Abstract
Immunological unresponsiveness established by the elimination or anergy of self-reactive lymphocyte clones is of importance to immunization against tumor-associated antigens. In this study, we have investigated induction of immunity against the human MUC1 carcinoma-associated antigen in MUC1 transgenic mice unresponsive to MUC1 antigen. Immunization of adult MUC1 transgenic mice with irradiated MUC1-positive tumor cells was unsuccessful in reversing unresponsiveness to MUC1. By contrast, fusions of dendritic cells with MUC1-positive tumor cells induced cellular and humoral immunity against MUC1. Immunization with the dendritic cell fusions that express MUC1 resulted in the rejection of established metastases and no apparent autoimmunity against normal tissues. These findings demonstrate that unresponsiveness to the MUC1 tumor-associated antigen is reversible by immunization with heterokaryons of dendritic cells and MUC1-positive carcinoma cells.
The human DF3/MUC1 glycoprotein is overexpressed and aberrantly glycosylated in breast and other carcinomas (1–4). The finding that lymphocytes from certain patients with carcinomas recognize and lyse MUC1-positive tumor cells (5, 6) has suggested that this antigen is a potential target for anticancer vaccines. Whereas MUC1 is expressed on the apical borders of normal epithelium (1–3) and unresponsiveness to self-antigens is an obstacle to the development of antitumor immunity, MUC1 transgenic (MUC1.Tg) mice provide a potential model to assess the induction of anti-MUC1 immune responses. In this context, MUC1.Tg C57BL6 mice express MUC1 in a pattern and at a level similar to that found in humans (7). Significantly, the MUC1.Tg mice are tolerant to stimulation by MUC1 antigen (7).
Dendritic cells (DC) are potent antigen-presenting cells (8) that sensitize CD4+ T cells to specific antigens in a major histocompatibility complex-restricted manner (9, 10) and generate antigen-specific cytotoxic T lymphocytes (CTLs) from naive T cells in vitro (11, 12). Moreover, DCs are the only antigen-presenting cells known to prime naive CTLs and to induce antigen-specific CTLs in vivo (13). DCs pulsed with tumor antigens or synthetic peptides derived from such antigens have been effective as vaccines in the induction of CTL responses and antitumor activity (14–17). Other studies have demonstrated that transduction of DC with recombinant viral vectors expressing tumor antigens generates vaccines that induce antigen-specific antitumor immune responses (18–20). Fusions resulting in heterokaryons of DC and carcinoma cells as vaccines have provided an alternative strategy for inducing immunity against both known and unidentified tumor antigens (21).
The present studies demonstrate that MUC1.Tg mice respond to fusions of DC and MUC1-positive MC-38 carcinoma cells with induction of anti-MUC1 immunity. The findings demonstrate that a DC fusion cell vaccine can reverse unresponsiveness to a tumor-associated antigen and induce the rejection of established metastases.
MATERIALS AND METHODS
MUC1 Transgenic Mice.
A C57BL/6 mouse strain transgenic for human MUC1 was established as described (7). Tail DNA (500 ng) was subjected to PCR amplification by using MUC1 primers (bp 745–765 and bp 1,086–1,065) to confirm the presence of MUC1 sequences. The PCR product was detected by electrophoresis in a 1% agarose gel (7).
Cell Culture and Fusion.
Murine (C57BL/6) MC-38 and MB49 carcinoma cells were stably transfected with a MUC1 cDNA (22–24). Cells were maintained in DMEM supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. DCs were obtained from bone marrow culture and fused to the carcinoma cells as described (21).
Immunizations.
MUC1.Tg mice were injected subcutaneously on day 0 and day 7 with 1 × 106 MC-38/MUC1 cells exposed to 100 Gy ionizing radiation (Gammacell 1000; Atomic Energy of Canada, Ottawa). FC/MUC1 fusion cells (5 × 105) were administered subcutaneously on day 0 and day 7 for the tumor prevention studies. The FC/MUC1 cells (1 × 106) were given intravenously on days 2 and 9 or days 4 and 11 after injection of MC-38/MUC1 tumor cells in the treatment studies.
