Summary
Oligomerization is important for the structure and function of many proteins, but frequently complicates their characterization. It is often desirable to obtain the protein in monomeric form. Here we report a strategy that allows the generation of monomers from weakly associated oligomers but which does not require knowledge of the three dimensional structure of the protein. The dynamics of protein association are used in solution NMR spectroscopy to identify regions of the polypeptide chain that are likely to be responsible for the interaction. Protein sequence analysis further refines the selection, as conserved sites with moderate hydrophobicity are targeted for modification. Gel filtration and activity assays straightforwardly reveal the consequences of the change and are used to screen for the desired mutants. The strategy is demonstrated for the Rac1 binding domain of plexin-B1. A monomeric variant is generated which preserves the Rac1 binding activity and the wild type protein structure.
Keywords: Protein-protein interactions, site directed mutagenesis, NMR spectroscopy, resonance line-broadening, chemical exchange
Introduction
Proteins predominantly function as oligomers in cells. For example, a survey of 372 E. coli proteins in the SWISS-PROT database showed that monomeric proteins account for only about one fifth of the gene products (Goodsell and Olson, 2000). Oligomerization is known to modulate the energetics and function of most proteins. Firstly, associated species are generally more stable than the individual units. For example, several enzymes exist as stable oligomers in hyperthermophilic organisms while monomeric forms, either engineered variants or mesophilic homologues, are thermodynamically labile at high temperature (Thoma et al., 2000). Secondly, oligomerization often provides proteins with novel activity or regulatory mechanisms, as is the case for multivalent binding and allostery that are formed by protein association (e.g. Changeux and Edelstein, 1998).
An important aspect of oligomers is, however, that these protein forms are often challenging to characterize. This is the case particularly when the association affinity between the subunits is weak in vitro (e.g. Kd in the range of 1 μM to greater than mM) as its actual strength and possible functional role will be highly context dependent in vivo. In many systems, the biological role of oligomerization is not clear and may be an artifact of the solution conditions, principally the high protein concentration used in crystallography and NMR. However, the characterization of relatively weak associations is important as the interacting surfaces are easily tuned to increase or decrease affinity by several orders of magnitude. Monomerization of proteins presents opportunities for their structural and functional characterization. The changes in conformation and activity that occur upon association can often be understood with reference to these simpler protein units. Thus, the generation of monomers tests our understanding of the particular system and our knowledge of protein biophysics. Along these lines, commercial production of proteins aims at high in vitro folding efficiency and catalytic stability, thus the generation of functional monomers from their oligomeric counterparts has been an objective of protein engineering for many years. In summary, monomeric forms of proteins are of considerable interest from a biological, basic science and from a commercial perspective.
It is generally thought that oligomeric proteins evolved from monomeric ancestors (Bennett et al., 1994; Bennett et al., 1995; Xu et al., 1998; D’Alessio, 1995; D’Alessio, 1999; Goodsell and Olson, 2000). Eisenberg and co-workers proposed the three-dimensional domain-swapping model (Bennett et al., 1994; Bennett et al., 1995) to explain the origin of a significant number of oligomers. In this model, the hinge loop linking the swapped domains is considered to be most critical for oligomerization. D’Alessio proposed that oligomerization, in general, is the outcome of a ‘primary’ mutational event that dramatically changes the stability of the ancestral monomer, leading to association of the protein for greater stability (D’Alessio, 1999). ‘Secondary’ mutational events further stabilize the metastable oligomers, but by themself are not sufficient to promote oligomerization. These views are consistent with the finding that mutations at different sites have diverse effects on the protein structure, function and/or stability. Sites which exert the most profound effects are called “hot spots” and are residues that have been highly selected in the proteins evolution (Taverna and Goldstein, 2002; DeLano, 2002). Thus, change in a single sidechain could be sufficient to disrupt oligomerization and there is considerable interest in the development of methods that identify residues that are responsible for protein association.
High-resolution solution NMR is a versatile tool for the characterization of protein structure, dynamics and also for the delineation of biomolecular interactions. Structure determination of proteins of size less than 25 kDa has become routine when the dynamics of the protein are favorable (Arrowsmith and Wu, 1998) and chemical shift assignments of an 81 kDa monomeric enzyme could be made when techniques such as perdeuteration, TROSY, and specific labeling are used (reviewed in Fernandez and Wider, 2003). However, protein oligomerization poses several challenges for NMR. Molecular size is increased several-fold, thus tumbling of the protein is slowed down compared to the monomer, leading to broadening of the resonances, greater signal overlap and reduced signal to noise. Resonance degeneracy hinders the distinction of inter- and intra-molecular signals and thus complicates structure calculation. Furthermore, in the case of a weak association between protein units, the dynamics of the monomer-oligomer equilibrium is typically unfavorable for NMR studies. Intermediate rate chemical exchange leads to a broadening, or even disappearance of resonances for the residues that experience different chemical environments between the monomer and the oligomer (Cavanagh et al., 1996). Such exchange property, although detrimental for the measurement of most NMR parameters, has been frequently used to map the location of residues involved the association interface. Thus, in our strategy, regions with missing assignments delineate possible interface regions of the polypeptide chain which, together with an analysis of the protein sequence, guides the selection of sites for mutagenesis. It should be noted that sites which are not affected by chemical exchange are not involved in the interface and usually can be assigned straightforwardly because the signals are intense. Our strategy utilizes the information that is the most straightforward to obtain—the regions of the protein that are easy to identify by NMR. Importantly, if the association interface and conformation of the oligomeric protein is to be studied by NMR, the assignment step is a necessary part of the analysis.
