Abstract
Aim
To evaluate the clinical value of serum high mobility group box chromosomal protein 1 (HMGB1) levels in making the early diagnosis of recurrent cervical squamous cell carcinomas (CSCC) and compare it with the value of serum squamous cell carcinoma antigen (SCCA), cytokeratin fragment (CYFRA) 21-1, and carcinoembryonic antigen (CEA) levels.
Methods
Immunohistochemical staining of tissue from 64 patients with recurrent CSCCs, 72 patients with non-recurrent carcinoma, and 28 healthy participants was performed to determine the expression of HMGB1 protein. The serum levels of the 4 markers in 112 patients with recurrent CSCC, 174 patients with non-recurrent disease, and 128 healthy participants were measured by enzyme-linked immunosorbent assay. The receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) was calculated.
Results
Higher immunostaining score was found in recurrent CSCC tissue sections than in non-recurrent CSCC sections. Serum HMGB1 levels in patients with recurrent CSCC were significantly higher than in patients with non-recurrent disease and healthy controls. The AUC of HMGB1, SCCA, CYFRA21-1, and CEA was 0.816, 0.768, 0.703, and 0.625, respectively. HMGB1 had the best specificity and positive likelihood ratio (78.0% and 3.25, respectively), whereas SCCA had the best sensitivity and negative likelihood ratio (76.3% and 0.34, respectively). Parallel combined measurements increased the diagnostic sensitivity and serial combination increased the specificity. High serum HMGB1 levels were inversely correlated with disease-free survival (P = 0.009, Pearson χ2 test) and overall survival (P = 0.018).
Conclusion
HMGB1 was overexpressed in recurrent CSCCs. Serum HMGB1 level could be a useful and specific marker for evaluating the disease recurrence and predicting prognosis in patients with CSCC. Serial combined measurements of serum HMGB1, SCCA, and CYFRA21-1 increased the diagnostic specificity, and parallel combined testing increased the diagnostic sensitivity.
Cervical carcinoma, one of the most frequent malignant tumors of the female reproductive system, remains to be among the leading causes of cancer-related death among women globally (1,2). Approximately 30% of International Federation of Gynecology and Obstetrics (FIGO) stage IB2 to stage IV cases will ultimately recur, despite modern multimodality treatment (3,4). Mostly, recurrence and metastasis occur in the local cervix, pelvic wall, and retroperitoneal lymph node. It is difficult to distinguish those diseases, except local relapse from radiation reaction or organized lymphocyst. One of the methods to identify early recurrence and prolong overall survival is screening of asymptomatic patients (5). In clinical practice, some tumor markers which can improve the sensitivity and specificity of diagnosis and prognostic prediction of recurrent cervical cancer have been used (6-9). Although recent advances have revealed different pathways in cervical carcinogenesis, no biomarker has yet been found to be significantly associated with the disease recurrence or clinical outcome in cases of cervical cancer. Therefore, finding new serum tumor markers of recurrent cervical squamous cell carcinomas (CSCC) for evaluating the cut-off value has become a frequent subject of investigation in the recent years.
Nuclear protein, high mobility group box-1 (HMGB1), has been found to play an important role in tumor development, growth, and spread. Increased expression of HMGB1 has been reported in several different tumor types, including breast carcinoma (10), colorectal cancer (11), prostate cancer (12), pancreatic cancer (13), and hepatocellular carcinoma (14). HMGB1 plays a role in metastasis development, and thus links it to poor prognosis in a variety of cancers (15). In this study, we hypothesized that patients with recurrent cervical cancer would overexpress HMGB1 in tissue and serum.
Patients and methods
Patients
All patient-derived specimens were collected and archived under protocols approved by the institutional review boards of Shandong Cancer hospital and institution. Archival tissues in paraffin blocks were retrieved from the Department of Pathology at Shandong Cancer Hospital. Written informed consents were obtained from the patients. Immunohistochemical staining of 66 recurrent CSCC paraffin block tissues, 70 non-recurrent CSCC tissues, and 28 healthy cervical tissues from the archive of the Department of Pathology, was performed to analyze the cellular localization of HMGB1 expression.
Blood samples were obtained with the written informed consent from 112 women with recurrent CSCC and from 174 women with non-recurrent carcinoma of the uterine cervix at Shandong Cancer Hospital between January 2000 and September 2008. All of the cervical cancer patients were clinically staged according to the FIGO staging system. Tumor size was clinically measured by magnetic resonance imaging scan. Plasma samples were collected before and after the initiation of treatment and centrifuged at 1500 g at 4°C for 10 minutes. The separated plasma was aliquoted and stored at -80°C for future analysis. Control plasma specimens (n = 128) were derived from age-matched examinees receiving health checks at Shandong Cancer Hospital and showing no history of disease and no abnormalities in laboratory examinations. The description of all patients involved in this trial is presented in Table 1.
