Abstract
Context
A wide variety of oral diabetes medications are currently available for the treatment of type 2 diabetes, but it is unclear how these agents compare with respect to long-term cardiovascular risk.
Objective
To systematically review the peer-reviewed literature on cardiovascular risk associated with oral agents (second-generation sulfonylureas, biguanides, thiazolidinediones, and meglitinides) for treating adults with type 2 diabetes mellitus.
Data Sources
MEDLINE®, EMBASE®, and the Cochrane Central Register of Controlled Trials, from inception through January 2006.
Study Selection
40 publications of controlled trials that reported information on cardiovascular events (primarily myocardial infarction and stroke).
Data Extraction
Using standardized protocols, 2 reviewers serially abstracted data for each article. Trials were first described qualitatively. For comparisons with four or more independent trials, results were quantitatively pooled using the Mantel- Haenszel method. Results were presented as odds ratios and corresponding 95% confidence intervals.
Results
Treatment with metformin was associated with a decreased risk of cardiovascular mortality(pooled odds ratio(OR)=0.74, 95%CI 0.62-0.89) compared with any other oral diabetes agent or placebo; the results for cardiovascular morbidity and all-cause mortality were similar but not statistically significant. No other significant associations of oral diabetes agents with fatal or non-fatal cardiovascular disease or all-cause mortality were observed. When compared to any other agent or placebo, rosiglitazone was the only diabetes agent associated with an increased risk of cardiovascular morbidity or mortality, but this result was not statistically significant(OR 1.68 95%CI 0.92-3.06).
Conclusions
Meta-analysis suggested that compared to other oral diabetes agents and placebo, metformin was moderately protective and rosiglitazone possibly harmful, but lack of power prohibited firmer conclusions. Larger, long-term studies taken to hard endpoints and better reporting of cardiovascular events in short term studies will be required to draw firm conclusions about major clinical benefits and risks related to oral diabetes agents.
A wide variety of oral diabetes medications are currently available for the treatment of type 2 diabetes. With the addition of newer oral therapies to the market in the late 1990s (e.g., thiazolidinediones and meglitinides), it is critical to evaluate how these agents compare to older medications. This is particularly important in light of the expense of many of the newer therapies. Clinical trials examining the efficacy of these different therapies have largely focused on intermediate clinical outcomes such as change in hemoglobin A1c(HbA1c), serum lipid levels, and blood pressure. Improvements in glucose control per se have been shown to reduce the incidence of microvascular disease(1) and there is accumulating evidence of potential macrovascular benefits(2–5). Nonetheless, specific effects of oral diabetes agents on cardiovascular risks are unclear.
An important clinical question is whether the different oral medications for type 2 diabetes variously affect hard clinical outcomes including cardiovascular morbidity and mortality and all-cause mortality. These outcomes have unequivocal clinical relevance. There has been recent controversy regarding possible cardiovascular risk associated with rosiglitazone(6–9). The debate surrounding rosiglitazone highlights the need for a comprehensive examination of all oral diabetes medications, alone and in combination. The objective of this study was to conduct a systematic review of all published peer-reviewed, randomized clinical trials of oral diabetes agents(second-generation sulfonlyureas, biguanides, thiazolidinediones, and meglitinides) to evaluate the risk of fatal and non-fatal cardiovascular disease and all-cause mortality. We hypothesized that the newer medications(thiazolidinediones and meglitinides) would be similar to the older medications(metformin and second-generation sulfonlyureas) with respect to cardiovascular risk, given that these medications had similar effects on hemoglobin A1c in a previous systematic review (10).
METHODS
Data Sources and Searches
We searched MEDLINE®, EMBASE®, and the Cochrane Central Register of Controlled Trials databases for original articles from inception to January 2006. Details regarding our search strategy have been previously published(10). We selected studies from the peer-reviewed literature that assessed benefits or harms of Federal Food and Drug Administration (FDA)-approved oral diabetes agents available in the United States as of January 2006. Studies must have reported original data in adults with type 2 diabetes. We included studies of combination of therapies that are commonly used, such as combinations of metformin, second-generation sulfonylureas, and thiazolidinediones. We excluded studies that evaluated combinations of three oral diabetes agents, and studies of first-generation sulfonlyureas since few clinicians prescribe these medications. We also excluded the alpha-glucosidase inhibitors as they have been reviewed previously(11) and are not commonly used in clinical practice in the U.S. Studies which did not report all-cause mortality or cardiovascular morbidity or mortality anywhere in the article were excluded from the present review. We also excluded studies which were less than 3 months in duration or where the total sample size was less than 40. We focus here on the peer-reviewed literature as it provides the strongest levels of evidence.
This study was conducted by the Johns Hopkins Evidenced-based Practice Center as part of a larger project commissioned by the Agency for Healthcare Research and Quality. The full Technical Report provides a detailed description of the study methods(12).
Data Extraction and Quality Assessment
Two investigators used standardized data abstraction forms to independently abstract all data. Disagreements were resolved by consensus. The Jadad scale was used to assess study quality(13). If cardiovascular disease was not a primary end point of the study, we separately rated the quality of the adverse event reporting for cardiovascular outcomes in each trial using a 4 point scale based on the FDA and the Consolidated Standards of Reporting Trials(CONSORT) guidelines for adverse event reporting(see Web Appendix, Table B)(14–16). For data abstraction, we relied on definitions of cardiovascular morbidity and mortality as defined in the respective studies(see Web Appendix, Table A). We excluded congestive heart failure cases when possible, but there were instances where studies reported combined endpoints in which heart failure cases could not be separated. We assumed events and deaths were reported for all arms if they were reported for one arm. Events were recorded as “not reported” for those studies which did not indicate the occurrence (or lack thereof) of events or deaths for that particular outcome. We used outcome definitions which were inclusive(for instance, in one study(17), “chest pain” was included with cardiovascular events as no other information was provided).
Data Synthesis and Analysis
We first summarized the trials qualitatively. In the quantitative synthesis, all analyses were conducted following the principle of intention-to-treat. Trials with no cardiovascular events in any treatment arm were excluded from the quantitative analysis. We conducted meta-analyses of comparisons for which there were at least four relatively homogenous trials. We combined the comparator arms to create an “any other” comparator group (drug or placebo). The comparisons of interest were: metformin vs any comparator(oral agent or placebo/diet); metformin vs any sulfonylurea combined with metformin; any sulfonylurea vs any comparator; any sulfonylurea vs any sulfonylurea combined with metformin; rosiglitazone vs any comparator; rosiglitazone plus metformin vs metformin alone; pioglitazone vs any comparator; and either of the meglitinides vs any comparator.
For trials with more than one dosing arm, we combined the dosing arms as long as the doses were consistent with current clinical practice. For trials with more than one comparison group, we combined groups when appropriate. Odds ratios were calculated and pooled both using a Mantel-Haenszel fixed effects model(with a 0.5 continuity correction)(18;19) and Peto’s method(20). Statistical heterogeneity was assessed with the I2 statistic(21). Sensitivity analyses were conducted to examine the effect of inclusion/exclusion of influential studies (e.g., United Kingdom Prospective Diabetes Study(UKPDS) and the PROspective pioglitAzone Clinical Trial In macroVascular Events(PROactive)) and different dosing and control group arms. All analyses were conducted using Stata SE Version 10.0(College Station, TX).
RESULTS
Search Results
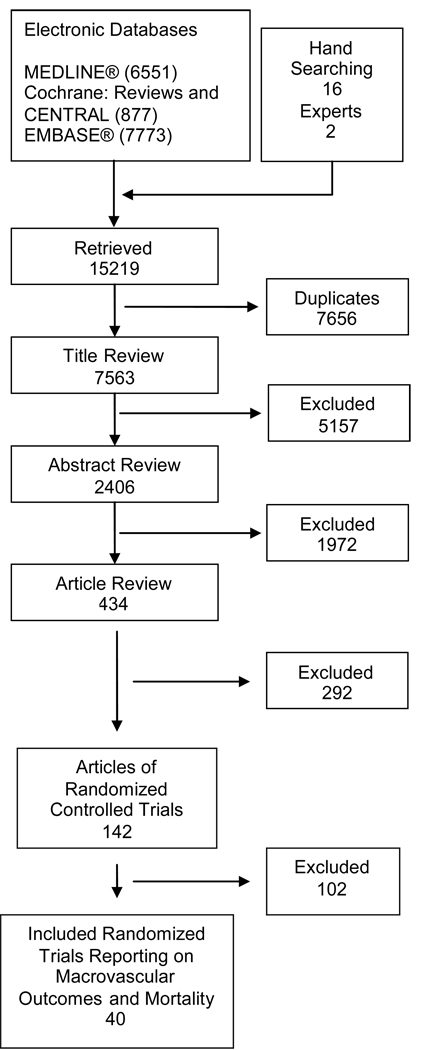
Figure 1 details the search and selection process; more details are found in the full Technical Report(12). Briefly, of the 7,563 unique citations retrieved, 434 were determined to be relevant to our study questions and were identified for full-text article review. One hundred and forty-two of these publications were of randomized controlled trials and only 40 reported data on cardiovascular events and/or mortality. The main findings from UKPDS were reported in two separate publications(1;22). For the purposes of our study, UKPDS 33 and 34 are considered separate trials and UKPDS 34 is further divided into two separate comparisons so that the sulfonylurea, metformin, and the early addition of metformin to sulfonylurea arms are analyzed separately as was done in the originally published reports. Forty articles made up our final study population.