The Rac1 binding domain (RBD) of plexin-B1 was chosen as the model system to illustrate our strategy. Plexins are receptors that detect guidance cues and communicate signal path-finding decisions in axons in the developing nervous system. Plexins are the first known receptors whose cytoplasmic domain interacts directly with a small Rho family GTPase, Rac1 (Vikis et al., 2000), serving as a functional readout in this case. Like many transmembrane receptors, plexins are presumed to form dimers in vivo. The crystal structure of semaphorin, a ligand for plexin, suggests that the dimer-monomer transition of plexins may play a role in their signaling mechanism (Antipenko et al., 2003). Consistent with this inference, we find that the cytosplasmic Rac1 binding domain alone exists as a weak dimer with a dissociation constant around 25 μM. No experimentally determined structure or homology model for the RBD of the plexin family exists at present, and thus this protein served a test case for our strategy.
In cases where no three-dimensional structural data are available, finding a monomer-generating mutation has been regarded a “lucky hit” amongst many “misses”. Here we propose a two component strategy to guide the selection of sites to be mutated. The first component is based upon the frequently observed broadening of resonances that arise from residues located at the protein-protein interface in solution NMR, as outlined above. The second, discussed below, is based upon sequence alignments and the identification of conserved residues and moderately hydrophobic regions. The results illustrate that many outcomes are possible, and also caution that some of the changes may disrupt protein function. Straightforward detection and interpretation of the outcomes form a cornerstone of the strategy. We believe this strategy is widely applicable to other systems, whose three dimensional structure is still unknown.
Results
Identification of weak dimerization by gel filtration and NMR
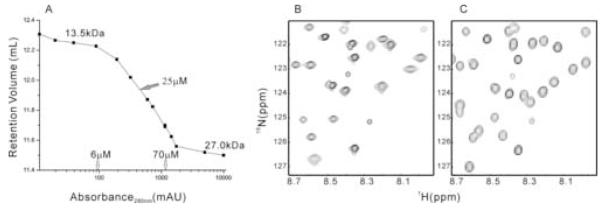
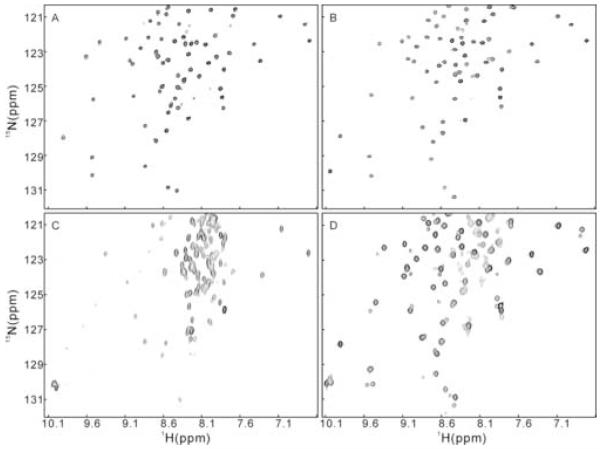
We used a sephadex-75 gel filtration column to assess the association status of the plexin-B1 RBD (res. 1743-1862). The retention time of the wild type protein was found to be concentration dependent (Figure 1A). At typical NMR concentration (0.3 to 1~2 mM), the RBD domain exists largely as a dimer judging from a molecular weight twice that expected for a monomer with reference to molecular weight standards. When the concentration is decreased to micromolar level (< 10 μM), the protein exists predominantly as a monomer of 13.5 kDa. At intermediate concentrations, monomer and dimer exist in equilibrium in the solution that is fast on the gel filtration time scale, giving population-averaged molecular weights. Taking the midpoint of the transition from monomer to dimer as a rough estimate of the dissociation constant, the value of 25 μM obtained is typical of weak interaction between proteins. Inspection of the 15N-1H HSQC spectrum of the wild type protein at NMR concentration (Figure 1B) reveals an uneven distribution of resonance intensities and signal line widths. Moreover, the number of cross-peaks corresponding to the backbone amide groups is up to 18 less than that is expected for this protein, not counting the prolines. The exact number depends on the concentration of the samples. In principle, resonance broadening can also arise from intermediate time scale internal protein dynamics. This is distinguished from association-dependent line broadening by the concentration dependence of the latter. At concentration of 10 μM, the missing resonances appeared in the HSQC spectrum recorded with a cryoprobe (Figure 1C). Thus, the results are consistent with a weak association of the protein. The missing resonances presumably correspond to those residues located at the dimer interface.
Figure 1.
Wild type plexin-B1 RBD at different protein concentrations
(A) Retention volume of plexin-B1 RBD on sephadex-75 gel filtration column at different concentrations, measured by UV absorbance at 280 nm, 276K. (B) 15N-1H HSQC of wild type plexin RBD at 1.4 mM, 298 K. (C) 15N-1H HSQC of wild type plexin RBD at 10 μM, 298 K. Each signal in the displayed regions arises from a different amide.
Resonance assignments suggest the regions to target for point mutations
Except for signals at the association interface, NMR spectra of the plexin RBD are highly resolved, suggesting well defined structure for the monomeric and dimeric forms of the protein. If the protein were to form non-specific aggregates, the quality of the spectrum would suffer more uniformly. Changing solution conditions (pH, temperature, salt concentration, addition of cosolvents, for example, low concentrations of TFE, DMSO, acetonitrile) can frequently dissociate weak protein oligomers. In the case of plexin, however, none of the changes in solution conditions yielded a significant population of monomeric protein at high protein concentration.
