jankovic (2005) concluded that there is insufficient human data to link chronic, low-level Mn exposure with neurological effects.
The National Institute for Occupational Safety and Health (NIOSH) is currently reviewing its previous recommendations for worker exposure in light of these new data and its own studies highlighting occupational exposures associated with neurodegenerative disease (Park et al., 2005; Park et al., 2006). Novel components of this updated risk assessment include an attempt to identify genetically based biomarkers of exposure, disease and susceptibility. This is consistent with NIOSH efforts to incorporate genetics and genomics into the field of occupational health (Schulte, 2007) and the goals of the Manganese Health Research Program (Aschner et al., 2006). This report explains the risk assessment process and discusses the steps required to move from epidemiological data to enforceable standards and the many challenges along the way.
2. Regulation vs. recommendation
The mission of the NIOSH is distinct from that of the U.S. Occupational Safety and Health Administration (OSHA), even though both agencies were created by the same 1970 federal legislation. OSHA is part of the U.S. Department of Labor and is responsible for developing and enforcing workplace safety and health regulations. NIOSH is in the U.S. Department of Health and Human Services and focuses on research, information, education, and training www.cdc.gov/NIOSH/about.html. The current recommended and regulatory limits for occupational exposure to inhaled Mn vary greatly across agencies (Table 1). The federal OSHA limit is a ceiling for exposures lasting 15 min or less. All other agencies use an 8-h time-weighted average (8 h TWA) calculating exposures over a standard work day. OSHA and California OSHA occupational exposure limits have the power of law whereas NIOSH and American Conference of Governmental Industrial Hygienists (ACGIH) values are recommendations only. Therefore, the NIOSH risk assessment described here could only result in a change for the Recommended Exposure Limit (REL) and not a new enforceable standard.
Table 1.
Occupational exposure limits for inhaled manganese.
| Agency | Exposure limit | Exposure period | Enforcement status |
|---|---|---|---|
| OSHA | 5 mg/m3 | Ceiling | Regulatory |
| California OSHA | 200 μg/m3 | 8 h TWA | Regulatory |
| NIOSH | 1 mg/m3 | 8 h TWA | Recommended |
| ACGIH | 200 μg/m3 | 8 h TWA | Recommended |
3. Determining Mn exposure in the workplace
Exposure assessments for inhaled Mn include both environmental air sampling and biological sampling. Blood manganese levels (MnB) appear to represent a body burden resulting from exposures in the preceding months while urinary levels (MnU) were highly correlated with more recent exposures with a half-life measured at 30 h (Roels et al., 1987). The association between MnB and cumulative exposure was stronger for the respirable fraction than for total airborne Mn (Lucchini et al., 1997). In a ferroalloy industry study, the relationship between MnB and cumulative exposure became stronger 2 weeks after a temporary exposure cessation occurred due to plant shut-down, at which time the MnU levels more closely reflected MnB and cumulative exposure (Lucchini et al., 1995). Smith et al. (2007) reported difficulties in using MnB and MnU to estimate chronic, low-level exposures in welders. A key confounder is the different pharmacokinetics in blood and other target tissues (Smith et al., 2007; Aschner and Dorman, 2006). Alternatively, a cumulative exposure index can be developed using detailed information about work histories and environmental samples (Park et al., 2006).
4. The risk assessment paradigm in occupational health
Risk assessment is the foundation for recommended occupational exposure limits (OEL) designed to protect the safety and health of workers. Current models rely heavily on uncertainty factors and other extrapolations (e.g. benchmark dose and benchmark response) to provide a reasonable margin of safety when using animal data or when detailed human dose response data is not available. However, these extrapolations present two potential problems. If the risks are underestimated, the OELs may not provide sufficient protection for the most susceptible workers. If the risks are overestimated, the resulting OELS may affect the economic viability of the workers’ employer without providing a commensurate benefit in return.
The risk assessment process is well-defined and incorporates four components in a specific chronological order: (1) hazard identification, (2) dose–response assessment, (3) exposure assessment, and (4) risk characterization (Fig. 1). The classic paradigm has been modified to identify the places where improved information on genetic susceptibility could lead to improved decision-making on appropriate regulatory exposure limits.
Fig. 1.
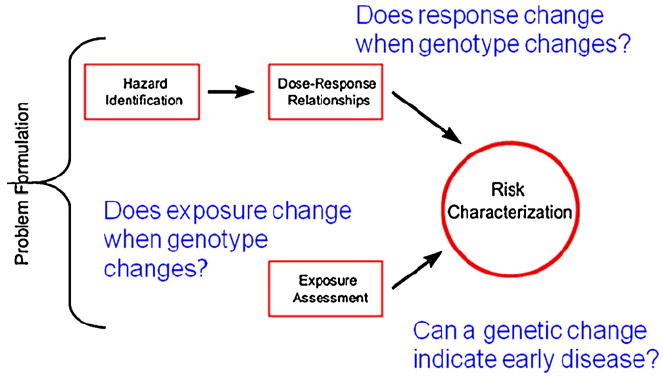
Risk Assessment Paradigm. The standard Risk Assessment Paradigm (National Research Council, 1983) includes four phases moving from Hazard Identification through Risk Characterization. The paradigm was modified to show how genetics and genomics can be incorporated to improve policy development and decision-making.
5. Incorporating genetic susceptibility into a Mn risk assessment
Both human studies and animal studies demonstrate inter-individual differences in susceptibility to Mn overexposure. Roels et al. (1992) found no significant correlations between an individual’s current Mn exposure and blood or urine Mn levels in dust-exposed battery production workers. Iregren (1990) observed performance deficits on a battery of neuropsychological tests in Mn-exposed foundry workers, but the deficits exhibited no correlation with current Mn exposure levels. Wide inter-individual differences were also reported in studies of Mn-exposed non-human primates (Schneider et al., 2006; Newland and Weiss, 1992; Olanow et al., 1996). This suggests that genetic differences may play a role in Mn-induced neurotoxicity. For example, genetic differences in uptake, distribution and excretion of Mn could help explain the wide variation seen in human MnB levels compared with environmental samples.