Figure 1. Summary of Literature Search (number of articles).
Qualitative Summary
Characteristics of the 40 included clinical trials are summarized in Table 1. The majority of trials were conducted in the U.S. or the U.K. The mean age of participants ranged from 52 to 69 years and mean HbA1c level at baseline ranged from 6.2% in UKPDS(1) to 10.2% in two small, short-term studies(23;24). The majority of trials (70%) were less than 1 year in duration. More than half of the studies reported receiving support from the pharmaceutical industry (see Web Appendix, Table A).
Table 1.
Characteristics of Included Randomized Clinical Trials of Oral Diabetes Medications
| Baseline Characteristics | Outcome(s), N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year (Reference) |
Duration of follow- up, months |
Intervention Arms | Dosage (esc), mg |
Sample size, N |
Mean age, years |
% Male |
Mean HbA1c, % |
CVD morbidity | CVD mortality |
All-cause mortality |
| Aronoff, 2000 (23) | 6.5 | Pioglitazone | range 7.5 to 45 | 329 | 54¶ | 58¶ | 10.2 | 12 | NR | NR |
| Placebo | NA | 79 | 54¶ | 58¶ | 10.4 | 5 | NR | NR | ||
| Bailey, 2005 (42) | 6 | Metformin | 2500 (esc) 3000 | 280 | 58 | 57 | 7.5 | NR | 0 | 0 |
| Rosiglitazone + Metformin | 4 (esc) 8 2000 (fixed) | 288 | 58 | 58 | 7.4 | NR | 1 | 1 | ||
| Baksi, 2004 (43) | 6.5 | Glyclazide | 160 (esc) to 320 | 241 | 62 | 63 | 8.6 | NR | 0 | 0 |
| Glyclazide+Rosiglitazone | 4 (fixed) + 160 | 225 | 61 | 57 | 8.5 | NR | 1 | 1 | ||
| Barnett, 2003 (44) | 6.5 | Rosiglitazone + existing unspecified SU | 4 bid (fixed) NR (NR) | 84 | 54 | 80 | 9.2 | 5 | NR | NR |
| Placebo + existing unspecified SU | NR (NR) | 87 | 54 | 75 | 9.1 | 0 | NR | NR | ||
| Carlson, 1993 (32) | 3 | Glyburide (Reformulated) | 3 (fixed) | 104 | 59 | 59 | 7.6 | unclear | 1 | 1 |
| Glyburide (Original) | 5 (fixed) | 102 | 60 | 61 | 7.6 | unclear | 0 | 0 | ||
| Choi, 2004 (26) | 6 | Rosiglitazone + existing medications | 8 (decreased) to 4 | 38 | 61 | 63 | 7.8 | 4 (revascularization); 9 (restenosis) | 0 | 0 |
| Uptitration of existing diabetes medications | NR (esc) | 45 | 60 | 76 | 7.7 | 9 (revascularization); 21 (restenosis) | 0 | 0 | ||
| Cryer, 2005 (COSMIC trial) (45) | 12 | Metformin* | 500 (esc) 2500 | 7227 | 58 | 49 | NR | 237 | 506 | 80 |
| Usual care* | NA | 1505 | 59 | 50 | NR | 49 | 136 | 20 | ||
| DeFronzo, 1995 (Protoctol 2) (46) | 7.25 | Metformin | 500 (esc) 2500 | 210 | 55 | 55 | 8.9 | NR | 1 | 1 |
| Glyburide | 10 (esc) 20 500 (esc) | 209 | 56 | 56 | 8.5 | NR | 0 | 0 | ||
| Metformin+glyburide | 2500 + 10 (esc) 20 | 213 | 55 | 55 | 8.8 | NR | 0 | 0 | ||
| Dormandy, 2005 (25) | 48 | Thiazolidinedione (Pioglitazone)* | 15 (esc) 45 | 2605 | 62 | 67 | 7.8 (median) | 514† | 127 | 177 |
| Placebo* | NR | 2633 | 62 | 66 | 7.9 (median) | 572† | 136 | 186 | ||
| Draeger, 1996 (33) | 12 | Glimepiride | 1 (esc) 8 | 524 | 60 | 62 | 8.1 | NR | 5 | 11 |
| Glibenclamide | 2.5 (esc) 20 | 520 | 61 | 65 | 8.1 | NR | 3 | 5 | ||
| Fonseca, 2000 (47) | 6.5 | Metformin+Placebo | 2500 (fixed) | 113 | 59 | 74 | 8.6 | NR | 0 | 0 |
| Metformin+Rosiglitazone | 2500 (fixed) + 4 (fixed) | 116 | 58 | 62 | 8.9 | NR | 1 | 1 | ||
| Metformin+Rosiglitazone | 2500 (fixed) + 8 (fixed) | 110 | 58 | 68 | 8.9 | NR | 0 | 0 | ||
| Fujioka, 2003 (31) | 6 | Metformin (immediate release) | 1000 (500 bid) (fixed) | 71 | 54 | 44 | 7.1 | 0 | 0 | 0 |
| Metformin (extended release) | 1000 (qd) (fixed) | 75 | 54 | 45 | 7.0 | unclear (0 to 4?) | 0 | 0 | ||
| Metformin (extended release) | 1500 (qd)(fixed) | 71 | 55 | 39 | 7.0 | unclear (0 to 4?) | 0 | 1 | ||
| Garber, 2003 (48) | Metformin | 500 (esc) 2000 | 164 | 55 | 43 | 8.5 | NR | NR | 0 | |
| Glyburide | 2.5 (esc) 10 | 151 | 55 | 44 | 8.7 | NR | NR | 0 | ||
| Glyburide+metformin | 1.25 + 250 (esc) 5 + 1000 | 171 | 56 | 44 | 8.8 | NR | NR | 2 | ||
| Goldberg, 1998 (49) | 4.5 | Placebo | NA | 33 | 56 | 76 | 8.1 | 0 | 0 | 0 |
| Repaglinide | 0.25 (esc) to 8.0 | 67 | 59 | 74 | 8.3 | 1 | 0 | 0 | ||
| Goldstein, 2003 (50) | 4.5 | Glipizide | 30 | 84 | 57 | 64 | 8.9 | NR | 0 | 0 |
| Metformin | 500 (esc) 2000 | 76 | 57 | 62 | 8.7 | NR | 0 | 0 | ||
| Glipizide+metformin | 5 (esc) 20 + 500 (esc) 2000 | 87 | 55 | 59 | 8.7 | NR | 0 | 0 | ||
| Gomez-Perez, 2002 (24) | 6.5 | Metformin + placebo | 2500 (fixed) | 34 | 53 | 29 | 9.8** | 1 | 0 | NR |
| Rosiglitazone + metformin | 2 bid (fixed) + 2500 (fixed) | 35 | 52 | 29 | 10.2** | 1 | 0 | NR | ||
| Rosiglitazone + metformin | 4 bid (fixed) + 2500 (fixed) | 36 | 54 | 19 | 9.7** | 2 | 0 | NR | ||
| Hanefeld, 2004 (51) | 12 | Pioglitazone* | 15 (esc) 45 | 319 | 60 | 54 | 8.8 | 10 | NR | 1 |
| Metformin* | 850 (esc) 2550 | 320 | 60 | 55 | 8.8 | 13 | NR | 2 | ||
| Hanefeld, 2000 (52) | 3 | Placebo | NA | 60 | 57 | 60 | 8.5 | NR | 0 | 0 |
| Nateglinide | 30 | 51 | 58 | 71 | 8.4 | NR | 1 | 1 | ||
| Nateglinide | 60 | 58 | 56 | 71 | 8.3 | NR | 0 | 0 | ||
| Nateglinide | 120 | 63 | 54 | 70 | 8.3 | NR | 0 | 0 | ||
| Nateglinide | 180 | 57 | 57 | 63 | 8.5 | NR | 0 | 0 | ||
