Abstract
Recent findings established that primary targets of HIV/SIV are lymphoid cells within the gastrointestinal (GI) tract. Focus has therefore shifted to T-cells expressing α4β7 integrin which facilitates trafficking to the GI tract via binding to MAdCAM-1. Approaches to better understand the role of α4β7+ T-cells in HIV/SIV pathogenesis include their depletion or blockade of their synthesis, binding and/or homing capabilities in vivo. Such studies can ideally be conducted in rhesus macaques (RM), the non-human primate model of AIDS. Characterization of α4β7 expression on cell lineages in RM blood and GI tissues reveal low densities of expression by NK cells, B-cells, naïve and TEM (effector memory) T-cells. High densities were observed on TCM (central memory) T-cells. Intravenous administration of a single 50 mg/kg dose of recombinant rhesus α4β7 antibody resulted in significant initial decline of α4β7+ lymphocytes and sustained coating of the α4β7 receptor in both the periphery and GI tissues.
Keywords: Cell trafficking, antibodies, AIDS, T-cells, rhesus macaque, Act1, alpha4beta7 integrin
Introduction
It is now established that the gut-associated lymphoid tissue (GALT) is the major initial target of pathology during acute infection of humans with HIV-1 or rhesus macaques (RM) with SIV [1–9]. During this period of primary infection, a significant frequency of CD4+ memory T-cells, which are CCR5+ and already in a state of activation due in part to exposure and response to intestinal flora, serve as prime targets for the virus leading to their infection and subsequent depletion via direct cytopathic effects and/or indirect mechanisms of apoptosis. The degree of impact of this major localized effect has been hypothesized to significantly influence the course of disease and has therefore led to a more detailed study of the mechanisms associated with T-cells that migrate and/or reside within the gastrointestinal tract. The intestinal homing receptors CCR9 and α4β7 play one of the central roles in promoting the migration of lymphocytes into intestinal mucosal tissue via binding to CCL25 and mucosal addressin cell adhesion molecule-1 (MAdCAM), respectively [10–15]. The α4β7 cell surface receptor has received much attention particularly in light of a recent study by Arthos et al which demonstrated that the HIV-1 envelope protein gp120 binds to an active form of α4β7 on CD4+ T-cells and initiates LFA-1 activation that facilitates formation of a viral synapse leading to cell-to cell spreading further facilitating viral infection [16]. Thus, the ability of the host to defend itself against lentiviral infection is likely to depend on the nature (such as phenotype and frequency) of these gut-homing lymphocytes. For instance, gut-homing virus-specific NK cells and CD8+ CTLs may contribute to the containment of HIV/SIV viral replication while gut-homing CD4+ T-cells besides their expected T helper cell activity may simply provide additional targets for the virus and sustain its replication. A detailed understanding of the immune responses in mucosal sites particularly during early stages of infection is therefore critical and because there is increasing evidence to support a significant contributing role for α4β7 + cells in HIV pathogenesis, it is important to fully understand the part played by these cells in early viral infection and subsequent disease progression. One approach that can be taken to accomplish this aim is by conducting in vivo studies that utilize an anti-α4β7 monoclonal antibody to either block α4β7 receptor and trafficking or to deplete α4β7+ cells prior to or during acute viral infection. This can best be studied in RM recognized to be the optimal non-human primate model for the study of AIDS. When infected with SIV, this species exhibits CD4+ T-cell depletion, chronic immune activation, immune exhaustion and disease remarkably similar to HIV infection in humans [17–24]. Furthermore, the GI pathology observed in acutely HIV-infected patients is similar to the pathology exhibited by SIV-infected RM [3, 7–9, 25]. However, while the expression of α4β7 on major cell lineages in humans has been documented, there is a paucity of data with regards to α4β7 expressing cells and the effect of SIV infection on this gut-homing marker in RM. In humans, flow cytometry utilizing Act I, a murine monoclonal antibody specific for human α4β7 integrin (henceforth referred to as murine α4β7 mAb), showed expression of both low and high density α4β7 (α4β7low and α4β7high) on adult T-cells and B-cells while NK cells, eosinophils, and neonatal T- and B-cells exhibited a α4β7low pattern of expression [10, 12, 26]. Furthermore, while α4β7low was expressed by naïve T- and B- cells, α4β7high was observed on memory T and B cells. Cell subsets with an α4β7high phenotype are believed to express this receptor in an active form and are thought to be those that preferentially migrate to and following binding to their cognate MAdCAM ligand, reside within the GI tract. Several studies primarily conducted utilizing murine models have shown that the induction of α4β7high expression on T-cells is attributed to retinoic acid (RA), which is a vitamin A metabolite catabolized specifically by either mucosal dendritic and/or stromal cells [11, 15, 27–32].
Thus, it was reasoned that baseline studies on the cell lineages that express α4β7 in tissues from RM would be a pre-requisite prior to pursuing in vivo α4β7+ cell-depleting and/or blocking studies in SIV infected macaques. The purpose of the current study was therefore twofold; first, to characterize and compare α4β7 expression levels on the major cell lineages involved in innate and adaptive immunity from healthy uninfected RM by multiparameter flow cytometry and to evaluate the in vitro and in vivo effects of RA and SIV infection, respectively, on α4β7 induction and/or mobilization of α4β7+ lymphocyte subsets. Second, after acquiring a sound understanding of these factors, to conduct a preliminary safety and efficacy study of the in vivo administration of a monoclonal rhesus α4β7+ antibody in RM. The results of our studies show a differential pattern of α4β7 expression among the major cell lineages and their subsets which is similar to what has been reported for human lymphocytes. In vitro incubation with RA was also found to significantly induce α4β7 expression on activated T-cells. Furthermore, while significant decreases in the frequency of α4β7+ lymphocytes were noted in rectal biopsy tissues, no significant changes in the frequency of α4β7+ cells were noted in the periphery of chronically SIV-infected RM. Of interest was the finding that there was a rapid disappearance of select subsets of α4β7+ NK and α4β7+ CD4+ T-cells in the periphery during the acute infection period. Finally, a preliminary study was conducted to define the potential in vivo depletion and/or blocking activity of a novel α4β7 monoclonal antibody (modified to create a less immunogenic rhesus recombinant construct Rh-α4β7) which was administered intravenously as a single bolus dose to healthy RM. The infusion of a single dose (50 mg/kg) of Rh-α4β7 mAb was found to be non-toxic and lead to an initial significant decline followed by a failure to detect (up to 5 weeks) α4β7+ lymphocytes in both peripheral and GI compartments. Collectively these data provides the foundation for in vitro and in vivo manipulation of α4β7+ lymphocytes for potential mechanistic-based experiments in SIV-infected animals. The implications of these current findings for future studies are discussed.
Materials and Methods
Animals
Healthy uninfected and SIV-infected RM were housed at the Yerkes National Primate Research Center (YNPRC) of Emory University. Their housing, care, diet and maintenance was in conformance to the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council and the Health and Human Services guidelines “Guide for the Care and Use of Laboratory Animals.” The RM involved in the cross-sectional and longitudinal study were infected intravenously with 200 TCID50 of SIVmac239. All uninfected and SIV-infected RM used in the study were male and age matched adults.
Specimen collection and blood processing
Peripheral blood mononuclear cells (PBMC) were isolated by standard Ficoll–Hypaque gradient centrifugation from heparinized whole blood. This procedure in addition to those for specimen collection of and lymphocyte isolation from colon, jejunum tissues, rectal biopsies and bronchial alveolar lavage (BAL) were performed as described previously [33–35].