The development of neurological symptoms following occupational or environmental Mn exposure can occur over many years following an initial exposure; therefore, identifying workers at highest risk working backward to develop early biomarkers of exposure and disease are important NIOSH research goals. Funding was provided for a pilot study to examine the feasibility of incorporating genetics and genomics into a Mn risk assessment. The three major challenges encountered were (1) finding an appropriate Mn-exposed cohort, (2) narrowing the list of candidate genes and (3) determining which genetics and genomics tools would provide the most useful data.
6. Finding a cohort
Occupational epidemiology studies offer opportunities for genetics investigations. As part of its mission, the NIOSH Hazard Evaluations and Technical Assistance Branch (HETAB) conducts evaluations in response to requests from workers or employers with concerns about occupational exposures to known and suspected toxicants www.cdc.gov/NIOSH/hhe/default.html. A typical evaluation includes an exposure assessment and medical evaluations of workers as well as a review of information collected on-site by the employer. Although Health Hazard Evaluations (HHE) can uncover new and important threats to human health, they have limited value for risk assessment. First, the HETAB mission is not primarily a research activity, so collection of DNA samples is not part of the routine protocols. Secondly, there is often a time lag between the first reports of workplace illnesses and the on-site investigation while the appropriate expertise is organized into a field team. This could potentially affect the relevance of the exposure assessment if changes in work practices have been made (e.g. improved ventilation). There can also be a significant time lag between the onset of exposure and neurological effects, with the lag time increasing as dose decreases (Newland, 1999). Finally, there is no guarantee of worker participation. For example, only 66% of employees participated in an HHE looking at Mn exposure in a Marietta, OH battery plant (HETA 90-0214-2523). Other existing potential cohorts included welders on the Bay Bridge reconstruction project (Bowler et al., 2007a,b; Park et al., 2006) and welders in the ship-building industry. Unfortunately, these cohorts were not large enough to provide meaningful data and blood samples taken were not optimized for DNA extraction. Recommendations for enhancing data collection from Mn-exposed cohorts are provided later in this report.
Ultimately, the decision was made to focus on a community cohort in Marietta, Ohio living within 10 miles of a ferromanganese alloy production facility. The plant has been in operation for more than 50 years, and the environmental exposures have been well characterized. Ambient exposures closest to the plant ranged from 0.10 to 2.0 μg/m3 (ATSDR, 2007). Those levels are well below occupational exposure limits, providing important information on chronic, low-level Mn exposure and increasing the opportunity to identify the most susceptible individuals. In addition, data and DNA samples are available from a preliminary study comparing blood and hair Mn levels in residents living near the plant with the results of postural sway tests (Standridge et al., 2008). Cohort variables are summarized in Table 2. Unlike the welding cohorts, the Marietta cohort has fewer potential confounders. For example, welders are exposed to mixtures of metals in welding fumes and other neurotoxicants such as carbon monoxide, making it difficult to analyze the impact of Mn alone while Mn appears to be the most significant exposure of the Marietta cohort. In addition, the Marietta cohort allows stratification by age and sex. Welding cohorts are almost exclusively male.
Table 2.
Overview of data from Marietta cohort.
| Subject data | Age | Sex | |
|---|---|---|---|
| Medical information | Height | Weight | Prescription medication |
| Biological measures | Blood Mn | Blood Pb | Hair Mn |
| Neurological measures | Postural sway | Tremor | |
| Environmental measures | Drinking water source | Years of residence | Proximity of home to refinery |
7. Extreme Discordant Phenotyping
The application of Extreme Discordant Phentoype (EDP) methodology (Nebert, 2000) provides an effective strategy for identifying key gene–gene and gene–environment interactions in complex traits. By focusing on the most highly resistant and most susceptible subsets of the population, the sample size can be reduced significantly without sacrificing sensitivity. EDP methodology has already proved successful in several pharmacology applications (Reist et al., 2007), so it is reasonable to expect similar success applying EDP methods to a toxicogenetics problem. Defining “most resistant” and “most susceptible” will require considerable debate, however. One possible strategy would identify those with similar outcomes in neurological measures, but extreme differences in hair and blood Mn levels. Alternatively, samples could be chosen from individuals with very similar hair and blood Mn levels, but widely varying results on neurological tests.
8. Identifying genes of interest
In contrast to the limited number of blood or DNA samples available from Mn-exposed workers, there is no shortage of potential candidate genes. The challenge is to select a manageable number and prioritize based on their potential to modify susceptibility to inhaled Mn or their potential to serve as early biomarkers of Mn-induced neurotoxicity (Table 3). It is quite likely that numerous genes affect susceptibility to Mn-induced neurotoxicity with the best candidates involved in metal homeostasis, divalent cation transport, the oxidative stress response and inflammation. It is interesting to note that many of these genes have been linked to an increased risk of Parkinson’s Disease (PD), including the familial hemochromatosis gene (HFE) (Akbas et al., 2006; Guerreiro et al., 2006) CYP2D6 (Bon et al., 1999), and glutathione-S-transferases (Wahner et al., 2007).
Table 3.