In Vitro T Cell Proliferation.
Single-cell preparations of spleen and lymph nodes were suspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cells were stimulated with 5 units/ml purified MUC1 antigen (25). After 1, 3, and 5 days of culture, the cells were pulsed with 1 μCi [3H]thymidine per well for 12 h and then collected on filters with a semiautomatic cell harvester. Radioactivity was quantitated by liquid scintillation.
Generation of CD8+ T Cell Lines.
Lymph node cells (LNC) were suspended in complete RPMI 1640 medium containing 5 units/ml MUC1 antigen. Murine interleukin 2 (10 units/ml) was added after 5 days of culture. On days 10 and 15 the cells were restimulated with 5 units/ml MUC1 antigen and 1:5 irradiated (30 Gy) syngeneic spleen cells as antigen-presenting cells. T cell cultures were analyzed after Ficoll centrifugation to remove dead cells and passage through nylon wool to deplete residual antigen-presenting cells. The T cells were stained with fluorescein isothiocyanate-conjugated antibodies against CD3e (145–2C11), CD4 (H129, 19), CD8 (53–6.7), αβTcR (H57–597), and γδ TcR (UC7–13D5) (PharMingen). After incubation on ice for 1 h, the cells were washed, fixed, and analyzed by FACScan (Becton Dickinson).
Cytotoxicity Assays.
In vitro cytotoxicity was measured in a standard 51Cr-release assay. Briefly, cells were labeled with 51Cr for 60 min at 37°C and then washed to remove unincorporated isotope. The cell targets (1 × 104) were added to wells of 96-well v-bottom plates and incubated with effector cells for 5 h at 37°C. The supernatants were assayed for 51Cr in a gamma counter. Spontaneous release of 51Cr was assessed by incubation of targets in the absence of effectors, and maximum or total release of 51Cr was determined by incubation of targets in 0.1% Triton X-100. Percentage of specific 51Cr release was determined by the following equation: percent specific release = [(experimental − spontaneous)/(maximum − spontaneous)] × 100.
Humoral Immune Responses.
Microtiter plates were coated overnight at 4°C with 5 units per well purified MUC1 antigen. The wells were washed with PBS containing 5% horse serum albumin and then incubated for 1 h with 4-fold dilutions of mouse sera. After washing and incubation with goat anti-mouse IgG conjugated to horseradish peroxidase (Amersham), antibody complexes were detected by development with o-phenylenediamine (Sigma) and measurement in an ELISA microplate autoreader EL310 at an OD of 490 nm.
Immunohistology.
Freshly removed tissues were frozen in liquid nitrogen. Tissue sections (5 μm) were prepared in a cryostat and fixed in acetone for 10 min. Sections were incubated with mAb DF3 (anti MUC1), anti-CD4 (H129, 19), or anti-CD8 (53–6.7) for 30 min at room temperature and then subjected to indirect immunoperoxidase staining by using the Vectastain ABC kit (Vector Laboratories).