| Hermann, 1994 (53) | 6 | Metformin + diet†† | 1000 (esc) 3000 | 38 | 60¶ | 63¶ | 7.3 | 2 | NR | NR |
| Glyburide + diet†† | 3.5 (esc) 10.5 500 (esc) 1500 | 34 | 60¶ | 63¶ | 7.1 | 3 | NR | NR | ||
| Metformin + glyburide | 1.75 (esc) 5.25 | 72 | 60¶ | 63¶ | 7.2 | 10 | NR | NR | ||
| Horton, 2000 (54) | 6 | Nateglinide | 360 (fixed) | 179 | 59 | 62 | 8.3 | NR | 0 | 0 |
| Metformin | 1500 (fixed) | 178 | 57 | 68 | 8.4 | NR | 1 | 1 | ||
| Placebo | NA | 172 | 60 | 61 | 8.3 | NR | 0 | 0 | ||
| Jovanovic, 2000 (17)[ | 6 | Repaglinide | 1 tid (fixed) | 140 | 58 | 69 | 8.9 | 1 (MI); 4 (chest pain) | NR | NR |
| Repaglinide | 4 tid (fixed) | 146 | 58 | 60 | 8.7 | 1 (MI); 4 (chest pain) | NR | NR | ||
| Placebo | NA | 75 | 59 | 65 | 8.6 | 0 (MI); 1 (chest pain) | NR | NR | ||
| Kipnes, 2001 (55) | 4 | Pioglitazone + existing unspecified SU | 15 (fixed) + NR | 184 | 57 | 59 | 10.0 | 22 (for both pioglitazone groups combined) | NR | NR |
| Pioglitazone + existing unspecified SU | 30 (fixed) + NR | 189 | 57 | 60 | 9.9 | NR | NR | |||
| Placebo + existing unspecified SU | NR (fixed) | 187 | 57 | 58 | 9.9 | 10 | NR | NR | ||
| Lawrence, 2004 (56) | 6 | Pioglitazone | 30 (esc) 45 | 21‡‡ | 60 | 70 | 7.4 | 0 | 0 | 0 |
| Metformin | 500 (esc) 1500 | 21‡‡ | 60 | 60 | 8.0 | 0 | 1 | 1 | ||
| Gliclazide | 80 (esc) 160 bid | 22‡‡ | 64 | 65 | 7.9 | 1 | 0 | 0 | ||
| Nishio, 2006 (27) | 6 | Control | NA | 28 | 68 | 71 | 6.9 | 17 (primary end point); 1 (MI) | 0 | 0 |
| Pioglitazone | 30 (fixed) | 26 | 66 | 73 | 7.7 | 2 (primary end point); 0 (MI) | 0 | 0 | ||
| Marbury, 1999 (57) | 12 | Repaglinide | 0.5 (esc) 12 | 362 | 58 | 67 | 8.7 | 19 | 2 | 3 |
| Glyburide | 2.5 (esc) 15 | 182 | 59 | 66 | 8.9 | 4 | 1 | 1 | ||
| Rachmani, 2002 (29) | 48 | Stopped Metformin* | NR | 198 | 64 | 52 | 8.6 | 53 | 52 | 64 |
| Continued Metformin* | NR | 195 | 65 | 53 | 8.6 | 51 | 50 | 62 | ||
| Rosenstock, 1996 (58) | 3.5 | Placebo | NA | 79 | 61 | 67 | 8.0 | NR | 0 | 0 |
| Glimepiride | 8 (once a day) | 88 | 62 | 74 | 8.1 | NR | 0 | 0 | ||
| Glimepiride | 4 (twice daily) | 81 | 59 | 70 | 8.1 | NR | 1 | 1 | ||
| Glimepiride | 16 (once a day) | 83 | 60 | 66 | 8 | NR | 0 | 0 | ||
| Glimepiride | 8 (twice daily) | 85 | 62 | 72 | 8.3 | NR | 0 | 0 | ||
| Rosenstock, 2006 (59) | 24 | Placebo+ uptitration Glipizide‡ | 10 (esc) to 20 | 111 | 69 | 72 | 7.7 | NR | 2 | 2 |
| Glipizide+Rosiglitazone‡ | 4 + 10 (esc) to 20 | 116 | 68 | 74 | 7.7 | NR | 0 | 0 | ||
| Schernthaner, 2004 (60) | 12 | Pioglitazone + placebo + diet | 30 (esc) 45 | 597 | 57 | 53 | 8.7 | 12 | NR | 3 |
| Metformin + placebo + diet | 850 (esc) 2550 | 597 | 56 | 58 | 8.7 | 13 | NR | 2 | ||
| Simonson, 1997 (61) | 4 | Placebo | NA | 69 | 60 | 77 | 8.3 | NR | NR | 0 |
| Glipizide (all dosing arms combined) | 5 to 60 (fixed) | 278 | 58 | 65 | 8.6 | NR | NR | 1 | ||
| Sonnenberg, 1997 (30) | 3.75 | Glimepiride | 3 (fixed) | 48 | NR | NR | NR | unclear | 0 | 0 |
| Glimepiride | 6 (fixed) | 46 | NR | NR | NR | unclear | 0 | 0 | ||
| St John Sutton, 2002 (62) | 12 | Rosiglitazone | 4 bid (fixed) | 104 | 55 | 72 | 9.1 | 16 | NR | NR |
| Sulfonlyurea (Glyburide) | NR (esc) 20 | 99 | 56 | 72 | 9.5 | 12 | NR | NR | ||
| Takagi, 2003 (28) | 6 | Pioglitazone + existing medications | 30 (fixed) | 23 | 64 | 87 | 6.8 | 5 | NR | NR |
| Control | NR (esc) to target | 21 | 65 | 67 | 6.7 | 11 | NR | NR | ||
| UKPDS 33, 1998 (1) | 133 | Glibenclamide +Diet | 2.5 (esc) 20 | 615 | 54 | 62 | 6.3 | 90 (MI), 45 (stroke) 162 (MI), 47 | 73§ | 121 |
| Diet | NR | 896 | 54 | 62 | 6.2 | (stroke) | 113§ | 190 | ||
| Comparison 1: UKPDS 34, 1998 (22) | 128 | Metformin + diet | 850 (esc) 2550 | 342 | 53 | 46 | 7.3 | 39 (MI), 12 (stroke) | 28§ | 50 |
| Diet | NA | 411 | 53 | 47 | 7.1 | 73 (MI), 23 (stroke) | 55§ | 89 | ||
| Comparison 2: UKPDS 34, 1998 (22) | 128 | Unspecified SU +Diet | NA | 269 | 58 | 61 | 7.6 | 31 (MI) | 14§ | 31 |
| Unspecified SU + Metformin +Diet | NA+850(esc) 2550 | 268 | 59 | 59 | 7.5 | 33 (MI) | 26§ | 47 | ||
| Virtanen, 2003 (63) | 6.5 | Rosiglitazone + diet | 2 bid (esc) 4 bid | 15 | 58 | 71 | 6.8 | 0 | NR | NR |
| same trial as Hallsten, 2002 (64) | Metformin + diet | 500 bid (esc) 1000 bid | 15 | 58 | 61 | 6.9 | 1 | NR | NR | |
| Placebo | NA | 14 | 58 | 71 | 6.3 | 0 | NR | NR | ||
| Weissman, 2005 (65) | 6 | Existing Metformin +Rosiglitazone | 1000+4 (esc) 8 | 382 | 56 | NR | 8.1 | 7 | 0 | 1 |
| Uptitration of existing Metformin | 1000 (esc) 2000 | 384 | 56 | NR | 8.0 | 4 | 0 | 0 | ||
| Wolffenbuttel, 1999 (66) | 12 | Repaglinide | 1.5 (esc) 12 | 286 | 61 | 62 | 7.1 | NR | NR | NR║ |
| Placebo + glyburide | 1.75 (esc) 10.5 | 139 | 61 | 68 | 7.0 | NR | NR | NR║ | ||
| Zhu, 2003 (67) | 6 | Placebo(+existing SU) | NA | 112 | 59 | 46 | 9.8 | 0 | 0 | 0 |
| Rosiglitazone(+existing SU) | 2 bid(fixed) | 221 | 59 | 41 | 9.8 | 0 | 0 | 0 | ||
| Rosiglitazone(+existing SU) | 4 bid (fixed) | 221 | 59 | 48 | 9.9 | 0 | 1 | 1 | ||
Abbreviations: CVD = cardiovascular disease; esc = escalated; DNS = dose not specified; patients on medication prior to trial and continued on existing dose.