Candidate genes identified from the literature and a review of genetics and genomics databases.
| Oxidative stress response | Biomarker of susceptibility | Biomarker of disease |
|---|---|---|
| GCLM (glutamate-cysteine ligase modifier subunit) | CYP2D6 (Cytochrome P450) | GABRA2 (GABA-A receptor) |
| GCLC (glutamate-cysteine ligase catalytic subunit) | SLC11A2/DMT1 (Solute carrier family 11/Divalent metal transporter) | GAD1 (Glutamate decarboxylase 1) |
| GSTM1 (glutathione-S-transferase mu) | TF (transferrin) | HOXA1 (Homeobox transcription factor) |
| GSTT1 (glutathione-S-transferase theta) | TFR1, TFR2 (transferrin receptor) | NEUROD1 (Neurogenic differentiation) |
| HO1 (heme oxygenase) | HFE (familial hemochromatosis) | NEUROD2 (Neurogenic differentiation) |
| NQO1 (NAD(P)H dehydrogenase quinone) | SLC39A4 (ZIP4) Solute carrier family 39 | PGR (progesterone receptor) |
| MT (metallothionein) | SLC39A8 (ZIP8) | STK11 (serine/threonine kinase) |
| SLC39A14 (ZIP14) |
9. Oxidative stress response genes
Numerous studies have linked Mn toxicity with oxidative stress; therefore genes associated with the oxidative stress response are logical candidate genes (Table 3; Column 1). Glutathione is the most abundant intracellular antioxidant, found in millimolar concentrations in many cells and tissues. Several genes associated with glutathione synthesis and transport are known to be polymorphic in the human population, and specific polymorphisms have been linked to an increased risk of human disease. These include GCLC, GCLM, GSTM1 and GSTT1 (Dalton et al., 2004; Romieu et al., 2006; Park et al., 2008; Wang et al., 2008). Since many toxicant exposures and deleterious effects are closely associated with depleted glutathione levels, polymorphisms that reduce levels or impair glutathione transport are likely to increase susceptibility to manganese-induced neurotoxicity. Mitochondria are also a well-known target of Mn toxicity (Malecki, 2001; Morello et al., 2008), and they would become more susceptible in the absence of a robust response to oxidative stress. However, there is ongoing debate concerning the relative importance of oxidative stress in Mn-induced neurotoxicity (Taylor et al., 2006); therefore, we wanted to expand our list of candidate genes to include other potential modes of action.
10. Potential biomarkers of susceptibility
Multiple genes are known to affect absorption, transport and deposition of metals in the brain (Table 3; Column 2). Both iron (Fe) and Mn can be found in the body in multiple oxidation states—most frequently Fe2+ ↔ Fe3+ and Mn2+ ↔ Mn3+, and numerous iron transporters can ferry Mn as well as Fe in the body (Aschner, 2000). The interplay between Fe and Mn is most apparent when nutritional status is considered. Iron status determines net absorption as increased uptake of Mn is associated with anemia (Rossander-Hulten et al., 1991). Iron deficiency in animals and humans has also been shown to increase Mn uptake in the brain while Fe overload decreases it (Aschner and Aschner, 1990; Mena, 1974; Anderson et al., 2007; Thompson et al., 2007; Fitsanakis et al., 2008; Heilig et al., 2006). Therefore, workers with iron deficiency may be at greater risk of manganism compared to workers with normal-to-high iron levels. This highlights the importance of measuring Fe status when trying to estimate an individual’s actual Mn exposure and the potential importance of polymorphisms in iron transporters in Mn-induced neurotoxicity.
The mechanisms by which Mn is transported across the blood–brain barrier are not well understood. However, a number of processes may be involved including facilitated diffusion, active transport, and two distinct carrier-mediated transport systems—transferrin-dependent and transferrin-independent pathways. Both carrier-mediated transport systems utilize a divalent metal transporter (DMT1) as the transport protein (Aschner, 1999; Aschner, 2000; Roth and Garrick, 2003). Both transferrin receptors and DMT1 have been identified within endothelial cells lining the capillary beds of the brain, and Mn increases DMT1 expression in the choroid plexus (Wang et al., 2006). Other genes which may play a role in genetic susceptibility or resistance include the Solute Carrier (SLC) family members SLC39A4, SLC39A8 and SLC39A14 which encode the ZIP4, ZIP8 and ZIP14 proteins, respectively. ZIP4 is a zinc transporter with polymorphisms linked to human disease (Mao et al., 2007). ZIP8 and ZIP14 are divalent metal transporters with a high affinity for manganese (Girijashanker etal., 2008; He et al., 2006). Changes in ZIP 8 regulation are associated with increased heavy metal toxicity (Dalton et al., 2005). Therefore, abnormal up-regulation or down-regulation of these candidate genes could affect the amount of manganese absorbed, excreted or deposited in the brain.
11. Potential biomarkers of disease
DNA methylation increases during normal aging in many human tissues, including the brain (Chu et al., 2007). Budovsky et al. (2006) proposed that epigenetic changes in gene regulation are a central mechanism underlying aging and age-related pathologies. All genes listed as potential biomarkers of disease (Table 3, Column 3) are expressed in the cerebral cortex and are associated with neurological functioning. Siegmund et al. (2007) reported that all of these genes are regulated by DNA methylation with progressively increased DNA methylation across the human lifespan. Therefore, abnormal methylation of these genes at younger ages could indicate accelerated aging and the early onset of neurological disease.
Studies in Mn-exposed rhesus monkeys identified changes in protein levels of two glutamate transporters (GLAST-1 and GLT-1) and tyrosine hydroxylase (TH), a key marker of dopaminergic neurons (Erikson et al., 2007, 2008). The most significant decreases were in the globus pallidus, which is uniquely susceptible to Mn neurotoxicity. Although gene expression may be altered in humans as well, imaging techniques would appear to provide more useful data than a genetics-based approach.
More than a dozen genes have been linked to inherited forms of Parkinson’s Disease (PD), including PARK1 (α-synuclein), PARK2 (parkin), PARK6/PINK1, and PRK8/LRKK2 (reviewed in Gasser, 2007; Deng et al., 2008). Inheritance patterns include autosomal dominant, autosomal recessive, and X-linked inheritance (Chase, 1997; Riess et al., 2002; Warner and Schapira, 2003; Gasser, 2007). Genes associated with the development of Parkinson’s were not included in the candidate gene list, because PD pathology is different from manganese-induced toxicity although functional deficits may overlap. Magnetic resonance imaging clearly reveals Mn deposition and damage is greater in the globus pallidus compared with the substantia nigra which is most affected in PD (Shinotoh et al., 1995; Kim, 2004; Fitsanakis et al., 2008). Patients suffering from Mn-induced neurotoxicity also do not respond to levodopa, the most common treatment for idiopathic PD (Lu et al., 1994; Chu, 2004; Koller et al., 2004). Therefore, these genes were considered much weaker candidates for detailed genetic studies.
