ABSTRACT
We present a case of a giant sellar and suprasellar skull base-invasive metastasis from a medullary carcinoma of the thyroid gland. Radiographic features were similar to atypical/malignant meningioma or pituitary macroadenoma. Intracranial metastases from medullary thyroid carcinoma are very rare. Unusual features of our case are discussed.
Keywords: Medullary thyroid carcinoma, metastasis, sella turcica, skull base
In general, metastatic intracranial involvement of thyroid carcinoma is very rare. Intracranial metastases occur in ~1% of all cases.1,2 The overall reported survival after diagnosis is usually less than 1 year.2,3 However, with respect to the medullary carcinoma in particular, the specific incidence of intracranial metastasis and survival after diagnosis is probably even more rare, as evidenced by a paucity of reported cases.
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor of the parafollicular thyroid C cells. It comprises ~5 to 10% of all thyroid cancers and may be sporadic or hereditary in origin, having an association with multiple endocrine neoplasia syndrome types 2A and 2B. It displays a variety of clinical behaviors, ranging from indolent to aggressive. Regional lymphatic spread occurs to the cervical and upper mediastinal lymph nodes. Distant metastases may occur in the liver, lungs, and bone. Medullary thyroid carcinoma may also invade the trachea, cervical and mediastinal vessels, and nerves.
We report a case of a giant intracranial sellar and suprasellar skull base metastasis from a medullary carcinoma of the thyroid gland. An apoplexic event was the initial presentation of this primary thyroid neoplasm. Its radiographic features were similar to atypical/malignant meningioma or pituitary macroadenoma. A single case of pituitary metastasis from medullary carcinoma of the thyroid has recently been reported in the literature.4 We discuss the unusual features of our case and contribute further to the clinical evidence base for intracranial metastasis in MTC.
CASE REPORT
A 46-year-old woman was transferred to our hospital from an outside facility with presumptive aneurysmal subarachnoid hemorrhage after she suddenly deteriorated at home and was found obtunded by her family. She required intubation in the Emergency Room. Before the ictus, the patient had several months of headaches, memory loss, and progressive decline in vision. Past medical history was unremarkable.
Neurological examination revealed fixed pupils, at 4 on the left and at 3 on the right. Her blood pressure was 124/80; her Glasgow Coma Scale score (GCS) was 3. She had disconjugate gaze; absent ocular-cephalic, gag, and cough reflexes; and a positive corneal reflex.
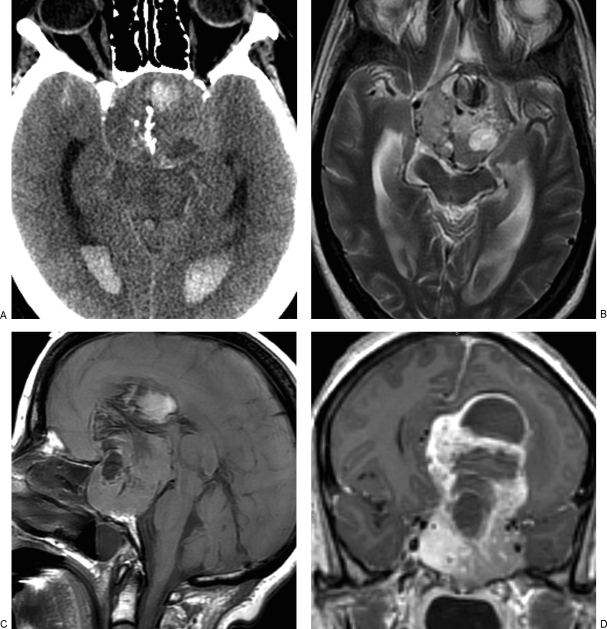
Computed tomography (CT) of the brain demonstrated a large heterogeneous, hemorrhagic, partially calcified suprasellar mass extending to the sella and resulting in marked enlargement and erosion of the skull base and tuberculum sellae (Fig. 1A). The mass produced marked compression of both frontal horns, with resulting mild acute hydrocephalus and a 6-mm midline shift. In addition, there was a small amount of subarachnoid hemorrhage bilaterally with extension into the lateral ventricles.
