Abstract
Background
Anticoagulation management services (AMSs) are widely used for anticoagulation management in many countries. Our AMS is a pharmacist-run ambulatory clinic with a physician advisory committee that manages patients referred with complicated anticoagulation histories. This paper assesses the adequacy of anticoagulation, rates of anticoagulant-related events and associated health care resource utilization for patients before and after referral to our AMS.
Methods
Consecutive patients referred to the AMS with 4 months of prior anticoagulation management who also had anticoagulation management for 4 months within the AMS were included in the evaluation. The primary endpoint was adequacy of anticoagulation (target international normalized ratio [INR] ± 0.5). Secondary outcomes included adverse events requiring an emergency department (ED) visit or hospital stay. These were classified by International Classification of Diseases (ICD) codes as thromboembolic, hemorrhagic, or non-anticoagulant related. Health care system resource consumption data were collected as number of hours spent in an ED and hospitalization costs.
Results
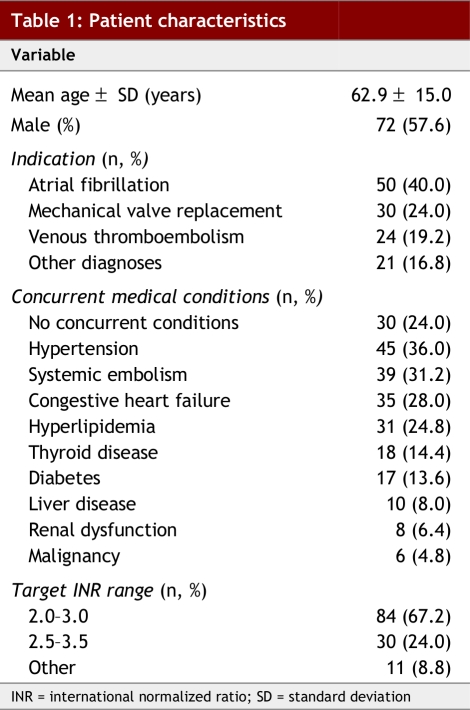
A total of 125 patients were included: 57.6% were male, with a mean age of 62.9 (standard deviation [SD]) ± 15.0 years. Indications for warfarin therapy were atrial fibrillation (40.0%), mechanical valve replacement (24.0%) and venous thromboembolism (19.2%). The adequacy of anticoagulant control was significantly greater during AMS care compared with the period before referral; patients were in the target INR range 66.5% versus 48.8% of the time, respectively (95% confidence interval [CI] 13.4%–22.0%; p < 0.0001). The relative risk of a thromboembolic event before referral to AMS care was 17.6 (95% CI 6.0–51.9; p < 0.0001), while the relative risk of a hemorrhagic event before AMS care was 1.6 (95% CI 0.7–3.7; p = 0.25). During AMS care, savings included 572 hours in the ED and Cdn$122,145.40 in hospitalization costs.
Conclusions
A pharmacist-directed, physician-supported AMS program achieved significantly better INR control and reduced rates of thromboembolic complications compared with standard care. Resource utilization was substantially reduced during AMS care.
Introduction
Warfarin therapy for the prevention and treatment of thromboembolic diseases is safe and effective only when it is maintained within a narrow therapeutic window, as measured by the international normalized ratio (INR).1 Failure to provide adequate anticoagulation consistently predicts thromboembolic events (e.g., stroke or pulmonary embolism), while patients who receive excessive anticoagulation are at risk of bleeding.2,3 The management of therapy within this narrow window is complicated by numerous factors, including drug interactions, comorbid acute and chronic diseases, diet and the variability of patients’ responses to warfarin therapy.
Given the complexities of its effective delivery, it is not surprising that warfarin therapy is underused4,5 and often suboptimally managed.6 Coordinated, systematic approaches to delivering this therapy, as offered by an anticoagulation management service (AMS), have improved both access to and management of warfarin therapy.7-12
AMSs are common in the United States and Europe, but have only recently begun to gain acceptance in Canada. Our AMS at the University of Alberta Hospital, Edmonton, was initiated in 2001 as a pilot project and is unique in the Canadian setting, as all direct patient care is provided by pharmacists who have an extended scope of practice and who work in consultation with specialist physicians.13 In fostering an interdisciplinary care model, it was important to conduct a thorough evaluation of the impact of the AMS on anticoagulant control, clinical events and resource utilization. This paper assesses the adequacy of anticoagulation, rates of anticoagulant-related events and associated health care resource utilization for patients before and after referral to our AMS.