Only one research group has reported a direct link between genetic polymorphisms and the risk of parkinsonism in manganese-exposed workers. (Zheng et al., 2002) reported that Chinese workers with a common polymorphism in CYP2D6 were at higher risk, although the authors concede the polymorphism they identified may only be a genetic marker with no direct physiological relevance to Mn neurotoxicity. Indeed, Dick et al. (2007a,b) reported no association between CYP2D6 genotype and the development of parkinsonism in manganese-exposed workers studied in the European-based Geoparkinson study. The availability of a simple RFLP protocol to determine CYP2D6 genotype (Zheng et al., 2002) provides a way to quickly screen large numbers of samples from the Marietta cohort, so we included this traditional technique in the pilot study. We also included CYP2D6 as a candidate gene because the putative susceptibility allele has a high prevalence in the human population.
12. Choosing the best molecular approach: omics overload
The rush to sequence the human genome produced huge (and even HUGO) databases filled with sequence data, but deciphering the significance of the nucleotide sequences proved more difficult than originally expected. As a result, researchers looking at genetic susceptibility to environmental toxicants must consider a vast array of omics technologies and platforms before deciding on the most informative experimental approach. The founding field of genomics has spawned transcriptomics, proteomics, metabonomics and metabolomics among others. All of these offer the advantage of being able to examine the impact of hundreds of genes in the same experiment and the opportunity to identify nonintuitive candidate genes. Epigenomics, for example, is making important progress in identifying DNA methylation changes that affect an individual’s risk of developing cancer (Ho et al., 2006; Russo et al., 2005), asthma (Miller and Ho, 2008; Perera et al., 2009), and even obesity (Waterland, 2009). The disadvantages of these techniques include their higher costs which can often limit sample size and the necessity of using a confirmatory technique such as real-time Q-PCR for gene expression microarrays.
In contrast, a more focused approach examining the impact of a few candidate genes can greatly reduce expenses thereby expanding the possible number of individual samples to be included in the study. Standard techniques range from RFLP analysis to the increasingly popular SNP-typing using customized molecular probes and primers. A disadvantage to these techniques is the growing number of reported SNPs with little to no information available concerning the functional significance of the sequence variation. This dilemma helps to explain the large number of studies reporting gene–environmental interactions which are never replicated in other cohorts (Wilson et al., 2000; Tamer et al., 2004; Masson et al., 2005).
In our attempt to understand genetic aspects of Mn toxicity, we propose to use promoter arrays to compare DNA methylation patterns in the most highly affected and least affected individuals in the Marietta community cohort. This will allow us to look at all three categories of susceptibility genes (Table 3). DNA methylation patterns change with age, and these changes are associated with neurological deficits (Liu et al., 2007; Siegmund et al., 2007). In addition, these epigenetic changes affect proteins directly involved in neurological processes (Ravindran and Ticku, 2005). The proposed DNA methylation tests should reveal whether the most affected individuals show a pattern of accelerated aging. Conversely, the array data may reveal epigenetic changes that affect manganese transport and metabolism. This differential gene regulation could potentially explain inter-individual variation in susceptibility given similar Mn exposures.
13. Potential biomarkers of exposure
There is increasing evidence that exposure to environmental toxicants changes global DNA methylation patterns (Salnikow and Costa, 2000; Russo et al., 2005; Ho et al., 2006; Salnikow and Zhitkovich, 2008). Specifically, oxidative stress – a known outcome of manganese toxicity (Chen and Liao, 2002; Barhoumi et al., 2004; Chen et al., 2006) – increases the frequency of mutations at methylated CpG islands (Lee et al., 2002). In addition, Hamet and Tremblay (2003) concluded that abnormal DNA methylation is a biomarker of genetic damage. DNA methylation arrays provide a global view of epigenetic changes across the genome; therefore, large-scale methylation patterns can be compared between highly affected and highly resistant individuals. In this case, no candidate genes have been identified a priori because this mechanism is unlikely to have gene-specific effects.
14. Recommendations for advancing genetics and genomics in risk assessment
No single workplace cohort study can provide sufficient data to reach clear conclusions concerning genetic susceptibility to Mn-induced neurotoxicity; however, pooling data across studies would increase power for the necessary genetic analyses. Incorporating data from community-based studies with good exposure data further increases the sample size and allows for stratification by age and sex. To improve data collection and analysis, we recommend the following steps be considered by the appropriate funding agencies and governmental entities:
Expedite exposure assessments in workplaces where neurological impairments are reported.
Include both environmental and biologically based measurements of Mn exposure.
Provide incentives to include DNA sampling in all studies of Mn-exposed populations.
Adopt standardized protocols for assessing Mn-exposed populations (e.g. Fe and folate status, MRI, neuropsychological, cognitive and motor function).
Develop a centralized and standardized system for collecting, storing and analyzing DNA samples and sharing data with collaborating researchers.
Develop informational and educational materials to encourage participation in genetics studies of susceptibility to Mn-induced neurotoxicity.
Beyond the scope of these studies is the issue of nutritional status during early development. While the nutritional status of exposed adults can be measured, and differences have been reported in Fe status among Mn alloy workers (Ellingsen et al., 2003), children appear at higher risk. Sahni et al. (2007) reports a case of pediatric manganism and iron deficiency in a 6-year-old exposed to high Mn in drinking water while an older sibling and relatives showed no impairments. Rodent studies have also confirmed that nutritional status during development can affect Mn transport into the developing brain (Garcia et al., 2006; Garcia et al., 2007; Fitsinakis et al., 2008). Further animal studies will prove quite valuable in understanding the specific mechanisms that increase susceptibility in adulthood.