Viral load determination
Plasma viral loads were determined using a competitive reverse transcriptase polymerase chain reaction assay by the Virology Core Lab supported by the Emory University CFAR. To determine if the α4β7 subset of CD4+ T cells were preferentially infected with SIV, PBMC were isolated from the peripheral blood of 6 SIV infected rhesus macaques during the chronic stage of infection. All monkeys were asymptomatic at the time of blood sampling. We selected 3 monkeys that had high (> 100,000 copies/ml) and 3 monkeys that had low plasma viral loads (< 10,000 viral copies/ml) to determine the potential role of plasma viral load on cellular viral loads in the CD4+ T cell subsets. CD4+ T cells were first enriched by depleting all cell lineages except CD4+ T cells using a cocktail of monoclonal antibodies. This was followed by incubating the remaining enriched population of CD4+ T cells with murine α4β7 mAb at 5 ug/ml per million cells at 4°C for 30 min. The cells were then washed and resuspended in PBS and incubated with anti-mouse Ig conjugated immuno-beads following concentrations as recommended by the commercial vendor. The enriched population of CD4+ α4β7 + and the remaining CD4+ α4β7 - cells were then used to determine the levels of SIV. An aliquot of the CD4+ α4β7- isolated population was subjected to flow cytometric analysis to determine degree of purity and found to contain > 92% CD4+ and < 0.01% α4β7+ cells. RNA was isolated from 2 million cells from each of the subsets from each of the monkeys using GuHCl/Proteinase K viral lysis solution and guanidium thiocyanate carrier solution. Viral RNA was eluted in 20 ul RNAse free water and stored at −80°C until use. Viral RNA from all the samples was then individually reverse transcribed using enhanced avian RT first strand synthesis kit and RNAse free oligoprimers (SIVgagrt). Level of viral copies were quantified in each of the cDNA sample using real time PCR using SYBR greenER for iCycler kit and the following RNAse free primer pairs:
SIV gagrt F: TTA TGG TGT ACC AGC TTG GAG GAA TGC
SIV gagrt R: CCA AAC CAA GTA GAA GTC TGT GTC TGT TCC ATC
The sensitivity of the assay was determined to be 10 viral copies/ml.
Flow cytometry
Multiple clones of monoclonal antibodies with specificity for human CD3, CD8alpha, CD8beta, CD95, CD28, alpha4 integrin (CD49d), beta7 integrin, CD16, CD14, CD20, CD56, and NKG2A, were first screened to identify those that provided optimal cross-reactivity for the identification of various T-cell, B-cell and NK cell lineages and subsets of cells from RM as described previously [33–35]. Our in-house purified and biotinylated murine α4β7 mAb was incubated with cells for 15 min followed by 10 min staining with PE-Cy7 or APC conjugated streptavidin for the determination of the frequency and absolute numbers of α4β7+ lymphocytes. Stained cells fixed with 1% paraformaldehyde were analyzed on either a FACS Calibur or a LSRII flow cytometer (BD Biosciences, San Jose CA). Flow cytometric acquisition and analysis of samples as well as the gating strategy for identifying total NK cells and its subsets, the CD4+ and CD8+ T cells subsets in lymphoid cells from rhesus macaques has been described previously [33, 34].
In vitro effects of Retinoic acid (RA) on α4β7expression
PBMC or isolated CD4+ T-cells purified by magnetic beads (Dynal Invitrogen) were cultured in RPMI 1640 media containing antibiotics and 10% fetal calf serum (heretofore referred to as media). Aliquots of such cells were cultured in media containing anti-CD3/CD28 antibody-conjugated magnetic beads [35] and/or 50 U IL-2 for 5 days at 37°C, 5% CO2 in the presence or absence of 10 nM all-trans RA (Sigma-Aldrich). The cells were then washed and analyzed for the frequency and relative density of α4β7+ expression by standard flow cytometry using the FACS Calibur system.
Generation and production of rhesus recombinant α4β7 monoclonal antibody (Rh-α4β7 mAb)
Immunoglobulin heavy and light chain variable regions were synthesized as minigenes comprising complementarity determining regions of murine α4β7 mAb [26], and human heavy and light chain variable region framework sequences. Synthesized variable region minigenes were subcloned into expression vectors containing rhesus IgG1 heavy chain or rhesus kappa light chain constant region sequences.
For large scale production of recombinant antibody, recombinant heavy and light chain vectors were packaged in retroviral vectors and used to infect CHO cells using the GPEx® expression technology (Catalent Pharma Solutions, Middleton, WI). A pool of transduced cells was grown in serum free medium and secreted antibody purified by protein A affinity chromatography. The purified Rh-α4β7 mAb was placed in phosphate buffer, pH 6.5, and confirmed to contain <1 EU/mg of antibody.
Characterization of recombinant Rh-α4β7 mAb
Specificity of Rh-α4β7 was confirmed by cross-blocking experiments. Briefly, α4β7+ expressing Hut 78 cells were incubated with varying concentrations of Act I or a control mouse antibody. After washing, cells were stained with the recombinant Rh-α4β7 conjugated to the fluorophore APC. Affinity of recombinant Rh-α4β7 was compared to the murine α4β7 mAb by incubating serial dilutions of both antibodies with a fixed number of Hut 78 cells and measuring antibody concentration before and after incubation. The affinity constant (Kd) for each antibody was calculated as previously described [36]. Similar cross-blocking experiments were performed on PBMC isolated from uninfected RM.
Detection of cell bound Rh-α4β7 following in vivo treatment of RM
Aliquots of PBMC and intra-epithelial lymphocytes isolated from heparinized blood and rectal biopsy samples, respectively, were stained with the biotinylated murine α4β7 mAb followed by streptavidin PE-Cy7 to determine if the murine α4β7 mAb binding was blocked by the Rh-α4β7 mAb in vivo. To determine if decreases in levels of α4β7+ lymphocytes as detected by the murine α4β7 mAb were due to depletion or blocking, an aliquot of the same cells were also stained with anti-α4 integrin-PE or anti-β7 integrin-APC which recognize epitopes distinct from the murine α4β7 and Rh-α4β7 mAbs. Since α4 integrin-PE+ lymphocytes also include α4β1+ lymphocyte populations, the data shown herein includes staining with β7 integrin-APC only, since cells bound by this Ab would most likely represent the same population detected by the murine α4β7 and the Rh-α4β7 mAbs.
Measurement of plasma levels of Rh-α4β7
Levels of rhesus recombinant Rh-α4β7 antibody in monkey plasma were measured using the α4β7+ expressing CD8+ human T cell line, HuT 78 in a flow cytometry-based assay. Plasma obtained from each monkey before and after Rh-α4β7 mAb administration was serially diluted in PBS/2% FBS and incubated with 106 HuT 78 cells for 30 min. Cells were then washed twice with PBS and incubated for 30 min with polyclonal goat anti-human IgG-PE (Jackson ImmunoResearch, West Grove, PA) that had been shown to cross react with rhesus IgG. Cells were washed twice with PBS and fixed with PBS/2% formalin. Stained cells were analyzed on a FACS Calibur flow cytometer for PE fluorescence. The mean channel fluorescence intensity (MFI) of cells stained with monkey serum was compared to MFI of cells stained with known concentrations of the Rh-α4β7 mAb. Whenever possible, the mean of two measurements made at different serum dilutions was used. The sensitivity of this assay was <4 ug/ml.
Statistical analysis
Data are represented as means ± standard deviation (S.D.) and were analyzed by using the two-tailed Student’s t test. A P value of <0.05 was considered to be statistically significant.