Table 1.
Clinicopathologic characteristics of the study participants (n = 414)*
| Healthy controls (n = 128) | Patients with non-recurrent cervical cancer (n = 174) | Patients recurrent cervical cancer (n = 112) | |
|---|---|---|---|
| Age, year (median, range) | 52 (23-77) | 58 (25-82) | 56 (32-79) |
| FIGO stage:* | - | ||
| I-II | 128 (73.6%) | 37 (33.0%) | |
| III-IV | 46 (26.4%) | 75 (67.0%) | |
| Treatment: | - | ||
| surgery only | 11 (6.3%) | 9 (8.0%) | |
| surgery plus adjuvant radiotherapy | 30 (17.2%) | 20 (17.9%) | |
| radiotherapy alone | 35 (20.1%) | 26 (23.2%) | |
| radiochemotherapy | 52 (29.9%) | 35 (31.3%) | |
| radiochemotherapy plus surgery | 46 (26.5%) | 22 (19.6%) |
*FIGO – International Federation of Gynecology and Obstetrics (16).
Diagnosis of local recurrence was mainly based on imaging examination accompanied with pelvioscopy and colpocytology, with or without biopsy pathologic examination. Local recurrence occurred in 96 (85.7%), liver metastases in 7 (6.3%), lung metastasis in 4 (3.6%), and bone metastasis in 5 cases (4.4%). Diagnosis of pelvic recurrence was based on masses or parametrial invasion and nodal involvement found by pelvioscopy, progressively aggravated pain, and symptoms or signs accompanied with gradual edema of legs and confirmed by ultrasonography (45/96), computed tomography (32/96), and magnetic resonance imaging (19/96). The symptoms or signs of fibrosis caused by pelvic irradiation will not progressively aggravate, which can act as a distinction. Distant metastases were confirmed by clinical examination accompanied with ultrasonography (3/16), computed tomography (11/16), and bone scanning (2/16).
The present study and the experiments were approved by the ethics committee of the Shandong Tumor Hospital and Institute, and all participants gave written informed consent prior to participation.
Enzyme-linked immunosorbent assay
Levels of HMGB1, serum squamous cell carcinoma antigen (SCCA), cytokeratin fragment (CYFRA) 21-1, and carcinoembryonic antigen (CEA) in plasma samples were measured with a solid phase sandwich enzyme-linked immunosorbent assay (ELISA) using commercially available human HMGB1, CYFRA21-1, and CEA ELISA assay kits (Immuno-Biological Laboratories, Gunma, Japan). Plasma and human protein standard was diluted with EIA buffer (1% BSA, 0.05% Tween 20 in phosphate buffer) and incubated for 1 hour at 37°C. After 7 washes with EIA buffer, horse radish peroxidase-conjugated antibodies were added and incubated for 30 minutes at 4°C. After 9 washes, 100 µL of tetramethyl benzidine solution was added and the signal was allowed to develop for 30 minutes at room temperature. The reaction was stopped with 100 µL of 1 N sulfuric acid and the absorbance at 450 nm was measured by an automatic ELISA reader. Results were converted from the mean absorbance of duplicate wells after subtraction of background values. Recombinant human HMGB1, SCCA, CYFRA21-1, or CEA protein was used as a standard. The standard curve was prepared simultaneously with the measurement of test samples. Reagent blank, test sample blank, and internal controls of plasma samples were used to normalize values obtained from each experiment.
Immunohistochemistry
Archived paraffin blocks were derived from patients who had surgery at Shandong Cancer Hospital. Paraffin sections were antigen-retrieved, blocked in normal horse serum, and incubated in mouse anti-HMGB1 at 1:200 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) overnight at 4°C. After 3 washes in phosphate-buffered saline, sections were incubated with secondary antibody for 1 hour at 37°C. Positive reactions were detected by incubating the slides with stable 3,3-diaminobenzidine for 3 to 5 minutes. Sections were counterstained with Gill’s hematoxylin for 3 to 6 minutes. The intensity of protein expression was evaluated using OPTIMAS 6.5 software (Optimas Corp., Seattle, WA, USA).
To assess the results, we used the immunostaining score corresponding to the sum of staining intensity (strong positive staining in most cells – 3+; moderate staining – 2+; weak staining – 1+; and no evidence of staining – 0) and the percentage of positive cells (more than 50% of cells staining positive – 3+; 5-50% of cells staining positive – 2+; fewer than 5% of cells staining positive – 1+; and no cells staining positive – 0). Slides were scored in the absence of any clinical data, and the average score of 3 observers was reported as the final immunostaining score.