RESULTS AND DISCUSSION
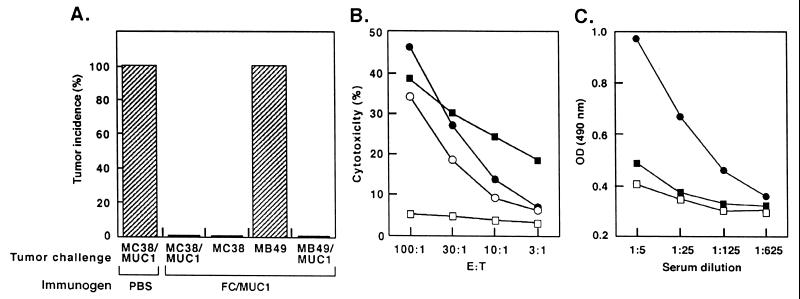
Recent studies have demonstrated that vaccines derived from fusions of DC and MC-38/MUC1 carcinoma cells (FC/MUC1) induce potent antitumor immunity (21). To assess the effects of vaccinating MUC1.Tg mice with FC/MUC1, we immunized twice with 5 × 105 FC/MUC1 and, as controls, with 106 irradiated MC-38/MUC1 cells or PBS. After challenge with 106 MC-38 or MC-38/MUC1 cells, all mice immunized with irradiated MC-38/MUC1 cells or PBS developed tumors (Fig. 1A and data not shown). By contrast, no tumor growth was observed in mice immunized with FC/MUC1 (Fig. 1A). Immunization of the MUC1.Tg mice with FC/MUC1 had no effect on growth of the unrelated MB49 bladder carcinoma (24) (Fig. 1A). However, MB49 cells that express MUC1 (MB49/MUC1) failed to grow in the FC/MUC1-immunized mice (Fig. 1A). To extend these results, CTLs from the FC/MUC1-immunized mice were assayed for lysis of target cells. CTLs from MUC1.Tg mice immunized with irradiated MC-38/MUC1 cells or PBS exhibited little if any reactivity against MC-38/MUC1 cells (data not shown). By contrast, CTLs from the mice immunized with FC/MUC1 induced lysis of MC-38, MC-38/MUC1, and MB49/MUC1, but not MB49, cells (Fig. 1B). As shown previously in wild-type mice (21), immunization with FC/MUC1 induces immunity against MUC1 and other unknown antigens on MC-38 cells. Thus, the demonstration that MB49/MUC1, and not MB49, cells are lysed by the CTLs confirms that FC/MUC1 induces a MUC1-specific response. Moreover, the finding that human MUC1-positive MCF-7 cells are not lysed by these CTLs indicates that the response is major histocompatibility complex restricted. Immunization of the MUC1.Tg mice with FC/MUC1 also induced a specific antibody response against MUC1 compared with that obtained in mice immunized with irradiated MC-38/MUC1 cells or PBS (Fig. 1C).
Figure 1.
Reversal of unresponsiveness to MUC1 antigen in MUC1 transgenic mice immunized with FC/MUC1. (A) MUC1.Tg mice were immunized twice (7-day interval) with PBS or 5 × 105 FC/MUC1 cells. The mice (10 per group) were challenged with 1 × 106 MC-38 cells, 1 × 106 MC-38/MUC1 cells, 5 × 105 MB49 bladder carcinoma, or 5 × 105 MB49/MUC1 cells. Tumors >3 mm in diameter were scored as positive. Similar results were obtained in three separate experiments. (B) MUC1.Tg mice were immunized twice (7-day interval) with 5 × 105 FC/MUC1 cells. After 20 days, splenocytes were isolated and incubated at the indicated effector:target ratios with 51Cr-labeled MC-38 (○), MC-38/MUC1 (•), MB49 □, and MB49/MUC1 (▪) target cells. CTL activity was determined by 51Cr release. (C) MUC1.Tg mice were immunized twice with 5 × 105 FC/MUC1 (•), irradiated MC38/MUC1 (▪), or PBS (□). Serum samples were collected at 14 days after the last immunization and analyzed for the presence of antibodies to MUC1 by an enzyme-linked immunoabsorbent assay.
