Abstract
The effects of estrogen on osteoclast survival and differentiation were studied using CD14-selected mononuclear osteoclast precursors from peripheral blood. Estradiol at ~1 nM reduced RANKL-dependent osteoclast differentiation by 40–50%. Osteoclast differentiation was suppressed 14 days after addition of RANKL even when estradiol was withdrawn after 18 hours. In CD14+ cells apoptosis was rare and was not augmented by RANKL or by 17-β-estradiol. Estrogen receptor-α (ERα) expression was strongly down-regulated by RANKL, whether or not estradiol was present. Mature human osteoclasts thus cannot respond to estrogen via ERα. However, ERα was present in CD14+ osteoclast progenitors, and a scaffolding protein, BCAR1, which binds ERα in the presence of estrogen, was abundant. Immunoprecipitation showed rapid (~5 minute) estrogen-dependent formation of ERα-BCAR1 complexes, which were increased by RANKL co-treatment. The RANKL-signaling intermediate Traf6, which regulates NF-κB activity, precipitated with this complex. Reduction of NF-κB nuclear localization occurred within 30 minutes of RANKL stimulation, and estradiol inhibited the phosphorylation of IκB in response to RANKL. Inhibition by estradiol was abolished by siRNA knockdown of BCAR1. We conclude that estrogen directly, but only partially, curtails human osteoclast formation. This effect requires BCAR1 and involves a non-genomic interaction with ERα.
Keywords: Estrogen receptor-α, Breast cancer antiestrogen resistance-1, p130Cas, Nuclear factor-κB, TNF-receptor associated factors
Osteoclasts are multinucleated bone-degrading cells that differentiate from monocytic precursors. They are critical to bone modeling and maintenance, and mediate the release of skeletal mineral for calcium and pH homeostasis. Consequently, osteoclast differentiation and activity are regulated by several systems. Because bone loss occurs when estrogen declines at the menopause, the modulation of osteoclast formation and activity by estrogen is an important issue. The mechanisms by which estrogen regulates bone turnover remain unclear [1]. Unresolved issues include whether estrogen causes apoptosis of osteoclasts or osteoclast precursors, and whether estrogen suppresses osteoclast formation directly or whether effects are mediated via other types of cells, including osteoblasts, which secondarily regulate osteoclast formation and activity.
The canonical mechanism for osteoclast differentiation depends on activation of a TNF-family receptor, receptor activator of nuclear factor-κB (RANK) by its ligand, RANKL. Signals from immune-related receptors and activity of CSF-1 (M-CSF) tyrosine kinase receptor fms are also required [2]. Estrogen probably affects osteoclast differentiation, in part, indirectly through its modulation of osteoblast RANKL and osteoprotegerin expression [3]. There is also evidence of estrogen effects on bone mediated by T-cells and their products [4]. In the bone, RANKL is expressed in osteoblasts and related cells mainly as a membrane-bound protein; however, use of soluble recombinant RANKL permits osteoclast formation to be studied in the absence of supporting cells.
It has also been suggested that estrogen may regulate bone resorption through direct effects on osteoclasts that may be mediated, at least in part, by rapid nongenomic mechanisms [5,6,7,8]. Nongenomic estrogen effects include activation of signaling intermediates through novel membrane-bound estrogen receptors [9,10,11] or through extranuclear actions of classical estrogen receptors including estrogen receptor-α [12,13,14,15,16]. Studies in rabbits and rodents suggested that estrogen regulates bone resorption by promoting osteoclast apoptosis [17,18]. Ablation of estrogen receptor-α (ERα) in cathepsin K-expressing cells of mice caused a loss of estrogen inhibition of bone resorption with increased numbers of apoptotic osteoclasts [19] through upregulation of Fas ligand. Other studies suggest that enhanced osteoclast apoptosis may be an indirect effect mediated by upregulation of Fas in osteoblasts [20], although analysis of Fas or Fas ligand-deficient mice did not suggest this [21]. Difficulties in resolving these controversies reflect in part the complexity of the animal models, and the limitations of in vitro studies: either those using transformed cells to model osteoclast precursors, or using osteoclast precursors from bone marrow that can contain osteoblasts and other cells that may mediate indirect estrogen effects on osteoclasts.
In the present studies we investigated whether estrogen directly induces apoptosis in osteoclasts or their precursor cells, and whether estrogen directly modulates osteoclast differentiation. To avoid contamination by bone marrow mesenchymal cells, affinity-isolated CD14-selected human monocytes from peripheral blood were treated with soluble recombinant cytokines to induce osteoclast differentiation. The use of primary cells avoids the artifacts due to transformation that complicate the use of cell lines, and the use of human cells avoids pitfalls due to differences in steroid responses even amongst mammals [22]. We find that estrogen reduces osteoclast formation from monocytic precursors in the absence of osteoblasts. The inhibitory effects of estrogen occur, at least in major part, through a rapid nongenomic mechanism that involves the interaction of estrogen receptor-α with the breast cancer anti-estrogen resistance protein-1 (BCAR1) [23]. BCAR1 was identified in a screen for proteins associated with antiestrogen resistance in breast cancer [24] but is also expressed by osteoclasts [25]; it is the human homolog of the rodent adaptor protein p130Cas, a substrate of Src necessary for v-Src oncogenic transformation [26,27,28]. While estrogen affects the survival of many cell types, we found that, at physiological concentrations, estrogen did not stimulate apoptosis of human osteoclasts or their monocytic precursors.
Materials and Methods
Cell culture and analysis of differentiation and survival
Human monocytic osteoclast precursors were isolated by anti-CD14 immuno-magnetic selection from human blood buffy coat cells after density gradient centrifugation [29] and maintained in culture in Dulbecco’s Modified Eagle’s Medium (DMEM, Mediatech, Herndon, VA) with 10% fetal bovine serum (Hyclone, Logan, UT), penicillin (100 units/ml)/streptomycin (100 μg/ml) (Invitrogen, Carlsbad, CA) and 10 ng/ml of CSF-1 (R&D, Minneapolis, MN). Use of human cells was approved by the institutional review board. Donors were de-labeled but were limited to adults aged 21 to 65. Murine monocytic RAW264.7 cells were from the American Type Culture Collection (Rockville MD) grown as described [6]. All media used were phenol-red free, to avoid its weak estrogen agonist effects, and charcoal stripped to remove serum steroids. From CD14-selected cells in culture at 2 × 105 cells/cm2, osteoclasts were generated by addition of recombinant soluble human RANKL (40 ng/ml or concentrations stated). Seventeen-β-estradiol (estradiol) was from Sigma-Aldrich (St Louis, MO) and the ERα selective ligand Y134 from Tocris Biosciences (Ellisville, Missouri). Tartrate resistant acid phosphatase activity, characteristic of osteoclasts, was assayed in cultures fixed 5 minutes in 40% acetone in citrate buffer at pH 5. Degradation of naphthol AS-BI phosphate substrate in acetate buffer, pH 5, with 10 mM tartrate added to inhibit other acid phosphatases, was detected by addition of fast garnet diazonium salt, which was coupled with degraded substrate to form a red precipitate. The product was measured by quantifying red channel absorbance on a 24 bit RGB scan. To evaluate osteoclast resorptive activity, osteoclasts formed from CD14-positive cells were grown on dentine matrix, which was subsequently stained with toluidine blue for detection of resorption pits [30]. Apoptosis assays used AlexaFluor 488-conjugated annexin V (Invitrogen) to detect phosphatidylserine translocation from the inner to the outer membrane lipid layer, with propidium iodide added to identify necrotic cells. As a positive control for apoptosis, parallel cultures were treated with 0.5 μM etoposide for 2 hours before annexin V labeling.
Flow cytometry
A FACScalibur instrument (Becton Dickinson, San Diego, CA) was used. Aliquots of 3 × 105 cells were washed in Hank’s balanced salt solution containing 0.1% bovine serum albumin and 0.1% NaN3 and labeled with appropriately-diluted antibodies directly conjugated with fluors, for 30 minutes, followed by three washes and fixation in 2% paraformaldehyde. Mouse monoclonal antibodies were used, FITC-, Cy5- or PE-labeled anti-human CD14 (BD Biosciences PharMingen, San Diego, CA), Cy-5 labeled CD19 (Invitrogen, Carlsbad, CA), and PE-labeled CD11b or CD3 (Caltag, Burlingame, CA), or PE-labeled CD1a (Serotec, Raleigh, NC). Appropriate isotype controls were from Caltag or BD. Data analysis used Cell Quest Software (Becton Dickinson).
Quantitative PCR
Total RNA was isolated (RNEasy; Qiagen, Valencia CA). First strand cDNA synthesis from 1 μg total RNA used random hexamer primers and Moloney murine leukemia virus reverse transcriptase (Superscript; Invitrogen). Real-time PCR used brilliant SYBR green fluorescent DNA intercalating dye as the analyte, purchased in a master mixture containing nucleotides and buffer (Stratagene, La Jolla, CA), adding 2.5 mM Mg, 100 nM oligonucleotide primers, and first strand cDNA. After 10 min at 95 °C, cycles of 15 sec at 95 °C and 1 min at 60 °C were done an MX3000P (Stratagene). For estrogen receptor-α and GAPDH, standard curves were prepared using total RNA from the human breast cancer cell line MCF7 to confirm linearity of threshold cycle (Ct) to log(initial copies) and equivalence of amplification efficiency. Mean Ct for replicates were used to calculate the ratio of cDNA to match GAPDH controls [31]. Primer sequences for estrogen receptor-α (GenBank NM_000125.3) were forward/reverse 5′-TGATGAAAGGTGGGATACGA-3′/5′-AAGGTTGGCAGCTCTCATGT-3′, and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, GenBank NM_002046.3) forward/reverse 5′-GAGTCAACGGATTTGGTCGT-3′/5′-TTGATTTTGGAGGGATCTCG-3′. PCR product sizes were verified by agarose gel electrophoresis.