Statistical analysis
One-way ANOVA was used for comparison of marker serum levels between the 3 groups, and a P-value lower than 0.05 was considered significant. In addition, ROC analysis was performed and the area under the curve (AUC) was calculated separately for each of the 4 markers of interest and for the combinations of markers. The receiver-operating characteristic (ROC) curve, which is defined as a plot of testing sensitivity, as the “y” coordinate vs its 1-specificity or false positive rate, and as the “x” coordinate, has been widely accepted as the standard method for describing and comparing the accuracy of medical diagnostic tests (17,18). The overall survival and disease-free survival curves were calculated using the Kaplan-Meier method and the log-rank test. All analyses were performed using SPSS version, 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Immunohistochemistry for HMGB1 in cervical cancer patients
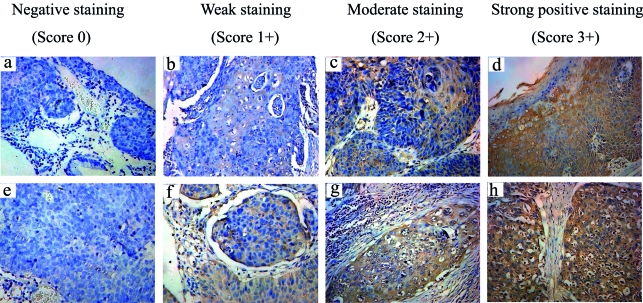
HMGB1 immunoreactivity in paraffin block sections of 66 archival recurrent CSCCs, 70 non-recurrent CSCCs, and 28 healthy cervical tissues was assessed by immunohistochemistry. HMGB1 immunoreactivity was detected in 47/66 (71.2%) recurrent cases and 33/70 (41.7%) non-recurrent cases. Immunohistochemical staining for HMGB1 in representative cases of non-recurrent and recurrent cervical cancer (Figure 1) demonstrated that HMGB1 was mostly expressed in the nuclei and cytoplasm of carcinoma cells and some fibroblasts. HMGB1 staining was weaker in normal adjacent epithelial cells than in cervical cancer tissues.
Figure 1.
Immunohistochemistry for high-mobility group box-1 (HMGB1) in cervical cancer clinical samples. (A)-(D) HMGB1 expression in non-recurrent cervical cancer; (E)-(H) HMGB1 expression in recurrent cervical cancer. Original magnification: ×200. An immunostaining score was used to compare each group corresponding to the sum of staining intensity.
Significantly higher mean immunostaining score was found in the recurrent CSCC tissue sections than in non-recurrent CSCC sections (2.96; 95% CI, 2.10-5.42 vs 1.57; 95% CI, 1.10-3. 27). There was no significant difference in immunostaining scores according to age, tumor size, and differentiation grade (P = 0.133). However, according to Pearson χ2 test in patients with cervical cancer, HMGB1 levels correlated with FIGO stage (P = 0.012), lymph node metastases (P = 0.028), local relapse (P = 0.008), and distant metastasis (P < 0.001) (Table 2).
Table 2.
Association between the clinicopathological features and immunostaning score of HMGB1 in 66 recurrent CSCC and 70 non-recurrent CSCC paraffin block tissues*
| Characteristics | HMGB1 (%) |
P† | |
|---|---|---|---|
| low expression number | high expression number | ||
| Age (years): | 0.736 | ||
| ≤45 (n = 59) | 25 (42.4) | 34 (57.6) | |
| >45 (n = 77) | 31 (40.3) | 46 (59.7) | |
| FIGO stage: | 0.012 | ||
| I-II (n = 85) | 43 (50.6) | 42 (49.4) | |
| III-IV (n = 51) | 13 (25.5) | 38 (74.5) | |
| Differentiation grades: | 0.146 | ||
| well/ moderately differentiated (n = 102) | 43 (42.2) | 59 (57.8) | |
| poorly differentiated (n = 34) | 13 (38.2) | 21 (61.8) | |
| Relapse: | 0.008 | ||
| yes (n = 66) | 19 (28.8) | 47 (71.2) | |
| no (n = 70) | 37 (52.9) | 33 (47.1) | |
| Lymph node metastases: | 0.028 | ||
| yes (n = 71) | 26 (32.4) | 45 (67.6) | |
| no (n = 65) | 30 (46.2) | 35 (53.8) | |
| Distant metastasis | <0.001 | ||
| yes (n = 22) | 4 (18.2) | 18 (81.8) | |
| no (n = 114) | 52 (45.6) | 62 (54.4) | |
*Abbreviations: HMGB1 – high mobility group box chromosomal protein 1; CSCC – cervical squamous cell carcinoma; FIGO – International Federation of Gynecology and Obstetrics (16).
†Pearson χ2 test.