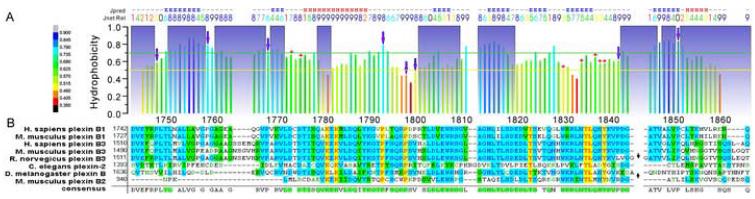
Assignment experiments of proteins are not feasible at micromolar concentrations, principally due to the intrinsic low sensitivity of current NMR techniques. Thus, in the first instance we recorded NMR experiments (Sattler et al., 1999) for assignment of the dimeric protein at 1.4 mM concentration in order to identify all resonances belonging to the mainchain regions which are not involved in the association. Close to 60% of the cross-peaks in the 15N-1H HSQC spectrum of the backbone amide groups were assigned in the initial step. Except unassigned residues in short sequence stretches (less than 4 consecutive residues), there remain three major regions unassigned, i.e. residues 1772-1778, 1782-1800, 1820-1842 (Figure 2A). Discounting the borders of these regions (one residue on either side), the regions represent 43 candidates for mutagenesis in the 120 residue protein and an additional selection of candidate sites was deemed necessary.
Figure 2.
NMR backbone assignment and sequence analysis of the plexin-B1 RBD
(A) Hydrophobicity scale (Eisenberg et al., 1984) as a measure of polarity and Jnet secondary structure prediction (Cuff and Barton, 2000) of the plexin RBD. For hydrophobicity calculation, a window size of 5 was chosen. A lower value indicates a locally more polar region. Other hydrophobicity scales give nearly identical results. “Jpred” indicates the consensus prediction results of Jnet method, where H stands for helical and E stands for extended secondary structure. “Jnet Rel” is the prediction accuracy, scaling from 0 to 9. Residues assigned initially from spectra of the dimer are shadowed. Prolines are indicated by arrows. A yellow line and a green line are drawn at hydrophobicity values of 0.5 and 0.7 respectively. Sites chosen for mutagenesis are labeled with +. (B) Alignment of the RBD of plexin-B1 with members of the protein family from different species. Non-conserved inserts of 26 residues in R. norvegicus plexin B3 and 7 residues in D. melanogaster plexin B, indicated by black arrows, are omitted in the plot for clarity. Conserved sites with 5 or more residues identical in the alignment are shadowed green in the consensus.
Point mutations are targeted to conservative residues and moderately hydrophobic regions
We targeted residues that are rather conservative among different species of plexins (identical in at least 5 of 8 plexin-B sub-family members as seen in Figure 2B), as we presume that oligomerization occurs across the family. Evolution will have resulted in a decreased variance of sidechains at sites that are critical for the interaction. However, residues are highly conserved for a multitude of reasons in proteins, depending on their importance in protein structure, stability and/or function. Thus, 8 out of 43 candidate sites were excluded, due to their lack of conservation (18, if a more stringent criterion- at least 6 of 8 identical, is used). It should be noted that with this example, we make no distinction between conservative and non-conservative mutations. Furthermore, a selection cut-off based on conservation will depend on the number of family members and their divergence in the alignment.
The subset of sites which are typically conserved because they comprise the hydrophobic cores of proteins may be excluded by a second selection criterion based on local residue hydrophobicity (shown in Figure 2A). While highly polar regions of the polypeptide chain are unlikely to be responsible for oligomerization, involvement of completely hydrophobic regions would argue against the notion of a stable monomeric precursor. Sites in regions with an average hydrophobicity of 0.5-0.7 were targeted (excluding 11 of the 43 candidate sites; 17 when combined with conservation; leaving 26 candidate sites). In some instances, several different substitutions were chosen for the same site, ranging from “conservative” mutations, which would replace presumably a hydrophobic contact, a hydrogen bond or charge, or change the residue size, to mutations that combine such changes and are envisaged to be more disruptive to the interface. Thirteen mutants (Table 1) were designed for residues located at 7 sites in two regions (residues 1772-1778 and 1820-1842) of the protein which were difficult to assign. Since change of the sidechain at a single site can be sufficient to disrupt oligomerization, it does not matter whether the candidate sites are widely distributed and whether residues are mutated in all regions.
Table 1.
Mutagenesis, oligomeric status and activity of plexin-B1 RBD
| Number | Mutation | Oligomeric statusa | Activityb |
|---|---|---|---|
| 1 | D1773N/D1775N | D | -c |
| 2 | W1830Y | M | Y |
| 3 | W1830S | M | Y |
| 4 | W1830F | M | Y |
| 5 | N1834D | D | N |
| 6 | Q1837M | D | Y |
| 7 | Q1837E | D | N |
| 8 | H1838S | M | Y |
| 9 | H1838W | M | - |
| 10 | H1838S/Y1839S | M | N |
| 11 | Y1839S | - | N |
| 12 | Y1839F | M | N |
| 13 | Y1839E | M | N |
Oligomeric status, D for dimer, M for monomer.