15. Summary
We have initiated a pilot study to help address well-known inconsistencies between Mn exposures (environmental and biological) and neurological outcomes in adults. Specifically, controversy continues concerning the role of inhaled Mn in the causation of the neurological effects associated with occupational exposures. There are diverse opinions on whether neurobehavioral and neurological signs and symptoms in Mn-exposed welders constitute adverse health effects, whether the effects are reversible or progress after the cessation of exposure, and whether these conditions are early manifestations of manganism. Santamaria et al. (2007), in a review of the available literature, concluded that although manganism was observed in highly exposed workers, the scant exposure–response data available for welders did not support a conclusion that welding is associated with clinical neurotoxicity. Olanow(2004), indistinguishing the diagnosis of frank manganism from PD, dismisses as insignificant the evidence of “preclinical” effects in Mn-exposed welders. Jankovic (2005) pointed to the methodological problems of many of the cross-sectional studies and considers some published reports with positive findings to be negative, such as those of Myers et al. (2003) and Bowler et al. (2003). Jankovic (2005) rejects neuropsychological testing for assessing adverse exposure effects. These views are not universally held (Bellinger, 2003; Anger, 2003; Mergler and Baldwin, 1997). Martin (2006) concluded that the current neuropsychological and clinical evidence is sufficient to justify preventive action to reduce the adverse effects of Mn exposure.
Given the strong likelihood that genetic background alters Mn pharmacokinetics and pharmacodynamics and an individual’s response to Mn exposure, incorporating genetic susceptibility into the risk assessment for inhaled Mn may help resolve these opposing viewpoints. Although pharmacogenetics and pharmacogenomics have not yet fulfilled their promise of “personalized medicine,” (Nebert and Vesell, 2006; Nebert et al., 2008), the goal of identifying those at highest risk of inhaled Mn exposure remains valid. The pilot study underway, using data and DNA from an exposed community population in Marietta, OH, begins to address this important research need. In addition, the inclusion of genes known to be differentially methylated in the brain and during normal aging could help identify important biomarkers of disease in exposed individuals.
We readily acknowledge that the limited number of DNA samples available and limited neurological endpoints measured will constrain the interpretation of the pilot study data. Therefore, we have recommended changes to improve and expedite the collection of critical data from ongoing studies of Mn-exposed workers and communities. If implemented successfully, these recommendations could become a model for similar studies of gene-environment interactions both in the workplace and in affected communities nationwide.
Acknowledgments
The authors gratefully acknowledge stipend support for postdoctoral research under the National Research Council’s Research Apprenticeship Program and pilot research funding from the NIOSH Education and Information Division and Risk Evaluation Branch. We thank our colleagues Dr. Kristi Haik, Dr. Janelle Crossgrove and Dr. Glenn Talaska for their helpful comments and careful review of this manuscript.
Footnotes
Conflicts of interest We certify that we have no conflicts of interest in the projects discussed herein.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Agency for Toxic Substances and Disease Registry. Health Consultation: Washington County Air Quality, Marietta, Ohio, 18-6-2007. Atlanta, GA: U.S. Department of Health and Human Services; Mar 31, 2009. [Google Scholar]
- Akbas N, Hochstrasser H, Deplazes J, Tomiuk J, Bauer P, Walter U, et al. Screening for mutations of the HFE gene in Parkinson’s disease patients with hyperechogenicity of the substantia nigra. Neurosci Lett. 2006;407(1):16–9. doi: 10.1016/j.neulet.2006.07.070. [DOI] [PubMed] [Google Scholar]
- Anderson JG, Cooney PT, Erikson KM. Inhibition of DAT function attenuates manganese accumulation in the globus pallidus. Environ Toxicol Pharmacol. 2007;23(2):179–84. doi: 10.1016/j.etap.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anger WK. Neurobehavioural tests and systems to assess neurotoxic exposures in the workplace and community. Occup Environ Med. 2003;60(7):531–8. 474. doi: 10.1136/oem.60.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. Manganese homeostasis in the CNS. Environ Res. 1999;80(2 Pt 1):105–9. doi: 10.1006/enrs.1998.3918. [DOI] [PubMed] [Google Scholar]
- Aschner M. Manganese: brain transport and emerging research needs. Environ Health Perspect. 2000;108(Suppl 3):429–32. doi: 10.1289/ehp.00108s3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Manganese transport across the blood–brain barrier: relationship to iron homeostasis. Brain Res Bull. 1990;24(6):857–60. doi: 10.1016/0361-9230(90)90152-p. [DOI] [PubMed] [Google Scholar]
- Aschner M, Dorman DC. Manganese: pharmacokinetics and molecular mechanisms of brain uptake. Toxicol Rev. 2006;25(3):147–54. doi: 10.2165/00139709-200625030-00002. [DOI] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221(2):131–47. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Lukey B, Tremblay A. The Manganese Health Research Program (MHRP): status report and future research needs and directions. Neurotoxicology. 2006;27(5):733–6. doi: 10.1016/j.neuro.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial-dependent activation of nuclear factor kappaB. Brain Res Mol Brain Res. 2004;122(2):167–79. doi: 10.1016/j.molbrainres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Perspectives on incorporating human neurobehavioral end points in risk assessments. Risk Anal. 2003;23(1):163–74. [PubMed] [Google Scholar]