Serum HMGB1, SCCA, CYFRA21-1, and CEA levels in CSCCs
Serum levels of HMGB1, SCCA, CYFRA21-1, and CEA are shown in Figure 2. Mean HMGB1 levels in women with recurrent CSCC (50.8 ng/mL; 95% CI, 34.4-87.l) were significantly higher (P = 0.027) than in women with non-recurrent disease (15.1 ng/mL; 95% CI, 10.1-18.9) and healthy controls (7.6 ng/mL; 95% CI, 3.1-11.4). There was a significant difference in serum CYFRA21-1 between patients with recurrent CSCC (8.4 ng/mL; 95% CI, 2.4-22.3) and patients with non-recurrent disease (5.1 ng/mL; 95% CI, 2.1-13.4). Higher serum SCCA and CEA were also observed in recurrent CSCC group, but the difference did not reach significance (P = 0.086).
Figure 2.
Serum levels of high mobility group box chromosomal protein 1 (HMGB1) (A), squamous cell carcinoma (B), cytokeratin fragment 21-1 (C), and carcinoembryonic antigen (D) in healthy control (n = 128), patients who remained disease free (n = 174), and patients whose disease relapsed (n = 112). Box – 95% confidence interval of HMGB1 level; line – mean of 3 independent experiments; asterisk – P < 0.05 vs control.
ROC curves
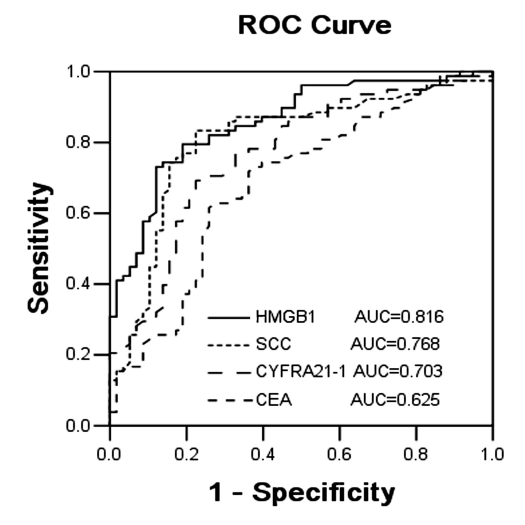
The serum levels of tumor markers HMGB1, SCCA, CYFRA21-1, and CEA were evaluated by ROC. The ROC curves of HMGB1 and SCCA were located closer to the theoretical 100% sensitivity and specificity values than the ROC curves of other investigated markers (Figure 3). Also, the AUC of every tumor marker was calculated. The AUC of HMGB1, SCCA, CYFRA21-1, and CEA was 0.816, 0.768, 0.703, and 0.625, respectively, suggesting clinical usefulness of the first 3 tumor markers for diagnosing recurrent cervical cancer. HMGB1 had the strongest integrative ability to differentially diagnose cervical cancer relapse (Figure 3). The point closest to the upper left-hand corner of the graph was chosen as the cut-off point. Markers’ cut-off levels were as follows: HMGB1 < 21.8 ng/mL, SCCA<2.6 ng/mL, CYFRA21-1 < 6.2 ng/mL, and CEA<5.0 ng/mL.
Figure 3.
Receiver operator characteristic (ROC) curves of high-mobility group box-1 (HMGB1), squamous cell carcinoma antigen (SCCA antigen), cytokeratin fragment 21-1 (CYFRA21-1), and carcinoembryonic antigen (CEA) in patients with recurrent cervical SCCA. The clinical relevance of HMGB1, SCCA antigen, CYFRA21-1, and CEA was compared by establishing ROC curves. The ROC curves of HMGB1 and SCCA antigen were located closer to the theoretical 100% sensitivity and specificity values than the ROC curves of other investigated markers.
We also calculated the sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) of the markers. PLR is the ratio of real positive rate to false positive rate, while NLR is the ratio of false negative rate to real negative rate. Tests with higher PLR have greater ability to confirm the disease, while tests with lower NLR have greater ability to exclude the disease. Our results showed that when comparing single tumor markers, HMGB1 had the best specificity and PLR (78.0% and 3.25, respectively), whereas SCCA had the best sensitivity and NLR (76.3% and 0.34, respectively).
Combined measurement
A result of parallel combined testing was considered positive if the value of any of the 4 tumor markers was higher than the cut-off value. This test increased the diagnostic sensitivity to 91.48%, but decreased the specificity and PLR priority. In serial combined testing, a positive result was defined only when the values of the 4 tumor markers were all higher than the cut-off value. This test decreased the diagnostic sensitivity, but increased the specificity and PLR to 92.2% and 6.3, respectively (Table 3).
Table 3.