Western blotting, immunoprecipitation and antibody reagents
Cell lysis, Western blotting and immunoprecipitation were performed as previously described [32] with minor modifications. Briefly, cells were lysed on ice in 150 mM NaCl, 1 mM EDTA, 50 mM Tris pH 8, 1% nonyl phenoxylpolyethoxylethanol (NP-40) and 10% glycerol, plus phosphatase and proteinase inhibitors (all from Sigma-Aldrich), then lysates were centrifuged to remove insoluble material. Protein concentrations were determined using the Biorad DC Protein Assay (Biorad, Hercules, CA). For Western blotting, lysates were diluted with 3x sample buffer [33], denatured by boiling for 5 minutes, and analyzed by SDS-PAGE. For immunoprecipitation, lysates were pre-cleared by incubation with protein A/G agarose (Santa Cruz Biotechnology, Santa Cruz, CA) at 4 °C for 1 hour. Cleared lysates were then incubated overnight at 4°C with primary antibody (anti-ERα at 1:250 dilution or anti-BCAR1 at 1:500) (Santa Cruz); immunoprecipitated proteins were collected by centrifugation 1 hour after addition of protein A/G agarose. The bound immune complexes were then washed 3 times with lysis buffer and eluted by boiling in sample buffer [33]. Proteins were separated by SDS-denaturing polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. Mouse monoclonal anti-ERα, rabbit polyclonal anti-BCAR1 or anti-Traf6 antibodies (Santa Cruz) were used for Western blots at 1:1000; mouse monoclonal anti-actin (Sigma-Aldrich) was used at 1:500. Rabbit polyclonal anti-IκB and anti-phospho-IκB (Cell Signaling, Danvers, MA) were used at 1:1000. Labeled proteins were detected with peroxidase-linked secondary antibodies (anti-rabbit IgG and anti-mouse IgG, Amersham, Piscataway, NJ) by enhanced chemiluminescence (ECL plus, Amersham). Where indicated, blots were reprobed with additional antibodies after the membrane was stripped for 40 minutes at 50°C in 62.5 mM Tris pH 6.6 with 2% SDS and 100 mM 2-mercaptoethanol.
Image acquisition and analysis
Cells were grown on chambered coverslips (Nunc, Fisher Scientific, Pittsburgh, PA). After treatments indicated, cells were fixed in 3.7% formaldehyde in phosphate-buffered saline for 10 minutes then placed in ethanol at −20 °C. Labeling was performed at room temperature after 10 minutes in 0.2% polyoxyethylene octyl phenyl ether (Triton X-100, Sigma-Aldrich) in phosphate-buffered saline for permeabilization. After 1 hour in blocking buffer (1% bovine serum albumin and 5% goat serum in phosphate-buffered saline with 0.05% polyoxyethylene sorbitol (Tween 20)), cells were incubated for one hour with primary antibodies in blocking buffer: rabbit polyclonal anti-NF-κB, goat polyclonal anti-RANK, mouse monoclonal anti-BCAR1, rabbit polyclonal anti-BCAR1 and mouse monoclonal anti-ERα (Santa Cruz) were used at 1:50 dilution in blocking buffer. Cells were washed, then incubated for 1 hour with secondary antibodies in blocking buffer: Cy3-labeled donkey anti-rabbit IgG at 1:500 and AlexaFluor488-labeled donkey anti-mouse IgG at 1:250 or FITC-labeled donkey anti-goat IgG at 1:500 (Invitrogen). Controls omitting primary antibody were performed to identify non-specific staining. For nuclear labeling we used Hoechst 33342 blue (Invitrogen), 10 ng/ml in 140 mM NaCl. Images were acquired on a Nikon TE2000 inverted fluorescence microscope using a Spot 12 bit 1600 × 1200 pixel charge-coupled device; green fluorescence used excitation 450–490 nm, a 510 nm dichroic filter, and a 520 nm barrier filter; red fluorescence used excitation 536–556 nm, a 580 nm dichroic filter, and a 590 nm barrier filter; and blue fluorescence used excitation at 330–380 nm, a 400 nm dichroic filter, and a 420 nm barrier filter; or where indicated by an Olympus FluoView FV1000 laser scanning confocal microscope with tunable excitation at the appropriate absorption maxima for the same fluors. Fluorescent labels were photographed using 1.3 NA 40x or 100x oil objectives.
Results
Selection and characterization of osteoclast precursors
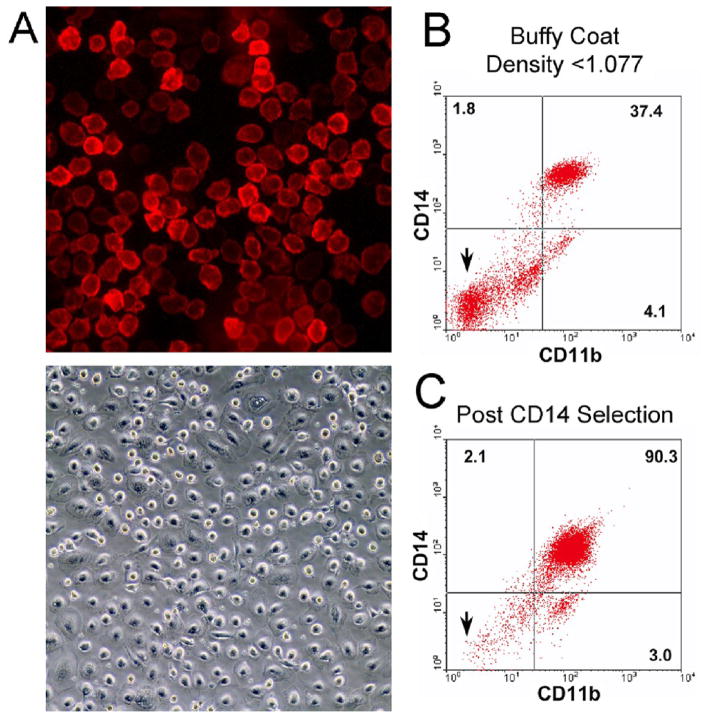
The response of osteoclasts and their precursor cells to estrogen is controversial. Conflicting results may reflect species differences and the variable purity of the osteoclast or osteoclast progenitors studied. To reduce the risk of artifacts, we used adult human peripheral blood as the source of osteoclast precursors, and did two-stage purification, first isolating cells by density gradient centrifugation (density <1.077), followed by immuno-magnetic separation using anti-CD14 coated magnetic beads. The resulting cells labeled almost completely with fluorescent anti-CD14 and, in the absence of RANKL, produced monotonous monolayers of mononuclear cells (Figure 1A). Flow cytometry confirmed substantial elimination of contaminating cells, with over 95% of selected cells labeling with anti-CD14 or anti CD11b (Figure 1B and C), a cell-surface antigen immediately downstream of CD14 in osteoclast precursor differentiation (Figure 1B and C) [34]. Additional flow assays showed only 0.5% CD19-labeled cells (B cells) and 1% CD3-labeled cells (T cells) after CD14-immunomagnetic separation (data not shown). Small numbers of red cell ghosts and red cells were also present (not illustrated). This approach reduces the risk that observed effects on osteoclasts may be indirect, depending on secondary mediators from other cell types. While bone marrow is an excellent source of osteoclast precursors, it is particularly difficult to eliminate from such preparations all osteoblasts, fibroblasts and other mesenchymal cells with the potential to produce cytokines that modulate osteoclast differentiation or survival. While contamination of cultures of CD14-selected cells from peripheral blood with mesenchymal cells could theoretically still occur, the chance of this is very small [35,36].
Figure 1. Isolation of osteoclast precursors from human peripheral blood.
A. Cells isolated by anti-CD14 immuno-magnetic separation from the mononuclear fraction of human peripheral blood. Fluorescence micrograph (top frame) of CD14-selected cells labeled with Cy5-conjugated anti-CD14 antibody (field 220 μm square) four hours after isolation, and a phase micrograph (lower frame) of 6 day culture (field 880 μm square). In each case, non-adherent cells were washed from the cultures after plating.
B,C. Expression of CD14 and CD11b by human peripheral blood mononuclear cells and cells isolated by immuno-magnetic separation. Mononuclear cells (B), isolated by density gradient centrifugation from human buffy coat cells, and cells (C) from the same buffy coat after CD14 immuno-magnetic selection are analyzed by flow cytometry as described in Methods. Compared to the results for unselected peripheral blood mononuclear cells (B), after immuno-magnetic selection (C), the fraction of cells not labeled by either CD14 or CD11b is greatly reduced, as expected (arrows). After selection, over 95% of cells labeled with CD14 or CD11b, while lymphocyte markers were present on <2% of cells (see text).
Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes, and effects on osteoclast differentiation are retained after withdrawal of estradiol
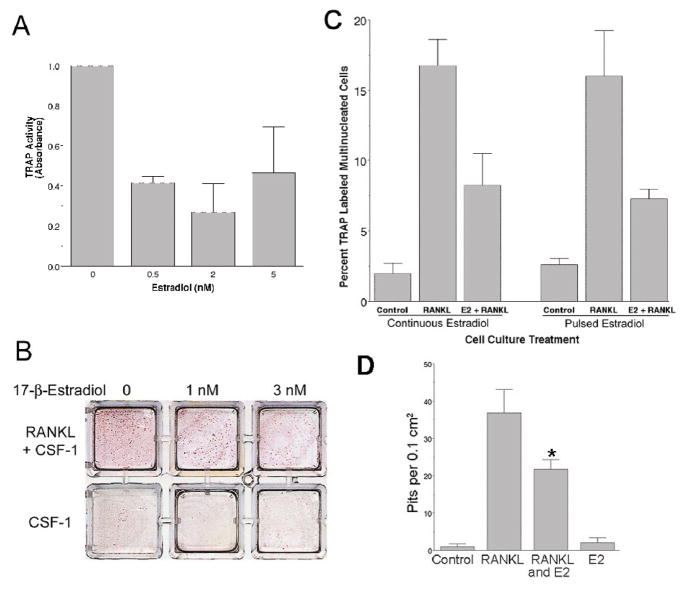
Tartrate-resistant acid phosphatase (TRAP) activity was evaluated first as a measure of osteoclastic differentiation in response to RANKL (40 ng/ml), in the presence and absence of estradiol, from CD14-positive precursor cells. After two weeks, cultures cotreated with estradiol showed significantly lower TRAP activity, compared to cultures treated with RANKL alone (Figure 2A). Analysis of variance demonstrated a significant difference between results in the absence versus the presence of estradiol (p<0.5). At estradiol concentrations of 0.5–5 nM, significant differences were not found amongst the estradiol treated cultures; at the concentrations tested, estradiol reduced TRAP by approximately 50%. Concentrations up to 10 nM produced similar effects (data not shown). The decrease in RANKL-stimulated TRAP positivity was visually apparent in cultures cotreated for two weeks with estradiol and RANKL, when compared to RANKL treatment alone (Figure 2B). Comparison of estradiol treated cultures without and with RANKL, however, revealed that TRAP still exceeded controls when RANKL was present. The estradiol concentrations tested reflect the physiological range of estrogen in humans. In premenopausal adult women, estrogen cycles at 0.2–0.4 nM; concentrations are higher in pregnancy, reaching 10–30 nM in the third trimester [37]. After menopause, serum estrogen levels are typically <0.1 nM, as are serum estrogen concentrations in men. Osteoclastic differentiation was further evaluated by determining the numbers of TRAP positive multinucleated cell numbers formed in the cultures of CD14 positive cells. RANKL markedly increased the numbers of TRAP positive multinucleated cells, as expected, and this effect was significantly inhibited by co-treatment with estradiol (Figure 2C, bars 2 and 3, p<0.05). We then evaluated whether sustained exposure to estradiol was necessary for the observed inhibition of RANKL-dependent osteoclastic differentiation. Parallel cultures were co-treated with estradiol and RANKL but only for the initial ~18 hours; estradiol was then removed while RANKL treatment continued for the remaining 9 days. Evaluation of TRAP positive multinucleated cell numbers in these cultures revealed that inhibition of RANKL-stimulated osteoclastic differentiation also occurred with only initial, transient exposure to estrogen (Figure 2C, bars 5 and 6, p<0.05). Furthermore, the inhibition produced by transient estradiol treatment was of the same magnitude (approximately 50%) as inhibition with continuous estradiol cotreatment. We also evaluated the effect of estrogen on mineral resorption by osteoclasts generated from human CD14-positive cells. These cells were grown on dentine sections in charcoal stripped medium with CSF-1 and 40 ng/ml RANKL for 14 days, with or without estradiol. Cells were then removed, and the dentine was stained with toluidine blue to demonstrate resorption pits. In keeping with the effect of estradiol on differentiation, there was a significant decrement in pit formation in cultures treated with 10 nM estradiol (Figure 2D). The decrease in resorption was similar to the decrease in numbers of TRAP-positive multinucleated cells
Figure 2. The effect of estrogen on in vitro osteoclast differentiation and activity using recombinant RANKL and nontransformed human CD14+ cells.
A. Effect of estradiol cotreatment on TRAP activity. CD14-selected human peripheral blood cells were treated in duplicate with 40 ng/ml of soluble RANKL and the indicated concentrations of 17-β-estradiol. Cultures not treated with estradiol received an equivalent volume of vehicle (ethanol). After two weeks in culture, tartrate-resistant acid phosphatase (TRAP) activity was assayed as described in Methods. Activity is normalized to control (=1); the difference of control relative to the estradiol treated cultures is significant by analysis of variance, p<0.05. Similar effects were observed in experiments using alternative osteoclast assays (see below). Estradiol at concentrations ranging from 0.5 to 5 nM significantly reduced, but did not eliminate, all TRAP activity in the RANKL-treated cultures. N=2, mean ± range.
B. Effects of estradiol on TRAP positive osteoclast formation. Six wells with identical isolates of CD14-selected cells treated with 40 ng/ml of RANKL (upper row) or not (Control, lower row) and indicated concentrations of estradiol. Cultures not treated with estradiol received equivalent vehicle (ethanol, 0.1%). After two weeks in culture, cells were fixed, and evaluated for TRAP. TRAP-positive cells are maroon; negative cells are yellow-brown due to staining by the naphthol phosphate substrate. Individual giant cells are not resolved well at this magnification but overall reduction in TRAP-positivity is clear in RANKL-stimulated cultures co-treated with estradiol at 1 and 3 nM, consistent with the results in (A). When compared to the control cultures, however, the inhibition of TRAP by estradiol is partial. Each well is 10 mm square.
C. Sustained inhibition of RANKL-induced osteoclast formation in cultures after overnight cotreatment with estradiol. CD14-selected human monocytes were treated with vehicle alone (Control, first and fourth bars), with 30 ng/ml RANKL plus vehicle (RANKL, second and fifth bars), or with 30 ng/ml RANKL plus 10 nM estradiol (E2 + RANKL, third and sixth bars). In the first set (bars 1–3) estradiol and RANKL treatments were continuous for 10 days. For the second set, estradiol was washed from the cultures (bar 6) after overnight incubation with estradiol (~18 hours treatment), but RANKL treatment was continued for the remaining 9 days. Cultures were then fixed, and TRAP-positive multinucleated cells counted. Cells continuously treated with both estradiol and RANKL showed significant inhibition of the RANKL-dependent increase in TRAP-positive multinucleated osteoclastic cells compared to cultures treated with RANKL alone (compare bars 2 and 3). Further, in cultures only initially cotreated with estradiol (bar 6), equivalent inhibition of RANKL-stimulated osteoclast formation occurred, compared to cultures continuously exposed to estradiol (bar 3). With both estradiol treatment conditions, the reduction in TRAP-positive multinucleated cells numbers compared to RANKL treatment alone was statistically significant (p<0.05). N = 6 (pulsed) or 4 (continuous), mean ± SEM.
D. Effect of estradiol cotreatment on resorption. CD14-selected human peripheral blood cells were grown on a dentine substrate without RANKL (Control), with 40 ng/ml RANKL (RANKL), with 40 ng/ml RANKL and 10 nM estradiol (RANKL and E2), or with 10 nM estradiol alone (E2). Cultures not treated with estradiol received an equal volume of vehicle. After 10 days, cells were removed and the dentine slices were stained with toluidine blue to show resorption pits, which were counted. Co-treatment with estradiol significantly reduced the numbers of resorption pits per cm2, compared to cultures treated only with RANKL (p <0.05). N=4. mean ± SEM.
Estrogen reduces RANKL-dependent NF-κB nuclear translocation at short time points
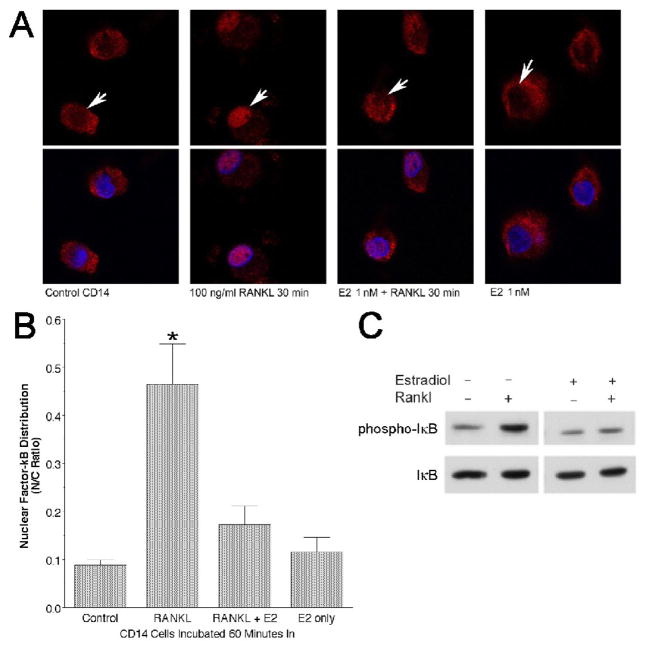
Our earlier studies in the murine monocytic cell line RAW264.7 [6] suggested that estradiol may inhibit early events in RANKL signaling. This observation, and studies of others using avian osteoclasts [38], raised the possibility that estradiol effects on osteoclasts may be mediated by extranuclear nongenomic mechanisms. Whether human cells respond similarly to estradiol was uncertain. NF-κB activation and translocation to the nucleus is a key step in RANKL signal transduction. We measured nuclear localization of NF-κB using laser scanning confocal imaging of cultures of nontransformed human CD14-selected cells treated with RANKL in the presence or absence of 1 nM estradiol (representative fields in Figure 3A, quantitative results in Figure 3B). Increased nuclear NF-κB was clear after 30 minutes treatment with RANKL, but nuclear localization of NF-κB was significantly inhibited when cultures were treated with estradiol as well as RANKL. In these experiments, a strong RANKL stimulus (100 ng/ml) was used; at RANKL concentrations of 10–20 ng/ml, the RANKL-dependent nuclear localization of NF-κB was less consistent and complete (not shown), hampering assessment of estradiol effects.