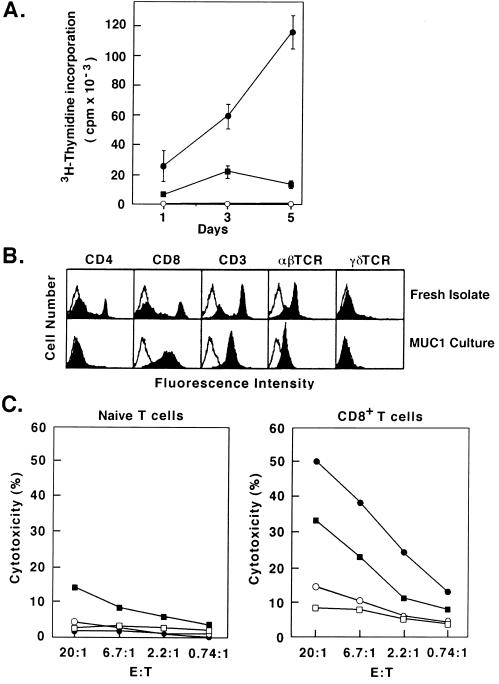
To determine whether T cells from the MUC1.Tg mice can be primed to induce an anti-MUC1 response, draining LNCs were isolated from mice immunized with irradiated MC-38/MUC1 cells or FC/MUC1. The LNC were stimulated with MUC1 antigen in vitro. The results demonstrate that LNC from mice immunized with PBS or irradiated MC-38/MUC1 cells fail to proliferate in the presence of MUC1 antigen (Fig. 2A). By contrast, LNC from mice immunized with FC/MUC1 responded to MUC1 with proliferation (Fig. 2A). To confirm the induction of CTLs against MUC1, draining LNC were isolated from MUC1 transgenic mice immunized with FC/MUC1 and cultured in the presence of MUC1 antigen and irradiated splenocytes. Cells were analyzed by FACScan at the beginning and at 10–15 days of culture. The results demonstrate the selection of a predominantly CD8+ T cell population after incubation with MUC1 antigen (Fig. 2B). Moreover, the CD8+ T cells exhibited specific CTL activity against MC-38/MUC1 and MB49/MUC1 targets compared with naive T cells from unimmunized MUC1.Tg mice (Fig. 2C). Collectively, these findings demonstrate that immunization with FC/MUC1 reverses unresponsiveness to MUC1 in the MUC1.Tg mice.
Figure 2.
Induction of MUC1-specific CTLs by FC/MUC1. (A) MUC1.Tg mice were immunized twice (7-day interval) with 5 × 105 FC/MUC1 cells (•), 1 × 106 irradiated MC-38/MUC1 cells (▪), or PBS (○). Two weeks later, draining LNCs were isolated and stimulated with 5 units/ml MUC1 antigen. Cells were cocultured for 1, 2, and 5 days. Uptake of [3H]thymidine was measured 6 h after a pulse with 1 μCi per well (1 Ci = 37 GBq). T cell proliferation was expressed as the mean of three determinations. Similar results were obtained in three separate experiments. (B) Draining LNCs were isolated from MUC1.Tg mice immunized twice (7-day interval) with 5 × 105 FC/MUC1 cells and cocultured in the presence of 5 units/ml purified MUC1 antigen. After 1 week, the LNC were restimulated with 5 units/ml MUC1 antigen and irradiated splenocytes. At day 10–15, the LNC were collected by Ficoll centrifugation and analyzed by flow cytometry. (C) Naive LNC isolated from unimmunized MUC1.Tg mice or CD8+ T cells isolated from FC/MUC1-immunized MUC1.Tg mice were incubated at the indicated effector: target ratios with 51Cr-labeled MC-38 (○), MC-38/MUC1 (•), MB49 (□), and MB49/MUC1 (▪) target cells. CTL activity was determined by 51Cr release.