Sensitivity, specificity, positive likelihood ratio (PLR) and negative likelihood ratio (NLR) of HMGB1, SCCA, CYFRA21-1, and CEA in parallel and serial combinations*
| Tumor marker | Sensitivity (%) | Specificity (%) | PLR | NLR |
|---|---|---|---|---|
| HMGB1 | 71.6 | 78.0 | 3.25 | 0.36 |
| SCCA | 76.3 | 70.6 | 2.60 | 0.34 |
| CYFRA21-1 | 58.3 | 66.9 | 1.76 | 0.62 |
| CEA | 48.7 | 75.6 | 1.99 | 0.68 |
| Parallel combination | 91.5 | 51.8 | 1.90 | 0.16 |
| Serial combination | 49.4 | 92.2 | 6.29 | 0.55 |
*Abbreviations: HMGB1 – high mobility group box chromosomal protein 1; SCCA – squamous cell carcinoma antigen; CYFRA21-1 – cytokeratin fragment 21-1; CEA – carcinoembryonic antigen.
Sensitivity and specificity values obtained in multiparametric tumor marker analysis are given in Table 4. When 2 markers were determined in identical blood samples, combined sensitivity values showed that the combination of HMGB1 and SCCA (with a sensitivity of 86.65% in parallel combination and a specificity of 80.48% in serial combination) was superior to the combinations of other markers. Parallel and serial combinations of 3 markers (HMGB1 in combination with SCCA and CYFRA21-1) lead to a superior sensitivity and specificity of 91.48% and 87.59%, respectively. Parallel combinations of 4 markers did not lead to a further increase in sensitivity, however, serial combination increased the specificity to 92.16%.
Table 4.
Sensitivity and specificity values of HMGB1, SCCA, CYFRA21-1, and CEA during multiparametric tumor marker analysis in recurrent CSCC*
| Tumor marker | Parallel combination† | Serial combination‡ | ||
|---|---|---|---|---|
| sensitivity (%) | specificity (%) | sensitivity (%) | specificity (%) | |
| HMGB1+SCCA | 86.7 | 68.5 | 64.5 | 80.5 |
| HMGB1+CYFRA21-1 | 74.5 | 63.3 | 59.2 | 71.6 |
| HMGB1+CEA | 69.1 | 71.5 | 58.7 | 80.2 |
| SCCA+CEA | 66.7 | 48.7 | 31.0 | 70.4 |
| SCCA+CYFRA21-1 | 61.2 | 41.6 | 40.3 | 62.3 |
| CEA+CYFRA21-1 | 53.6 | 50.9 | 36.6 | 59.5 |
| HMGB1+SCCA+CYFRA21-1 | 91.5 | 54.4 | 55.8 | 87.6 |
| HMGB1+SCCA+CEA | 88.7 | 60.6 | 54.3 | 82.4 |
| HMGB1+CYFRA21-1+CEA | 82.4 | 57.8 | 51.4 | 81.8 |
| SCCA +CYFRA21-1+CEA | 71.6 | 53.4 | 48.8 | 72.4 |
| HMGB1+SCCA +CYFRA21-1+CEA | 91.5 | 51.8 | 49.4 | 92.2 |
*Abbreviations: HMGB1 – high mobility group box chromosomal protein 1; SCCA – squamous cell carcinoma antigen; CYFRA21-1 – cytokeratin fragment 21-1; CEA – carcinoembryonic antigen.
†Parallel combined testing, a positive result was defined if the value of any of the tumor markers was higher than the cut-off value.
‡Serial combined testing, a positive result was defined only when the values of the tumor markers were all higher than the cut-off value.
HMGB1 expression is correlated with prognosis in cervical cancer patients
HMGB1 expression in cervical cancer was significantly correlated with the overall survival (P = 0.018) and disease-free survival (P = 0.009; Figures 4A and 4B), indicating that the high serum level of HMGB1 was correlated with a shorter survival. Multivariate survival analysis, which included age, FIGO stage, differentiation grades, disease relapse, and HMGB1 expression, was carried out to determine whether the serum level of HMGB1 was an independent prognostic factor of outcomes (Table 5). Serum HMGB1 level had a significant correlation with prognosis and was an independent prognostic factor of outcomes of recurrent cervical cancer.
Figure 4.
Survival curve (Kaplan-Meier method): Overall survival and disease-free survival for uterine cervical cancers according to serum high-mobility group box-1 (HMGB1) level. Broken line – high HMGB1 > 21.8ng/mL; dotted line – low HMGB1 ≤ 21.8ng/mL.
Table 5.