Results
Characterization of α4β7 expression in cell lineages from RM
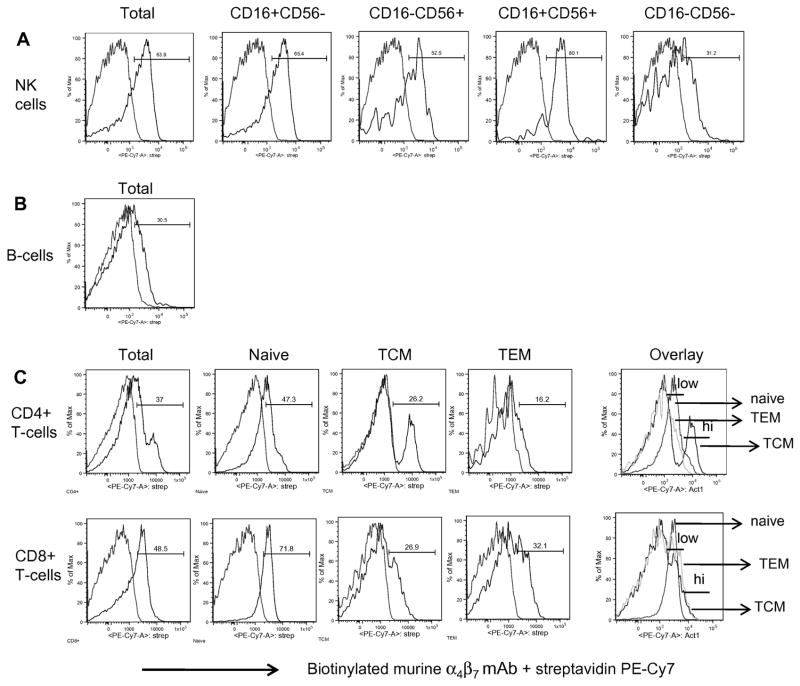
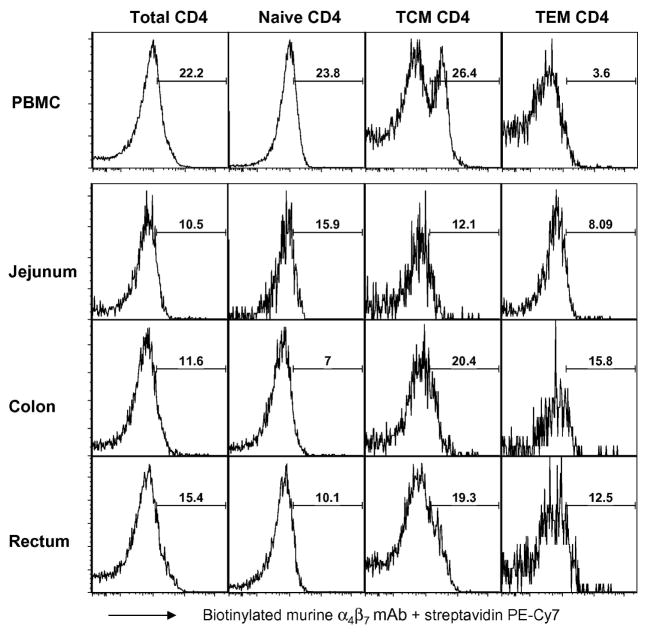
Previous characterization of α4β7 on human leukocytes using the murine Act1 mAb has shown this mAb to specifically recognize the α4β7 heterodimer [10, 26]. These studies showed that while T-cells and B-cells express both α4β7low and α4β7high populations, NK cells exhibited a predominantly α4β7low phenotype. Previous studies have also examined the induction of α4β7 expression on SIV-specific CD8+ T-cells in RM [37, 38], but to date, a comprehensive analysis of profiles of α4β7 expressing cell lineages and their subsets from RM has been lacking. Our laboratory therefore set out to first characterize α4β7 expression profiles on T-cell and its subsets, B-cells and NK cell subsets isolated from healthy uninfected RM. Using multi-parameter flow cytometry, the biotinylated murine α4β7 mAb was tested for reactivity along with a panel of mAb reagents that have previously been shown by several labs including ours to be optimal for the identification of the aforementioned cell populations in the PBMC samples from 9 uninfected RM. It should be noted that high and low densities of α4β7+ expression were more discernable on the FACS Calibur flow cytometer while an overlay of histograms was necessary to better identify these two subpopulations on the LSRII flow cytometer. This is likely due to basic system differences between these two flow cytometry instruments such as laser voltages or compensation settings, but it is important to note that the frequencies of α4β7+ lymphocytes acquired by the two flow cytometers was still similar, if not identical. As shown by the representative FACS profiles in Figure 1A, the analysis of NK cells (defined as CD3−CD8+CD14−CD20−NKG2A+) and their subsets based on CD16 and CD56 expression [34] revealed that, in agreement with the previous characterization of human NK cells, >50% total RM NK cells generally expressed low mean densities of α4β7 with the majority of α4β7+ cells being the cytokine-producing CD16+/CD56+ and the cytolytic CD16+CD56− NK cell subsets (Table 1). Low relative densities of α4β7 expression were also observed on the CD16-CD56- NK cell subset in RM. In contrast to what has been reported for human B-cells, α4β7 expression on the majority of B-cells (Figure 1B, Table 1) in RM was found to be α4β7low. With regard to T-cells, greater than 35% of both CD4+ and CD8+ T-cells from healthy uninfected RM were found to express α4β7 with CD4+ T-cells exhibiting both α4β7low and α4β7high phenotypes (Figure 1C). Further gating based on CD28 and CD95 expression to distinguish naïve (CD28+CD95−), TCM (CD28+CD95+) and TEM (CD28−CD95+) subsets in the periphery revealed that while there was clearly a discrete sub-population of α4β7high expressing CD4+ TCM cells, the naïve and TEM CD4+ T cells were α4β7low. All three (naïve, TCM and TEM) CD8+ T-cell subsets appeared to exhibit a α4β7low phenotype (Figure 1C). The average frequencies of α4β7+ cells for each cell lineage and its subsets in the periphery of uninfected RM are summarized in Table 1. The examination of α4β7 expression in intraepithelial lymphocytes isolated from jejunum, colon and rectal tissue samples from 2 healthy uninfected RM (Figure 2, representative data) revealed a predominantly α4β7low phenotype on CD4+ T-cells, with the exception of CD4+ memory cells, which expressed heterogeneous levels of α4β7high and were higher in frequency in the cells from the colon and rectum as compared with jejunum biopsies. CD8+ T-cells and its subsets from these tissue samples exhibited a predominantly α4β7low phenotype (not shown).
Figure 1.
Representative LSR II facilitated flow cytometric profiles of α4β7 expression on total and subset populations of (A) NK cells, (B) B-cells and (C) T-cell lineages in PBMC from 9 healthy uninfected RM. For detection of α4β7+ cells, PBMC from 9 RM were surface stained with biotinylated murine α4β7 mAb followed by secondary staining with streptavidin PE-Cy7. Shown is representative data (n= 9 RM) with gating indicating α4β7+ populations in comparison to background levels. To better indicate α4β7low and α4β7high densities, Figure 1(C) also includes an overlay of α4β7+ naïve, α4β7+ TCM and α4β7+ TEM for CD4+ and CD8+ T-cells.
Table 1.
Frequency (Mean +/− SD) of α4β7+ cells in major cell lineages and their subsets* in PBMC isolated from healthy uninfected RM (n=9).
| Cell subpopulation | Frequency of α4β7+ lymphocytes detected using murine α4β7 mAb |
|---|---|
| CD3+CD4+ T-cells | 51.9 ± 1.5 |
| Naïve | 66.7 ± 20.9 |
| TCM | 31.6 ± 6.2 |
| TEM | 18.9 ± 6.7 |
| CD3+CD8+ T-cells | 60.6 ± 16.8 |
| Naïve | 83.1 ± 13.4 |
| TCM | 34.4 ± 12.6 |
| TEM | 44.8 ± 15.2 |
| CD3−CD20+ B-cells | 48.5 ± 12.8 |
| CD3−CD8+NKG2A+ NK cells | 68.2 ± 14.8 |
| CD16−CD56+ | 68.7 ± 17.6 |
| CD16+CD56− | 77.4 ± 19.6 |
| CD16−CD56− | 47.0 ± 29.8 |
| CD16+CD56+ | 73.2 ± 9.8 |
RM lymphocytes were gated for the specific population (such as gated on CD3+CD4+ T cells) for analysis of the frequency of α4β7+ cells within each subpopulation.
Figure 2.
Representative flow cytometric profiles of α4β7 expression on total and subset populations of CD4+ T-cells in the gut of healthy RM. Intraepithelial lymphocytes were isolated from colon, jejunum and rectal tissue samples from 2 healthy uninfected RM. The frequency of α4β7+ lymphocytes were determined on the LSR II as described in the text. The number noted within each profile indicates only the frequency of cells expressing α4β7high densities. Shown for comparison are α4β7high populations in CD4+ T-cell subsets from uninfected RM PBMC.