Figure 3. Estrogen cotreatment inhibits RANKL signaling consequences.
A. Confocal images of NF-κB subcellular localization in CD14-positive cells after treatment with RANKL, with and without estradiol co-treatment. CD14 selected cells without treatment (control), treated with 100 ng/ml RANKL (RANKL), with 1 nM estradiol and 100 ng/ml RANKL (E2+RANKL), or with 1 nM estradiol alone (E2 only) for 30 minutes. Cells were then fixed and labeled with anti-p65 antibody to show the distribution of NF-κB (red), with Hoechst 33342 blue to label nuclei (blue). The top row of frames shows the red signal only. Note that addition of RANKL causes strong nuclear localization of NF-κB (arrows, second versus first columns) but this effect is markedly reduced by cotreatment with estradiol (third column), while estradiol alone had no discernable effect (fourth column).
B. Effect of estradiol on NF-κB subcellular localization analyzed by laser scanning confocal microscopy. After 30 minute treatment of CD14-positive cells with RANKL, nuclear localization of NF-κB is markedly increased as expected. This increase was inhibited significantly (*p<0.01) in cultures cotreated with estradiol and RANKL (RANKL+E2). N = 6 or 7, mean ± SD.
C. Estradiol inhibits RANKL-stimulated IκB phosphorylation rapidly. CD14-positive cells were treated with 40 ng/ml RANKL, 10 nM estradiol, or both. After 5 minutes, cells were lysed and lysates evaluated by SDS-PAGE and Western blotting for phosphorylated IκB (phospho-IκB, above) and for total IκB protein (below). All lanes are from a single blot; an irrelevant lane has been omitted. Cotreatment with estradiol inhibited rapid, RANKL-dependent IκB phosphorylation.
The inhibition of NF-κB nuclear localization by estradiol might reflect inhibition of RANKL-stimulated signaling events upstream from NF-κB, rather than a direct action on NF-κB. The increase in nuclear NF-κB reflects release from bound IκB, following serine phosphorylation of IκB by RANKL-activated IκB kinase [39,40]. We therefore examined IκB phosphorylation in human CD14-positive cells treated with RANKL in the presence or absence of estradiol. Western blotting using a phospho-specific antibody recognizing only the IKK-phosphorylated IκB revealed an increase in phospho-IκB after 5 minutes treatment with RANKL alone, compared to untreated control; however, cotreatment with estradiol appeared to inhibit RANKL-stimulation of IκB phosphorylation (Figure 3C). The blots were reprobed with antibody to total IκB protein. After 5 minute of RANKL treatment, significant degradation of IκB would not be expected, and in fact we found equivalent amounts of IκB protein in all samples; this result confirmed that the changes in the anti-phospho-IκB signal did reflect differences in the amounts of total IκB protein loaded. Thus, cotreatment of human monocytes with estradiol appears to inhibit both RANKL-stimulated IκB phosphorylation and nuclear localization of NF-κB.
Differences in apoptosis were not observed for isolated human osteoclast precursors or osteoclasts with addition of estradiol at any physiological concentration
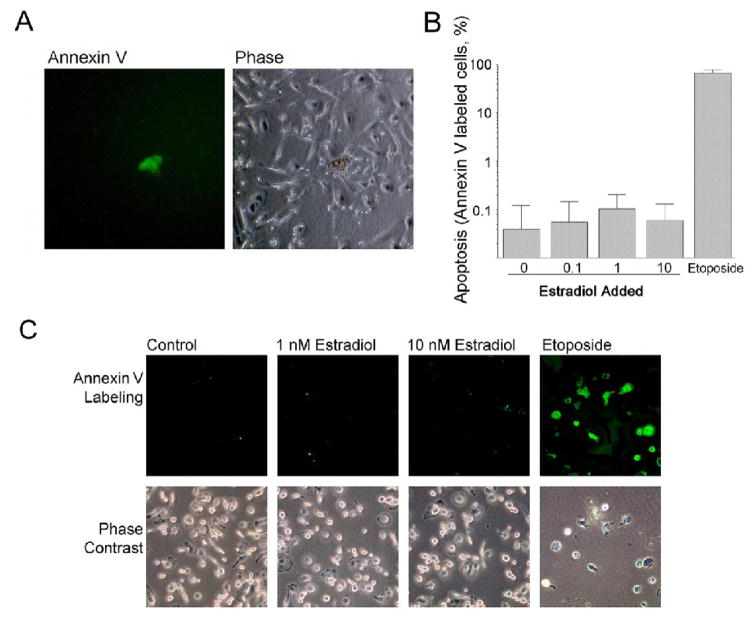
Whether osteoclasts respond to estrogen by undergoing estrogen-dependent apoptosis is controversial. We addressed whether estrogen directly induces apoptosis in human osteoclast precursors or osteoclasts using highly purified human cell preparations, as shown in Figure 1. Human CD14-selected cells were differentiated by treatment with RANKL or RANKL plus estradiol at 0.1, 1, and 10 nM, and the preparations were evaluated for apoptosis at after 2, 6, and 24 hours, 7 and 21 days. As a positive control, 0.5 μM etoposide was added 2 hours before annexin V labeling to induce efficient apoptosis. Results of these experiments were similar, and showed very low rates of apoptosis that did not vary with estradiol. Figure 4A shows a culture in 1 nM estradiol at 7 days. Annexin V labeling showed very few positive cells in these cultures treated with either RANKL alone or RANKL plus estradiol; similar results were obtained with treatment times of up to 3 weeks. Figure 4B shows quantitative results of annexin V labeling at 7 days; note that a log scale is used to allow comparisons of the wide range between very low rates of apoptosis and the efficient apoptosis induced by etoposide. The percentage of annexin V labeled cells showed no significant differences between cultures without estradiol and those cotreated with estradiol at concentrations up to 10 nM at any time tested. Experiments were also performed in cultures differentiated with RANKL, and then treated with estradiol for short periods (Fig 4C); here also, only positive controls with etoposide had significant apoptosis. These results show that the decrease in osteoclast numbers in the presence of estrogen does not reflect direct estrogen-driven apoptosis, but are compatible with decreased osteoclast formation as the mechanism for reduction by estrogen of osteoclast numbers. On the other hand, it should be noted that estrogen-dependent apoptosis in mice may occur, as there are often radical differences in steroid response between different species of mammals.
Figure 4. Effects of estradiol on survival of human osteoclasts.
A. Rare apoptotic cells in estradiol and RANKL treated cultures. In cultures of CD14-selected cells treated for 5 days with 1 nM estradiol and 40 ng/ml RANKL (upper row), apoptotic cells were rare. Apoptotic cells were identified by labeling with AlexaFluor 488-conjugated annexin V (green); necrotic cells were distinguished by nuclear labeling with propidium iodide (red). The appearance of cultures at times from 2 hours to three weeks was similar, with very few annexin V labeled cells (see text; quantification of differences at 5 days is shown in Figure 4B, below). A fluorescence micrograph (left frame) and phase image (right frame) of the same field are shown. Fields are 220 μm square.
B. Estradiol does not produce a concentration dependent increase apoptosis in RANKL-treated cells. CD14-selected cells were treated with RANKL without estradiol or with indicated concentrations of estradiol added, for 5 days. Positive controls were produced by inducing apoptosis with 0.5 μM etoposide for 2 hours (right bar). Apoptotic cells were identified by labeling with AlexaFluor 488-conjugated annexin V as in (A). The percentage of apoptotic cells present is shown. Note that a log scale is used for the y-axis to allow the wide range in apoptosis to be displayed. At all estradiol concentrations, annexin V-labeled cells represented less than 0.2%, while essentially quantitative high apoptosis resulted from etoposide treatment. Mean ± SEM for four separate assays.
C. Estradiol did not affect survival after RANKL-induced differentiation. CD14-selected human osteoclast precursors were differentiated in RANKL for 5 days then: left untreated as a negative control (column 1), treated with 1 or 10 nM Estradiol (columns 2 and3), or treated with 0.5 μM etoposide as a positive control (column 4). Annexin V labeling was used to demonstrate apoptotic cells; in the untreated and estrogen treated cultures, no cells were labeled by annexin V in most fields. In each case, a phase image is also shown for comparison (bottom row). As in long-term estradiol treatment, apoptosis was rare except for the positive control with etoposide, which induced essentially quantitative apoptosis. Fields are 220 μm square.