Summary of survival analyses*
| Risk factor or classification | Overall survival† |
Disease-free survival‡ |
||||
|---|---|---|---|---|---|---|
| P | hazard ratio | 95% CI | P | hazard ratio | 95% CI | |
| Age: ≤45 vs >45 y | 0.573 | 1.176 | 0.386-1.745 | 0.373 | 0.886 | 0.432-1.323 |
| FIGO stage: I-II vs III-IV | 0.031 | 2.041 | 1.074-5.605 | 0.018 | 2.542 | 1.350-5.882 |
| Differentiation grades: well/moderately vs poorly | 0.038 | 1.943 | 0.986-4.203 | 0.009 | 2.382 | 1.155-5.013 |
| Relapse: yes vs no | 0.004 | 3.252 | 1.744-6.945 | 0.001 | 3.869 | 1.863-8.013 |
| HMGB1 expression: <21.8 vs >21.8 ng/mL | 0.021 | 2.124 | 1.089-4.526 | 0.007 | 2.928 | 1.454-6.325 |
*Abbreviations: CI – confidence interval; FIGO – International Federation of Gynecology and Obstetrics (16); HMGB1 – high mobility group box chromosomal protein 1.
†For overall survival, the event considered was death from any cause. Hazard ratios above 1.0 indicate a worse outcome.
‡For disease-free survival, the event considered was death or recurrence. Hazard ratios above 1.0 indicate a worse outcome.
Discussion
We showed that HMGB1 protein was overexpressed in 71.2% of CSCC tissues and only in 41.7% of non-recurrent cervical cancer tissues. HMGB1 overexpression was significantly associated with FIGO stage, disease relapse, lymph node metastases, and distant metastasis. We also measured the levels of serum HMGB1 by ELISA and demonstrated that serum HMGB1 levels were significantly higher in patients with recurrent cervical cancer than in patients with non-recurrent disease or healthy women. Comparing single tumor markers, our results showed that HMGB1 had the best specificity and PLR, whereas SCCA had the best sensitivity and NLR. ROC curve analysis showed that HMGB1 had the strongest integrative ability to differentially diagnose cervical cancer relapse, stronger than SCCA, CYFRA21-1, or CEA. When 2 markers were determined in combination in identical blood samples, sensitivity values showed that the combination of HMGB1 and SCCA was superior to the combinations of other markers. HMGB1 combined with SCCA and CYFRA21-1 lead to a superior sensitivity and specificity. Parallel combination of 4 markers did not lead to a further increase in sensitivity, which suggested a low clinical usefulness for CEA in the diagnosis of CSCC.
Furthermore, we found that serum HMGB1 level was inversely correlated with overall survival and disease-free survival. The patients with higher expression of HMGB1 had a shorter survival. In multivariate analyses, high expression of HMGB1 was a significant predictor of poor prognosis for recurrent CSCC patients and was an independent prognostic factor of outcomes of recurrent cervical cancer. Thus, our study showed that HMGB1, as a potentially oncogenic protein, might play an important role in the recurrence of CSCC and poor outcome. These observations provide new insight into the understanding of HMGB1 protein in cancer. In the present study, we did not find a significant difference between differentiation grades in cervical cancer, as we did for FIGO stage, disease relapse, and lymph node metastases. This may be due to the small sample size of poor differentiation (n = 34). However, further studies are needed to validate this observation.
It is well known that tumor markers play an important role in the diagnosis of recurrence of cancers. Cervical carcinoma is the most frequent disease of the reproductive organs and its recurrence rates are still high. Many tumor markers have been used in the diagnosis and monitoring of the relapse of CSCC. Serum SCCA is a commonly used serum marker for CSCCs. Previous studies have reported that increasing serum SCCA can precede the clinical diagnosis of relapse in 46-92% of cases (19-21). However, it is still a matter of debate whether serum SCCA assay may represent a prognostic variable and be useful for earlier diagnosis of relapse (22,23). Most reports found CYFRA 21-1 to be a less sensitive serum tumor marker for squamous cell cervical cancer than SCCA (24-26). One-third of SCCA-negative patients are CYFRA 21-1 positive, and thus CYFRA 21-1 assay appears to be a useful supplementary test in a small subset of patients with squamous cell cervical cancer. CEA has also been correlated with clinical stage, parametrial invasion, tumor size, and lymph follicle metastasis in squamous cervical cancer (27,28).
On the other hand, measurement of these tumor markers has limited clinical application due to a lack of evidence on their specificity and accuracy. New tumor markers useful in the early diagnosis and in monitoring of the treatment and recurrence of the cervical cancer are still being sought. To the best of our knowledge, there has been no report about the relationship of HMGB1 and recurrent CSCC until now. In this study, we focused on the role of new tumor marker, HMGB1, in comparison with typical tumor markers in the diagnostics of cervical cancer relapse, including SCCA, CYFRA 21-1, and CEA.
Increased expression of HMGB1 has been reported to be strongly correlated with tumor genesis and tumor metastasis (10-15). However, until now, there has been very little information about HMGB1 activity and its role in patients with cervical cancer. Hao et al (29) reported that the mRNA and protein expression of HMGB1 was significantly higher in CSCC than in normal cervical tissue and that it correlated with stage, invasion, and metastasis. The levels of both RAGE mRNA and HMGB1 mRNA were significantly higher in metastatic CSCC than in non-metastatic cases. We found significantly higher immunostaining score in the recurrent CSCC tissue sections than in non-recurrent disease. HMGB1 levels correlated with FIGO stage, lymph node metastases, local relapse, and distant metastasis.