Medication was added to existing therapy.
The composite primary endpoint of the PROActive trial included death, nonfatal MI, silent MI, stroke, major leg amputation, acute coronary symdrome; coronary revascularization, and leg revascularization
Both groups were on glipizide (10 mg bid) prior to enrollment.
Any diabetes-related death (death from MI, stroke, peripheral vascular disease, renal disease, hyperglycemia, hypoglycemia, or sudden death)
Author states, "cardiac events were reported at similar frequencies in both treatment groups"
Not reported separately for the study arms; overall estimates recorded here.
Baseline HbA1c were derived from figure
Metformin or glyburide was added if glycemic target was not reached
Baseline sample size is unclear as three subjects withdrew at 6 weeks due to hyperglycemia, but respective treatment arms not identified.
In most studies, cardiovascular outcomes were recorded as adverse events and were not a primary or secondary outcome of the trial, with the exception of two major studies: PROactive and UKPDS(1;22;25). With approximately 4,000 participants and a mean of 10.7 years of follow-up, the UKPDS is the longest trial of oral diabetes medications in the published literature. UKPDS 33 compared the effects of intensive glucose control with either sulfonylurea or insulin and conventional treatment on the risk of microvascular and macrovascular complications(1). A median difference in HbA1c of 0.9% was achieved between the two arms and intensive control with either sulfonylurea or insulin was shown to substantially decrease the risk of microvascular outcomes compared to conventional treatment. The results for macrovascular outcomes were more equivocal, with no significant differences observed for stroke or a combined endpoint of amputation or death from peripheral vascular disease but a borderline significant 16% reduction in myocardial infarctions(p-value=0.052). When the intensive therapy group was further subdivided into glibenclamide versus conventional treatment, the observed effect was similar, specifically a borderline 22% reduction in myocardial infarctions was seen with glibenclamide compared to conventional treatment(p-value=0.056).
In UKPDS 34, metformin, chlorpropamide, glibenclamide, and insulin were compared in one analysis and a second supplementary analysis compared the sulfonylurea arm to early addition of metformin added to sulfonylurea therapy(22). The main findings of UKPDS 34 suggested no difference in cardiovascular outcomes when comparing the different therapies indirectly. Only metformin therapy compared with conventional treatment in overweight individuals showed a significant 36% and 39% reduction in all-cause mortality and myocardial infarction, respectively. Additionally, the early addition of metformin to sulfonylurea unexpectedly showed a 60% significantly increased risk of all-cause mortality compared with the sulfonylurea arm where metformin or insulin was added only if the participant was markedly hyperglycemic. UKPDS was conducted prior to the emergence of thiazolidinediones.
The PROactive study of over 5,000 participants followed for an average of just under 3 years (34.5 months) was designed to investigate whether treatment with the thiazolidinedione pioglitazone would be associated with a reduced risk of cardiovascular endpoints compared to treatment with placebo (taken in addition to existing diabetes medications)(25). PROactive showed a non-significant reduction in the primary composite endpoint (10% relative risk reduction, p-value=0.095) and a significant reduction in the main secondary endpoint of all-cause mortality, non-fatal myocardial infarction, and stroke (16% relative risk reduction, p-value= 0.027). The median decrease in percent HbA1c in the pioglitazone arm was 0.8 compared to 0.3 in the control arm.