- Bon MA, Jansen Steur EN, de Vos RA, Vermes I. Neurogenetic correlates of Parkinson’s disease: apolipoprotein-E and cytochrome P450 2D6 genetic polymorphism. Neurosci Lett. 1999;266(2):149–51. doi: 10.1016/s0304-3940(99)00278-5. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Booty A, Hartney C, Roels HA. Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. Int J Hyg Environ Health. 2003;206(6):517–29. doi: 10.1078/1438-4639-00249. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Nakagawa S, Drezgic M, Roels HA, Park RM, Diamond E, et al. Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology. 2007a;28(2):298–311. doi: 10.1016/j.neuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007b;64(3):167–77. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovsky A, Muradian KK, Fraifeld VE. From disease-oriented to aging/longevity-oriented studies. Rejuvenation Res. 2006;9(2):207–10. doi: 10.1089/rej.2006.9.207. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. Occupational Employment Statistics: National Occupational Employment and Wage Estimates, 2006. Welders, Cutters, Solders, and Brazers U.S. Department of Labor; May, 2006. [31-3-09]. Available at: http://www.bls.gov/oes/2006/may/oes514121.htm. [Google Scholar]
- Chase TN. A gene for Parkinson disease. Arch Neurol. 1997;54(9):1156–7. doi: 10.1001/archneur.1997.00550210084017. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liao SL. Oxidative stress involves in astrocytic alterations induced by manganese. Exp Neurol. 2002;175(1):216–25. doi: 10.1006/exnr.2002.7894. [DOI] [PubMed] [Google Scholar]
- Chen MT, Cheng GW, Lin CC, Chen BH, Huang YL. Effects of acute manganese chloride exposure on lipid peroxidation and alteration of trace metals in rat brain. Biol Trace Elem Res. 2006;110(2):163–78. doi: 10.1385/BTER:110:2:163. [DOI] [PubMed] [Google Scholar]
- Chu MW, Siegmund KD, Eckstam CL, Kim JY, Yang AS, Kanel GC, et al. Lack of increases in methylation at three CpG-rich genomic loci in non-mitotic adult tissues during aging. BMC Med Genet. 2007;8:50. doi: 10.1186/1471-2350-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu NS. Effect of levodopa treatment for parkinsonism in welders: a double-blind study. Neurology. 2004;63(8):1541. doi: 10.1212/wnl.63.8.1541. [DOI] [PubMed] [Google Scholar]
- Clewell HJ, Lawrence GA, Calne DB, Crump KS. Determination of an occupational exposure guideline for manganese using the benchmark method. Risk Anal. 2003;23(5):1031–46. doi: 10.1111/1539-6924.00379. [DOI] [PubMed] [Google Scholar]
- Cooper WC. The health implications of increased manganese in the environment resulting from the combustion of fuel additives: a review of the literature. J Toxicol Environ Health. 1984;14(1):23–46. doi: 10.1080/15287398409530561. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37(10):1511–26. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, et al. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA. 2005;102(9):3401–6. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Jarabek AM, Mage DT, Graham JA. The EPA health risk assessment of methyl-cyclopentadienyl manganese tricarbonyl (MMT) Risk Anal. 1998;18(1):57–70. doi: 10.1111/j.1539-6924.1998.tb00916.x. [DOI] [PubMed] [Google Scholar]
- Deng H, Le W, Shahed J, Xie W, Jankovic J. Mutation analysis of the parkin and PINK1 genes in American Caucasian early-onset Parkinson disease families. Neurosci Lett. 2008;430(1):18–22. doi: 10.1016/j.neulet.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Dick FD, De PG, Ahmadi A, Osborne A, Scott NW, Prescott GJ, et al. Gene-environment interactions in parkinsonism and Parkinson’s disease: the Geoparkinson study. Occup Environ Med. 2007a;64(10):673–80. doi: 10.1136/oem.2006.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FD, De PG, Ahmadi A, Scott NW, Prescott GJ, Bennett J, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007b;64(10):666–72. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–28. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Haug E, Ulvik RJ, Thomassen Y. Iron status in manganese alloy production workers. J Appl Toxicol. 2003;23:239–47. doi: 10.1002/jat.913. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Manganese neurotoxicity and glutamate–GABA interaction. Neurochem Int. 2003;43(4–5):475–80. doi: 10.1016/s0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicol Sci. 2007;97(2):459–66. doi: 10.1093/toxsci/kfm044. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dorman DC, Lash LH, Aschner M. Duration of airborne-manganese exposure in rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Neurotoxicology. 2008;29(3):377–85. doi: 10.1016/j.neuro.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Feldman RG. Manganese Occupational and environmental neurotoxicology. New York, NY: Lippincott-Raven Press; 1999. pp. 168–188. [Google Scholar]
- Fell JM, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G, et al. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet. 1996;347(9010):1218–21. doi: 10.1016/s0140-6736(96)90735-7. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Au C, Erikson KM, Aschner M. The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int. 2006;48(6–7):426–33. doi: 10.1016/j.neuint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, et al. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci. 2008;103(1):116–24. doi: 10.1093/toxsci/kfn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci. 2006;92:516–25. doi: 10.1093/toxsci/kfl017. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci. 2007;95:205–14. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Gasser T. Update on the genetics of Parkinson’s disease. Mov Disord. 2007;22(Suppl 17):S343–50. doi: 10.1002/mds.21676. [DOI] [PubMed] [Google Scholar]
- Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, et al. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008 doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Bras JM, Santana I, Januario C, Santiago B, Morgadinho AS, et al. Association of HFE common mutations with Parkinson’s disease, Alzheimer’s disease and mild cognitive impairment in a Portuguese cohort. BMC Neurol. 2006;6:24. doi: 10.1186/1471-2377-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamet P, Tremblay J. Genes of aging. Metabolism. 2003;52(10 Suppl 2):5–9. doi: 10.1016/s0026-0495(03)00294-4. [DOI] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, et al. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70(1):171–80. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M. Manganese and iron transport across pulmonary epithelium. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1247–59. doi: 10.1152/ajplung.00450.2005. [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de FJ, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iregren A. Psychological test performance in foundry workers exposed to low levels of manganese. Neurotoxicol Teratol. 1990;12(6):673–5. doi: 10.1016/0892-0362(90)90085-q. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64(12):2021–8. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Manganese: a high-octane dispute. Science. 2003;300(5621):926–8. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- Kim Y. High signal intensities on T1-weighted MRI as a biomarker of exposure to manganese. Ind Health. 2004;42(2):111–5. doi: 10.2486/indhealth.42.111. [DOI] [PubMed] [Google Scholar]