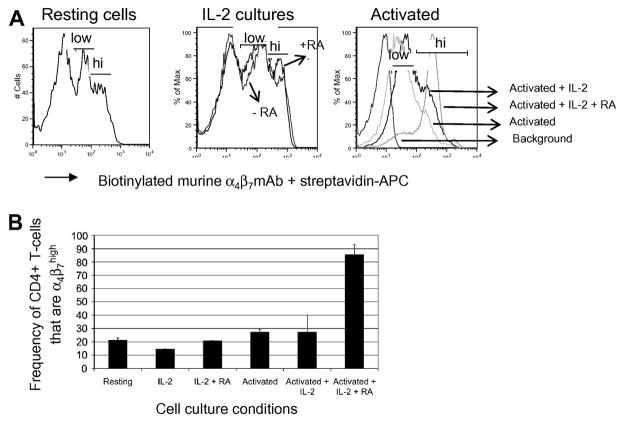
The expression of α4β7high on CD4+ memory T-cells has been described before and more recent studies have demonstrated that the frequency of CD4+ T-cells that express α4β7high can be significantly increased in vitro via activation in the presence of retinoic acid [27, 29, 31], which is a vitamin A metabolite believed to be responsible for promoting the gut-homing of lymphocytes in vivo. In order to determine if this held true for RM T-cells, purified CD4+ T-cells were cultured in vitro with low levels of IL-2 and/or activated with anti-CD3/CD28 Ab-conjugated magnetic beads in the presence or absence of all-trans RA. As shown in Figures 3A and 3B, flow cytometric analysis on the FACS Calibur revealed that when compared to resting CD4+ T-cells, the effect of RA on α4β7 expression was minimally enhanced when CD4+ T-cells were cultured with only IL-2. However, a dramatic increase (~4-fold) in the frequency of α4β7high CD4+ T-cells was observed when these lymphocytes were activated in the presence of RA for 5 days. The induction of α4β7 on CD8+ T-cells following incubation with RA was also examined but was not found to be significant, with activated cells exhibiting a less than twofold increase in the frequency of α4β7high CD8+ T-cells in the presence of RA for 5 days (data not shown). Thus, these data collectively demonstrate that α4β7 expression on RM lymphocytes can be readily identified and results largely reflect α4β7 expression patterns similar to those noted for human lymphocytes. Furthermore, RA can be utilized successfully to manipulate and upregulate and potentially prepare large numbers of α4β7+ expressing CD4+ T for autologous therapeutic transfusion studies.
Figure 3.
Retinoic acid (RA) induces α4β7 expression on CD4+ T-cells. (A) Representative flow cytometry profiles (acquired by the FACS Calibur) of α4β7 expression on resting CD4+ T cells and CD4+ T cells cultured or activated in media containing 50 U/ml IL-2 or 10nM retinoic acid (RA). Gating was performed on viable cells only. (B) Bar chart illustrating the frequency (Mean +/− SD) of α4β7high CD4+ T-cells under indicated conditions. The assay was repeated three times and performed using purified CD4+ T-cells from 2 RM each time.
In vivo administration of Rh-α4β7 mAb results in a significant decline in the level of α4β7+ lymphocytes in the periphery and GI tissues
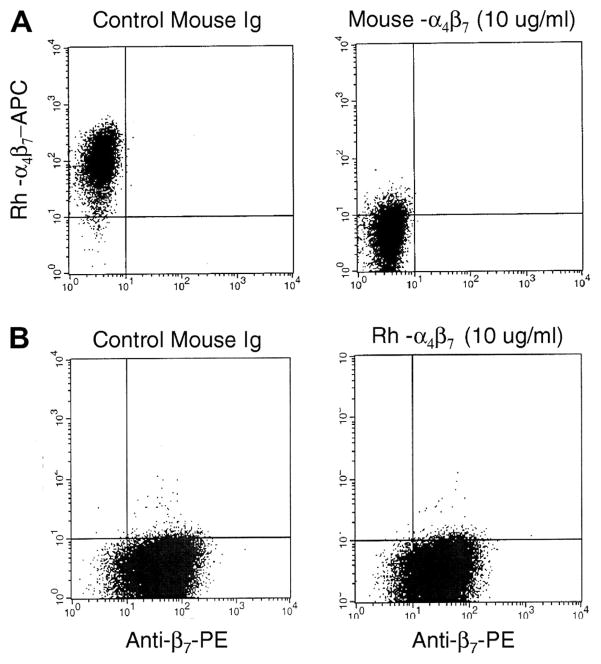
The findings that the murine α4β7 mAb effectively cross-reacts with α4β7+ lymphocytes from RM prompted us to determine whether α4β7-expressing lymphocytes could be targeted in vivo. In order to minimize the immunogenicity of the murine α4β7 mAb in vivo, a rhesus recombinant IgG1 antibody was generated as noted in the Methods section. The recombinant Rh-α4β7 mAb was then confirmed by flow cytometry to exhibit specificity that was similar to murine α4β7 mAb. The human α4β7-expressing cell line, Hut 78, was used to characterize Rh-α4β7 mAb. The Rh-α4β7 mAb bound to Hut 78 cells and could be completely cross-blocked by the pre-incubation of cells with the parent murine α4β7 mAb (Figure 4A). The affinity constant for Rh-α4β7 was similar to the murine α4β7 mAb when measured by binding to Hut 78 cells with Kd values of 6.4 × 10−10 and 1.8 × 10−10 for Rh-α4β7 mAb and murine α4β7 mAb, respectively. The specificity of Rh-α4β7 mAb was further confirmed by the finding that pre-incubation of RM PBMC with Rh-α4β7 was cross-blocked the binding of murine α4β7 mAb by almost 100% in vitro (and vice versa). Of interest was the finding that the pre-incubation with Rh-α4β7 resulted in minimal cross-blocking of anti-β7 integrin mAb (Figure 4B) or anti-CD49d (α4, data not shown) single chain specific mAb. Also of interest is our observation that staining with anti-β7 integrin mAb alone did not reveal distinct low and high density β7+ expressing CD4+ T cell sub-populations as seen by staining with Rh-α4β7 or murine α4β7 mAbs. Collectively, these data suggest that the Rh-α4β7 mAb is directed at an epitope formed by the α4β7 heterodimer which is distinct from those that are recognized by the individual α4 or β7 mAbs. This finding was exploited for the detection of cells bound by Rh-α4β7 in vivo (see Methods section and below).
Figure 4.
Recombinant rhesus α4β7 (Rh-α4β7) is cross-blocked by murine α4β7 mAb but not β7 mAb. (A) Hut-78 cells were pre-incubated with mouse isotype control antibody (left panel), or with murine α4β7 mAb followed by staining with Rh-α4β7-APC. (B) Hut-78 cells were pre-incubated with mouse isotype control antibody (left panel) or with rhesus α4β7 followed by staining with β7-PE mAb.
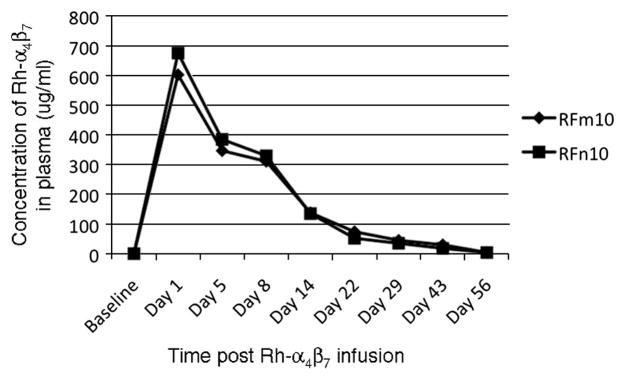
Having characterized the binding and specificity properties of Rh-α4β7, an acute in vivo administration study was then initiated in two healthy uninfected RM RFm10 and RFn10 (6.49 kg and 7.17 kg respectively). The Rh-α4β7 mAb was diluted in sterile infusion-grade saline and was gradually administered intravenously (IV) to each of the two animals at a dose of 50 mg/kg, which equates to a starting dose of 0.85 mg/ml of plasma based on the assumption that each RM has approximately 60 mls blood/kg. The monkeys demonstrated no adverse effects during or after the antibody infusion. Blood samples used for PBMC isolation, cell blood counts (CBC) and blood chemistries were collected at baseline and at days 1, 5, 8, 14, 22, 29, 36, 43, 56 and 63 post infusion. As evident by the representative blood chemistries results (Supplemental data, Table 1), Rh-α4β7 was well tolerated by both animals and did not lead to any significant physiological changes. The plasma levels of Rh-α4β7 were also determined over the course of the study (see Figure 5) and were found to be at a maximum as expected on Day 1 (603 ug/ml and 676 ug/ml in RFm10 and RFn10, respectively) followed by a decline thereafter with an alpha half life of approximately 8 days. By day 29, notable concentrations were still detected in plasma from both RFm10 (45 ug/ml) and RFn10 (35 ug/ml) but undetectable levels were noted by day 56.
Figure 5.