Rac1 binding activity as revealed by pull-down assay, Y: mutant binds Rac1, N: mutant does not binds Rac1
data not available
Characterization of the mutants by gel filteration, pull-down assay and NMR
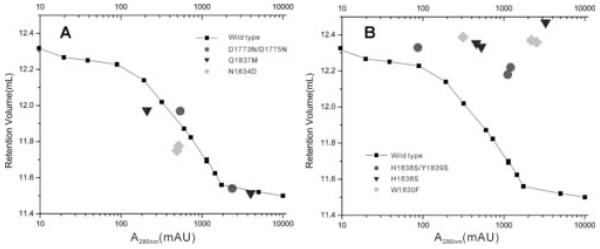
All the mutants were over-expressed and for efficiency purified utilizing the same procedure as used for the wild type protein. Interestingly, not all mutants can be purified equally well; several did not express as well and one precipitated during purification (i.e. Y1839S), providing us with a first selection screen (further inspection of these mutants by NMR revealed that aggregation was increased or that the protein fold was significantly destabilized, e.g. Y1839E, as might be expected since some of the conserved sites targeted may have critical structural roles). The oligomerization state of the mutants was examined by gel filtration at least at two different concentrations (Figure 3A, 3B). Mutations at residues 1773/1775 and at residues 1834 or 1837 do not appear to weaken the tendency of the proteins to associate, although it is possible that the mutations were too conservative. The mutants still undergo weak association, leading to a concentration-dependent elution profile on the gel filtration column. However, mutations at two other sites, residue 1830 and residues 1838/1839, lead to monomerization of the domain.
Figure 3.
Association status of representative mutants
Retention volumes of (A) dimeric and (B) monomeric mutants on gel filtration shown in comparison with the retention volume profile of wild type plexin-B1 RBD.
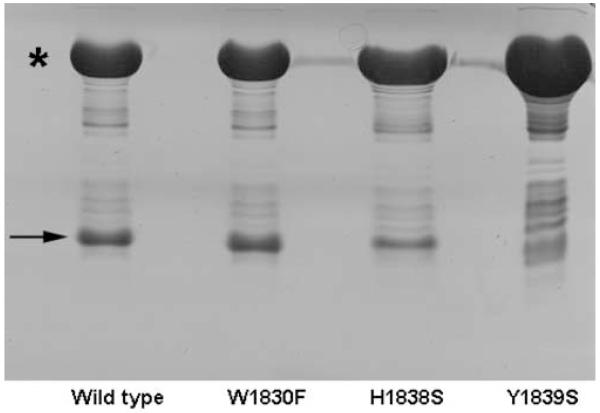
Pull-down assays of the mutants with the plexin RBD binding partner, activated Rac1 (Q61L mutant) were carried out to test the binding affinity of the mutants as a functional readout. Shown in Figure 4 are the results for mutants W1830F, H1838S and Y1839S. W1830F shows binding affinity with RacQ61L comparable to that of the wild type protein, while H1838S shows reduced binding affinity and Y1839S show no binding affinity to RacQ61L in this assay at all. To check the effect of mutation on the structure of the mutants, a 15N-1H HSQC spectrum was acquired for each protein. Interestingly, while most of the resonances in the HSQC spectrum of W1830F (Figure 5A) coincide well with those of the wild type protein at low concentration (Figure 1C), more than 40 signals in the HSQC spectrum of H1838S (Figure 5B) are not traceable to those in the spectrum of the wild type protein. However, the resonances are still well dispersed, indicating a major conformational change in certain parts of the protein structure. Not only protein plasticity, but also examples of more profound conformational changes that can occur as the result of a single residue mutation have been noted in other systems (e.g. Cordes et al., 1999). The dispersion pattern of the 15N-1H HSQC spectrum of Y1839E (Figure 5C) bears a resemblance to that of the wild type protein but also shows many resonances near random coil values, which arise from a population of protein in the unfolded state, indicating that the structure is greatly destabilized upon the mutation at site 1839. The HSQC spectrum of mutant N1834D (Figure 5D) reveals coexistence of two folded conformations in the solution. A rationalization of the varied outcomes for some of the mutations will need to await determination of the structure of the plexin RBD. However, it is interesting that all four possible outcomes (dimer/monomer both with and without binding affinity) are observed, suggesting a partial overlap between the dimerization and the binding interfaces. For this reason, we carried out further analysis of the interaction surfaces in this system.
Figure 4.
Rac1 binding activity of plexin-B1 RBDs
Pull down assay of untagged wild type plexin-B1 RBD, W1830F, H1838S, and Y1839S with GST-tagged Rac1.Q61L show different binding activities. Star (*) and arrow (→) indicate the position of GST-Rac1.Q61L and the plexin RBDs respectively.
Figure 5.
15N-1H HSQC spectra of plexin-B1 RBD mutants at 298 K
(A) Spectrum of RBD-W1830F shows nearly identical distribution of peaks to that of the wild type RBD. (B) Spectrum of RBD-H1838S is also well dispersed but quite different from that of RBD-W1830F. (C) Spectrum of RBD-Y1839E shows of population of unfolded protein. (D) Spectrum of RBD-N1834D shows co-existence of two conformations.
Assignment of a monomeric mutant confirms the interface residues of the wild type protein and shows that this interface partially overlaps with the regions involved in Rac1 binding
Generation of a well defined monomeric protein has been an important step in the determination of the structure of the plexin-B1 RBD (in progress). In the case of W1830F, for example, complete backbone resonance assignment was possible for this monomeric protein in an efficient manner. When comparing the initial assignment of the mainchain of the dimeric form with the assignment of the monomeric species, we find that of the 50 missing assignments, 18 were due to missing resonances (excluding 8 prolines), 8 were due to overlap of the resonances, the rest resonances were unassigned due to interruption of sequential connection by either prolines or missing resonances. Together these account for the reasons why certain regions of the mainchain are refractory to assignment. Translation of the completed assignment from the monomeric W1830F to the monomeric wild type protein at 10 μM was also straightforward as comparison of the HSQC spectra shows that the chemical shift perturbation introduced by the mutation is small and local in the monomer, limited to 4 amide groups.