Tumor cells overexpressing HMGB1 might release it in the extracellular medium, and extracellular HMGB1 might mediate many important functions, including development of a chronic inflammatory response, promotion of tumor cell survival, expansion, and metastasis through activation of intracellular signaling via binding to its receptor RAGE (30-33). Signaling through RAGE leads to the activation of the nuclear factor-kB pathway, mitogen activated protein kinase, type-IV collagenase, and Rac/Cdc42, which are important for cancer cell growth, invasion, and metastasis (34-36). HMGB1 could act as an oncoprotein because of its anti-apoptotic properties (10). In addition, overexpression of HMGB1 has been shown to cause modulation of the transcriptional expressing of many groups of genes reported to play key roles in different biological processes of neoplasm progression and metastasis (15). Moreover, blockade of HMGB1-RAGE signaling has been shown to suppress tumor growth and metastasis (37).
In conclusion, this study demonstrated that the level of expression of HMGB1 was highly increased in recurrent CSCC. HMGB1 could be a useful and specific marker for evaluating the disease recurrence and predicting prognosis in patients with CSCC. Serial combined measurements of serum HMGB1, SCCA, and CYFRA21-1 increased the diagnostic specificity, and parallel combined testing increased the diagnostic sensitivity. The findings in this study may provide an insight into the development of new approaches for effective diagnosis and therapy of CSCC.
Reference
- 1.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/S0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 2.Goldie SJ, Kuhn L, Denny L, Pollack A, Wright TC. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. JAMA. 2001;285:3107–15. doi: 10.1001/jama.285.24.3107. [DOI] [PubMed] [Google Scholar]
- 3.Lai CH, Hong JH, Hsueh S, Ng KK, Chang TC, Tseng CJ, et al. Preoperative prognostic variables and the impact of postoperative adjuvant therapy on the outcomes of Stage IB or II cervical carcinoma patients with or without pelvic lymph node metastases: an analysis of 891 cases. Cancer. 1999;85:1537–46. doi: 10.1002/(SICI)1097-0142(19990401)85:7<1537::AID-CNCR15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–25. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 5.Bodurka-Bevers D, Morris M, Eifel PJ, Levenback C, Bevers MW, Lucas KR, et al. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecol Oncol. 2000;78:187–93. doi: 10.1006/gyno.2000.5860. [DOI] [PubMed] [Google Scholar]
- 6.Maiman M. The clinical application of serum squamous cell carcinoma antigen level monitoring in invasive cervical carcinoma. Gynecol Oncol. 2002;84:4–6. doi: 10.1006/gyno.2001.6532. [DOI] [PubMed] [Google Scholar]
- 7.Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66:10–20. doi: 10.1016/j.critrevonc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Ferrandina G, Macchia G, Legge F, Deodato F, Forni F, Digesu C, et al. Squamous cell carcinoma antigen in patients with locally advanced cervical carcinoma undergoing preoperative radiochemotherapy: association with pathological response to treatment and clinical outcome. Oncology. 2008;74:42–9. doi: 10.1159/000138979. [DOI] [PubMed] [Google Scholar]
- 9.Tabata T, Takeshima N, Tanaka N, Hirai Y, Hasumi K. Clinical value of tumor markers for early detection of recurrence in patients with cervical adenocarcinoma and adenosquamous carcinoma. Tumour Biol. 2000;21:375–80. doi: 10.1159/000030143. [DOI] [PubMed] [Google Scholar]
- 10.Brezniceanu ML, Volp K, Bosser S, Solbach C, Lichter P, Joos S, et al. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003;17:1295–7. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- 11.Volp K, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D, et al. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut. 2006;55:234–42. doi: 10.1136/gut.2004.062729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuniyasu H, Chihara Y, Kondo H, Ohmori H, Ukai R. Amphoterin induction in prostatic stromal cells by androgen deprivation is associated with metastatic prostate cancer. Oncol Rep. 2003;10:1863–8. [PubMed] [Google Scholar]
- 13.Takada M, Hirata K, Ajiki T, Suzuki Y, Kuroda Y. Expression of receptor for advanced glycation end products (RAGE) and MMP-9 in human pancreatic cancer cells. Hepatogastroenterology. 2004;51:928–30. [PubMed] [Google Scholar]