Plasma concentrations of Rh-α4β7 following in vivo infusion. Plasma was isolated from heparinized blood at the indicated time points from animals RFm10 and RFn10 and the levels of Rh-α4β7 mAb were measured using the α4β7+ expressing CD8+ human T cell line HuT 78 in a flow cytometry-based assay. The concentration of Rh-α4β7 in each plasma sample was determined by comparing the MFI of HuT 78 cells stained with monkey plasma to the MFI of HuT 78 cells stained with known concentrations of Rh-α4β7 mAb.
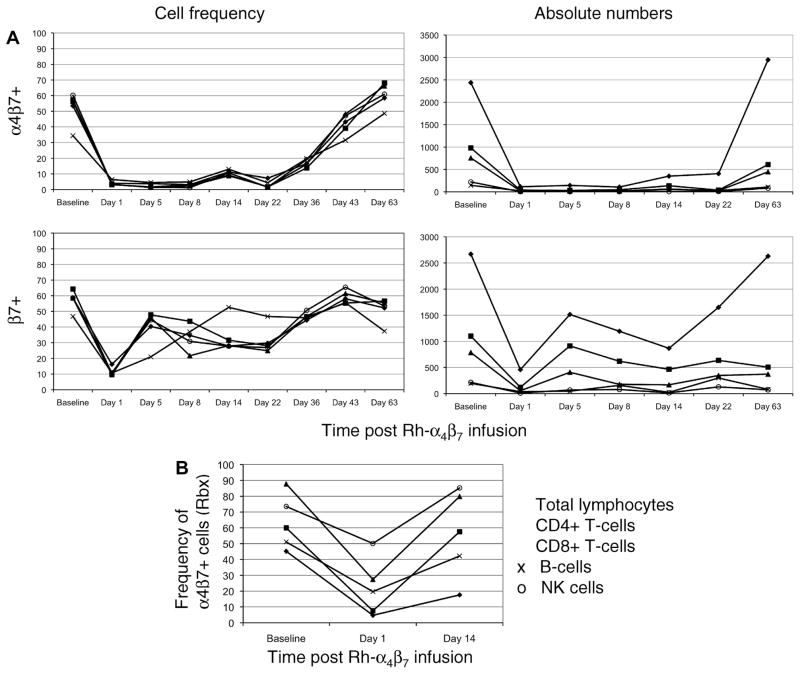
PBMC were analyzed by flow cytometry to determine the frequency of major α4β7+ cell lineages and their subsets. Detection of α4β7+ cells following in vivo administration of the Rh-α4β7 using the parent murine α4β7 mAb alone would not be feasible due to cross-blocking. Attempts were made to directly detect Rh-α4β7 on PBMC utilizing PE-conjugated anti-rhesus IgG. However, despite Fc blocking prior to in vitro staining, the background level with this secondary antibody was too high to allow for clear identification of cells bound by the Rh-α4β7 (data not shown). Based on the findings that the Rh- α4β7 mAb does not block the reactivity of mAb against either the α4 intergrin or β7 integrin single chain, it was reasoned that use of the anti-β7 integrin mAb would be preferable since the anti-α4 integrin mAb would not only bind to cells with bound Rh-α4β7 but would also bind to cells that express α4β1. It was thus reasoned that the detection of cells that are β7+ would likely represent cells that are bound with the Rh-α4β7 mAb in vivo. Thus, the frequency of α4β7+ cells was determined by staining aliquots of cells with the parent murine α4β7 mAb as well as with anti-β7 chain specific mAb. Results revealed that on Day 1 post infusion, there was significant cross-blocking of α4β7+ T-cells and α4β7+ NK cells by Rh-α4β7 mAb because staining of PBMC with the parent murine α4β7 mAb showed a decline in frequency by ~ 95% while the decline in the frequency of α4β7+ B-cells was by ~ 80% in both the monkeys (Figure 6A, upper left panel). This decline occurred in both α4β7low and α4β7high subsets. The frequency of α4β7+ lymphocytes remained low through Day 22 until a significant increase to levels near baseline were noted by Day 43.
Figure 6.
In vivo administration of Rh-α4β7 leads to significant decline and subsequent cross-blocking of α4β7+ lymphocytes. Rh-α4β7 was infused IV at a dose of 50 mg/kg to 2 RM and the frequency and ABS of α4β7+ and β7+ lymphocytes for each cell lineage in (A) the periphery and (B) Rbx, were determined at the indicated points post infusion. Shown is representative data for total α4β7+ lymphocytes (◆), α4β7+ CD4+ T-cells (■), α4β7+ CD8+ T-cells (▲), α4β7+ B-cells (x), and α4β7+ NK cells (o).
To distinguish between cross-blocking and a true decline in α4β7+ lymphocytes, staining with anti-β7 mAb was performed and also revealed a similar decline in β7+ lymphocytes on Day 1 post infusion (Figure 6A, lower left panel). However, this was followed by a rebound to ~60–70% of baseline on Day 5. These increased values were maintained until day 22 with values returning near baseline by Day 36. These data suggest that while there is an initial and partial decline in the α4β7+ expressing cells, this is followed by a recovery of cells that express α4β7+ but are blocked by the in vivo presence of the Rh-α4β7 mAb. The absolute numbers (ABS) of total lymphocytes, CD4+ T-cells, CD8+ T-cells, B-cells and NK cells expressing α4β7+ and β7+ (Figure 6A, right panels) were also calculated in order to distinguish whether the observed decreases in cell frequency were due to the cross-blocking effects of Rh-α4β7 mAb or was due to a true decrease (depletion or re-distribution). As shown by the representative data in Figure 6A, the analysis of ABS of α4β7+ and β7+ T-cells, B-cells and NK cells revealed a true decline in these cell populations in the periphery on Day 1 which reflects the cell frequency data. By day 63, the ABS of all these lymphocyte subsets returned to baseline levels.
Since α4β7+ lymphocytes home to the GI tract which is the target site of interest, the frequency of α4β7+ and β7+ lymphocytes was also determined in rectal biopsy (Rbx) samples from each animal at baseline, Day 1 and Day 14 post infusion with the Rh-α4β7 mAb. A similar analysis of BM and BAL samples was also performed to determine the extent of Rh-α4β7 bio-distribution in vivo. As shown by the results in Figure 6B, staining of isolated day 1 Rbx intra-epithileal lymphocytes with either the murine α4β7 mAb or the anti-β7 integrin mAb revealed a 10-fold and 3-fold decline in the frequency of α4β7+ CD4+ T-cells and α4β7+ CD8+ T-cells, respectively. An approximately 2-fold decrease was also noted for α4β7+ B-cells and α4β7+ NK cells in such Rbx tissues. By Day 14, the frequencies of all α4β7+ lymphocyte populations were at or near baseline. A similar pattern of decrease in the frequency of α4β7+ lymphocytes was noted for bone marrow samples in that significant declines in α4β7+ lymphocytes were observed on Day 1 while a recovery to levels near baseline were noted on Day 14. Data obtained from BAL samples did not reveal any clear trend (data not shown). In summary, in vivo administration of a single dose of 50 mg/kg of Rh-α4β7 was well tolerated and resulted in significant initial decline and a prolonged blocking of the α4β7 molecule on peripheral α4β7+ T-cells, B-cells and NK cells for a significant period of time (upto 5 weeks) as well as a substantial decrease in the frequency of these lymphocyte subsets in Rbx and BM samples, but for a more transient time period.