The majority of the trials included in the present study were not designed (nor powered) to examine cardiovascular events. A history of cardiovascular disease was an exclusion criteria for most studies, but four studies specifically examined the effects of oral agents in populations of persons with a history of cardiovascular disease(26–29). Choi et al, Nishio et al, and Takagi et al each assessed restenosis rates in small, 6 month trials of oral diabetes therapy in persons with type 2 diabetes. Because it is unclear whether these three studies are generalizable to the general population of persons with type 2 diabetes, they were excluded from our quantitative analysis. A study by Rachmani et al(29) was unusual in that it aimed to examine the “safety” of metformin in patients with contraindications. Because this study was not typical in its design and included participants with contraindications, it was excluded from our analysis.
The bulk of other studies identified by our search were more typical randomized clinical trials comparing the efficacy or effectiveness of various oral medications on intermediate clinical measures (e.g., change in HbA1c, blood pressure, lipids) and also reported collecting data on adverse events, including cardiovascular events. A meta-analysis of the effects of oral diabetes drugs on intermediate measures has been previously published by our research group(10). Our search identified two dose-response studies(30;31), one study comparing two different formulations of glyburide(32), and one study comparing two different sulfonylurea therapies(33). These 4 trials are included in Table 1, but were excluded from our quantitative analyses since they did not contribute to our comparisons of interest.
Across all trials, the average Jadad quality score was a 3(out of 5). More detailed information regarding the quality of these trials is presented in the complete report(12). Of the relevant 142 randomized trials initially identified in our search, only 40 indicating collecting data on serious adverse events including mortality or cardiovascular events. Of the 40 trials reviewed here, 8 included cardiovascular events in the primary or secondary endpoint. The quality of serious adverse event reporting among the 32 trials where cardiovascular events were not included in the primary or secondary outcome, was fair with an average quality score of 3 (out of 4). Only 12 trials scored a perfect 4, indicating that all serious adverse events, withdrawals and drop-outs were reported and clear definitions of serious adverse events were provided in the manuscript. Serious adverse events, such as fatal and non-fatal cardiovascular events, may be underreported in this literature; however, we were unable to directly evaluate this phenomenon.
Quantitative Summary
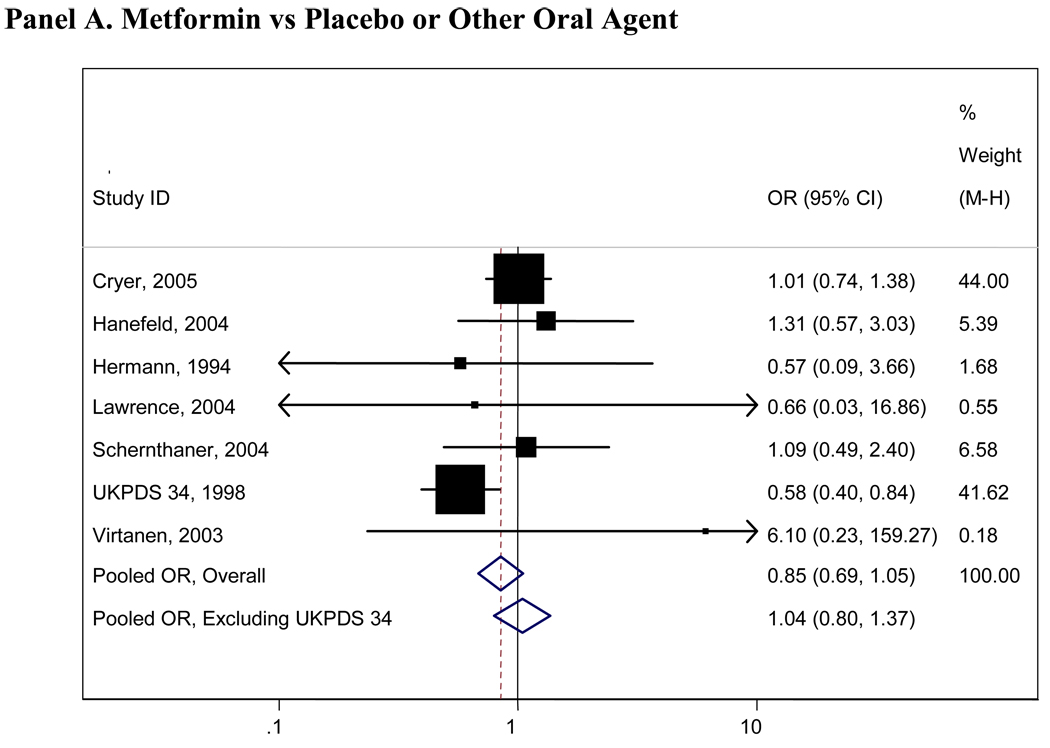
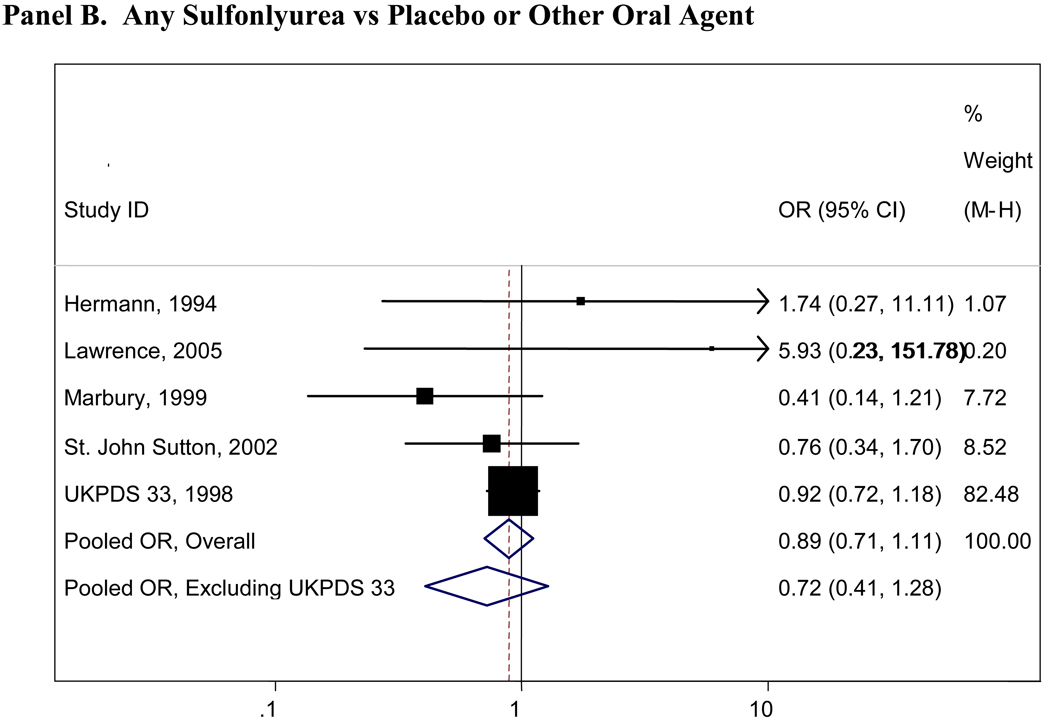
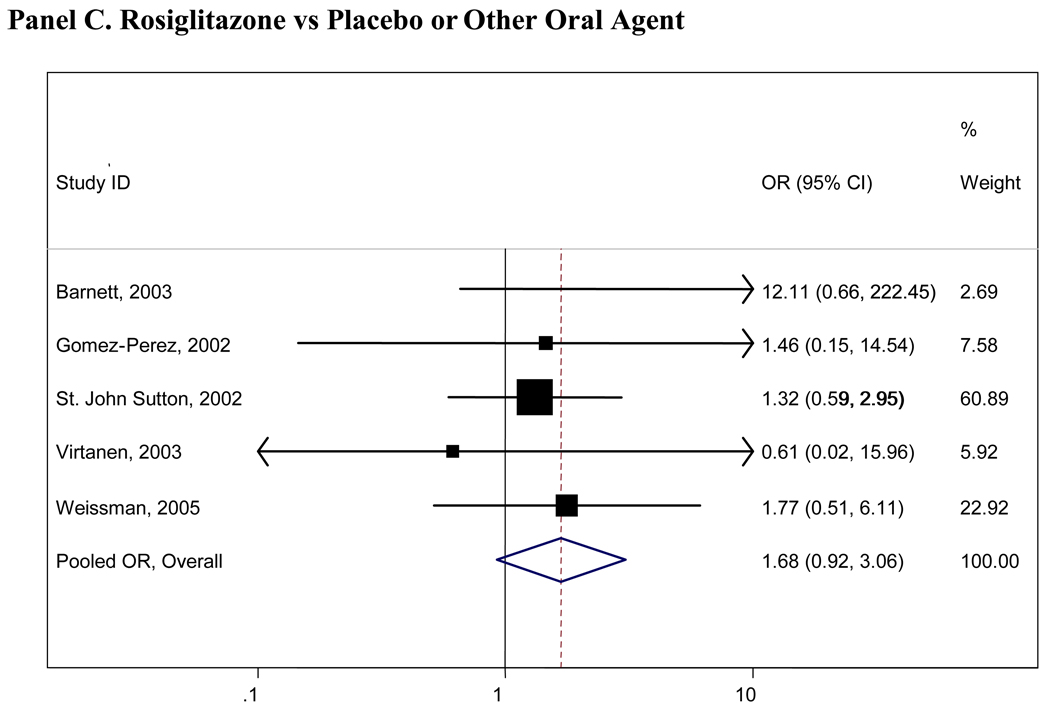
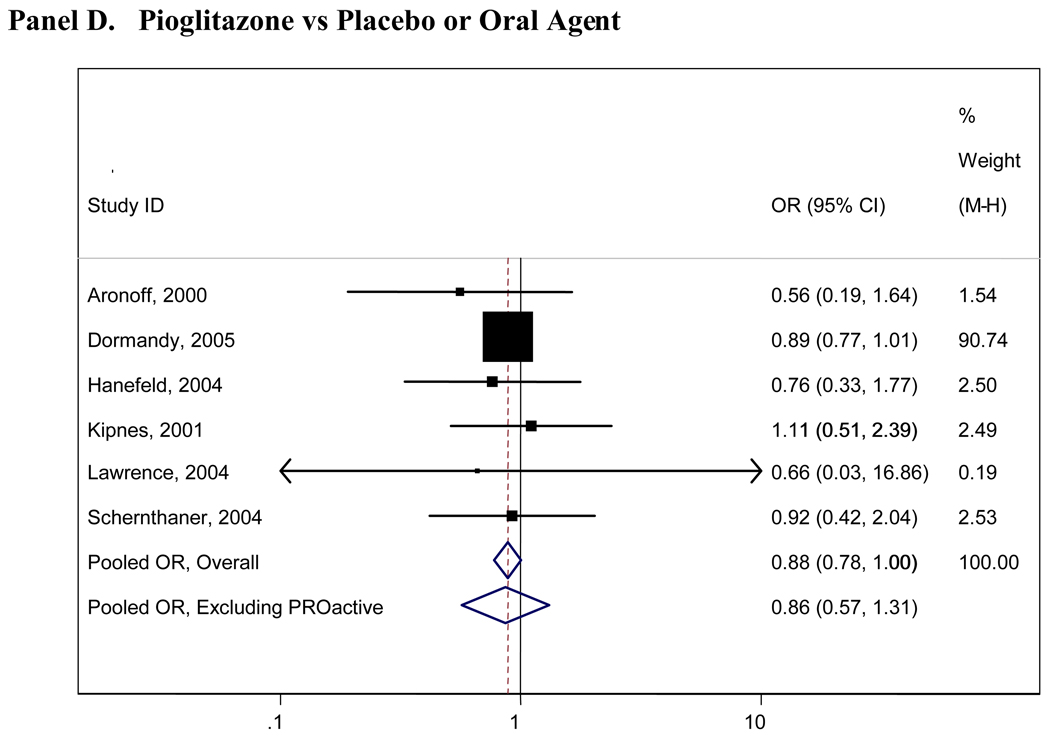
Pooled results for all comparisons of interest are presented in Table 2. Pooled analyses using the Peto and Mantel- Haenszel methods did not appreciably differ and thus only the Mantel- Haenszel results are presented here. There were insufficient numbers of trials (<4 studies) for many of the comparisons and we were thus unable to pool these data (Table 2). Figure 2 presents graphical displays (forest plots) of the pooled and individual ORs for cardiovascular morbidity for the following comparisons: metformin vs any comparator, any sulfonylurea vs any comparator; rosiglitazone vs any comparator; and pioglitazone vs any comparator. Our comparisons for metformin vs any comparator (other oral agent or placebo/diet) were the most robust, with 7 trials with 11,986 total participants who contributed to the pooled estimate for cardiovascular morbidity(OR=0.85, 95%CI 0.69-1.05), 6 trials with 11,385 individuals for cardiovascular mortality(OR=0.74, 95%CI 0.62-0.89) and 9 trials with 13,046 individuals contributing to the pooled OR for all-cause mortality(OR=0.81, 95% CI 0.60-1.08). No other significant associations were observed for any oral agent with cardiovascular morbidity, mortality, or all-cause mortality. In the analysis of the sulfonlyureas, the UKPDS trial was highly influential(accounting for over 500 participants and the majority of events). When UKPDS was excluded from these comparisons, the results remained non-significant, but the confidence intervals were substantially wider reflecting the imprecision of the remaining studies(see Figure 2). Similarly, the PROactive trial was highly influential in our analyses of pioglitazone and when this trial was excluded, the results remained non-significant but far less precise(see Figure 2).