Figure 1.
Large intracranial metastasis from medullary carcinoma of the thyroid gland. (A) Noncontrast head computed tomography, (B) axial T2-weighted, (C) sagittal precontrast, and (D) coronal postcontrast images demonstrate heterogeneous, hemorrhagic, partially calcified, and avidly enhancing sellar/suprasellar mass.
Magnetic resonance imaging (MRI) of the brain, also obtained on admission, demonstrated a large (6.6 × 5.1 × 8.5 cm) heterogeneously and avidly enhancing hemorrhagic, partially calcified sellar/suprasellar mass (Fig. 1B–D). Partial invasion of the cavernous sinuses bilaterally was suspected, but there was no evidence of occlusion of the internal carotid arteries. Both anterior cerebral arteries (ACAs) were encased by tumor, with possible occlusion of the left ACA. Posterior extension of the mass resulted in compression of the midbrain and displacement of the distal basilar and both posterior cerebral arteries.
The preoperative differential diagnosis included macroadenoma, atypical/malignant meningioma, and craniopharyngioma. A left frontotemporal craniotomy with microdissection was performed to debulk the tumor and decrease intracranial pressure. Although the mass had a dense capsule and separated easily from the brain parenchyma superiorly, it was adherent to adjacent structures more inferiorly, especially at the skull base.
Frozen section at the time of surgery demonstrated a poorly differentiated neoplasm. Further histological evaluation of the tumor showed a highly anaplastic, giant-cell neoplasm with features of medullary carcinoma of the thyroid. Immunohistochemical stains that supported this diagnosis included TTF-1, calcitonin, and chromogranin. Subsequently, CT of the neck, chest, and abdomen demonstrated a heterogeneous mass (4.9 × 2.6 cm) in the right lobe of the thyroid. There was no radiographically pathological adenopathy in the neck. Evaluation of the chest, abdomen, and pelvis showed no additional pathology.
She slowly improved after 5 weeks and on discharge was awake and alert, following commands, with equal and reactive pupils (GCS E4M6V3) and right hemiparesis. After extensive discussion with the family, no further surgical treatment was elected. She was discharged to hospice and expired 12 weeks after the initial presentation to our hospital.
DISCUSSION
There are four types of thyroid carcinoma: papillary, follicular, anaplastic, and medullary.
Medullary thyroid carcinoma arises from parafollicular C cells that are derived from neural crest tissue in the ultimobranchial bodies. They are usually solitary lesions and have a higher mortality rate than well-differentiated papillary and follicular carcinomas. Although they may invade locally, they also spread to regional lymph nodes, and may result in distant metastases, most commonly to the lungs, bones, and liver. Intracranial metastases are only occasionally reported.4,5,6,7,8,9,10
Patients with intracranial metastasis from MTC present with known distant metastases in other sites.2 The prognosis is poor after the diagnosis of intracranial thyroid carcinoma, with a reported median survival of 4.7 months.2 Most reported cases of intracranial thyroid carcinoma were intraparenchymal, with two exceptions: Kabir et al10 report an extra-axial mass arising from the petrous apex and invading the cavernous sinus; Bhatoe et al4 report a case of intrapituitary metastasis in a patient who had been operated on for pituitary adenoma 5 years earlier. Our case is the third reported extra-axial and second intrasellar metastasis from MTC. In contrast to prior cases, our patient presented with a large intracranial mass (6.6 × 5.1 × 8.5 cm) and increased intracranial pressure from tumor hemorrhage as the initial presentation of thyroid carcinoma. Prior reports described intracranial metastasis in patients with a known and treated MTC. Most of the reported cases of intracranial metastasis occurred many years after the initial diagnosis of the primary thyroid neoplasm. This asymptomatic period ranged from 3 to 4 years5,7 to 19 years9 to 25 years.6,10
Medullary thyroid carcinoma can have an indolent course, but in this case, intratumoral hemorrhage resulted in an apoplexic presentation and significant neurological morbidity, even after debulking surgery. There are no established protocols for treatment of intracranial metastasis from MTC because of the small number of reported cases. Some authors suggest that resection of brain metastasis could be of symptomatic, but not survival value.2,8 In patients with small solitary lesions, gamma-knife radiosurgery may be effective.8 The role of whole brain radiation is not established, and chemotherapy is not used for the treatment of medullary carcinomas.2,7,8,11 Chiu et al2 reported that neither radiotherapy nor chemotherapy significantly increased disease-specific survival (median survival was 2.4 months compared with 25.2 months after surgical resection of brain metastasis). Orlandi et al11 described low response rates to chemotherapy (doxorubicin alone or combined with other cytotoxic agents), not exceeding 20 to 30% of patients. In their experience, response was usually partial and short lasting. Because these tumors do not take up iodine, radioactive iodine therapy is not used.