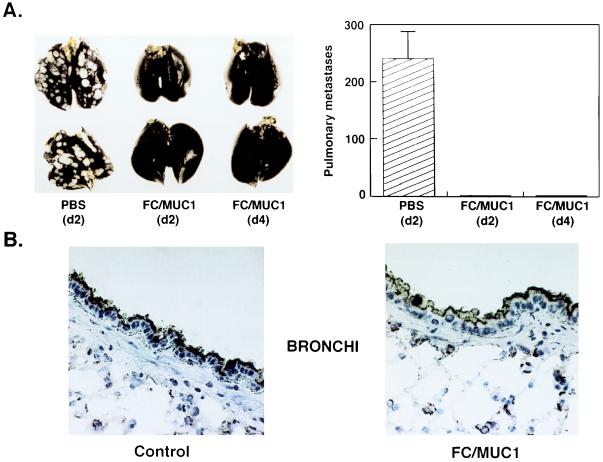
The finding that unresponsiveness to MUC1 can be reversed by immunization with FC/MUC1 suggested that this vaccine could be used to treat disseminated disease in a background of MUC1 expression by normal epithelia. In a treatment model, MC-38/MUC1 pulmonary metastases were established by tail vein injection of MC-38/MUC1 cells into the MUC1.Tg mice. Whereas control mice treated with vehicle developed pulmonary metastases, mice immunized with FC/MUC1 on days 2 or 4 had no detectable metastases (Fig. 3A). These findings indicated that FC/MUC1 immunizations can be used to treat metastatic disease in the MUC1.Tg mice. Importantly, mice protected against MC-38/MUC1 tumor exhibited persistent expression of MUC1 antigen in normal bronchial epithelium (Fig. 3B) and other tissues that express the transgene (7). Also, staining of MUC1-positive tissues with anti-CD4 and anti-CD8 antibodies failed to demonstrate T cell infiltration (data not shown).
Figure 3.
Treatment of established pulmonary metastases in MUC1.Tg mice with FC/MUC1. (A) MUC1.Tg mice were injected intravenously (tail vein) with 1 × 106 MC-38/MUC1 cells. Control mice were immunized with PBS (eight per group) on days 2 and 9 after tumor challenge. Groups of 10 mice were immunized with 1 × 106 FC/MUC1 on days 2 and 9 or days 4 and 11. The mice were sacrificed 10 days after the last immunization. Pulmonary metastases were highlighted by staining the lungs with India ink (Left). The data for all mice in the three groups is presented as metastases (mean ± SE) enumerated by sectioning the lungs (Right). (B) Sections of bronchi from MUC1.Tg mice immunized twice (7-day interval) with PBS (Left) or 1 × 106 FC/MUC1 (Right) and sacrificed 15 days later. The sections were stained with the anti-MUC1 (mAb DF3) antibody. MUC1 antigen is detectable along the apical borders of secretory epithelium lining the bronchi.
The early work of Medawar and colleagues (26) and Burnet (27) led to the hypothesis that lymphocytes are rendered tolerant by antigenic recognition in neonatal life. However, more recent work has shown that neonatal T cells can be activated by varying the dose of antigen (28), the adjuvant (29) and the type of antigen presenting cell (30). In the present studies using adult MUC1.Tg mice that are tolerant to MUC1 antigen (7), immunization with the DC-tumor cell fusions expressing MUC1 was highly effective in inducing cellular and humoral immunity against MUC1. The results support a mechanism of clonal anergy, that is the presence of reactive clones against MUC1 that are functionally inactive, in the MUC1 transgenic mice. Immunization with the DC-based vaccine activates these clones and thereby reverses anergy. Alternatively, the vaccine may have induced new anti-MUC1 CTLs that had been removed from the repertoire by clonal deletion. Reversal of unresponsiveness against a self-antigen in adult mice has potential importance, given the limitations of animal models, to the field of antitumor immunotherapy. In this context, the present studies demonstrate that immunization with the DC-tumor cell fusions induces an immune response that is sufficient to achieve rejection of established metastases. Of further interest is the demonstration that induction of an anti-MUC1 response that confers antitumor immunity has little if any effect on normal secretory epithelia that express MUC1 at apical borders along ducts. One potential explanation for selectivity of the anti-MUC1 response against tumors could be low levels of MUC1 peptide/major histocompatibility complex class I complexes on normal cells. Although further studies are needed to define the basis for selectivity, our findings suggest that the induction of anti-MUC1 immunity may represent an effective strategy for the treatment of MUC1-positive human tumors.