Table 2.
Pooled Odds Ratios for Comparisons of Interest
| No. of studies | Total No. of Participants |
Pooled Odds Ratio (95% CI) |
p-value for Heterogeneity |
|
|---|---|---|---|---|
| Cardiovascular Morbidity | ||||
| Metformin vs Any comparator | 7 | 11,986 | 0.85 (0.69, 1.05) | 0.218 |
| Metformin vs Any Sulfonlyurea+Metformin | 2 | 831 | -* | -* |
| Any Sulfonlyurea vs Any Comparator | 5 | 2,795 | 0.89 (0.71, 1.11) | 0.400 |
| Any sulfonylurea vs Any Sulfonlyurea+Metformin | 1 | 577 | -* | -* |
| Rosiglitazone vs Any Comparator | 5 | 1,338 | 1.68 (0.92, 3.06) | 0.643 |
| Rosiglitazone+Metformin vs Metformin Alone | 2 | 886 | -* | -* |
| Pioglitazone vs Any Comparator | 6 | 9,287 | 0.88 (0.78, 1.00) | 0.884 |
| Meglitinide (neteglide or repaglinide) vs Any Comparator | 3 | 1,049 | -* | -* |
| Cardiovascular Mortality | ||||
| Metformin vs Any comparator | 6 | 11,385 | 0.74 (0.62, 0.89) | 0.274 |
| Metformin vs Any sulfonlyurea+metformin | 2 | 1,251 | -* | -* |
| Any Sulfonlyurea vs Any Comparator | 5 | 3,466 | 0.92 (0.68, 1.26) | 0.971 |
| Any sulfonylurea vs Any sulfonlyurea+metformin | 2 | 748 | -* | -* |
| Rosiglitazone vs Any Comparator | 5 | 3,202 | 1.03 (0.30, 3.53) | 0.702 |
| Rosiglitazone+Metformin vs Metformin Alone | 3 | 909 | -* | -* |
| Pioglitazone vs Any Comparator | 2 | 5,566 | -* | -* |
| Meglitinide (neteglide or repaglinide) vs Any Comparator | 2 | 1,256 | -* | -* |
| All-Cause Mortality | ||||
| Metformin vs Any comparator | 9 | 13,046 | 0.81 (0.60, 1.08) | 0.579 |
| Metformin vs Any sulfonlyurea+metformin | 3 | 1,631 | -* | -* |
| Any Sulfonlyurea vs Any Comparator | 6 | 4,255 | 0.90 (0.70, 1.15) | 0.989 |
| Any sulfonylurea vs Any sulfonlyurea+metformin | 2 | 939 | -* | -* |
| Rosiglitazone vs Any Comparator | 6 | 2,927 | 1.21 (0.39, 3.77) | 0.777 |
| Rosiglitazone+Metformin vs Metformin Alone | 4 | 1,676 | 2.52 (0.51, 12.52) | 0.988 |
| Pioglitazone vs Any Comparator | 4 | 7,507 | 0.96 (0.78, 1.18) | 0.902 |
| Meglitinide (neteglide or repaglinide) vs Any Comparator | 2 | 1,257 | -* | -* |
Data were only pooled for those comparisons with 4 or more trials
Figure 2. Forrest Plots of Odds Ratios of Cardiovascular Morbidity for Major Comparisons of Interest.
Panel A. Metformin vs Placebo or Other Oral Agent
Panel B. Any Sulfonlyurea vs Placebo or Other Oral Agent
Panel C. Rosiglitazone vs Placebo or Other Oral Agent
Panel D. Pioglitazone vs Placebo or Oral Agent
Boxes are the odds ratios estimated from each study; the horizontal bars are 95% confidence intervals. The size of the box is proportional to the weight of the study in the pooled analysis. The pooled Mantel- Haenszel odds ratios are represented by the diamonds; the width of the diamond represents the pooled 95% confidence interval. The vertical line at 1.0 indicates no effect.
Rosiglitazone was the only oral agent which was associated with an increased risk of cardiovascular morbidity, mortality, and all-cause mortality(all ORs >1.0); however, none of these estimates were statistically significant, possibly owing to the small sample sizes and limited number of included studies. Many small studies reported only one or two cardiovascular events or deaths in any arm; these studies provide imprecise estimates of cardiovascular risk and do not contribute substantially to our comparisons. For those comparisons with larger and longer duration studies(and corresponding higher numbers of events), the pooled estimates were most reliable, such as those for metformin. No significant quantitative heterogeneity was observed, although our formal tests for statistical heterogeneity were likely underpowered.
DISCUSSION
There have been few rigorous systematic reviews of hard clinical outcomes comparing oral diabetes agents. Recent meta-analyses have focused on possible cardiovascular effects of single drugs, particularly the newer thiazolidinediones, rosiglitazone and pioglitazone(6;7;34;35). We included the most common oral diabetes medications currently in use in the U.S. to provide a comprehensive picture of possible cardiovascular risk. When compared to any other treatment or placebo, we found that metformin was associated with a statistically significant decrease in cardiovascular mortality(OR=0.74, 95%CI 0.62-0.89). The point estimates for metformin with cardiovascular morbidity and all-cause mortality were similar, but not statistically significant. When compared to any other diabetes agent or placebo, rosiglitazone was the only therapy which was associated with a possible increase in risk of cardiovascular morbidity or mortality, but these results were not statistically significant. No other differences in cardiovascular risk between other commonly used oral diabetes medications were evident in this literature. Nonetheless, the poor quality and inconsistent reporting of adverse events and profound lack of long-term studies makes it difficult to draw firm conclusions.
The UKPDS was designed principally to examine the effect of absolute reductions in glucose on long-term outcomes. In UKPDS, the lack of difference in cardiovascular risk reduction when indirect comparisons were made across treatments but significant reduction observed when intensive control was compared to the conventional treatment group suggests that it is glycemic control per se which may be partially driving cardiovascular risk reduction. This is consistent with several other large, epidemiologic studies(2–4). Furthermore, in the PROactive study, the pioglitazone group had a 0.8 absolute percentage point decrease in HbA1c compared to a 0.3 absolute percentage point decrease in the control arm; this trial showed a corresponding moderate reduction in the secondary endpoint (all-cause mortality, non-fatal myocardial infarction, and stroke) in the pioglitazone-treated group compared to control.
Questions have recently been raised regarding possible “cardiotoxic” side-effects of rosiglitazone, a newer thiazolidinedione. The Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM), a large study in individuals with pre-diabetes published in 2006, showed that rosiglitazone was associated with a reduced risk of the composite outcome of incident diabetes (based on glucose levels) and death(36). The interpretation of this trial has been controversial because of a borderline statistically significant increase in cardiovascular events(RR=1.37, p-value=0.08) and a statistically significant increase in congestive heart failure cases in the treatment arm(RR=7.03, p-value = 0.01)(37). A second study in persons with type 2 diabetes, the A Diabetes Outcome Prevention Trial(ADOPT), was published after the completion of our literature search and so was not included in our analyses(38). The ADOPT trial showed a non-significant increase in fatal and non-fatal myocardial infarction in the rosiglitazone group compared with metformin or glyburide. A recent meta-analysis by Nissen et al(6) suggested a statistically significant excess of cardiovascular morbidity due to treatment with rosiglitazone in a pooled analysis that included a diverse population of published and unpublished studies of individuals with and without type 2 diabetes (including ADOPT and DREAM). An interim analysis of the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) trial showed no statistically significant elevation in cardiovascular risk (besides congestive heart failure) related to rosiglitazone treatment compared with metformin and sulfonlyureas (39). Some data relevant to the question of cardiovascular risk among persons taking oral diabetes medications exists outside the peer-reviewed literature and have been included in previous reviews including that by Nissen et al(6). In a sensitivity analysis in which we pooled data from the ADOPT, RECORD, and eligible unpublished trials analyzed by Nissen et al with our included studies resulted in a pooled ORs of 1.28(95%CI 0.95-1.76) for cardiovascular morbidity and 1.24(95%CI 0.87-1.79) for cardiovascular mortality when comparing rosiglitazone with any other comparator. Our main results, based exclusively on published data in persons with type 2 diabetes, are not inconsistent with an increase in cardiovascular risk with rosiglitazone treatment, but we had an insufficient number of studies to draw firm conclusions. The interpretation of the data on rosiglitazone remains controversial.