Methods
All patients who attended the University of Alberta Hospital AMS between April 2001 and December 2003 who were prescribed warfarin before being referred were considered for our study. To be eligible, patients had to be prescribed warfarin for at least 4 months before referral and to have continued warfarin therapy for a further 4 months or longer at the AMS. We used this criterion for patient selection rather than random assignment to avoid excluding medically complicated cases. The Human Research Ethics Board at the University of Alberta Hospital approved our research protocol.
The detailed design of the AMS program has been published previously.13 Funded by Alberta Health and Wellness through the Health Innovation Fund, the program allows any physician practising within the Capital Health Region to refer appropriate patients for management by the AMS. Capital Health is the largest health region in Canada, encompassing approximately 1.7 million people. Patients are accepted to the AMS provided they have a legitimate indication for warfarin therapy in accordance with the American College of Chest Physician guidelines,1 are able to attend at least one clinic appointment, can be reached for follow-up, and have the capacity (independently or through the agency of a caregiver) to understand the condition and implications of anticoagulant therapy.
Once referred to the AMS, patients receive a standardized one-on-one educational session and an information package. During this session, the patient is informed of the role of the AMS in his or her care, the need for compliance with therapy and blood tests, and the importance of contacting the AMS with any changes to his or her general health or medications that may affect the anticoagulant therapy. Further, the risks and benefits of warfarin therapy, as well as factors that may affect it (e.g., drugs, diet, alcohol), are explained. An awareness of signs and symptoms indicative of hemorrhagic and thromboembolic complications are also addressed. Anticoagulation management for the patient is assumed by the AMS after this initial visit. With each INR drawn, patients are contacted by telephone by a pharmacist, an assessment is performed, warfarin dosing instructions are given, and patients are scheduled for their next INR test date.
We assessed adequacy of anticoagulation by measuring the proportion of time patients spent within their indicated INR range (target INR ± 0.5 units) and expanded INR range (target INR ± 0.7), using the method developed by Rosendaal and colleagues.14 In brief, this method assumes linear pharmacokinetics between INR assessments and calculates the percentage of time spent within the desired INR range. We omitted from our analysis the first 30 days of INR management at the commencement of warfarin therapy before referral and the first 30 days of INR management at the AMS, to allow for initial periods of stabilization. INR results for patients before referral were derived from the Regional Laboratory Services database. We retrieved data on events from November 2000 (6 months before the AMS accepted the first patient for management) to December 2003, while data contained within the AMS (namely INRs) continued past this interval to July 2004, allowing for a more prolonged time frame for analysis of the adequacy of anticoagulation. Only patients who had INR tests within the Capital Health Region were captured for this analysis.
We collected hospital utilization data by coding all instances of emergency department (ED) presentations and hospital admissions according to the International Classification of Diseases (ICD-9 and ICD-10). For ED visits the primary diagnosis was used, whereas for hospital stays the most responsible diagnosis was used. These ICD codes were then further classified as hemorrhagic, thromboembolic and non-anticoagulant. At the time of event classification, the management strategy (before or during AMS care) was not known. Personal health numbers were used to track health care system utilization recorded in the Capital Health Region database. Data collected included admission and discharge dates, diagnoses, length of stay in the ED (hours) and inpatient hospital stay (days), and the resource intensity weight (RIW).15 The RIW is an indicator of typical resources consumed during a hospital stay for a given admission diagnosis. The Capital Health Region has allocated a cost of Cdn$3500 for one RIW.15 Using this benchmark, the costs of hospital care before and during AMS care were computed. All costing information reported throughout this paper is based on the RIW.
A paired t test was used to compare the adequacy of anticoagulation before and during AMS care. Event comparisons before and during AMS care were done using log-linear (Poisson) models for correlated data (generalized estimating equation [GEE] with exchangeable correlation matrix). This model takes into account the number of person-months of warfarin use. We used SAS version 9.1 (SAS Institute Inc. Cary, North Carolina, US) statistical software.
Results
Between April 2001 and December 2003, 388 patients were referred to the AMS. Of these, 265 were excluded from our analysis as they had fewer than 4 months of continuous warfarin therapy before AMS care. The remaining 125 patients had a mean age of 62.9 ± 15.0 years (± SD); 57.6% were male (Table 1). The primary indications for warfarin were atrial fibrillation (40.0%), mechanical valve replacement (24.0%) and venous thromboembolism (19.2%).
Table 1.