- Kirkey KL, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Gorell JM. Occupational categories at risk for Parkinson’s disease. Am J Ind Med. 2001;39(6):564–71. doi: 10.1002/ajim.1055. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Chandler M, Kumar N, Ahlskog JE, Josephs KA. Neuropsychological profiles of manganese neurotoxicity. Eur J Neurol. 2006;13(10):1139–41. doi: 10.1111/j.1468-1331.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W. Effect of levodopa treatment for Parkinsonism in welders: a double-blind study. Neurology. 2004;62(5):730–3. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet. 1995;346(8970):270–4. doi: 10.1016/s0140-6736(95)92164-8. [DOI] [PubMed] [Google Scholar]
- Leavens TL, Rao D, Andersen ME, Dorman DC. Evaluating transport of manganese from olfactory mucosa to striatum by pharmacokinetic modeling. Toxicol Sci. 2007;97(2):265–78. doi: 10.1093/toxsci/kfm061. [DOI] [PubMed] [Google Scholar]
- Lee DH, O’Connor TR, Pfeifer GP. Oxidative DNA damage induced by copper and hydrogen peroxide promotes CG–>TT tandem mutations at methylated CpG dinucleotides in nucleotide excision repair-deficient cells. Nucleic Acids Res. 2002;30(16):3566–73. doi: 10.1093/nar/gkf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, van GT, Kadish I, Tollefsbol TO. DNA methylation impacts on learning and memory in aging. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Vahter M. Time to re-evaluate the guideline value for manganese in drinking water? Environ Health Perspect. 2007;115(11):1533–8. doi: 10.1289/ehp.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger S, Zayed J. Environmental contamination and human exposure assessment to manganese in the St-Lawrence River ecozone (Quebec, Canada) using an environmental fate/exposure model: GEOTOX. SAR QSAR Environ Res. 1997;6(1–2):105–19. doi: 10.1080/10629369708031727. [DOI] [PubMed] [Google Scholar]
- Lu CS, Huang CC, Chu NS, Calne DB. Levodopa failure in chronic manganism. Neurology. 1994;44(9):1600–2. doi: 10.1212/wnl.44.9.1600. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Bergamaschi E, Smargiassi A, Festa D, Apostoli P. Motor function, olfactory threshold, and hematological indices in manganese-exposed ferroalloy workers. Environ Res. 1997;73(1–2):175–80. doi: 10.1006/enrs.1997.3702. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, et al. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health. 1995;21(2):143–9. doi: 10.5271/sjweh.1369. [DOI] [PubMed] [Google Scholar]
- Malecki EA. Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull. 2001;55(2):225–8. doi: 10.1016/s0361-9230(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem. 2007;282(10):6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- Martin CJ. Manganese neurotoxicity: connecting the dots along the continuum of dysfunction. Neurotoxicology. 2006;27(3):347–9. doi: 10.1016/j.neuro.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Masson LF, Sharp L, Cotton SC, Little J. Cytochrome P-450 1A1 gene polymorphisms and risk of breast cancer: a HuGE review. Am J Epidemiol. 2005;161(10):901–15. doi: 10.1093/aje/kwi121. [DOI] [PubMed] [Google Scholar]
- Mena I. The role of manganese in human disease. Ann Clin Lab Sci. 1974;4(6):487–91. [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73(1–2):92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008;177(6):567–73. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery EB., Jr Heavy metals and the etiology of Parkinson’s disease and other movement disorders. Toxicology. 1995;97(1–3):3–9. doi: 10.1016/0300-483x(94)02962-t. [DOI] [PubMed] [Google Scholar]
- Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, Bocca B, et al. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology. 2008;29(1):60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Myers JE, teWaterNaude J, Fourie M, Zogoe HB, Naik I, Theodorou P, et al. Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology. 2003;24(4–5):649–56. doi: 10.1016/S0161-813X(03)00035-4. [DOI] [PubMed] [Google Scholar]
- National Research Council. Risk assessment in the federal government: managing the process. Washington, DC: National Academy Press; 1983. [PubMed] [Google Scholar]
- Nebert DW. Extreme discordant phenotype methodology: an intuitive approach to clinical pharmacogenetics. Eur J Pharmacol. 2000;410(2–3):107–20. doi: 10.1016/s0014-2999(00)00809-8. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Vesell ES. Can personalized drug therapy be achieved? A closer look at pharmaco-metabonomics. Trends Pharmacol Sci. 2006;27(11):580–6. doi: 10.1016/j.tips.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Zhang G, Vesell ES. From human genetics and genomics to pharmacogenetics and pharmacogenomics: past lessons, future directions. Drug Metab Rev. 2008;40(2):187–224. doi: 10.1080/03602530801952864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese’s neurotoxicity. Neurotoxicology. 1999;20:415–32. [PubMed] [Google Scholar]