The limitations of this meta-analysis largely reflect the limitations of the published literature on oral diabetes medications. A major weakness is there have been few trials undertaken to examine the comparative effectiveness of oral diabetes medications on cardiovascular outcomes. Indeed, there were only two studies included in our quantitative analyses that had participant follow-up greater than 2 years. Importantly, despite combining multiple comparator groups together, the total number of events in each of our comparisons of interest was small and we only included studies which had at least one cardiovascular event in one arm. Studies which did not report collecting information on cardiovascular events were excluded from the review. Furthermore, while we attempted to exclude congestive heart failure cases from all analyses, we relied on the definitions in the individual studies and there were instances in which the reporting of cardiovascular events was ambiguous.
The current evidence of comparing specific oral diabetes medications for risk of cardiovascular morbidity and mortality is inconclusive. Our study demonstrates that there are few trials of oral diabetes therapies that last longer than 6 months, and adverse event reporting for cardiovascular disease is poor. As most medications have similar short-term efficacy(10) selecting appropriate oral therapy is largely based on patient and provider preferences, side effect profile, and cost. There is a critical need for studies of oral diabetes medications with long-term outcomes. The relatively modest differences in blood pressure, cholesterol, and weight observed with treatment of oral diabetes medications in short term trials may not translate to changes in long term cardiovascular risk. Only long-term trials can provide definitive conclusions regarding the comparative efficacy of oral diabetes medications and long term risks. Because individuals with diabetes are at a dramatically elevated risk of cardiovascular disease, trials of even one to two years duration with rigorous and standardized adverse event reporting can provide important information, especially when the results of separate studies can be pooled. One clear conclusion from the literature is that all clinical trials comparing oral diabetes medications, regardless of duration, should endeavor to rigorously collect and report adverse events including cardiovascular and all-cause mortality. This includes clear protocols for reporting adverse events and determining reasons for withdrawal of study participants. The development of the CONSORT standards for reporting of randomized clinical trials(14;40;41)—which requires that “all important adverse events or side effects in each intervention group” be reported—should help ameliorate this problem, but such standards need to be rigorously and consistently applied.
In conclusion, our meta-analysis suggested that compared to other oral diabetes agents and placebo, metformin appeared moderately protective and rosiglitazone possibly harmful, but lack of power prohibited firmer conclusions. Larger, long-term studies taken to hard endpoints and better reporting of cardiovascular events in short term studies will be required to draw firm conclusions about major clinical benefits and risks related to oral diabetes agents.
Acknowledgments
We thank Dr. Eliseo Guallar for helpful statistical advice.
Grant support: This article is based on research conducted by the Johns Hopkins Evidence Based Practice Center under contract number 290-02-0018 with the Agency for Healthcare Research and Quality, Rockville, Maryland. Drs. Selvin (grant K01 DK076595) and Brancati (grant K24 DK62222) were supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclaimer: The authors of this article are responsible for its contents, including any clinical or treatment recommendations. No statement in this article should be construed as an official position of the Agency for Healthcare Research and Quality or of the U.S. Department of Health and Human Services.
The authors have no conflicts of interest to declare.
Reference List
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 2.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic Control and Coronary Heart Disease Risk in Persons With and Without Diabetes: The Atherosclerosis Risk in Communities Study. Archives of Internal Medicine. 2005;165(16):1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 4.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Loke YK, Furberg CD. Long-term Risk of Cardiovascular Events With Rosiglitazone: A Meta-analysis. JAMA: The Journal of the American Medical Association. 2007;298(10):1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 8.Diamond GA, Bax L, Kaul S. Uncertain Effects of Rosiglitazone on the Risk for Myocardial Infarction and Cardiovascular Death. Ann Intern Med. 2007;147(8):578–581. doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- 9.Mulrow CD, Cornell J, Localio AR. Rosiglitazone: A Thunderstorm from Scarce and Fragile Data. Ann Intern Med. 2007;147(8):585–587. doi: 10.7326/0003-4819-147-8-200710160-00013. [DOI] [PubMed] [Google Scholar]
- 10.Bolen S, Feldman L, Vassy J, Wilson L, Yeh Hc, Marinopoulos S, et al. Systematic Review: Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus. Ann Intern Med. 2007;147(6):386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 11.Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van WC. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003639. doi: 10.1002/14651858.CD003639.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolen S, Wilson L, Vassy J, Feldman L, Yeh J, Marinopoulos S, et al. Comparative Effectiveness and Safety of Oral Diabetes Medications for Adults with Type 2 Diabetes. Comparative Effectiveness Review No. 8. (Prepared by Johns Hopkins Evidence-based Practice Center under Contract No. 290-02-0018.) Rockville, MD: Agency for Healthcare Research and Quality; 2007 available from http://effectivehealthcare.ahrq.gov/repFiles/OralFullReport.pdf. [PubMed]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285(15):1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 15.Consolidated Standards of Reporting Trials (CONSORT) [8-17-2007];Transparent Reporting of Trials - Reporting of Adverse Events. 2007 http://www.consort-statement.org/?o=1092 Ref Type: Electronic Citation.
- 16.Food and Drug Administration. [8-17-2007];Guideline for Industry: Structure and Content of Clinical Study Reports. 1996 http://www.fda.gov/cder/guidance/iche3.pdf Ref Type: Electronic Citation.
- 17.Jovanovic L, Dailey G, Huang WC, Strange P, Goldstein BJ. Repaglinide in type 2 diabetes: a 24-week, fixed-dose efficacy and safety study. J Clin Pharmacol. 2000;40(1):49–57. doi: 10.1177/00912700022008694. [DOI] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 19.Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol. 1986;124(5):719–723. doi: 10.1093/oxfordjournals.aje.a114447. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 23.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL The Pioglitazone 001 Study Group. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. Diabetes Care. 2000;23(11):1605–1611. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Perez FJ, Fanghanel-Salmon G, Antonio Barbosa J, Montes-Villarreal J, Berry RA, Warsi G, et al. Efficacy and safety of rosiglitazone plus metformin in Mexicans with type 2 diabetes. Diabetes Metab Res Rev. 2002;18(2):127–134. doi: 10.1002/dmrr.264. [DOI] [PubMed] [Google Scholar]
- 25.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 26.Choi D, Kim SK, Choi SH, Ko YG, Ahn CW, Jang Y, et al. Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care. 2004;27(11):2654–2660. doi: 10.2337/diacare.27.11.2654. [DOI] [PubMed] [Google Scholar]
- 27.Nishio K, Sakurai M, Kusuyama T, Shigemitsu M, Fukui T, Kawamura K, et al. A randomized comparison of pioglitazone to inhibit restenosis after coronary stenting in patients with type 2 diabetes. Diabetes Care. 2006;29(1):101–106. doi: 10.2337/diacare.29.01.06.dc05-1170. [DOI] [PubMed] [Google Scholar]
- 28.Takagi T, Yamamuro A, Tamita K, Yamabe K, Katayama M, Mizoguchi S, et al. Pioglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with type 2 diabetes mellitus: an intravascular ultrasound scanning study. Am Heart J. 2003;146(2):E5. doi: 10.1016/S0002-8703(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 29.Rachmani R, Slavachevski I, Levi Z, Zadok B, Kedar Y, Ravid M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med. 2002;13(7):428. doi: 10.1016/s0953-6205(02)00131-0. [DOI] [PubMed] [Google Scholar]
