Abstract
Background
We report the first case of atovaquone/proguanil treatment failure in severe Plasmodium falciparum malaria acquired by a non-immune traveller to the Indian subcontinent. Recrudescent infection was complicated by neurological involvement 14 days after directly observed therapy with atovaquone/proguanil. Sequence analysis of the plasmodial cytochrome b gene confirmed a contribution of atovaquone resistance to treatment failure. The recrudescent isolate had a single mutation at position 268 (Tyr268Cys). Video recordings illustrate dramatic but ephemeral manifestations of malaria with neurological involvement.
Severe Plasmodium falciparum (Pf) malaria can occur in non-immune travellers of all ages. Adults have a higher risk than children for renal failure and acute respiratory distress syndrome, while women are at particular risk for neurological involvement.1 Because severe or complicated malaria is rare in developed countries, even experienced physicians can be unfamiliar with its presentation and management.2,3 Suggested Canadian diagnostic criteria, based upon those of the World Health Organization, include a wide spectrum of clinical, physiological and biochemical abnormalities. Any one of these findings should be considered ominous (Textbox 1).
Textbox 1.
Suggested Canadian criteria for the diagnosis of severe falciparum malaria
The fixed drug combination of atovaquone/proguanil (Malarone) is approved by Health Canada for the prevention of Pf malaria and for the treatment of uncomplicated cases. Although atovaquone/proguanil remains highly effective, treatment failure has been confirmed among travellers returning from sub-Saharan Africa and South America4,5 and, in 2007, from Thailand.6
We report the first genetically confirmed case of atovaquone-resistant falciparum malaria acquired on the Indian subcontinent. In late 2007 a Canadian traveller acquired P. falciparum infection in northern India or southern Nepal. She received directly observed therapy with a standard 3-day course of atovaquone/proguanil for what was thought to be uncomplicated Pf malaria; however, the infection subsequently recrudesced. At the height of her illness, the patient provided informed consent for video recording; this consent was reconfirmed in writing after her recovery. She agreed to the publication of images that include her face, under the Creative Commons License specified by Open Medicine’s editorial policy.
Case report
A 21-year-old Canadian woman flew from Vancouver to Delhi in October 2007. Her subsequent itinerary included a river trip on the Ganges at Varanasi, India, and a 4-day jungle safari in Chitwan National Park, Nepal. She took no antimalarial chemoprophylaxis.
After travelling for 3 weeks through northern India and lowland Nepal she developed headaches, fever, and psychiatric/neurological symptoms. During a subsequent detailed neuropsychiatric interview, she described having experienced ataxia, sensory and motor problems affecting the fingers of both hands, speech difficulties, olfactory hallucinations, and perception of vivid colours.
She presented on 3 November 2007 to the CIWEC Clinic in Kathmandu, Nepal, complaining of fever, tiredness, headache, dizziness, “feeling strange,” and “hallucinating at times” (Table 1). Her temperature was 39.5°C, blood pressure 132/70 mmHg, and heart rate 100 beats/min. Apart from pallor and icterus, the findings on physical examination were unremarkable. Laboratory values (local normal range in parentheses) included: hematocrit 38% (36%–45%), white blood cells (WBC) 2.75 giga/L (4.5–10.5), platelets 40 giga/L (150–400), total bilirubin 48 μmol/L (3.4– 41.0), and a positive falciparum antigen test. Peripheral blood microscopy showed 3.4% of the red blood cells infected with P. falciparum. A Dengue immunoglobulin (IgM) immunochromatographic assay (Dengue Duo Cassette Kit, Panbio Diagnostics, Australia) was weakly positive.