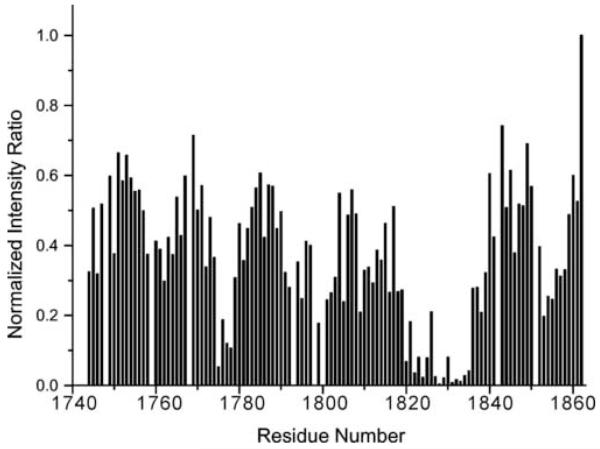
With the assignments completed, residues involved in the dimer interface can be deduced from the intensity ratio of the amide groups at two different concentrations (Figure 6). The analysis reveals that the dimer interface is predominantly located in two regions of the sequence, i.e. residues 1775-1778 and residues 1820-1835, consistent with the location of the mutant sites which yield monomeric proteins. A third region, residues 1791-1803 and a fourth region, residues 1852-1858 are less significantly broadened. Other work in our laboratory has shown that the principle interaction surface between the plexin-B1 RBD and activated Rac1 GTPase partially overlaps with the two regions involved in the dimerization interface (in preparation). This and the disruption of dimerization upon Rac1 binding is consistent with reports which suggest that the entire plexin-B1 receptor exists as a dimer and that receptor activation by extracellular ligand but also by intracellular GTPase binding is associated with a change in dimerization status (Antipenko et al., 2003; Turner et al., 2004). The set of monomerization mutations will be useful for testing the functional role of receptor dimerization at the intact receptor and the cellular level.
Figure 6.
Identification of the dimer interface of the plexin-B1 RBD
Change of signal intensities at different concentrations of wild type plexin-B1 RBD at 298 K are plotted against protein sequence. Intensity at 0.2 mM was divided by that of the corresponding signals at 10 μM protein concentration and normalized to the intensity ratio of the C-terminal amide Q1862, which is not affected by the association.
Discussion
Strategies for the monomerization of proteins
The great majority of efforts to monomerize oligomers to date are based on known three-dimensional protein structures. e.g. (Mossing and Sauer, 1990; Rajarathnam et al., 1994; Borchert et al., 1994; Breiter et al., 1994; Mullen and Jennings, 1998). Even when a three-dimensional structure is available, for example in the case of triosephosphate isomerase, rational design required extensive changes to the interface (Borchert et al., 1994), while a chance mutation showed that a single mutation can be sufficient for the generation of a fully active monomer (Maithal et al., 2002). Several studies have examined the physical and chemical properties of the subunit interfaces of oligomers (Janin et al., 1988; Jones and Thornton, 1995; Bahadur et al., 2003; Jones and Thornton, 1997). Yet interactions of thousands of atoms within a protein are often energetically networked (Lockless and Ranganathan, 1999; Hedstrom, 1996), making it difficult to determine which residues are the most important for a diverse range of interfaces (Ofran and Rost, 2003) even when these are analyzed based on detailed structural data.
However, considerable progress has been made (Kortemme and Baker, 2002; Guerois et al., 2002; Halperin et al., 2004) and a number of themes are beginning to emerge that are relevant for the case considered here; that is, they can also be applied to the prediction of the most important interface residues when three dimensional structural information is not available. Firstly, a higher than average evolutionary conservation has been found for residues at oligomer interfaces and this has been used in predicting which residues are the most important (Valdar and Thornton, 2001; Valencia and Pazos, 2002). In the case of the RBD domain of plexin-B1, mutagenesis sites were deliberately chosen to be those that are relatively well conserved. Secondly, a statistical analysis of the sidechains that are energetically most important for protein association has suggested that certain sidechain types (Trp, Tyr and Arg) are predominantly located in hot spots, while others (Leu, Met, Ser, Thr and Val) are represented much less than expected (Bogan and Thorn, 1998; Ma et al., 2003). Interestingly, as has been pointed out, these results show a strong correlation with hot spot residues which are usually moderately polar, suggesting that a modest dehydration distinguishes protein-protein interfaces from the protein core and protein surface. We note that the three sites that lead to the monomerization of the plexin RBD involve Trp, Tyr and His and that the regions involved in dimerization have medium polarity (Figure 2A). This is consistent with a statistical analysis of the interface of homodimeric proteins (Bahadur et al., 2003). A more exhaustive mutagenesis and structural study would be required in order to establish correlations between residue types and their importance for plexin dimerization and added to this statistical database, but we find that our site selection is consistent with the emerging themes in the field.