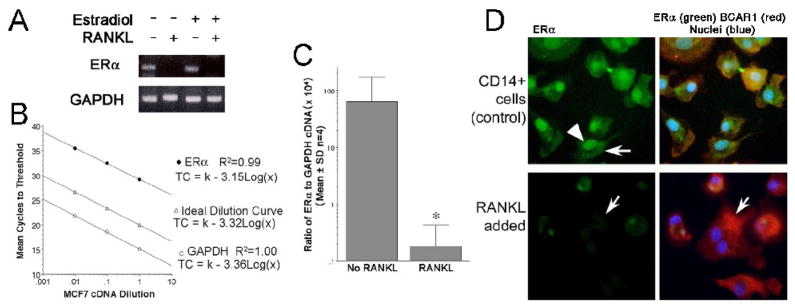
RANKL modulates expression of ERα by human osteoclast precursors
The surprising lack of estradiol effects on human osteoclast survival and the durable effect of short term-estradiol treatment on human osteoclast differentiation led us to examine the effect of RANKL on ERα expression by human osteoclastic cells and their precursors. Results from one hour RANKL treatment showed equivalent ERα in CD14 cells with or without RANKL, with or without estradiol (not illustrated). However, at 21 days cells treated with RANKL expressed essentially no ERα mRNA (Figure 5A). PCR products were evaluated by agarose gel electrophoresis and, where detectable, results confirmed amplification products of expected size. Additional cultures of CD14-selected cells were treated or not with RANKL for 1 hour or 21 days, and ERα expression was evaluated by quantitative real-time PCR, with results expressed as the ratio of ERα to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. Linearity of amplification of the target transcripts was verified using mRNA from MCF-7 cells (Figure 5B). Quantitative real-time PCR showed that the ERα was transcribed in CD14 cells, but the transcript was not produced at meaningful concentrations after RANKL-induced differentiation (Figure 5C). Fluorescence microscopy demonstrated ERα protein in nuclei and cytoplasm of cultures of CD14-selected cells maintained in culture for six days (Figure 5D, top panels; an arrowhead indicates a labeled nucleus; an arrow indicates extranuclear labeling). Our results are consistent with previously reported observations of localization of estrogen receptor-α at extranuclear as well as nuclear sites [41,42,43,44] In matched cell cultures treated in addition with 40 ng/ml RANKL, ERα was essentially undetectable (Figure 5D, lower panels; the arrow indicates a multinucleated cell, which shows essentially no ERα positivity).
Figure 5. RANKL regulates ERα expression during differentiation of CD14-positive osteoclast precursors.
A. Effect of RANKL on ERα mRNA in CD14 selected cells. RNA was isolated after three weeks from untreated cells (No RANKL) and from cells treated with 40 ng/ml RANKL. RNA from each cell culture was reverse transcribed and the resulting cDNA analyzed by PCR. From untreated CD14-selected cells, ERα products of the expected size (125 bp) were demonstrated by agarose gel electrophoresis (top image), but cells differentiated in RANKL did not produce detectible ERα. Quality of reverse-transcribed RNA was verified by amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 238 bp, bottom image) from the same cDNA samples. Results of cultures three weeks in media without or with 40 ng/ml RANKL and without or with 1 nM estradiol are shown.
B. Standard curves for ERα and GAPDH. To verify that amplification of ERα and GAPDH segments had similar efficiency, standard curves were run using cDNA reverse transcribed from MCF7 cells, used as well characterized control cells that transcribe the genes of interest (top line, ERα cycles to threshold versus log [concentration]; bottom line, GAPDH cycles to threshold versus log [concentration]), relative to a theoretical curve (middle line) demonstrating the expected relationship of concentration and threshold cycles. Linearity of the amplicons by threshold cycle over a 100 fold range of dilutions demonstrates the suitability of the probe-sets for quantitative PCR comparisons.
C. Quantitative Real Time PCR for ERα relative to GAPDH in human osteoclast precursors and osteoclasts. The ratio of ERα to GAPDH mRNA from cultures treated or not with RANKL is shown. The decrease in ERα expression was calculated from cycle threshold data shown in (B), relative to GAPDH. Note the log scale, used to allow data with several hundred-fold differences to be displayed on the same scale. The expression of ERα in RANKL-treated cells was negligible. N=4, mean ± SD.
D. ERα protein identified by immunofluorescence microscopy in osteoclast precursors. CD14-selected cells were treated or not with 20 ng/ml RANKL for 6 days then fixed and labeled for ERα (green), BCAR1 (red), and Hoechst 33342 to label nuclei (blue). Results for ERα alone are shown in the first column, and the three labels together in the second column. CD14-selected cells (top row) showed, as expected, nuclear (arrowhead) and non-nuclear ERα (arrow). Controls omitting the antibody to ERα were negative (not shown). After 6 days treatment with RANKL (bottom row), early conversion to multinucleated osteoclasts had begun, and there was a marked reduction of ERα with residual ERα essentially limited to the mononuclear cells. In multinucleated cells ERα was not visible (example, arrow). Images: 85 μm square.
Estrogen and RANKL stimulate the formation of signaling complexes containing ERα and BCAR1
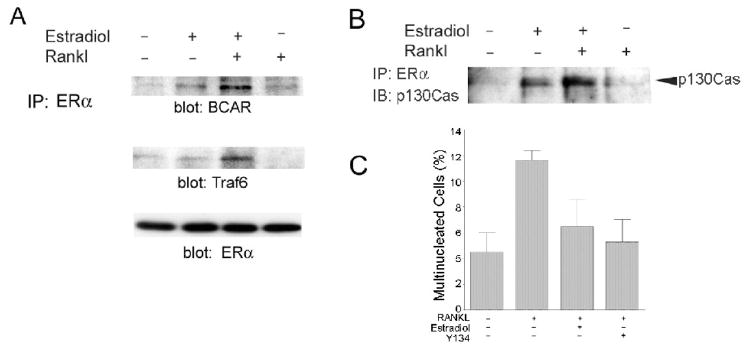
Cabodi et al. [23] found that rapid nongenomic estrogen effects in breast cancer cells were mediated by the interactions between ERα and a scaffolding protein, p130Cas, the murine equivalent of human BCAR1 (breast cancer anti-estrogen resistance protein 1) [24]. We observed that BCAR1 mRNA and protein were abundant in human CD14+ cells (not shown), and so investigated whether a similar mechanism might be involved in the rapid effects of estrogen on RANKL signaling in osteoclast precursors. CD14-positive human peripheral blood cells were treated for 5 minutes with RANKL with or without estradiol, and Western blotting was performed to detect proteins co-precipitated with anti-ERα. As in breast cancer cells, estradiol treatment of monocytic osteoclast precursors stimulated the association of BCAR1 with ERα; in osteoclast precursors, we further observed that the association of ERα with BCAR1 was enhanced by co-treatment with RANKL (Figure 6A). We then investigated whether components of RANKL-stimulated signaling pathways might also be associated. Reprobing the Western blot with antibodies to Traf6 revealed its co-precipitation after treatment with RANKL and estradiol, but not by either treatment alone (Figure 6A, middle panel). The importance of Traf6 for RANKL-stimulated osteoclast formation was shown by severe osteopetrosis in mice with targeted disruption of the Traf6 gene [45]. Equivalent recovery of ERα was confirmed by anti-ERα in serial Western blots (Figure 6A, bottom). Even more than estradiol effects on NF-κB localization, the effects of estradiol on early RANKL signaling were rapid, with maximum effects detected at 5 minutes and a decline evident by 15 minutes. The results in Figure 6A demonstrate effects of 5 minute ligand stimulation. Time courses revealed that by 15 minutes the association between ERα and BCAR1 was decreasing (not shown). In some experiments, RANKL alone appeared to stimulate some ERα-BCAR1 complex formation compared to untreated controls, but the effect was variable. The observation of ERα-BCAR1 complex formation was confirmed in studies using RAW264.7 murine cells, which showed that estradiol stimulated the association of ERα and p130Cas, and that formation of this complex was enhanced when cells were co-treated with RANKL (Figure 6B). We also investigated the role of ERα in the inhibition of osteoclast differentiation by estradiol using the ERα selective ligand Y134. It, like estradiol, significantly inhibited RANKL-dependent multinucleation (Figure 6C).
Figure 6. Estrogen and RANKL modulate interactions of ERα with BCAR1 and Traf6.
A. Effects of estrogen and RANKL on ERα protein interactions in human CD14+ cells. Adherent CD14-positive cells were treated for 5 minutes with vehicle (control), with 10nM estradiol, 10 nM estradiol and 40 ng/ml RANKL, or 40 ng/ml RANKL, then lysed. Anti-ERα immunoprecipitates from lysates were analyzed by Western blotting with antibodies to the scaffolding protein BCAR1 (top) and to the RANKL-NF-κB signaling intermediate Traf6 (middle). Estrogen stimulated co-immunoprecipitation of BCAR1 with ERα, and this association was enhanced by RANKL cotreatment. Cotreatment of cells with estradiol and RANKL also resulted in co-immunoprecipitation of Traf6. This shows interactions of estrogen and RANKL signaling intermediates in keeping with modulation of RANKL signaling by estrogen. Western blots reprobed with anti-ERα (bottom) confirmed equivalent recovery of ERα from each lysate.
B. p130Cas(BCAR1)-ERα complexes form in response to estrogen and are enhanced by RANKL in RAW 264.7 cells. RAW264.7 cells, a pre-osteoclastic murine cell line, were treated for 5 minutes with 10 nM estradiol with or without 40 ng/ml RANKL or with RANKL alone, lysed, and proteins were immunoprecipitated with antibody to ERα. The estrogen-stimulated association of p130Cas (the murine homolog of BCAR1) with ERα was again detected, and RANKL enhanced the association of BCAR1 with ERα.