- 14.Cheng BQ, Jia CQ, Liu CT, Lu XF, Zhong N, Zhang ZL, et al. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis. 2008;40:446–52. doi: 10.1016/j.dld.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Sasahira T, Akama Y, Fujii K, Kuniyasu H. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch. 2005;446:411–5. doi: 10.1007/s00428-005-1210-x. [DOI] [PubMed] [Google Scholar]
- 16.Pecorelli S, Odicino F. Cervical cancer staging. Cancer J. 2003;9:390–4. doi: 10.1097/00130404-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 18.Walter SD, Sinuff T. Studies reporting ROC curves of diagnostic and prediction data can be incorporated into meta-analyses using corresponding odds ratios. J Clin Epidemiol. 2007;60:530–4. doi: 10.1016/j.jclinepi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Micke O, Prott FJ, Schafer U, Tangerding S, Potter R, Willich N. The impact of squamous cell carcinoma (SCC) antigen in the follow-up after radiotherapy in patients with cervical cancer. Anticancer Res. 2000;20:5113–5. [PubMed] [Google Scholar]
- 20.Esajas MD, Duk JM, de Bruijn HW, Aalders JG, Willemse PH, Sluiter W, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervical cancer. J Clin Oncol. 2001;19:3960–6. doi: 10.1200/JCO.2001.19.19.3960. [DOI] [PubMed] [Google Scholar]
- 21.Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol. 2002;84:7–11. doi: 10.1006/gyno.2001.6497. [DOI] [PubMed] [Google Scholar]
- 22.Gaarenstroom KN, Kenter GG, Bonfrer JM, Korse CM, Van de Vijver MJ, Fleuren GJ, et al. Can initial serum cyfra 21-1, SCC antigen, and TPA levels in squamous cell cervical cancer predict lymph node metastases or prognosis? Gynecol Oncol. 2000;77:164–70. doi: 10.1006/gyno.2000.5732. [DOI] [PubMed] [Google Scholar]
- 23.Chang TC, Law KS, Hong JH, Lai CH, Ng KK, Hsueh S, et al. Positron emission tomography for unexplained elevation of serum squamous cell carcinoma antigen levels during follow-up for patients with cervical malignancies: a phase II study. Cancer. 2004;101:164–71. doi: 10.1002/cncr.20349. [DOI] [PubMed] [Google Scholar]
- 24.Pras E, Willemse PH, Canrinus AA, de Bruijn HW, Sluiter WJ, ten Hoor KA, et al. Serum squamous cell carcinoma antigen and CYFRA 21-1 in cervical cancer treatment. Int J Radiat Oncol Biol Phys. 2002;52:23–32. doi: 10.1016/s0360-3016(01)01805-3. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Nakano T, Ohno T, Abe A, Morita S, Tsujii H. Serum CYFRA 21-1 in cervical cancer patients treated with radiation therapy. J Cancer Res Clin Oncol. 2000;126:332–6. doi: 10.1007/s004320050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inal E, Lacin M, Asal K, Ceylan A, Koybasioglu A, Ileri F, et al. The significance of ferritin, lipid-associated sialic acid, CEA, squamous cell carcinoma (SCC) antigen, and CYFRA 21-1 levels in SCC of the head and neck. Kulak Burun Bogaz Ihtis Derg. 2004;12:23–30. [PubMed] [Google Scholar]
- 27.Molina R, Filella X, Lejarcegui JA, Pahisa J, Torne A, Rovirosa A, et al. Prospective evaluation of squamous cell carcinoma and carcinoembryonic antigen as prognostic factors in patients with cervical cancer. Tumour Biol. 2003;24:156–64. doi: 10.1159/000073846. [DOI] [PubMed] [Google Scholar]
- 28.Molina R, Filella X, Auge JM, Bosch E, Torne A, Pahisa J, et al. CYFRA 21.1 in patients with cervical cancer: comparison with SCC and CEA. Anticancer Res. 2005;25:1765–71. [PubMed] [Google Scholar]
- 29.Hao Q, Du XQ, Fu X, Tian J. Expression and clinical significance of HMGB1 and RAGE in cervical squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2008;30:292–5. [in Chinese] [PubMed] [Google Scholar]
- 30.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, et al. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–70. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- 32.Maeda S, Hikiba Y, Shibata W, Ohmae T, Yanai A, Ogura K, et al. Essential roles of high-mobility group box 1 in the development of murine colitis and colitis-associated cancer. Biochem Biophys Res Commun. 2007;360:394–400. doi: 10.1016/j.bbrc.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 33.Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–48. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 34.Kuniyasu H, Chihara Y, Takahashi T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep. 2003;10:445–8. [PubMed] [Google Scholar]
- 35.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–7. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Evans A, Lennard TW, Davies BR. High-mobility group protein 1(Y): metastasis-associated or metastasis-inducing? J Surg Oncol. 2004;88:86–99. doi: 10.1002/jso.20136. [DOI] [PubMed] [Google Scholar]
- 37.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]