Cross-sectional and longitudinal analyses of uninfected and SIV-infected RM reveals early changes in the frequency and absolute numbers (ABS) of select α4β7+ lymphocyte subsets during the acute stage of infection
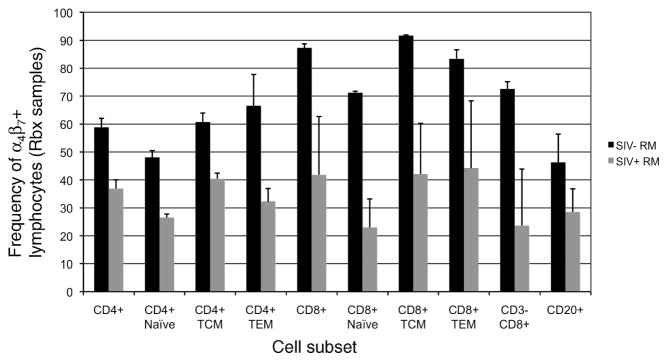
Since our main goal is to eventually administer the Rh-α4β7 mAb to RM prior to or during acute SIV-infection, it was deemed important to first characterize and understand the changes, if any, that occur in α4β7+ lymphocytes following SIV-infection. To this end, both longitudinal and cross-sectional studies were conducted to evaluate the acute and chronic effects, respectively, of SIV-infection on α4β7+ expression on lymphocytes from the periphery and GI tissues of uninfected and SIV-infected RM. The cross-sectional analysis of PBMC samples from SIVmac239 chronically infected RM (n=9) with either high VL (>100,000 vRNA copies/ml plasma, n=5) or low VL (<10,000 vRNA copiles/ml plasma, n=4) and for comparison uninfected RM (n = 5) failed to reveal any significant changes (p > 0.05) in the frequency of α4β7+ T-cell subsets, α4β7+ B-cells or α4β7+ NK cell subsets in samples from the SIV-infected animals during chronic infection as compared to the uninfected control animals (data not shown). However, the comparison of intra-epithelial lymphocytes isolated from Rbx from 2 uninfected and 2 SIV-infected RM 8–10 weeks post infection revealed a ≥ 2-fold decline in the frequency of α4β7+ NK cells and α4β7+ T-cells particularly among α4β7+ CD4+ TEM cells and α4β7+ CD8+ T-cells (Figure 7) while a relatively smaller decline was noted for α4β7+ B-cells in these Rbx tissues. The relative viral loads in enriched populations of α4β7+ versus α4β7− enriched CD4+ T-cell populations isolated from the peripheral blood of 6 chronically SIV-infected but asymptomatic RM with both high and low (n=3 each) plasma VL was also determined but results revealed no statistically significant differences in cellular VL copy number between the α4β7+ and α4β7− CD4+ T-cells (p > 0.05, data not shown).
Figure 7.
Chronic SIV infection in RM leads to a decline in the frequency of most α4β7+ lymphocytes in the GALT. Intra-epithelial lymphocytes isolated from rectal biopsy samples from uninfected (black bars, n=2) and SIV-infected rhesus macaques (grey bars, n=2, approximately 2 mths post infection) were stained with biotinylated murine α4β7 mAb and streptavidin-PE-Cy7 and analyzed by flow cytometry. Shown are the frequency of α4β7+cells within CD4+ and CD8+ T-cell subsets, NK cells and B-cells.
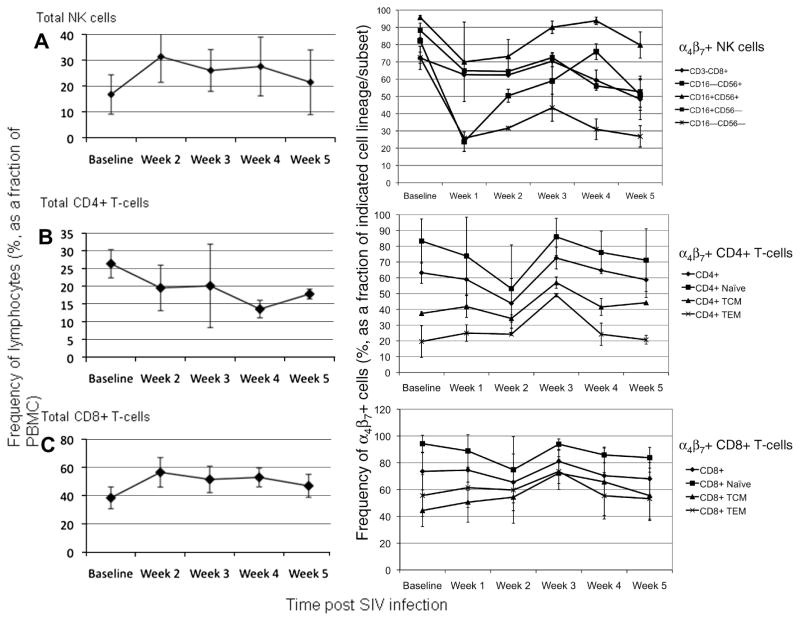
In contrast to chronically SIV-infected RM, significant changes in the frequency and ABS of select peripheral T-cell and NK cell subsets were noted during the acute phase of SIV-infection. Analysis of 4 RM prior to (following 2 consecutive baseline values) and during the first 5 weeks of infection with SIVmac239 revealed that a significant increase in both the frequency (Figure 8A, left panel) and ABS (not shown) of total NK cells occurred in the periphery which is similar to our previous findings [34]. Analysis of the specific α4β7+ NK cells and its subsets (Figure 8A, right panel) revealed that a nearly 4-fold decline occurred in the frequency of α4β7+ CD16−CD56+ and α4β7+ CD16−CD56− NK subsets at week 1 post infection while a less significant decline was also noted in the frequency of α4β7 expressing CD16+CD56− and CD16+CD56+ NK subsets. The determination of absolute numbers (ABS) revealed an approximate 2-fold decline by week 2 and the ABS of the α4β7+ CD16−CD56+ and α4β7+ CD16−CD56− NK subsets remained low through week 5 (not shown). However, a substantial increase in the ABS of α4β7+ CD16+CD56− and CD16+CD56+ NK subsets was observed, with a 4-fold and 2-fold increase, respectively, being noted at week 2 post infection. Levels of ABS returned to near baseline values for the cytolytic α4β7+ CD16+CD56− NK subset by week 5 but remained elevated for α4β7+ CD16+CD56+ NK cells.
Figure 8.
Acute SIV infection in RM leads to a decline and/or mobilization of select α4β7+ lymphocytes. Longitudinal analysis of the frequency of (A) total and α4β7+ NK cell subsets, (B) total and α4β7+ CD4+ T-cell subsets and (C) total and α4β7+ CD8+ T-cell subsets during the acute phase of SIV infection in RM. The frequency of total NK cells, CD4+ T-cells and CD8+ T-cells (left panels) was determined in 4 RM that were experimentally infected with SIVmac239. Isolated PBMC were also stained with biotinylated murine α4β7 mAb followed by streptavidin PE-Cy7 and the frequencies of indicated α4β7+ lymphocyte subsets (right panels) in the periphery were monitored by flow cytometry every week for 5 weeks post infection. Shown are the mean frequencies for each cell subset at the indicated time points. Changes, if any, in ABS are described in the text.
With regards to CD4+ T cells, a steady decline in the frequency of total CD4+ T-cells was observed (Figure 8B, left panel) and while a 20–30% decrease in the frequency of total α4β7+ CD4+ T-cells and the α4β7+ naïve subset was observed by week 2, no significant changes were noted in the frequency of α4β7+ TCM and α4β7+ TEM subsets (Figure 8B, right panel). However, the analysis of ABS at week 2 revealed a significant 2–3 fold decline in all three α4β7+ CD4+ T-cell subsets with the largest decrease being observed for α4β7+ naïve and α4β7+ TCM subsets (data not shown). The levels of these subsets remained low with a modest increase in ABS being observed at week 5 post infection. No significant changes in the frequency of α4β7+ CD8+ T-cells and its α4β7+ subsets were observed during the acute infection period (Figure 8C, right panel). However, in contrast to α4β7+ CD4+ T-cells, a slight increase in the ABS of α4β7+ CD8+ T-cells and its subsets were noted at week 2 post infection, with the largest increase (2-fold) being noted for the α4β7+ TEM subset (data not shown). The ABS of the α4β7+ CD8+ TCM subset remained steady but by weeks 4 and 5, an approximately 2-fold decline in the ABS of the α4β7+ CD8+ naïve and α4β7+ CD8+ TEM subsets were observed. There were no significant changes observed in the frequency of α4β7+ B-cells and there was no clear trend noted in ABS of B-cells during acute SIV-infection (data not shown).