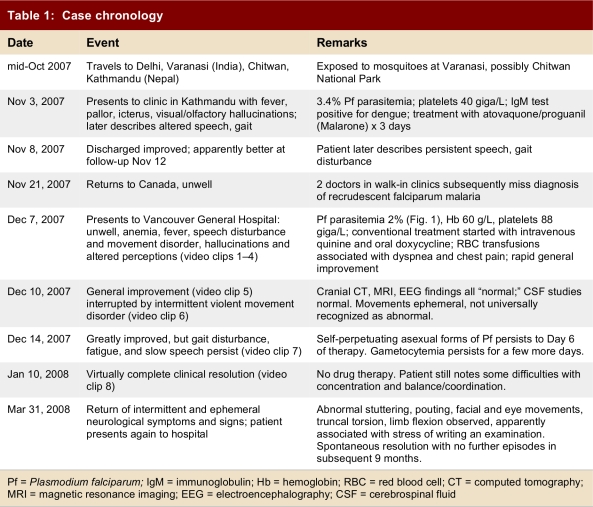
Table 1.
Case chronology
The patient was admitted to hospital and treated with atovaquone 250 mg / proguanil 100 mg (Malarone), 4 tablets daily for 3 days, given with food after promethazine to control vomiting. Intravenous fluids were administered, as her blood pressure dropped to 80/50 mmHg on the first day. Within 12 hours of the initiation of antimalarial chemotherapy, parasitemia had declined to 2%, the hematocrit was 34%, and the platelets had increased to 90 giga/L. By day 3 of treatment, and again on day 4, parasites were no longer seen in the blood smear. Oral ciprofloxacin was added from day 2 for probable bacterial diarrhea. The patient’s fever subsided and her blood pressure was 120/80 mmHg by day 5, although her heart rate was still 108. She was discharged, and appeared much improved at follow-up on 12 November. The final diagnosis was resolved Pf malaria and associated Dengue fever.
She returned to Canada on 21 November 2007 because she still felt unwell with myalgia, chills, and speech difficulties. She also reported intermittent movement disturbance, including unusual face and tongue movements, focal dystonia of her arm and neck, and gaze deviation. There was no prior psychiatric history. She reported that two physicians assessed her at walk-in clinics, before a third recommended repeat malaria smears.
On 7 December the patient presented to hospital in Vancouver with fever and rigors. On physical examination, she was obviously unwell (temperature 39.0°C, HR = 117). Her speech was slow, stuttering and repetitive, despite normal comprehension. She provided informed written consent to videography. Laboratory abnormalities included (local reference range in parentheses): hemoglobin 60 g/L (115–155), white blood cell count 2.8 giga/L (4–11), platelets 88 giga/L (150–400), lactate dehydrogenase 437 U/L (90–210). A smear showed 2% P. falciparum parasitemia with delicate ring forms and gametocytes (Fig. 1). Results of a cranial computed tomography (CT) scan were unremarkable. During 3 of 4 attempted transfusions of packed red blood cells, the patient experienced chest pain and dyspnea (video clips 1–3). No evidence for acute transfusion reaction was found.
Figure 1.
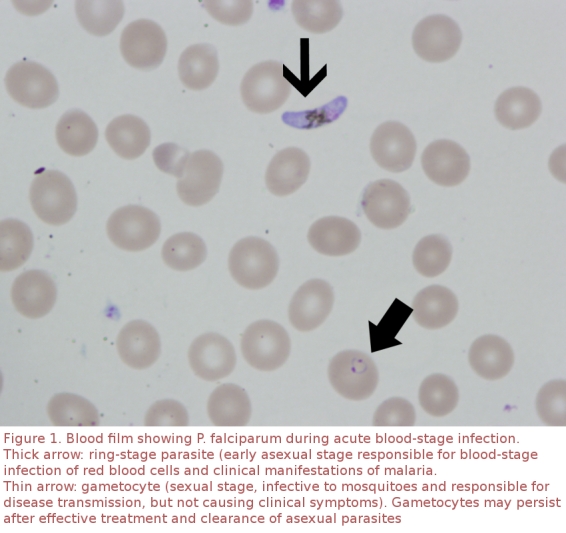
Blood film showing P. falciparum during acute blood stage infection
The patient was treated with conventional doses of intravenous quinine and oral doxycycline until she could take quinine reliably by mouth (Textbox 1). Clinical improvement was prompt (video clip 4). Electrocardiography and blood glucose monitoring showed no subsequent abnormality. However, on the third day in hospital, the patient noticed weakness of the left arm along with generalized motor slowing and unprovoked dystonic posturing lasting a few minutes (video clips 5, 6). Electroencephalography (EEG) performed 80 minutes later, magnetic resonance imaging, and cerebrospinal fluid analysis were all unremarkable.