Additionally, we noticed that several of the sites targeted for mutagenesis are located in regions of the sequence that are associated with weak or ambiguous secondary structure predictions (e.g. res. 1830 and 1838) and are predicted to be positioned at the termini of secondary structural elements. These sites may thus be primed for conformational change that accompanies protein association, a process that frequently involves formation of additional intra-protein hydrogen bonds (Betts and Sternberg, 1999; Fernandez and Scheraga, 2003). However, secondary structure predictions have to our knowledge not been utilized as a criterion for site selection and our limited survey of protein-protein interaction hot spots in other systems revealed no correlation. Nevertheless, residues that are important for oligomerization may be located at critical positions in secondary structures. The center position of edge β-strands, for example, has been targeted in negative design strategies (Richardson and Richardson, 2002). Furthermore, the importance of secondary structure, specifically of hinge loops has recently emerged with the discovery of domain swapping in proteins as a common means of oligomerization (Rousseau et al., 2003; Liu and Eisenberg, 2002). Although the structure of the plexin-B1 RBD dimer has not yet been determined, the weak association affinity and the kinetics of the monomer-dimer equilibrium, suggest that our protein is not domain swapped.
Limitations, variations and extensions of the NMR-based strategy
Here we report that NMR can be employed as an additional strategy for the monomerization of proteins in absence of three-dimensional structural information. NMR line broadening is used as a guide for the delineation of mainchain regions involved in the protein-protein association. A principal limitation is the requirement that the monomer-oligomer equilibrium has association-dissociation kinetics that are on the intermediate NMR μs-ms time scale. Although solution conditions, principally temperature, and NMR field strength may be varied to reach this range, many protein associations are also by nature either very weak (and often non-specific) or can be strong. These affinities affect protein kinetics and NMR signals in a way that makes resonance assignments more readily possible but no longer distinguishes interacting and non-interacting protein surfaces. In these cases, more elaborate NMR experiments need to be carried out, e.g. by monitoring chemical shift changes in titrations or by measurement of cross saturation (Zuiderweg, 2002). A second requirement is that the goal, a soluble, folded monomer is achievable by single or few multi-site mutations. This may not be the case for proteins that are only stable in the oligomeric form. Mutations at sites other than at the oligomerization surface may be needed to stabilize a monomeric structure. However, obtaining NMR spectra that suggest a locally defined interaction surface, as in the case of plexin-B1 RBD, already imply the monomeric protein has a stable fold and has only local conformational differences compared to the dimer. If this were not the case, larger scale or even global transitions upon association would likely obscure the regions actually located at the interface.
Chemical shift perturbations and resonance line broadening that occur as results of protein-protein or protein-ligand interactions have been used extensively as tools for the mapping of interaction sites (Gao et al., 2004). These are natural and already popular variants of the strategy proposed here. However, by contrast to such mapping experiments, typically requiring full assignments for the unbound state, our strategy considers the associated state of the protein as the starting point and can be applied to situations where only this state predominates. It therefore fills a gap in the repertoire of NMR experimental strategies.
It should be noted that in principle, the NMR part of our strategy could be replaced by simply choosing a wider range of sites to target for mutagenesis, based on sequence information alone, and by a more extensive mutagenesis. The balance of the two strategy components (NMR vs. site selection based on sequence information) may be adjusted depending on the number of resonances that are affected by the protein association. With the plexin RBD there is very little change in protein structure upon dimerization; in case of a more extensive change, assignment on the associated species is even more useful/necessary. Furthermore, it is anticipated that the protein surface region affected by the association is relatively decreased as the proteins become larger or unequal in size (less area from the perspective of the larger protein). Extension of the strategy should be possible to systems that are considerably larger, because, as mentioned above, perdeuteration and TROSY now enable assignment of proteins in complexes as large as 81 kDa. In addition, the strategy can be equally well applied to cases where the interacting proteins are different. It should be noted that while our strategy succeeded in the disruption of a weak dimer, a variant may well be useful in strengthening the protein-protein association (currently in progress). This strategy would use the same experimental techniques employed here but with different principles guiding the mutagenesis, because mutations which create higher affinity interactions are generally more challenging to devise. A tighter association would bring our system into slow exchange on the NMR time scale and allow a detailed structure determination for the oligomer.
Summary
The RBD domain of plexin-B1 exists in solution as a monomer-dimer equilibrium. A weak association leads to line broadening of NMR resonances belonging to the residues that are involved in the protein-protein interaction. This is generally seen as unfavorable for NMR studies, but the information obtained can be used in a strategic manner: Based on the initial assignments, we are able to identify regions which are difficult to observe because they are located at the association interface. No three-dimensional structural information or model for the protein is required to generate a monomer, but sequence conservation and local hydrophobicity is used to further narrow target sites for mutagenesis. Several mutations in the chosen regions disrupt the weak association, leading to a monomerization of the protein. Activity assays and 2D heteronuclear NMR are combined to screen for monomeric variants which preserve binding affinity and experience negligible structural perturbations. The combined use of molecular biology, biochemistry and NMR will be useful in overcoming the problem of weak association and will increase the number of proteins that are candidates for high throughput solution structure determination, principally by NMR. Furthermore, mutations that lead to monomerization, can be used to test the functional significance of the protein association.
Experimental procedures
The gene encoding the Rac1 binding domain (RBD) of human plexin-B1 (res. 1743-1862) was subcloned into pET11a (Novagen). Site-directed mutagenesis was carried out using QuickChange (Stratagene). Proteins were overexpressed in E.Coli BL21(DE3) strain grown in M9 minimal media. 15NH4Cl was supplemented as the sole nitrogen source, 13C-glucose as the sole carbon source when necessary. Weak ion exchange DEAE, hydrophobic interaction, and gel filtration (sephadex-75) chromatography were used for purification of the wild type RBDs and mutants. It should be noted that the dissociation constant obtained from gel filtration is an estimate at 276 K, as the protein is diluted on moving through the column. Final buffer conditions were 50 mM phosphate, pH 6.8, 100 mM NaCl, 4 mM DTT, 4 mM MgCl2 for all samples (10% D2O/90% H2O for NMR). For pull-down assays, GST-tagged Rac1 (constitutively active mutant Q61L) was obtained from the E.Coli lysate and bound to glutathione beads; lysate of the untagged plexin-B1 RBD domain or mutants was then loaded onto the beads. After washing, the beads were heated in the SDS-PAGE loading buffer and the supernatant analyzed using gel electrophoresis. Equivalent results were obtained when the binding experiments were carried out with GST-tagged plexins and untagged Rac1.Q61L. NMR experiments were carried out either on a Varian Inova 600M or a Bruker ICE600 spectrometer equipped with a cryoprobe. Experiments for assignment were carried out on a 66% deuterated, uniformly 15N, 13C labeled sample at 1.4 mM concentration, 298 K for the wild type RBD and on a uniformly 15N, 13C labeled sample at 2 mM for RBD-W1830F.