Discussion
The implications of α4β7 expression patterns on the ability of distinct cell populations to home to gut mucosal sites have led to efforts to understand both the mechanism behind the substantial decline in CD4+ T-cells in HIV/SIV pathogenesis as well as mechanisms underlying the trafficking of lymphocytes that would naturally replace and potentially help in the control of viral replication at this site of infection. A recent study by Arthos et al [16] demonstrated that not only was there a direct interaction between α4β7 and the HIV envelope protein gp120 but that depending on the viral isolate, there was considerable variability in the efficiency with which this binding occurred. Thus, in addition to offering one explanation for the rapid decline in mucosal memory CD4+ T-cells during acute HIV infection, these observations also raise the possibility that targeting gp120-α4β7 interactions in vivo may interfere with optimal binding, perhaps limiting and/or inhibiting the establishment of a successful viral infection. Studies of in vivo blocking studies in mice using antibodies against α4β7 have already laid the foundation for blocking such receptor ligand interactions in the trafficking of T cells to intestinal tissues [39, 40]. Several antagonists for the gut homing markers α4β7 and even CCR9 are currently in clinical development for the treatment of inflammatory diseases, which include humanized anti-α4β7 antibody and the use of a small molecule CCR9 inhibitor such as Traficet-EN™ (ChemoCentryx) for the treatment of Crohn’s disease and ulcerative colitis [41, 42]. Exploiting this approach in the context of HIV pathogenesis may prove to be effective since an anti-α4β7 blocking or depleting antibody may have preventative or therapeutic effects. Given the limitations of our knowledge of the potential consequences that such therapy would have in HIV patients, the studies presented herein therefore set out to first characterize α4β7 expression patterns in RM, the non-human primate model of AIDS, and then proceeded to evaluate the safety and efficacy of a Rh-α4β7 mAb which is a recombinant primatized construct of the original murine α4β7 mAb that has previously been shown to specifically recognize the α4β7 heterodimer.
While the analysis of α4β7 expression on various lymphocyte subsets from RM revealed expression patterns that are basically very similar to what has been reported for human lymphocytes, some notable differences were observed. First, α4β7 expression levels on CD8+ T-cells and particularly B-cells in RM were predominantly low which is in contrast to what has been observed on these two lymphocyte subsets in humans. This may possibly be due to a species-specific difference although it should be noted that even in humans, considerable variability in α4β7 density was reported at least for B-cells and this appeared to be age-related [10]. Second, our results showed that α4β7high is expressed on mostly peripheral CD4+ TCM T-cells (as defined by CD28, CD95 and CCR7 expression) with CD4+ naïve and TEM cells being predominantly α4β7low, and only a small frequency of α4β7high CD4+ cells were observed in GALT samples which was unexpected. These observations raise the question of whether high densities of α4β7 are truly required for mobilization to the gut or if significant downregulation of α4β7 expression occurs after α4β7+ lymphocytes have trafficked to this mucosal target site. More detailed trafficking studies of α4β7low and α4β7high lymphocytes will be required to better address this issue. Nonetheless, our observation of α4β7high expression levels on predominantly CD4+ memory T-cells in combination with the recent finding by Arthos et al regarding the binding of HIV gp120 to α4β7, offers further support for why this cell subset is relatively more susceptible to infection and depletion early in infection. However, our analysis of cellular VL in purified α4β7+ and α4β7− CD4+ T-cell populations from chronically SIV-infected RM suggest no preferential replication of SIV in α4β7+ cells over α4β7− subsets although studies are being pursued by us to determine cellular VL in more specific α4β7low and α4β7high subsets, particularly in acutely SIV-infected animals, to elucidate the susceptibility of these cell populations to infection and subsequent depletion. Replacing this depleted population via infusion methods is not implausible as our current study showed that high levels of α4β7 expression on CD4+ T-cells can be effectively induced in vitro with RA. Thus, expanding these cells ex vivo for possible in vivo infusion experiments as a means to replenish CD4+ T-cells and/or provide robust effector CD4+ T-cells that home preferentially to the affected GI compartment is feasible. In support of this, a recent study that involved the in vivo tracking of infused CFSE-labeled CD4+ α4β7+ T-cells that were expanded ex vivo with anti-CD3/CD28 Abs revealed that the gut tissues contained 2% of such labeled cells at one week following infusion (F. Villinger et al, manuscript in preparation). While results of the in vitro RA assay did not reveal any significant induction of α4β7 expression on CD8+ T-cells following a 5-day incubation period with RA, it is possible that the kinetics of α4β7 upregulation on this cell lineage may be delayed in comparison to CD4+ T-cells and prolonged treatment with this Vitamin A metabolite may lead to significant α4β7 induction on CD8+ T-cells.
Our analysis of α4β7 expression in the context of SIV infection revealed rapid peripheral declines in select lymphocyte subsets that include α4β7+ CD4+ T-cells and α4β7+ NK cells primarily during the acute phase. It is not clear whether these rapid peripheral declines in α4β7+ CD4+ T-cells are due to induced trafficking to the GALT, direct cytopathic effects of the virus, or both. Of importance, the declines in peripheral α4β7+ NK cells, particularly within the cytokine-producing α4β7+ CD16−CD56+ subset, suggest that their trafficking to the GI track is induced as early as week 1, setting in motion downstream SIV-specific adaptive immune responses at this major site of infection. In support of this view, a decline in the ABS of α4β7+ naïve and α4β7+ TEM CD8+ T-cells occurred in the periphery at weeks 4 and 5, after the noted decline in α4β7+ CD16−CD56+ NK cells. If trafficking of α4β7+ NK cells to the GALT is indeed occurring, this again raises the question of whether high densities of α4β7 expression are truly required for trafficking to this mucosal site since NK cells in RM were found to exhibit primarily a α4β7low phenotype, as did CD8+ TEM cells. It is possible that integrins other than α4β7 contribute to trafficking of certain cell lineages to the GI tract. It is of interest to note that there were minimal changes in the level of α4β7+ B-cells during acute SIV infection, suggesting a less prominent role for this cell lineage in the GALT or that other gut-homing receptors are upregulated on B-cells during acute SIV/HIV infection.
Prior to in vivo administration of Rh-α4β7 mAb, we confirmed that this mAb had limited immunogenicity to minimize cellular activation that could lead to additional cellular targets for SIV infection therefore counteracting the intended preventive or therapeutic effect of the antibody in future experiments with SIV-infected RM. Experiments to evaluate the effect of this antibody on general tyrosine phosphorylation in unfractionated PBMC or highly enriched population of CD4+ T-cells failed to reveal any detectable increase in phosphorylation when compared to untreated cells (data not shown). An additional in vitro 3H-thymidine-based cell proliferation assay revealed that Rh-α4β7 at varying concentrations (0.1 to 25 ug/ml) failed to induce detectable cell proliferation (data not shown), thus lending further support for the observation that Rh-α4β7 does not induce detectable levels of cell activation at least under these conditions. These data are distinct from a previous study [43] which reported that immobilized α4β7 mAb served to co-stimulate T cells when used in conjunction with sub-mitogenic doses of anti-CD3 mAb. Similar approaches are therefore being pursued in efforts to determine if in fact our Rh-α4β7 mAb has similar effects. The administration of Rh-α4β7 in vivo, even at a high dose of 50 mg/kg was however well tolerated. Importantly, the substantial decline in the frequency and ABS of α4β7+ lymphocytes in both the periphery and GALT on Day 1 suggests that Rh-α4β7 successfully exerted its effect in vivo. Although the reduction in ABS and frequency of α4β7+ lymphocytes in the periphery and GALT, respectively, suggests a true decline in these cell populations, the redistribution of these lymphocytes to other compartments cannot be ruled out. For future experiments, it would also be important to determine if such a decrease in ABS also occurs in the GALT following Rh-α4β7 administration.
During the course of this experiment, two additional observations were made which provided important information about both the mAb itself and the nature of the cells that it targets. First, a recovery of β7 integrin+ lymphocytes was noted in the periphery at Day 5 and thereafter but the level of α4β7+ lymphocytes detected by murine α4β7 mAb was still negligible for > 6 weeks after Rh-α4β7 administration, suggesting that circulating Rh-α4β7 cross-blocked newly produced and/or newly trafficking α4β7+ cells upon entry into the periphery thereby blocking their detection by the murine mAb. The finding that the primatized mAb remained in circulation for up to 40–50 days also suggests long-term in vivo stability and that it was unlikely that the monkeys generated an immune response to this antibody, which otherwise would have exhibited a faster in vivo clearance. Second, our results also revealed that the extent of decline was not uniform among the cell lineages, with a slightly less pronounced decrease being noted for α4β7+ B-cells in the periphery and GALT. It is possible that differences in the tissue specific redistribution of these cell lineages and/or rates of cell turnover account for this difference, since it has been reported that B-cell turnover in RM occurs at a rate faster than T-cells [44, 45]. Thus, the observed lower level of decline of α4β7+ B-cells may be due to quicker neogenesis/replacement of these lymphocytes and perhaps a more substantial decrease may have been observed in the hours immediately following Rh-α4β7 administration. Nonetheless, these data collectively demonstrate that Rh-α4β7 can be safely administered and results in remarkably efficient cross-blocking of the α4β7 receptor and perhaps even a decline due to redistribution and/or depletion of α4β7+ lymphocytes, particularly T-cells which ultimately is the target cell lineage of interest. These observations lay the foundation for future chronic dosing experiments with Rh-α4β7 in acutely and chronically SIV-infected animals. Whether chronic dosing of this mAb leads to safe and sustained cross-blocking, depletion and/or redistribution of α4β7+ lymphocytes remains to be determined and is the current focus of study in our laboratory, as are studies to determine the potential preventative and/or therapeutic effects of Rh-α4β7 during early viral infection.