Oral quinine and doxycycline were continued for 7 days, but the patient remained generally unwell (video clip 7) and parasitemic, with ring forms present up to day 6 of therapy. Gametocytes persisted for a few more days. She was discharged from hospital on day 11 and had recovered almost fully by day 35 (video clip 8). Findings of repeat serological testing for Dengue and related arboviruses were negative.
The patient returned to our emergency department 81 days later, after developing subtle and intermittent stuttering and involuntary facial twitching. This had progressed over 2 weeks after a presumed viral gastroenteritis to involuntary writhing movements of her entire body. Vital signs, including temperature, were normal. The admitting physicians hypothesized that anxiety related to a written examination exacerbated the abnormal movements that precipitated her repeat presentation. The neurology service observed pouting, eye closing, tongue and mouth contractions, episodes of limb flexion with truncal torsion, and bilateral writhing movements. (No video recording was obtained.) All laboratory studies, including repeat EEG, were normal. Physicians unfamiliar with this patient’s prior acute malarial illness requested psychiatric consultation and neuropsychiatric testing to evaluate the hypothesis of a “conversion disorder.” The neuropsychologist found “no obvious psychiatric overlay to her current presentation.” The abnormal movements subsided spontaneously, but isolated facial twitching recurred briefly after use of alcohol, 5 months later. One year after the definitive treatment in Vancouver, the patient was healthy and free of neurological sequelae.
This patient’s recrudescence of P. falciparum parasitemia at least 2 weeks after initial parasite clearance indicated late treatment failure, suggesting the possibility of acquired atovaquone resistance. DNA was extracted from the 7 December 2007 blood sample obtained in Vancouver using QIAGEN columns (QIAGEN, Chatsworth, CA) and the cytochrome b gene was amplified and sequenced to detect mutations as described.5,7,8 The patient’s isolate possessed a Tyr268Cys mutation previously associated with atovaquone/proguanil treatment failure and with in vitro atovaquone resistance, with a 9000-fold increase in the 50% minimal inhibitory concentration.4,5
Discussion
Clinical encounters with travellers offer important opportunities to detect emerging drug-resistant malaria.9 Here we report the first known case of atovaquone/proguanil-resistant P. falciparum malaria acquired by a short-term traveller to the Indian subcontinent. Atovaquone binds to the parasite’s mitochondrial cytochrome bc1 complex, inhibiting electron transport and collapsing mitochondrial membrane potential.10 Resistance has been linked to mutations in the plasmodial cytochrome b gene.11
This patient likely acquired Pf malaria during a river trip at Varanasi, during which she slept outdoors and was aware of exposure to mosquitoes. Malaria risk among travellers to Nepal, including Chitwan National Park, is very low. During a recent outbreak of febrile illness that affected 6000 residents of the Chitwan district, no malaria was detected.12,13
Malaria in travellers is almost completely preventable with precautions such as the use of bed nets, insect repellents and prophylactic medications when indicated. Health Canada14 and the United States Centers for Disease Control and Prevention15 recommend malaria prophylaxis for travel to most of India. Atovaquone/proguanil is a commonly prescribed first-line drug because of its efficacy and tolerability.16 However, increasing resistance may threaten the effectiveness of this safe and well-tolerated agent.
For our patient, initial treatment may have been suboptimal, insofar as atovaquone/proguanil is recommended only for the treatment of uncomplicated falciparum malaria.4 The neuropsychiatric symptoms noted at her initial presentation in Kathmandu may have been attributed to her febrile and dehydrated condition, in the context of suspected dual infections with Pf malaria and Dengue, since the Dengue rapid IgM test was positive. Dengue IgM tests are known to yield frequent false positive results, especially in the equivocal range.17 In retrospect, there may have been cerebral involvement by Pf at clinical onset, a criterion for more aggressive therapy (Textbox 2). The relatively high parasitemia of 3.4% at presentation may have contributed to the emergence of atovaquone resistance and recrudescent infection, given that higher parasite burdens are associated with higher risk of treatment failure.