C. Role of ERαand in estrogen inhibition of RANKL-stimulated multinucleation. Cells were treated on day 1 with RANKL (20 ng/ml), with estradiol (0.5 nM) or with the ERα specific ligand Y134 (5 μM) as specified. After 16 days of incubation, the percentage of multinucleated cells was counted. The cultures treated with RANKL alone have more multinucleated cells than the control (p<0.05), but the cultures with estradiol or Y134 do not. Note that the parameter analyzed is total multinucleated cells rather than TRAP-expressing multinucleated cells, and that some multinucleation (without osteoclast differentiation) occurs in the absence of RANKL. N=2, Mean ± range.
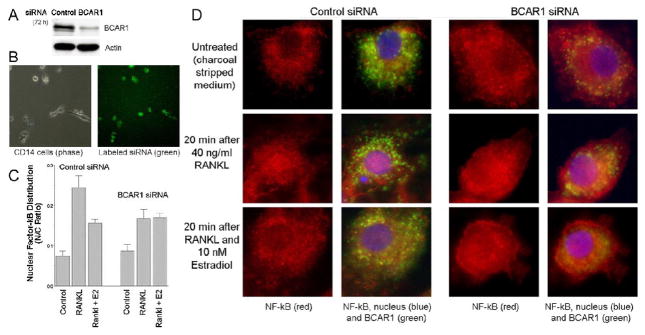
BCAR1 suppression blocks effects of estrogen on NF-κB translocation
Results of antibody precipitation studies indicated that ERα forms a complex with BCAR1, and that their association is regulated by estrogen and by RANKL. This suggested that the ERα-BCAR1 complex might represent the convergence point of RANKL and nongenomic estrogen signaling. To test this hypothesis, we analyzed the effects of inhibiting BCAR1 protein expression by RNA interference using transfected double-stranded siRNA. CD14-selected human monocytes were transfected with siRNA duplexes targeting human BCAR1 (Dharmacon SmartPool siRNA) or with control siRNA (Ambion). Transfected cells were analyzed for BCAR1 by Western blot. There was marked inhibition of BCAR1 expression. Suppression of BCAR1 was evident from 48 to 96 hours after siRNA transfection (not shown) with maximal effects at 72 hours (Figure 7A). We evaluated the efficiency of siRNA transfection using fluorescently labeled of siRNA. This showed > 90% transfection efficiency (Figure 7B). Using BCAR1 deficient and control siRNA-treated cells, the effect of RANKL and estradiol on NF-κB nuclear localization was determined in cells using anti-NF-κB antibodies, as in Figure 2. Fluorescence intensity was measured for nucleus and cytoplasm, and the ratio of nuclear to non-nuclear signal was calculated (Figure 7C). Measurements in cells fixed at 30 min after RANKL addition demonstrated RANKL induced nuclear localization of NF-κB. Consistent with our previous results (Figure 2), 10 nM estradiol cotreatment reduced the RANKL effect on NF-κB subcellular localization (Figure 7C bars 2 versus 3). In contrast, when BCAR1 was suppressed, estradiol had no effect on RANKL-stimulated NF-κB nuclear localization (Figure 7C, bars 5 versus 6). This supported our hypothesis that nongenomic effects of estrogen on RANKL signaling are dependent on BCAR1. Unexpectedly, reduction of BCAR1 protein itself inhibited RANKL-induced NF-κB nuclear translocation (Figure 7C, bars 2 versus 5). Examples of fluorescent antibody labeling for BCAR1 and NF-κB, and Hoechst nuclear staining, for cells from each of the treatment conditions studied are shown in Figure 7D.
Figure 7. Effect of inhibition of BCAR1 expression on estrogen and RANKL signaling.
A. Inhibition of BCAR1 expression by targeted siRNA. CD14-positive cells transfected with control or BCAR1 siRNA as described in Methods were lysed after 72 hours. Lysates were analyzed by Western blotting for BCAR1 (upper) and Actin (lower).
B. Efficiency of siRNA transfection. CD14-positive cells were transfected with siRNA labeled with a fluorescent tag (Ambion). Fluorescence microscopy was used to evaluate transfected cells at 48 hours. Fluorescence (right) and phase (left) micrographs of a representative field are shown; over 90% of cells were labeled.
C. Effects of BCAR1 suppression on estrogen inhibition of RANKL signaling. CD14-positive cells were transfected with control or BCAR1 siRNA. After 72 hours, cells were treated for 30 minutes with vehicle (Control), with 40 ng/ml RANKL (RANKL), or with 10 nM estradiol and 40 ng/ml RANKL (RANKL + E2). Cultures were then fixed and labeled for NF-κB (red) and BCAR1 (green), plus Hoechst dye to label the nuclei. The fluorescence signal for NF-κB was measured in the nucleus and cytoplasm; the nuclear fraction was calculated and plotted (mean ± SEM, N=4). The first three bars show the results for cells treated with control siRNA; in these cells, estradiol significantly inhibited the RANKL-stimulated increase in nuclear localization of NF-κB, as expected (Figure 3). The second set of three bars show results for cells treated with BCAR1 siRNA; in these cells lacking BCAR1, estradiol no longer reduced NF-κB nuclear localization.
D. Immunofluorescent detection of BCAR1 protein and subcellular localization of NF-κB. Representative fields from cultures analyzed in 3C are shown: results for cells transfected with control siRNA in columns 1 and 2, and results for cells transfected with BCAR1 siRNA columns 3 and 4. Columns 1 and 3 shows results with anti-NF-κB antibodies alone (red); columns 2 and 4 show the same field with merged images for anti-NF-κB (red), anti-BCAR1 (green) and the Hoechst 33342 blue to label nuclei. The top row shows controls, the middle row shows RANKL-treated cells, and the bottom row shows cells cotreated RANKL plus estradiol.
Discussion
Mechanisms by which estrogen reduces osteoclast activity are controversial. We show that estrogen inhibits of nuclear translocation of NF-κB during early signaling. Our results support the hypothesis that rapid nongenomic estrogen effects on osteoclast maturation depend, in major part, on interaction of ERα with the scaffolding protein BCAR1. RANKL signaling in human and murine osteoclast precursors was also reduced when BCAR1 was absent. Thus, in the osteoclast, BCAR1 is critical for the function of intermediate complexes that balance three key control pathways, the tyrosine kinase signal from CSF-1 [46], the TNF-receptor signal from RANK, and the estrogen signal via ERα.
The reduction of osteoclast formation by estrogen in nontransformed human cells (Figures 1–2) is in keeping with the effect of estrogen on differentiation of transformed mouse Raw264.7 monocytic cells [6]. The suppression of osteoclast differentiation in our isolated nontransformed CD14+ cells was similar to the effect of estrogen in murine marrow osteoclast precursors [8], and in osteoclast formation in bone marrow cells of postmenopausal women [47]. Sorensen et al. [48] reported slightly greater inhibition by estrogen of osteoclast formation than we observed, but aside from minor differences, reduction by estrogen of osteoclast differentiation in human or rodent precursor cells appear consistent. In a few cases, insensitivity of osteoclast differentiation, including human osteoclast differentiation, to estrogen has been reported [20]. This might reflect the loss of ERα expression during differentiation of pre-osteoclastic cells in vitro (Fig 5).
Effects on osteoclast differentiation did not require the long term presence of estrogen. Indeed, in keeping with previous reports of a lack of estrogen effects on fully differentiated human osteoclasts [20,48], we found that ERα, though present in CD14-expressing osteoclast precursors, was strongly down-regulated by RANKL (Figure 5). Earlier studies by others concluded that rapid nongenomic estrogen effects on avian osteoclast activity exist [38], although a specific mechanism was not identified, but estrogen responses in human cells appear to be limited to osteoclast precursors. Some studies in mice and murine cell lines also implicated non-genomic mechanisms in estrogen effects on osteoclastic differentiation [8,49]. Here we show that, at least at initial stages, the estrogen effect on human osteoclast precursors depends on the association of the estrogen receptor with BCAR1 (in rodents called p130Cas), which mediates nongenomic effects of estrogen in breast cancer cells [23]. In breast cancer cells, p130Cas forms complexes including Src and PI-3-kinase. In human CD14-positive cells, in the presence of estrogen and RANKL, a BCAR1 complex also precipitates Traf6. This is likely to be critical to the observation that estrogen reduces the rate of osteoclast differentiation, but does not eliminate osteoclast formation even at high concentrations of estrogen (10 nM), which occur in women only during the third trimester of pregnancy. The interaction between Traf6 and BCAR1 may also affect degradation of proteins in the complex, analogous to the effect of TRAF6 binding to the protein MAST205 [50]. In particular, in the presence of RANKL the complex of ERα and BCAR1 was enhanced (Fig 6A–B).