ABBREVIATIONS
- CTL
cytotoxic T lymphocyte
- LNC
lymph node cell
- DC
dendritic cell
- FC
fusion cell
References
- 1.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 2.Burchell J, Gendler S, Taylor-Papadimitriou J, Girling A, Lewis A, Millis R, Lamport D. Cancer Res. 1987;47:5476–5482. [PubMed] [Google Scholar]
- 3.Perey L, Hayes D F, Maimonis P, Abe M, O’Hara C, Kufe D W. Cancer Res. 1992;52:2563–3568. [PubMed] [Google Scholar]
- 4.Hull S, Bright A, Carraway K, Abe M, Hayes D, Kufe D W. Cancer Commun. 1989;1:261–267. [PubMed] [Google Scholar]
- 5.Barnd D L, Lan M S, Metzgar R S, Finn O J. Proc Natl Acad Sci USA. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerome K R, Barnd D L, Bendt K M, Boyer C M, Taylor-Papdimitriou J, McKenzie I F C, Bast R C, Jr, Finn O J. Cancer Res. 1991;51:2908–2916. [PubMed] [Google Scholar]
- 7.Rowse G J, Tempero R M, VanLith M L, Hollingsworth M A, Gendler S J. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- 8.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Metlay J P, Crowley M T, Steinman R M. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L M, MacPherson G G. J Exp Med. 1993;177:1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macatonia S E, Taylor P M, Knight S C, Askonas B A. J Exp Med. 1988;169:1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta-Damani A, Markowicz S, Engleman E G. J Immunol. 1994;153:996–1003. [PubMed] [Google Scholar]
- 13.Porgador A, Gilboa E. J Exp Med. 1995;182:255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young J W, Inaba K. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayordomo J I, Zorina T, Storkus W J, Zitvogel L, Celluzzi C, Falo L D, Melief C J, Ildstad S T, Kast W M, Deleo A B, et al. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 16.Bakkar A B H, Marland G, de Boer A J, Huijbens J F, Danen E H J, Adema G J, Figdor C G. Cancer Res. 1995;55:5330–5334. [PubMed] [Google Scholar]
- 17.Flamand V, Sornasse T, Thielemans K, Demanet C, Bakkus M, Bazin H, Tielemans F, Leo O, Urbain J, Moser M. Eur J Immunol. 1994;24:605–610. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Chen L, Kashiwaba M, Manome Y, Tanaka T, Kufe D. Gene Ther. 1997;4:1023–1028. doi: 10.1038/sj.gt.3300496. [DOI] [PubMed] [Google Scholar]
- 19.Song W, Kong H L, Carpenter H, Torii H, Granstein R, Rafii S, Moore M A S, Crystal R G. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Specht J M, Wang G, Do M T, Lam J S, Royal R E, Reeves M E, Rosenberg S A, Hwu P. J Exp Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong J, Chen D, Kufe D. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Proc Natl Acad Sci USA. 1988;85:2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akagi J, Hodge J V, McLaughlin J P, Fritz L, Panicali D, Kufe D, Schlom J, Kantor J A. J Immunother. 1997;20:38–47. doi: 10.1097/00002371-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Chen D, Block E, O′Donnell M, Kufe D W, Clinton S. J Immunol. 1997;159:351–359. [PubMed] [Google Scholar]
- 25.Sekine H, Kufe D. J Immunol. 1985;135:3610–3616. [PubMed] [Google Scholar]
- 26.Billingham R E, Brent L, Medawar P B. Nature (London) 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 27.Burnet F M. The Clonal Selection Theory of Acquired Immunity. Nashville, TN: Vanderbilt Univ. Press; 1959. [Google Scholar]
- 28.Sarzotti M, Robbins D S, Hoffman P M. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 29.Forsthuber T, Yip H C, Lehmann P V. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 30.Ridge J P, Fuchs E J, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]