Textbox 2.
Chemotherapy of severe or complicated Plasmodium falciparum malaria
Health care workers in non-endemic areas may not recognize the symptoms and seriousness of severe malaria (Textbox 1). Falciparum malaria has a 1% overall mortality, but up to 22% of severe cases end in death, even with appropriate treatment.18 It can also cause a spectrum of neurological abnormalities extending well beyond the established but narrow World Health Organization definition of “cerebral malaria” as “unrousable coma not attributable to any other cause.”19 Some symptoms or signs reflecting central nervous system involvement may be subtle, or missed by a physician not previously familiar with the patient.
The pathophysiology of cerebral malaria involves a poorly understood multi-system disorder resulting from a dynamic interplay between the host immune response and determinants of parasite virulence. Activation of cytokines, chemokines and complement cascades contributes to the metabolic derangement and tissue injury that characterize severe malaria. Pf-infected red blood cells sequester in the microvasculature of vital organs of all infected individuals, but only occasionally induce overt neurological symptoms or sequelae.20
Physicians with experience involving more than 9000 neurologically affected Kenyan children recently proposed the term “malaria with neurological involvement” to describe the large majority of patients who presented with neurological illness attributable to malaria but did not fulfill the official WHO definition of “cerebral malaria.” They emphasize that “the public health importance of such neurological involvement is enormous,” not only for acute treatment costs but also because long-term neurological and cognitive impairments have been described in 24% of children with a history of cerebral malaria or of malaria with multiple seizures. Children also suffer an increased risk for sequelar epilepsy.21
In our patient, diverse and ephemeral symptoms and signs reflected diffuse but temporally variable encephalopathy resulting from a life-threatening Pf infection. Her clinical instability confused experienced physicians, leading some to hypothesize a “functional” (psychiatric) cause for a clinical presentation rarely seen in Nepal or Canada. Her initial syndrome presumably reflected dynamic microvascular and biochemical changes in cerebral, cardiopulmonary and, perhaps, other circulations, in the absence of abnormalities detectable by conventional diagnostic imaging and tests. Attempted transfusion of packed red cells correlated with onset of chest pain and shortness of breath, which was likely another manifestation of her diffuse but rapidly changing intravascular pathophysiology. Transfusion may have been unwise, since even in more severe malaria there is no clear evidence that transfusion improves outcome.22
Reflection on the complex pathophysiology of Pf malaria suggests a novel answer to the classic neurological question: “Where is the lesion?” In severe malaria, the apparent “lesion” (video clips 1–7) may vary within minutes from “nowhere” to “everywhere” in the body, including the brain. Our patient’s recapitulation of neurological symptoms and signs more than 3 months after resolution of her severe malaria may have reflected a lingering brain injury that was better appreciated by the patient and her family than by clinical observers unfamiliar with her normal appearance and personality (video clip 8).
For physicians working in nontropical regions, improved understanding of the gravity and variety of clinical presentations of severe malaria could reduce delay in diagnosis and encourage attention to optimal treatment. It is obviously preferable to arrest Pf infection before “malaria with neurological involvement” proceeds to prostration, frank coma, or death and qualifies the patient for the traditional WHO definition of “cerebral malaria.” Accepting the expanded definition proposed by Kenyan investigators could help emphasize the crucial importance of early and aggressive treatment of this disease.21
Video Clips
Video Clip 1.
22 year old with Falciparum malaria - cerebral manifestations
Video Clip 2.
Symptoms during RBC transfusion, dysphagia
Video Clip 3.
16 hours after start of IV quinine
Video Clip 4.
More alert than she had seemed?
Video Clips 5 and 6.
Day 3 of therapy - speech and motor function remain impaired
Video Clip 7.
Day 5 of therapy –- still parasitemic and sweats, but improved
Video Clip 8.
Almost fully recovered 30 days later
Acknowledgments
We thank our patient for her altruism in sharing videographic images of her experience with others who can learn from it. Dr. Diane Roscoe of the Vancouver Hospital and Health Science Centre assisted with specimen handling, and Mr. Chris Stephenson of the University of British Columbia and Dr. Tarek Loubani provided invaluable technical assistance with the video images.