In conclusion, we further contribute to the literature on the rare occurrence of intracranial MTC, and highlight the unusual presentation of the case as an apoplexic hemorrhagic event superimposed on giant sellar and suprasellar disease.
REFERENCES
- Girelli M E, Nacamulli D, Pelizzo M R, et al. Medullary thyroid carcinoma: clinical features and long-term follow-up of seventy-eight patients treated between 1969 and 1986. Thyroid. 1998;8:517–523. doi: 10.1089/thy.1998.8.517. [DOI] [PubMed] [Google Scholar]
- Chiu A C, Delpassand E S, Sherman S I. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997;82:3637–3642. doi: 10.1210/jcem.82.11.4386. [DOI] [PubMed] [Google Scholar]
- Venkatesh S, Leavens M E, Samaan N A. Brain metastases in patients with well-differentiated thyroid carcinoma: study of 11 cases. Eur J Surg Oncol. 1990;16:448–450. [PubMed] [Google Scholar]
- Bhatoe H S, Badwal S, Dutta V, Kannan N. Pituitary metastasis from medullary carcinoma of thyroid: case report and review of literature. J Neurooncol. 2008;89:63–67. doi: 10.1007/s11060-008-9586-5. [DOI] [PubMed] [Google Scholar]
- Timothy J, Kerawala C, Brazil L, Bartlett J, Doshi B. Medullary cell carcinoma of the thyroid: metastases to the central nervous system. Eur J Surg Oncol. 1995;21:329–330. doi: 10.1016/s0748-7983(95)91987-2. [DOI] [PubMed] [Google Scholar]
- Pitale S U, Melian E, Thomas C, Moley J F, Origitano T, Sizemore G W. Brain metastases from medullary thyroid carcinoma in a patient with multiple endocrine neoplasia type 2A. Thyroid. 1999;9:1123–1125. doi: 10.1089/thy.1999.9.1123. [DOI] [PubMed] [Google Scholar]
- Salvati M, Frati A, Rocchi G, et al. Single brain metastasis from thyroid cancer: report of twelve cases and review of the literature. J Neurooncol. 2001;51:33–40. doi: 10.1023/a:1006468527935. [DOI] [PubMed] [Google Scholar]
- McWilliams R R, Giannini C, Hay I D, Atkinson J L, Stafford S L, Buckner J C. Management of brain metastases from thyroid carcinoma: a study of 16 pathologically confirmed cases over 25 years. Cancer. 2003;98:356–362. doi: 10.1002/cncr.11488. [DOI] [PubMed] [Google Scholar]
- Mikami T, Niwa J, Kubota T, Chiba M. Solitary brain metastasis from medullary carcinoma of the thyroid. No Shinkei Geka. 2003;31:905–909. [PubMed] [Google Scholar]
- Kabir S M, Zafar M S, Brydon H L. Intracranial metastasis from medullary carcinoma of the thyroid 25 years after primary surgery. Br J Neurosurg. 2006;20:169–172. doi: 10.1080/02688690600777158. [DOI] [PubMed] [Google Scholar]
- Orlandi F, Caraci P, Mussa A, Saggiorato E, Pancani G, Angeli A. Treatment of medullary thyroid carcinoma: an update. Endocr Relat Cancer. 2001;8:135–147. doi: 10.1677/erc.0.0080135. [DOI] [PubMed] [Google Scholar]