The controversy as to estrogen and osteoclast apoptosis reflects differences in model systems. Isolated human nontransformed osteoclasts and their precursors show no estrogen-dependent apoptosis (Figure 3). Murine osteoclasts lose much of their ERα in differentiation [6], while human osteoclasts had for practical purpose no ERα at all after differentiation in RANKL. Other studies are consistent with no differences in osteoclast activity or survival with estrogen added [20,48,51]. But different outcomes are reported in some cell culture studies, such as primary isolated rabbit osteoclasts, where osteoclast apoptosis induced by estrogen was found [17]. These inconsistencies may represent species differences, or may reflect the effects of contaminating cells, such as osteoblasts, which are very difficult to eliminate completely from bone marrow-isolated cells. In addition, in female transgenic mice in which ERα was deleted by Cre recombinase driven by the cathepsin K promoter lost bone relative to wild type animals, an effect attributed to Fas ligand-dependent apoptosis in control animals [19]. This probably reflects interactions between multiple types of cells. Specifically, cathepsin K is expressed in monocytes and macrophages as well as in osteoclasts [52], and in human osteoblasts [53,54]. We assayed cathepsin K mRNA and did immune labeling in human CD14-positive cells and osteoblasts and results (not shown) were in accord with these reports. Regarding estrogen and osteoclasts, it should be noted that estrogen concentrations are highest during pregnancy, when human bone resorption is robust to the point that maternal skeleton is sacrificed to allow fetal skeletal growth if dietary calcium is inadequate [55]. Bone loss in pregnancy is a common finding even with good nutrition, although it is for the most part reversible. However, it is important to consider that estrogen-dependent osteoclast apoptosis may occur in mice and other species, but not human, osteoclasts; in particular several precedents exist for major differences in sex steroid response between humans and rodents (cf. [22]).
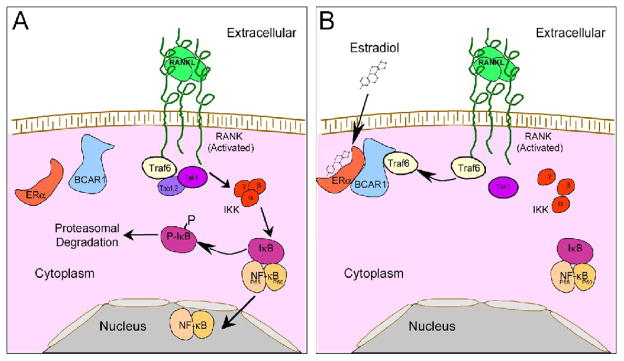
Our work supports a model in which estradiol regulates early stages of the response to RANKL in osteoclast precursors (Figure 8). This, at least in major part, reflects nongenomic interactions of ERα with intermediate proteins. An important estrogen-dependent protein complex includes BCAR1 (p130Cas) and ERα. Cabodi et al. [23] found that, in MCF7 breast cancer cells, p130Cas is important in modulating Src kinase-dependent cellular activity. Independently, BCAR1 was discovered to be important in osteoclastic cytoskeletal organization [25], and later that BCAR1 mediates CSF-1 and adhesion-related signals in osteoclasts [46]. Importantly, BCAR1 binds Traf6 [56], which is essential for RANK signaling in osteoclasts. Since effects of estradiol on Erk and tyrosine kinase activity in murine Raw264.7 cells follow estrogen-dependent suppression of NF-κB nuclear localization [6], it is logical that BCAR1 is, at least in part, an initial mediator of ERα-dependent effects on osteoclast differentiation. The association of BCAR1 with Traf6 is of key importance because it is an early intermediate protein involved signaling of a number of TNF receptors including RANK [57]. In osteoclast differentiation, Traf6 plays a major role in RANK signaling [34,45,57]. Traf6 stimulates the formation of a complex including Tab1, Tab2, and the kinase Tak1. This leads to the activation of Tak1 by a mechanism involving Traf-6 dependent non-classical ubiquitination of Tak1 [58,59,60]. Tak1 activates IκB kinase (IKK) leading to phosphorylation of IκB; phosphorylated IκB is degraded, removing its inhibitory effects on NF-κB. We saw as expected a decreased rate of IκB phosphorylation in the presence of estradiol, although these effects were much more difficult to demonstrate in human CD14-positive monocytes than in murine cells [6]. This probably reflects that human osteoclast differentiation in vitro in response to RANKL is typically 2–3 times slower than murine osteoclast formation. It may also reflect that costimulating factors of osteoclast formation [2] were present in the nontransformed human monocytes during their isolation from buffy coats, so that cells showed in most cases a less dramatic IκB phosphorylation response when RANKL was added than is typically seen in murine marrow cells or in cell lines.
Figure 8. Model for the role of BCAR1 in the non-genomic effects of estrogen on RANKL signaling.
A. RANKL signaling from RANK to NF-κB. RANKL binding to its receptor stimulates the association of Traf6 with RANK and the assembly of the Tab1-Tab2-Tak1 complex, leading to IκB kinase (IKK) activation. IKK phosphorylates IκB, which undergoes proteosomal degradation, releasing NF-κB from the IκB-NF-κB complex, resulting in nuclear localization and activation of NF-κB.
B. RANKL plus Estradiol signaling. Treatment with both estrogen and RANKL results in the association of ERα, BCAR1 and Traf6. We hypothesize that the association of Traf6 with ERα-BCAR1, which may also serve to stabilize the ERα-BCAR1 association (see text), inhibits Traf6 protein interactions necessary for formation of the Tab1-Tab2-Tak1 complex upstream from NF-κB; IKK activation and dissolution of the IκB-NF-κB complex are thus inhibited, blocking RANKL-stimulated NF-κB nuclear localization and activation.
Absence of BCAR1 is lethal during embryonic life [61]. Given the role of BCAR1 in integrin and CSF-1 signaling, this is not surprising. Inhibition of BCAR1 expression by siRNA reduced RANKL-stimulated NF-κB nuclear translocation, a critical step in RANKL signal transduction. It is likely that BCAR1 may also inhibit osteoclast differentiation or survival at longer time points due to its interaction with integrin and fms signaling. CD14-selected cells transfected with control or BCAR1 siRNA were maintained in RANKL and CSF-1 further times. In these cells, TRAP and multinucleation were decreased relative to control cells. However, survival of BCAR1 knockdown cells was compromised to varying extents, so it is likely that BCAR1 is required for both survival and differentiation in RANKL of CD14-positive cells, but the effects must be isolated to resolve the issue. Artificial BCAR1 constructs with specific partial BCAR1 deletions [62,63] may make it possible to address this issue.
In summary, BCAR1 is essential for the rapid estrogen effect on osteoclast differentiation. Estrogen-dependent complexes were shown by immune precipitation, and differences in NF-κB activation were abolished by RNA interference targeting BCAR1. An intriguing additional possibility is that BCAR1 interactions with ERα may influence the actions of TNF-family receptors other than RANK. We noted that MCF7 breast cancer cells, which do not express RANK but which are known to be modified by TRAIL or TNFα, would thus be candidates for ERα-BCAR1 complexes that modify TNF-family receptors other than RANKL. We performed antibody precipitation of ERα from BCAR1 cells after treatment with estradiol, and found that these complexes were, indeed, markedly increased if TRAIL or TNFα were added (not illustrated). Thus, although the meaning of the ERα-BCAR1 association in MCF7 cell survival and differentiation was not studied in our work, it may be useful to consider this in further studies of nongenomic estrogen effects on breast cancer cells. It remains to be determined how the association of Traf6 is involved in the reduced NF-κB signaling, although the effect appears to be upstream of the IκB complex in murine and human osteoclast precursors. It may involve direct Traf6 binding to an intermediate complex, which would delay IκB complex degradation, as modeled in Figure 8. However, this simple hypothesis, although attractive, will require additional work and the mechanism is likely to involve many other proteins. There may, for example, be effects on Src activity required for efficient RANK activation, and BCAR1 is an important Src target with this action largely, in the osteoclast, downstream of CSF-1 activity via its receptor Fms [46]. Furthermore, additional extranuclear interactions of ERα with other signaling intermediates may occur in osteoclasts. An adaptor protein that is present in osteoclasts and osteoclast precursors, Shc, forms estrogen-dependent complexes that include ERα [64] and are important for the localization of the estrogen receptor at the plasma membrane that is necessary for further signaling protein interactions. While not directly implicated in RANK signaling, pathways involving Shc could mediate apoptosis under some circumstances [65], although we observed no apoptosis in vitro.