- 30.Sonnenberg GE, Garg DC, Weidler DJ, Dixon RM, Jaber LA, Bowen AJ, et al. Short-term comparison of once- versus twice-daily administration of glimepiride in patients with non-insulin-dependent diabetes mellitus. Ann Pharmacother. 1997;31(6):671–676. doi: 10.1177/106002809703100601. [DOI] [PubMed] [Google Scholar]
- 31.Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529. doi: 10.1016/s0149-2918(03)80093-0. [DOI] [PubMed] [Google Scholar]
- 32.Carlson RF, Isley WL, Ogrinc FG, Klobucar TR. Efficacy and safety of reformulated, micronized glyburide tablets in patients with non-insulin-dependent diabetes mellitus: a multicenter, double-blind, randomized trial. Clin Ther. 1993;15(5):788–796. [PubMed] [Google Scholar]
- 33.Draeger KE, Wernicke-Panten K, Lomp HJ, Schuler E, Rosskamp R. Long-term treatment of type 2 diabetic patients with the new oral antidiabetic agent glimepiride (Amaryl): a double-blind comparison with glibenclamide. Horm Metab Res. 1996;28(9):419–425. doi: 10.1055/s-2007-979830. [DOI] [PubMed] [Google Scholar]
- 34.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and Risk of Cardiovascular Events in Patients With Type 2 Diabetes Mellitus: A Meta-analysis of Randomized Trials. JAMA: The Journal of the American Medical Association. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 35.Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim S. Rosiglitazone for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(3):CD006063. doi: 10.1002/14651858.CD006063.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. The Lancet. 368(9541):1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 37.Nissen SE. The DREAM trial. The Lancet. 368(9552):2049. doi: 10.1016/S0140-6736(06)69825-5. [DOI] [PubMed] [Google Scholar]
- 38.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 39.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, et al. Rosiglitazone Evaluated for Cardiovascular Outcomes -- An Interim Analysis. N Engl J Med. 2007;357(1):28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 40.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 41.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 42.Bailey CJ, Bagdonas A, Rubes J, McMorn SO, Donaldson J, Biswas N, et al. Rosiglitazone/metformin fixed-dose combination compared with uptitrated metformin alone in type 2 diabetes mellitus: a 24-week, multicenter, randomized, double-blind, parallel-group study. Clin Ther. 2005;27(10):1548–1561. doi: 10.1016/j.clinthera.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Baksi A, James RE, Zhou B, Nolan JJ. Comparison of uptitration of gliclazide with the addition of rosiglitazone to gliclazide in patients with type 2 diabetes inadequately controlled on half-maximal doses of a sulphonylurea. Acta Diabetol. 2004;41(2):63–69. doi: 10.1007/s00592-004-0146-y. [DOI] [PubMed] [Google Scholar]
- 44.Barnett AH, Grant PJ, Hitman GA, Mather H, Pawa M, Robertson L, et al. Rosiglitazone in Type 2 diabetes mellitus: an evaluation in British Indo-Asian patients. Diabet Med. 2003;20(5):387–393. doi: 10.1046/j.1464-5491.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 45.Cryer DR, Nicholas SP, Henry DH, Mills DJ, Stadel BV. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care. 2005;28(3):539–543. doi: 10.2337/diacare.28.3.539. [DOI] [PubMed] [Google Scholar]
- 46.DeFronzo RA, Goodman AM The Multicenter Metformin Study Group. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 47.Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA. 2000;283(13):1695–1702. doi: 10.1001/jama.283.13.1695. [DOI] [PubMed] [Google Scholar]
- 48.Garber AJ, Donovan DS, Dandona P, Bruce S, Park JS. Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab. 2003;88(8):3598–3604. doi: 10.1210/jc.2002-021225. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg RB, Einhorn D, Lucas CP, Rendell MS, Damsbo P, Huang WC, et al. A randomized placebo-controlled trial of repaglinide in the treatment of type 2 diabetes. Diabetes Care. 1998;21(11):1897–1903. doi: 10.2337/diacare.21.11.1897. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein BJ, Pans M, Rubin CJ. Multicenter, randomized, double-masked, parallel-group assessment of simultaneous glipizide/metformin as second-line pharmacologic treatment for patients with type 2 diabetes mellitus that is inadequately controlled by a sulfonylurea. Clin Ther. 2003;25(3):890–903. doi: 10.1016/s0149-2918(03)80112-1. [DOI] [PubMed] [Google Scholar]
- 51.Hanefeld M, Brunetti P, Schernthaner GH, Matthews DR, Charbonnel BH. One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27(1):141–147. doi: 10.2337/diacare.27.1.141. [DOI] [PubMed] [Google Scholar]
- 52.Hanefeld M, Bouter KP, Dickinson S, Guitard C. Rapid and short-acting mealtime insulin secretion with nateglinide controls both prandial and mean glycemia. Diabetes Care. 2000;23(2):202–207. doi: 10.2337/diacare.23.2.202. [DOI] [PubMed] [Google Scholar]
- 53.Hermann LS, Schersten B, Bitzen PO, Kjellstrom T, Lindgarde F, Melander A. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care. 1994;17(10):1100–1109. doi: 10.2337/diacare.17.10.1100. [DOI] [PubMed] [Google Scholar]
- 54.Horton ES, Clinkingbeard C, Gatlin M, Foley J, Mallows S, Shen S. Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care. 2000;23(11):1660–1665. doi: 10.2337/diacare.23.11.1660. [DOI] [PubMed] [Google Scholar]
- 55.Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111(1):10–17. doi: 10.1016/s0002-9343(01)00713-6. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence JM, Reid J, Taylor GJ, Stirling C, Reckless JP. Favorable effects of pioglitazone and metformin compared with gliclazide on lipoprotein subfractions in overweight patients with early type 2 diabetes. Diabetes Care. 2004;27(1):41–46. doi: 10.2337/diacare.27.1.41. [DOI] [PubMed] [Google Scholar]
- 57.Marbury T, Huang WC, Strange P, Lebovitz H. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract. 1999;43(3):155–166. doi: 10.1016/s0168-8227(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 58.Rosenstock J, Samols E, Muchmore DB, Schneider J Glimepiride Study Group. Glimepiride, a new once-daily sulfonylurea. A double-blind placebo-controlled study of NIDDM patients. Diabetes Care. 1996;19(11):1194–1199. doi: 10.2337/diacare.19.11.1194. [DOI] [PubMed] [Google Scholar]
- 59.Rosenstock J, Goldstein BJ, Vinik AI, O'neill MC, Porter LE, Heise MA, et al. Effect of early addition of rosiglitazone to sulphonylurea therapy in older type 2 diabetes patients (>60 years): the Rosiglitazone Early vs. SULphonylurea Titration (RESULT) study. Diabetes Obes Metab. 2006;8(1):49–57. doi: 10.1111/j.1463-1326.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 60.Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89(12):6068–6076. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 61.Simonson DC, Kourides IA, Feinglos M, Shamoon H, Fischette CT The Glipizide Gastrointestinal Therapeutic System Study Group. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. Diabetes Care. 1997;20(4):597–606. doi: 10.2337/diacare.20.4.597. [DOI] [PubMed] [Google Scholar]
- 62.St John Sutto M, Rendell M, Dandona P, Dole JF, Murphy K, Patwardhan R, et al. A comparison of the effects of rosiglitazone and glyburide on cardiovascular function and glycemic control in patients with type 2 diabetes. Diabetes Care. 2002;25(11):2058–2064. doi: 10.2337/diacare.25.11.2058. [DOI] [PubMed] [Google Scholar]
- 63.Virtanen KA, Hallsten K, Parkkola R, Janatuinen T, Lonnqvist F, Viljanen T, et al. Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects. Diabetes. 2003;52(2):283–290. doi: 10.2337/diabetes.52.2.283. [DOI] [PubMed] [Google Scholar]
- 64.Hallsten K, Virtanen KA, Lonnqvist F, Sipila H, Oksanen A, Viljanen T, et al. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes. 2002;51(12):3479–3485. doi: 10.2337/diabetes.51.12.3479. [DOI] [PubMed] [Google Scholar]
- 65.Weissman P, Goldstein BJ, Rosenstock J, Waterhouse B, Cobitz AR, Wooddell MJ, et al. Effects of rosiglitazone added to submaximal doses of metformin compared with dose escalation of metformin in type 2 diabetes: the EMPIRE Study. Curr Med Res Opin. 2005;21(12):2029–2035. doi: 10.1185/030079905x74844. [DOI] [PubMed] [Google Scholar]
- 66.Wolffenbuttel BH, Landgraf R Dutch and German Repaglinide Study Group. A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Diabetes Care. 1999;22(3):463–467. doi: 10.2337/diacare.22.3.463. [DOI] [PubMed] [Google Scholar]
- 67.Zhu XX, Pan CY, Li GW, Shi HL, Tian H, Yang WY, et al. Addition of rosiglitazone to existing sulfonylurea treatment in chinese patients with type 2 diabetes and exposure to hepatitis B or C. Diabetes Technol Ther. 2003;5(1):33–42. doi: 10.1089/152091503763816445. [DOI] [PubMed] [Google Scholar]