Acknowledgments
We thank Drs. Frank Sönnichsen and Witold Surewicz for critical reading and Dr. Jun Qin for helpful comments, Johanna Busch from U.Penn for her contribution to this project during her summer research experience. Drs. Alan Hall, UCL and Michael Rosen, UTSouthwestern for providing plasmids for plexin-B1 and Rac1 respectively. M.B. is a scholar of the Mt. Sinai Health Care Foundation, Cleveland, Ohio. This work is also partially funded by a Scientist Development Grant from the American Heart Foundation (National Center 0335353N). Y.T. is a postdoctoral fellow of American Heart Association, Ohio Valley Affiliate (0425678B).
References
- Antipenko A, Himanen JP, van Leyen K, Nardi-Dei V, Lesniak J, Barton WA, Rajashankar KR, Lu M, Hoemme C, Puschel AW, Nikolov DB. Structure of the semaphorin-3A receptor binding module. Neuron. 2003;39:589–598. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- Arrowsmith CH, Wu YS. NMR of large (> 25 kDa) proteins and protein complexes. Prog. NMR Spectrosc. 1998;32:277–286. [Google Scholar]
- Bahadur RP, Chakrabarti P, Rodier F, Janin J. Dissecting subunit interfaces in homodimeric proteins. Proteins. 2003;53:708–719. doi: 10.1002/prot.10461. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Choe S, Eisenberg D. Domain swapping: entangling alliances between proteins. Proc. Natl Acad. Sci. U. S. A. 1994;91:3127–3131. doi: 10.1073/pnas.91.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Schlunegger MP, Eisenberg D. 3D domain swapping: a mechanism for oligomer assembly. Protein Sci. 1995;4:2455–2468. doi: 10.1002/pro.5560041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MJ, Sternberg MJ. An analysis of conformational changes on protein-protein association: implications for predictive docking. Protein Eng. 1999;12:271–283. doi: 10.1093/protein/12.4.271. [DOI] [PubMed] [Google Scholar]
- Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 1998;280:1–9. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- Borchert TV, Abagyan R, Jaenicke R, Wierenga RK. Design, creation, and characterization of a stable, monomeric triosephosphate isomerase. Proc. Natl. Acad. Sci. U. S. A. 1994;91:1515–1518. doi: 10.1073/pnas.91.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter DR, Resnik E, Banaszak LJ. Engineering the quaternary structure of an enzyme: construction and analysis of a monomeric form of malate dehydrogenase from Escherichia coli. Protein Sci. 1994;3:2023–2032. doi: 10.1002/pro.5560031115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J, Fairbrother WJ, Palmer AG, III, Skelton NJ. Protein NMR Spectroscopy: Principles and Practice. Academic Press, Inc.; San Diego, CA: 1996. Relaxation and Dynamic Processes; pp. 243–300. [Google Scholar]
- Changeux JP, Edelstein SJ. Allosteric receptors after 30 years. Neuron. 1998;21:959–980. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- Cordes MH, Walsh NP, McKnight CJ, Sauer RT. Evolution of a protein fold in vitro. Science. 1999;284:325–328. doi: 10.1126/science.284.5412.325. [DOI] [PubMed] [Google Scholar]
- Cuff JA, Barton GJ. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins. 2000;40:502–511. doi: 10.1002/1097-0134(20000815)40:3<502::aid-prot170>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- D’Alessio G. Oligomer evolution in action? Nat. Struct. Biol. 1995;2:11–13. doi: 10.1038/nsb0195-11. [DOI] [PubMed] [Google Scholar]
- D’Alessio G. The evolutionary transition from monomeric to oligomeric proteins: tools, the environment, hypotheses. Prog. Biophys. Mol. Biol. 1999;72:271–298. doi: 10.1016/s0079-6107(99)00009-7. [DOI] [PubMed] [Google Scholar]
- DeLano WL. Unraveling hot spots in binding interfaces: progress and challenges. Curr. Opin. Struct. Biol. 2002;12:14–20. doi: 10.1016/s0959-440x(02)00283-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Schwarz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Scheraga HA. Insufficiently dehydrated hydrogen bonds as determinants of protein interactions. Proc. Natl. Acad. Sci. U. S. A. 2003;100:113–118. doi: 10.1073/pnas.0136888100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Wider G. TROSY in NMR studies of the structure and function of large biological macromolecules. Curr. Opin. Struct. Biol. 2003;13:570–580. doi: 10.1016/j.sbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Gao G, Williams JG, Campbell SL. Protein-protein interaction analysis by nuclear magnetic resonance spectroscopy. Methods Mol. Biol. 2004;261:79–92. doi: 10.1385/1-59259-762-9:079. [DOI] [PubMed] [Google Scholar]
- Goodsell DS, Olson AJ. Structural symmetry and protein function. Annu. Rev. Biophys. Biomol. Struct. 2000;29:105–153. doi: 10.1146/annurev.biophys.29.1.105. [DOI] [PubMed] [Google Scholar]
- Guerois R, Nielsen JE, Serrano L. Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J. Mol. Biol. 2002;320:369–387. doi: 10.1016/S0022-2836(02)00442-4. [DOI] [PubMed] [Google Scholar]