Biographies
Thomas L. Perry is a general internist/clinical pharmacologist at the Vancouver Hospital and Health Sciences Centre (VHHSC) and teaches clinical pharmacology at the University of British Columbia medical school, Vancouver, Canada.
Prativa Pandey is Medical Director of the CIWEC Clinic Travel Medicine Center in Kathmandu, Nepal.
Jennifer Grant is a medical microbiologist in the Division of Microbiology and Infection Control, VHHSC, Vancouver.
Kevin C. Kain is Director of the Centre for Travel and Tropical Medicine at Toronto General Hospital, Toronto, Canada.
Footnotes
Competing interests: None declared.
Funding source: Dr. Kain’s work on this report was supported by a CIHR Team Grant in Malaria, CIHR operating grant MT-13721, by Genome Canada through the Ontario Genomics Institute, and by the CIHR Canada Research Chair program.
References
- 1.Mühlberger N, Jelinek T, Behrens R H, Gjørup I, Coulaud J P, Clerinx J, Puente S, Burchard G, Gascon J, Grobusch M P, Weitzel T, Zoller T, Kollaritsch H, Beran J, Iversen J, Hatz C, Schmid M L, Björkman A, Fleischer K, Bisoffi Z, Guggemos W, Knobloch J, Matteelli A, Schulze M H, Laferl H, Kapaun A, McWhinney P, Lopez-Velez R, Fätkenheuer G, Kern P, Zieger B W, Kotlowski A, Fry G, Cuadros J, Myrvang B Surveillance importierter Infektionen in Deutschland Surveillance Networks. Age as a risk factor for severe manifestations and fatal outcome of falciparum malaria in European patients: observations from TropNetEurop and SIMPID Surveillance Data. Clin Infect Dis. 2003;36(8):990–995. doi: 10.1086/374224. http://www.journals.uchicago.edu/doi/abs/10.1086/374224. [DOI] [PubMed] [Google Scholar]
- 2.Kain Kevin C, Harrington Mary Anne, Tennyson Shan, Keystone Jay S. Imported malaria: prospective analysis of problems in diagnosis and management. Clin Infect Dis. 1998;27(1):142–149. doi: 10.1086/514616. http://www.journals.uchicago.edu/doi/abs/10.1086/514616. [DOI] [PubMed] [Google Scholar]
- 3.Kain K C, MacPherson D W, Kelton T, Keystone J S, Mendelson J, MacLean J D. Malaria deaths in visitors to Canada and in Canadian travellers: a case series. CMAJ. 2001;164(5):654–9. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=11258214. [PMC free article] [PubMed] [Google Scholar]
- 4.Boggild Andrea K, Parise Monica E, Lewis Linda S, Kain Kevin C. Atovaquone-proguanil: Report from the CDC expert meeting on malaria chemoprophylaxis (II) Am J Trop Med Hyg. 2007;76(2):208–23. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=17297027. [PubMed] [Google Scholar]
- 5.Rose Gregory W, Suh Kathryn N, Kain Kevin C, Le Saux Nicole, McCarthy Anne E. Atovaquone-proguanil resistance in imported falciparum malaria in a young child. Pediatr Infect Dis J. 2008;27(6):567–569. doi: 10.1097/INF.0b013e318167918d. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-200806000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Krudsood Srivicha, Patel Samir N, Tangpukdee Nopaddon, Thanachartwet Wipa, Leowattana Wattana, Pornpininworakij Karnchana, Boggild Andrea K, Looareesuwan Sornchai, Kain Kevin C. Efficacy of atovaquone-proguanil for treatment of acute multidrug-resistant Plasmodium falciparum malaria in Thailand. Am J Trop Med Hyg. 2007;76(4):655–8. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=17426165. [PubMed] [Google Scholar]
- 7.Schwartz Eli, Bujanover Shay, Kain Kevin C. Genetic confirmation of atovaquone-proguanil-resistant Plasmodium falciparum malaria acquired by a nonimmune traveler to East Africa. Clin Infect Dis. 2003;37(3):450–451. doi: 10.1086/375599. http://www.journals.uchicago.edu/doi/abs/10.1086/375599. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn Susan, Gill M John, Kain Kevin C. Emergence of atovaquone-proguanil resistance during treatment of Plasmodium falciparum malaria acquired by a non-immune north American traveller to west Africa. Am J Trop Med Hyg. 2005;72(4):407–9. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=15827276. [PubMed] [Google Scholar]