Supplementary Material
Acknowledgments
We would like to sincerely thank Dr. James T. Kurnick who provided us with the murine α4β7 mAb clone and also thank Stephanie Ehnert and the veterinary and support staff of the YNPRC for their efficient care and handling of the animals. We would also like to thank Ann Mayne, Susan Stephenson, Austin Lewis, Dawn Little and Keli Kolegraff for their help with initial characterization of the murine α4β7 mAb.
Footnotes
Supported by NIH RO1 AI 078773-01, N0I AI 040101, R24 RR016001, grants from the Thailand Research Fund and the Ministry of Health, Labor and Welfare, Japan.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dandekar S. Pathogenesis of HIV in the gastrointestinal tract. Current HIV/AIDS Reports. 2007;4:10–15. doi: 10.1007/s11904-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 2.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay ini restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heise C, Vogel P, Miller CJ, Halsted CH, Dandekar S. Simian immunideficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological and morphological changes. Am J Pathol. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RP. How HIV guts the immune system. N Engl J Med. 2008;358:2287–9. doi: 10.1056/NEJMcibr0802134. [DOI] [PubMed] [Google Scholar]
- 5.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:2335–2348. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talal AH, Irwin CE, Dieterich DT, Yee H, Zhang L. Effect of HIV-1 infection on lymphocyte proliferation in gut-associated lymphoid tissue. J Acquir Immune Defic Syndr. 2001;26:208–217. doi: 10.1097/00042560-200103010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Vajdy M, Veazey R, Tham I, deBakker C, Westmoreland S, Neutra M, Lackner A. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J Infect Dis. 2001;184:1007–1014. doi: 10.1086/323615. [DOI] [PubMed] [Google Scholar]
- 8.Vajdy M, Veazey RS, Knight HK, Lackner AA, Neutra MR. Differential effects of simian immunideficiency virus infection on immune inductive and effector sites in the rectal mucosa of rhesus macaques. Am J Pathol. 2000;157:485–495. doi: 10.1016/S0002-9440(10)64560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 10.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin α4β7, on human leukocytes. J Immunol. 1994;153:517–527. [PubMed] [Google Scholar]
- 11.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Andrian UHV. Selective imprinting of gut-homing T-cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 12.Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. J Immunol. 1996;156:3727–3736. [PubMed] [Google Scholar]
- 13.Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GEG, Lazarovits AI, Buck D, Shaw S. Selective expression of integrin α4β7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J Immunol. 1993;151:717–729. [PubMed] [Google Scholar]
- 14.Agace WW. T-cell recruitment to the intestinal mucosae. Trends Immunol. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2400. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arthos J, Cicala C, Martinelli E, Macleod K, Ryk DV, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 17.Kewenig S, Schneider T, Hohloch K, Lamps-Dreyer K, Ullrich R, Stolte N, Stahl-Hennig C, Kaup FJ, Stallmach A, Zeitz M. Rapid mucosal CD4(+)T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–1123. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 18.Letvin NL, King NW. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simiam immunodeficiency virus of macaques. J Acqui Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 19.Onlamoon N, Pattanapanyasat K, Ansari AA. Human and nonhuman primate lentiviral infection and autoimmunity. Ann N Y Acad Sci. 2005;1050:397–409. doi: 10.1196/annals.1313.091. [DOI] [PubMed] [Google Scholar]
- 20.Firpo PP, Axberg I, Scheibel M, Clark EA. Macaque CD4+ T-cell subsets: influence of activation on infection by simian immunodeficiency viruses (SIV) AIDS Res Hum Retroviruses. 1992;8:357–366. doi: 10.1089/aid.1992.8.357. [DOI] [PubMed] [Google Scholar]
- 21.Kaur A, Grant RM, Means RE, McClure H, Feinberg M, Johnson RP. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J Virol. 1998;72:9597–9611. doi: 10.1128/jvi.72.12.9597-9611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, Haase A, Watkins DI. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J Virol. 2005;79:9228–35. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: Insights from the SIV-macaque model. Annu Rev Med. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- 26.Lazarovits AI, Moscicki RA, Kurnik JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB. Lymphocyte activation antigens: A monoclonal antibody, anti-Act1, defines a new late lymphocyte activation antigen. J Immunol. 1984;133:1857–1862. [PubMed] [Google Scholar]
- 27.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coombes JL, Siddiqui KRR, Arancibia-Carcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. A functionally specialzed population of CD103+ DCs induces FoxP3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J Exp Med. 2007 doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:458–466. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 32.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 33.Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, Estes JD, Engram JC, Mayne A, Dunham RM, Lawson B, Sodora DL, Staprans SI, Reimann K, Silvestri G, Ansari AA. The availability of activated CD4+ T cells is a major determinant of set point viremia during natural SIV infection of sooty mangabeys. J Clin Invest. 2008;118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira LE, Johnson RP, Ansari AA. Sooty mangabeys and rhesus macaques exhibit significant divergent natural killer cell responses during both acute and chronic phases of SIV infection. Cell Immunol. 2008;254:10–19. doi: 10.1016/j.cellimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Villinger F, Brice GT, Mayne AE, Bostik P, Mori K, June CH, Ansari AA. Adoptive transfer of simian immunodeficiency virus (SIV) naive autologous CD4+ cells to macaques chronically infected with SIV is sufficient to induce long-term nonprogressor status. Blood. 2002;99:590–599. doi: 10.1182/blood.v99.2.590. [DOI] [PubMed] [Google Scholar]
- 36.Bator JM, Reading CL. Measurement of antibody affinity for cell surface antigens using an enzyme linked immunosorbent assay. J Immunol Meth. 1989;125:167–176. doi: 10.1016/0022-1759(89)90090-2. [DOI] [PubMed] [Google Scholar]
- 37.Cromwell MA, Veazey RS, Altman JD, Mansfield KG, Glickman R, Allen TM, Watkins DI, Lackner AA, Johnson RP. Induction of mucosal homing virus-specific CD8+ T lymphocytes by attenuated simian immunodeficiency virus. J Virol. 2000;74:8762–8766. doi: 10.1128/jvi.74.18.8762-8766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans DT, Chen LM, Gillis J, Lin KC, Harty B, Mazzara GP, Donis RO, Mansfield KG, Lifson JD, Desrosiers RC, Galan JE, Johnson RP. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J Virol. 2003;77:2400–2409. doi: 10.1128/JVI.77.4.2400-2409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reese SR, Kudsk KA, Genton L, Ikeda S. l-selectin and alpha4beta7 integrin, but not ICAM-1, regulate lymphocyte distribution in gut-associated lymphoid tissue of mice. Surgery. 2005;137:209–215. doi: 10.1016/j.surg.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 41.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Cohen A, Bitton A, Baker J, Dubé R, Landau SB, Vandervoort MK, Parikh A. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 Integrin. Clin Gastroenterol Hepatol. 2008;6:1370–1377. doi: 10.1016/j.cgh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, Dube R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 43.Teaugue TK, Lazarovits AI, McIntyre BW. Integrin alpha4beta7 co-stimulation of human peripheral blood T cell proliferation. Cell Adhes Commun. 1994;2:539–547. doi: 10.3109/15419069409014217. [DOI] [PubMed] [Google Scholar]
- 44.Boer RJD, Mohri H, Ho DD, Perelson AS. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J Immunol. 2003;170:2479–2487. doi: 10.4049/jimmunol.170.5.2479. [DOI] [PubMed] [Google Scholar]
- 45.Mohri H, Bonhoeffer S, Monard S, Perelson AS, Ho DD. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science. 1998;279:1223–1227. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.