- Halperin I, Wolfson H, Nussinov R. Protein-protein interactions; coupling of structurally conserved residues and of hot spots across interfaces. Implications for docking. Structure (Camb. ) 2004;12:1027–1038. doi: 10.1016/j.str.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Hedstrom L. Trypsin: a case study in the structural determinants of enzyme specificity. Biol. Chem. 1996;377:465–470. [PubMed] [Google Scholar]
- Janin J, Miller S, Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol. Biol. 1988;204:155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Jones S, Thornton JM. Protein-protein interactions: a review of protein dimer structures. Prog. Biophys. Mol. Biol. 1995;63:31–65. doi: 10.1016/0079-6107(94)00008-w. [DOI] [PubMed] [Google Scholar]
- Jones S, Thornton JM. Prediction of protein-protein interaction sites using patch analysis. J Mol. Biol. 1997;272:133–143. doi: 10.1006/jmbi.1997.1233. [DOI] [PubMed] [Google Scholar]
- Kortemme T, Baker D. A simple physical model for binding energy hot spots in protein-protein complexes. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14116–14121. doi: 10.1073/pnas.202485799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Eisenberg D. 3D domain swapping: as domains continue to swap. Protein Sci. 2002;11:1285–1299. doi: 10.1110/ps.0201402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockless SW, Ranganathan R. Evolutionarily conserved pathways of energetic connectivity in protein families. Science. 1999;286:295–299. doi: 10.1126/science.286.5438.295. [DOI] [PubMed] [Google Scholar]
- Ma B, Elkayam T, Wolfson H, Nussinov R. Protein-protein interactions: structurally conserved residues distinguish between binding sites and exposed protein surfaces. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5772–5777. doi: 10.1073/pnas.1030237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maithal K, Ravindra G, Nagaraj G, Singh SK, Balaram H, Balaram P. Subunit interface mutation disrupting an aromatic cluster in Plasmodium falciparum triosephosphate isomerase: effect on dimer stability. Protein Eng. 2002;15:575–584. doi: 10.1093/protein/15.7.575. [DOI] [PubMed] [Google Scholar]
- Mossing MC, Sauer RT. Stable, monomeric variants of lambda Cro obtained by insertion of a designed beta-hairpin sequence. Science. 1990;250:1712–1715. doi: 10.1126/science.2148648. [DOI] [PubMed] [Google Scholar]
- Mullen CA, Jennings PA. A single mutation disrupts the pH-dependent dimerization of glycinamide ribonucleotide transformylase. J. Mol. Biol. 1998;276:819–827. doi: 10.1006/jmbi.1997.1530. [DOI] [PubMed] [Google Scholar]
- Ofran Y, Rost B. Analysing six types of protein-protein interfaces. J. Mol. Biol. 2003;325:377–387. doi: 10.1016/s0022-2836(02)01223-8. [DOI] [PubMed] [Google Scholar]
- Rajarathnam K, Sykes BD, Kay CM, Dewald B, Geiser T, Baggiolini M, Clark-Lewis I. Neutrophil activation by monomeric interleukin-8. Science. 1994;264:90–92. doi: 10.1126/science.8140420. [DOI] [PubMed] [Google Scholar]
- Richardson JS, Richardson DC. Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2754–2759. doi: 10.1073/pnas.052706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Schymkowitz JW, Itzhaki LS. The unfolding story of three-dimensional domain swapping. Structure. (Camb.) 2003;11:243–251. doi: 10.1016/s0969-2126(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Sattler M, Schleucher J, Griesinger C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. NMR Spectrosc. 1999;34:93–158. [Google Scholar]
- Taverna DM, Goldstein RA. Why are proteins so robust to site mutations? J. Mol. Biol. 2002;315:479–484. doi: 10.1006/jmbi.2001.5226. [DOI] [PubMed] [Google Scholar]
- Thoma R, Hennig M, Sterner R, Kirschner K. Structure and function of mutationally generated monomers of dimeric phosphoribosylanthranilate isomerase from Thermotoga maritima. Structure. Fold. Des. 2000;8:265–276. doi: 10.1016/s0969-2126(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Turner LJ, Nicholls S, Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J. Biol. Chem. 2004;279:33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- Valdar WS, Thornton JM. Protein-protein interfaces: analysis of amino acid conservation in homodimers. Proteins. 2001;42:108–124. [PubMed] [Google Scholar]
- Valencia A, Pazos F. Computational methods for the prediction of protein interactions. Curr. Opin. Struct. Biol. 2002;12:368–373. doi: 10.1016/s0959-440x(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Vikis HG, Li W, He Z, Guan KL. The semaphorin receptor plexin-B1 specifically interacts with active Rac in a ligand-dependent manner. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12457–12462. doi: 10.1073/pnas.220421797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Tsai CJ, Nussinov R. Mechanism and evolution of protein dimerization. Protein Sci. 1998;7:533–544. doi: 10.1002/pro.5560070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuiderweg ER. Mapping protein-protein interactions in solution by NMR spectroscopy. Biochemistry. 2002;41:1–7. doi: 10.1021/bi011870b. [DOI] [PubMed] [Google Scholar]