- 9.Labbé Annie-Claude, Patel Samir, Crandall Ian, Kain Kevin C. A molecular surveillance system for global patterns of drug resistance in imported malaria. Emerg Infect Dis. 2003;9(1):33–6. doi: 10.3201/eid0901.020121. http://www.cdc.gov/ncidod/EID/vol9no1/02-0121.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava I K, Rottenberg H, Vaidya A B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J Biol Chem. 1997;272(7):3961–3966. doi: 10.1074/jbc.272.7.3961. http://www.jbc.org/cgi/doi/10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- 11.Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother. 2000;44(8):2100–2108. doi: 10.1128/AAC.44.8.2100-2108.2000. http://aac.asm.org/cgi/doi/10.1128/AAC.44.8.2100-2108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cave William, Pandey Prativa, Osrin David, Shlim David R. Chemoprophylaxis use and the risk of malaria in travelers to Nepal. J Travel Med. 2003;10(2):100–5. doi: 10.2310/7060.2003.31761. [DOI] [PubMed] [Google Scholar]
- 13.Lewis Michael D, Serichantalergs Oralak, Pitarangsi Chittima, Chuanak Niphon, Mason Carl J, Regmi Laxmi R, Pandey Prativa, Laskar Ranjan, Shrestha Chandrika D, Malla Sarala. Typhoid fever: a massive, single-point source, multidrug-resistant outbreak in Nepal. Clin Infect Dis. 2005;40(4):554–61. doi: 10.1086/427503. [DOI] [PubMed] [Google Scholar]
- 14.Health Canada. Canadian recommendations for the prevention and treatment of malaria among international travellers. Canadian Communicable Disease Report. 2004;30S1 http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/04vol30/30s1/index.html. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Malaria. In: Arguin Paul M, Kozarsky Phyllis E, Reed Christie., editors. Health Information for International Travel 2008. Atlanta: US Department of Health and Human Services, Public Health Service; 2007. http://wwwn.cdc.gov/travel/yellowBookCh4-Malaria.aspx. [Google Scholar]
- 16.Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg. 1996;54(1):62–6. doi: 10.4269/ajtmh.1996.54.62. [DOI] [PubMed] [Google Scholar]
- 17.Gubler D J. Dengue and Dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11(3):480–96. doi: 10.1128/cmr.11.3.480. http://cmr.asm.org/cgi/pmidlookup?view=long&pmid=9665979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dondorp Arjen, Nosten François, Stepniewska Kasia, Day Nick, White Nick South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366(9487):717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 19.Severe malaria: A practical handbook. Geneva: World Health Organization; 2008. http://www.who.int/malaria/docs/hbsm.pdf. [Google Scholar]
- 20.Mackintosh Claire L, Beeson James G, Marsh Kevin. Clinical features and pathogenesis of severe malaria. Trends in Parasitology. 2004;20(12):597–603. doi: 10.1016/j.pt.2004.09.006. http://linkinghub.elsevier.com/retrieve/pii/S147149220400251X. [DOI] [PubMed] [Google Scholar]
- 21.Idro Richard, Ndiritu Moses, Ogutu Bernhards, Mithwani Sadik, Maitland Kathryn, Berkley James, Crawley Jane, Fegan Gregory, Bauni Evasius, Peshu Norbert, Marsh Kevin, Neville Brian, Newton Charles. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA. 2007;297(20):2232–2240. doi: 10.1001/jama.297.20.2232. http://jama.ama-assn.org/cgi/doi/10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meremikwu M, Smith HJ. Blood transfusion for treating malarial anaemia. Cochrane Database of Systematic Reviews. 1999;(Issue 4) doi: 10.1002/14651858.CD001475. Art. No.: CD001475 www.cochrane.org/reviews/en/ab001475.html. [DOI] [PMC free article] [PubMed] [Google Scholar]