Acknowledgments
This work was supported in part by grants from the US National Institutes of Health AR053566, AR47700, and by the Department of Veterans Affairs (USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu BY, Wu PW, Bringhurst FR, Wang JT. Estrogen inhibition of PTH-stimulated osteoclast formation and attachment in vitro: involvement of both PKA and PKC. Endocrinology. 2002;143:627–635. doi: 10.1210/endo.143.2.8614. [DOI] [PubMed] [Google Scholar]
- 2.Blair HC, Zaidi M. Osteoclastic differentiation and function regulated by old and new pathways. Rev Endocr Metab Disord. 2006;7:23–32. doi: 10.1007/s11154-006-9010-4. [DOI] [PubMed] [Google Scholar]
- 3.Zallone A. Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann NY Acad Sci. 2006;1068:173–179. doi: 10.1196/annals.1346.019. [DOI] [PubMed] [Google Scholar]
- 4.Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252:68–80. doi: 10.1016/j.cellimm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Saintier D, Khanine V, Uzan B, Ea HK, de Vernejoul MC, Cohen-Solal ME. Estradiol inhibits adhesion and promotes apoptosis in murine osteoclasts in vitro. J Steroid Biochem Mol Biol. 2006;99:165–173. doi: 10.1016/j.jsbmb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.García Palacios V, Robinson LJ, Borysenko CW, Kalla SE, Blair HC. Negative regulation of RANKL induced osteoclastic differentiation in RAW264.7 cells by estrogen and phytoestrogens. J Biol Chem. 2005;280:13720–13727. doi: 10.1074/jbc.M410995200. [DOI] [PubMed] [Google Scholar]
- 7.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O’Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava S, Toraldo G, Weitzmann MN, Cenci S, Ross FP, Pacifici R. Estrogen decreases osteoclast formation by down-regulating receptor activator of NF-κB ligand (RANKL)-induced JNK activation. J Biol Chem. 2001;276:8836–8840. doi: 10.1074/jbc.M010764200. [DOI] [PubMed] [Google Scholar]
- 9.Ravenkar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 10.Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17-beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2005;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- 11.Heino TJ, Chagin AS, Sävendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J Endocrinol. 2008;197:R1–R6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- 12.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocrine Reviews. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 13.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 14.Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- 15.Song RXD, Zhang Z, Santen RJ. Estrogen rapid action via protein complex formation involving ERα and Src. Trends Endocrinol Metab. 2005;16:347–353. doi: 10.1016/j.tem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Moriarty K, Kim KH, Bender JR. Minireview: Estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 17.Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC. Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem. 2005;280:4632–4638. doi: 10.1074/jbc.M411530200. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor-α and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27:535–545. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacic N, Lukic IK, Grcevic D, Katavic V, Croucher P, Marusic A. The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol. 2007;178:3379–3389. doi: 10.4049/jimmunol.178.6.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo SW, Olive DL. Two unsuccessful clinical trials on endometriosis and a few lessons learned. Gynecol Obstet Invest. 2007;64:24–35. doi: 10.1159/000098413. [DOI] [PubMed] [Google Scholar]
- 23.Cabodi S, Moro L, Baj G, Smeriglio M, Di Stefano P, Gippone S, Surico N, Silengo L, Turco E, Tarone G, Defilippi P. p130Cas interacts with estrogen receptor-α and modulates non-genomic estrogen signaling in breast cancer cells. J Cell Sci. 2004;117:1603–1611. doi: 10.1242/jcs.01025. [DOI] [PubMed] [Google Scholar]
- 24.Dorssers LC, van Agthoven T, Dekker A, van Agthoven TL, Kok EM. Induction of antiestrogen resistance in human breast cancer cells by random insertional mutagenesis using defective retroviruses: identification of BCAR-1, a common integration site. Mol Endocrinol. 1993;7:870–878. doi: 10.1210/mend.7.7.8413311. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura I, Jimi E, Duong LT, Sasaki T, Takahashi N, Rodan GA, Suda T. Tyrosine phosphorylation of p130Cas is involved in actin organization in osteoclasts. J Biol Chem. 1998;273:11144–11149. doi: 10.1074/jbc.273.18.11144. [DOI] [PubMed] [Google Scholar]
- 26.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 28.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Yaroslavskiy BB, Sharrow AC, Wells A, Robinson LJ, Blair HC. Necessity of inositol-1,4,5-trisphosphate receptor-1 and μ-calpain in nitric oxide induced osteoclast motility. J Cell Sci. 2007;120:2884–2894. doi: 10.1242/jcs.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair HC, Borysenko CW, Villa A, Schlesinger PH, Kalla SE, Yaroslavskiy BB, Garcia Palacios V, Oakley JI, Orchard PJ. In vitro differentiation of CD14 cells from osteopetrotic subjects: contrasting phenotypes with TCIRG1, CLCN7, and attachment defects. J Bone Miner Res. 2004;19:1329–1338. doi: 10.1359/JBMR.040403. [DOI] [PubMed] [Google Scholar]
- 31.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Xue J, Zadorozny EV, Robinson LJ. G-CSF stimulates Jak2-dependent Gab2 phosphorylation leading to Erk1/2 activation and cell proliferation. Cell Signal. 2008;20:1890–1899. doi: 10.1016/j.cellsig.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Blair HC, Robinson LJ, Zaidi M. Osteoclast signaling pathways. Biochem Biophys Res Commun. 2005;328:728–738. doi: 10.1016/j.bbrc.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 35.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 36.Lazarus HM, Haynesworth SE, Gerson SL, Caplan AI. Human bone marrow-derived mesenchymal (stromal) progenitor cells (MPCs) cannot be recovered from peripheral blood progenitor cell collections. J Hematother. 1997;6:447–455. doi: 10.1089/scd.1.1997.6.447. [DOI] [PubMed] [Google Scholar]
- 37.Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and FSH across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–1561. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 38.Brubaker KD, Gay CV. Estrogen stimulates protein tyrosine phosphorylation and Src kinase activity in avian osteoclasts. J Cell Biochem. 1999;76:206–216. doi: 10.1002/(sici)1097-4644(20000201)76:2<206::aid-jcb5>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 41.Norfleet AM, Thomas ML, Gametchu B, Watson CS. CS 1999 Estrogen receptor- detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology. 1999;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- 42.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors derive from a single transcript: studies of ERα and ERβ expressed in CHO cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 43.Marquez D, Chen HW, Curran E, Welshons WV, Pietras RJ. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol. 2006;246:91–100. doi: 10.1016/j.mce.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2006. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 45.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura I, Rodan GA, Duong le T. Distinct roles of p130Cas and c-Cbl in adhesion-induced or macrophage colony-stimulating factor-mediated signaling pathways in prefusion osteoclasts. Endocrinology. 2003;144:4739–4741. doi: 10.1210/en.2003-0615. [DOI] [PubMed] [Google Scholar]
- 47.Taxel P, Kaneko H, Lee SK, Aguila HL, Raisz LG, Lorenzo JA. Estradiol rapidly inhibits osteoclastogenesis and RANKL expression in bone marrow cultures in postmenopausal women: a pilot study. Osteoporos Int. 2008;19:193–199. doi: 10.1007/s00198-007-0452-7. [DOI] [PubMed] [Google Scholar]
- 48.Sorensen MG, Henriksen K, Dziegiel MH, Tankó LB, Karsdal MA. Estrogen directly attenuates human osteoclastogenesis, but has no effect on resorption by mature osteoclasts. DNA Cell Biol. 2006;25:475–483. doi: 10.1089/dna.2006.25.475. [DOI] [PubMed] [Google Scholar]
- 49.Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–1664. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong H, Li H, Chen Y, Zhao J, Unkeless JC. Interaction of TRAF6 with MAST205 regulates NF-κB activation and MAST205 stability. J Biol Chem. 2004;279:43675–43683. doi: 10.1074/jbc.M404328200. [DOI] [PubMed] [Google Scholar]
- 51.Williams JP, Blair HC, McKenna MA, Jordan SE, McDonald JM. Regulation of avian osteoclastic H+-ATPase and bone resorption by tamoxifen and calmodulin antagonists. Effects independent of steroid receptors. J Biol Chem. 1996;271:12488–12495. doi: 10.1074/jbc.271.21.12488. [DOI] [PubMed] [Google Scholar]
- 52.Tamaki Y, Sasaki K, Sasaki A, Takakubo Y, Hasegawa H, Ogino T, Konttinen YT, Salo J, Takagi M. Enhanced osteolytic potential of monocytes/macrophages derived from bone marrow after particle stimulation. J Biomed Mater Res. 2008;84:191–204. doi: 10.1002/jbm.b.30861. [DOI] [PubMed] [Google Scholar]
- 53.Punturieri A, Filippov S, Allen E, Caras I, Murray R, Reddy V, Weiss SJ. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J Exp Med. 2000;192:789–799. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mandelin J, Hukkanen M, Li TF, Korhonen M, Liljeström M, Sillat T, Hanemaaijer R, Salo J, Santavirta S, Konttinen YT. Human osteoblasts produce cathepsin K. Bone. 2006;38:769–777. doi: 10.1016/j.bone.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Oliveri B, Parisi MS, Zeni S, Mautalen C. Mineral and bone mass changes during pregnancy and lactation. Nutrition. 2004;20:235–240. doi: 10.1016/j.nut.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura I, Kadono Y, Takayanagi H, Jimi E, Miyazaki T, Oda H, Nakamura K, Tanaka S, Rodan GA, le Duong T. IL-1 regulates cytoskeletal organization in osteoclasts via TNF receptor-associated factor 6/c-Src complex. J Immunol. 2002;168:5103–5109. doi: 10.4049/jimmunol.168.10.5103. [DOI] [PubMed] [Google Scholar]
- 57.Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-κB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-κB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- 58.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 59.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 60.Mizukami J, Takaesu G, Akatsuka H, Sakurai H, Ninomiya-Tsuji J, Matsumoto K, Sakurai N. RANKL activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol. 2002;22:992–1000. doi: 10.1128/MCB.22.4.992-1000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 62.Nakamoto T, Sakai R, Honda H, Ogawa S, Ueno H, Suzuki T, Aizawa S, Yazaki Y, Hirai H. Requirements for localization of p130Cas to focal adhesions. Mol Cell Biol. 1997;17:3884–3897. doi: 10.1128/mcb.17.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tazaki T, Miyazaki K, Hiyama E, Nakamoto T, Sakai R, Yamasaki N, Honda Z, Noda M, Miyasaka N, Sueda T, Honda H. Functional analysis of Src homology 3-encoding exon (exon 2) of p130Cas in primary fibroblasts derived from exon 2-specific knockout mice. Genes Cells. 2008;13:145–157. doi: 10.1111/j.1365-2443.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- 64.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor-α to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]