- Newland MC, Weiss B. Persistent effects of manganese on effortful responding and their relationship to manganese accumulation in the primate globus pallidus. Toxicol Appl Pharmacol. 1992;113(1):87–97. doi: 10.1016/0041-008x(92)90012-h. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann N Y Acad Sci. 2004;1012:209–23. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, et al. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46(2):492–8. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- Park EY, Hong YC, Lee KH, Im MW, Ha E, Kim YJ, et al. Maternal exposure to environmental tobacco smoke, GSTM1/T1 polymorphisms and oxidative stress. Reprod Toxicol. 2008;26(3–4):197–202. doi: 10.1016/j.reprotox.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Park RM, Bowler RM, Eggerth DE, Diamond E, Spencer KJ, Smith D, et al. Issues in neurological risk assessment for occupational exposures: the Bay Bridge welders. Neurotoxicology. 2006;27(3):373–84. doi: 10.1016/j.neuro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, et al. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48(1):63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, et al. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS ONE. 2009;4(2):e4488. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran CR, Ticku MK. Methylation of NMDA receptor NR2B gene as a function of age in the mouse brain. Neurosci Lett. 2005;380(3):223–8. doi: 10.1016/j.neulet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Reist C, Ozdemir V, Wang E, Hashemzadeh M, Mee S, Moyzis R. Novelty seeking and the dopamine D4 receptor gene (DRD4) revisited in Asians: haplotype characterization and relevance of the 2-repeat allele. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):453–7. doi: 10.1002/ajmg.b.30473. [DOI] [PubMed] [Google Scholar]
- Riess O, Kruger R, Schulz JB. Spectrum of phenotypes and genotypes in Parkinson’s disease. J Neurol. 2002;249(Suppl 3):III/15–III/20. doi: 10.1007/s00415-002-1303-2. [DOI] [PubMed] [Google Scholar]
- Roels H, Lauwerys R, Genet P, Sarhan MJ, de Fays M, Hanotiau I, et al. Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am J Ind Med. 1987;11(3):297–305. doi: 10.1002/ajim.4700110307. [DOI] [PubMed] [Google Scholar]
- Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med. 1992;49(1):25–34. doi: 10.1136/oem.49.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Ramirez-Aguilar M, Sienra-Monge JJ, Moreno-Macias H, del Rio-Navarro BE, David G, et al. GSTM1 and GSTP1 and respiratory health in asthmatic children exposed to ozone. Eur Respir J. 2006;28(5):953–9. doi: 10.1183/09031936.06.00114905. [DOI] [PubMed] [Google Scholar]
- Rossander-Hulten L, Brune M, Sandstrom B, Lonnerdal B, Hallberg L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr. 1991;54(1):152–6. doi: 10.1093/ajcn/54.1.152. [DOI] [PubMed] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66(1):1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Russo AL, Thiagalingam A, Pan H, Califano J, Cheng KH, Ponte JF, et al. Differential DNA hypermethylation of critical genes mediates the stage-specific tobacco smoke-induced neoplastic progression of lung cancer. Clin Cancer Res. 2005;11(7):2466–70. doi: 10.1158/1078-0432.CCR-04-1962. [DOI] [PubMed] [Google Scholar]
- Sahni V, Leger Y, Panaro L, Allen M, Giffin S, Fury D, et al. Case report: a metabolic disorder presenting as pediatric manganism. Environ Health Perspect. 2007;115:1776–9. doi: 10.1289/ehp.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikow K, Costa M. Epigenetic mechanisms of nickel carcinogenesis. J Environ Pathol Toxicol Oncol. 2000;19(3):307–18. [PubMed] [Google Scholar]
- Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008;21(1):28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS. State-of-the-science review: does manganese exposure during welding pose a neurological risk? J Toxicol Environ Health B Crit Rev. 2007;10(6):417–65. doi: 10.1080/15287390600975004. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, et al. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118(1):222–31. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA. The contributions of genetics and genomics to occupational safety and health. Occup Environ Med. 2007;64(11):717–8. doi: 10.1136/oem.2006.030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotoh H, Snow BJ, Hewitt KA, Pate BD, Doudet D, Nugent R, et al. MRI and PET studies of manganese-intoxicated monkeys. Neurology. 1995;45(6):1199–204. doi: 10.1212/wnl.45.6.1199. [DOI] [PubMed] [Google Scholar]
- Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, et al. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS ONE. 2007;2(9):e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, et al. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50(11):801–11. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Standridge JS, Bhattacharya A, Succop P, Cox C, Haynes E. Effect of chronic low level manganese exposure on postural balance: a pilot study of residents in southern ohio. J Occup Environ Med. 2008;50(12):1421–9. doi: 10.1097/JOM.0b013e3181896936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamer L, Ercan B, Camsari A, Yildirim H, Cicek D, Sucu N, et al. Glutathione S-transferase gene polymorphism as a susceptibility factor in smoking-related coronary artery disease. Basic Res Cardiol. 2004;99(3):223–9. doi: 10.1007/s00395-004-0465-8. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Erikson KM, Dobson AW, Fitsanakis VA, Dorman DC, Aschner M. Effects of inhaled manganese on biomarkers of oxidative stress in the rat brain. Neurotoxicology. 2006;27(5):788–97. doi: 10.1016/j.neuro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 2007;21(1):223–30. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahner AD, Glatt CE, Bronstein JM, Ritz B. Glutathione S-transferase mu, omega, pi, and theta class variants and smoking in Parkinson’s disease. Neurosci Lett. 2007;413(3):274–8. doi: 10.1016/j.neulet.2006.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Tang JJ, Tang NP, Wang MW, Yan JJ, Wang QM, et al. Association of GSTM1 and GSTT1 gene polymorphisms with coronary artery disease in relation to tobacco smoking. Clin Chem Lab Med. 2008;46(12):1720–5. doi: 10.1515/CCLM.2008.353. [DOI] [PubMed] [Google Scholar]
- Wang X, Li GJ, Zheng W. Upregulation of DMT1 expression in choroidal epithelia of the blood–CSF barrier following manganese exposure in vitro. Brain Res. 2006;1097(1):1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S16–23. doi: 10.1002/ana.10487. [DOI] [PubMed] [Google Scholar]
- Waterland RA. Is epigenetics an important link between early life events and adult disease? Horm Res. 2009;71(Suppl 1):13–6. doi: 10.1159/000178030. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Grant PJ, Hardie LJ, Wild CP. Glutathione S-transferase M1 null genotype is associated with a decreased risk of myocardial infarction. FASEB J. 2000;14(5):791–6. doi: 10.1096/fasebj.14.5.791. [DOI] [PubMed] [Google Scholar]
- Zheng YX, Chan P, Pan ZF, Shi NN, Wang ZX, Pan J, et al. Polymorphism of metabolic genes and susceptibility to occupational chronic manganism. Biomarkers. 2002;7(4):337–46. doi: 10.1080/13547500210146740. [DOI] [PubMed] [Google Scholar]