Patient characteristics
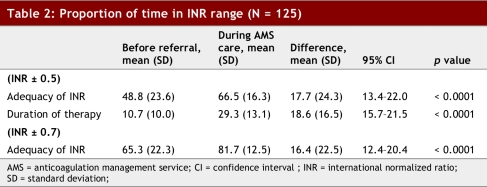
The mean proportion of time that patients were in their indicated INR range before referral was 48.8% ± 23.6%, whereas during AMS care the time in the target INR range was 66.5% ± 16.3% (95% CI 13.4%–22.0%; p < 0.0001) (Table 2). This assessment included a longer interval during AMS care as compared with the period before referral, as more data were available for the AMS care period. Using the expanded range, the adequacy of anticoagulation increased to 65.3% ± 22.3% prior to referral and 81.7% ± 12.5% during AMS care (95% CI 15.7%–21.5%; p < 0.0001).
Table 2.
Proportion of time in INR range (N = 125)
To address the question of whether the study group of 125 patients was representative of the larger population of patients requiring anticoagulant therapy, we compared this cohort of patients with those ineligible for the analysis (n = 502), finding similar demographic characteristics and almost identical distributions of time in the therapeutic range (data not shown).
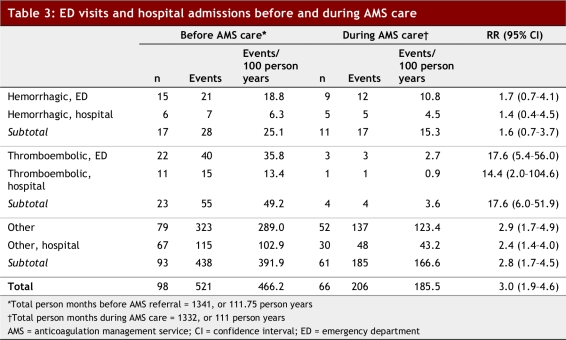
In the mean follow-up period of 10.7 months prior to AMS care, significantly more patients had ED presentations and hospital stays for thromboembolic events than during AMS care: 49.2 events/100 patient years versus 3.6 events/100 patient years (relative risk 17.6; 95% CI 6.0–51.9; p < 0.0001) (Table 3). Although fewer hemorrhagic ED presentations and hospital stays occurred during AMS care, this was not statistically significant.
Table 3.
ED visits and hospital admissions before and during AMS care
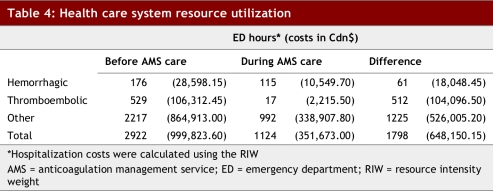
During AMS care, patients had fewer thromboembolic, hemorrhagic and non-anticoagulant ED presentations than during their prior care and spent fewer total hours in the ED (Table 4). Similarly, the cost of hospital stays for both thromboembolic and bleeding complications during AMS care were substantially lower ($12,765.20 v. $134,910.60).
Table 4.
Health care system resource utilization
Discussion
We demonstrated substantive improvement in anticoagulant care for patients during AMS care compared with their prior management. Patients were within their desired INR range significantly more often during AMS care and experienced fewer thromboembolic events. There was also a trend toward reduced hemorrhagic events.
Overall, AMS care substantially reduced the use of health care system resources. Patients included in this study consumed an additional 572 ED hours and $122,144.40 in hospital costs for thromboembolic and hemorrhagic events before management by the AMS. Given the average follow-up of 10.7 months before AMS care, the monthly savings conferred by AMS care for these anticoagulant-related events were 53 ED hours and $11,415.46 in hospital costs. Interestingly, there were also striking reductions in events not believed to be linked to anticoagulation therapy during AMS care, with 1225 fewer ED hours and $526,005.20 less spent in hospital costs. Although the AMS cannot claim causal inference for these non-anticoagulant related events, it is recognized that the clinic is in constant contact with these patients, and thus able to identify changes in general health and to suggest measures to mitigate progression.
The adequacy of anticoagulant control within our AMS population is comparable to other AMSs,8-12 where time in the therapeutic range varies from 59% to 89%, depending on the population evaluated. Our rates may be on the lower end of this range because we receive referrals largely from subspecialists for patients with poor anticoagulant-related outcomes or those whose anticoagulation therapy has been inadequate in the past. For anticoagulation and non-anticoagulation-related events combined, there were 4.7 events per patient year before referral, whereas during AMS care only 1.9 events per patient year occurred. Despite the impressive reduction observed under AMS care, these data highlight that each patient under AMS care still uses our acute health care system twice per year.
Rates of thromboembolic events were lower during AMS care and substantially higher before referral to our program, compared with prior studies of AMSs (rate of 4.8 events/100 patient years managed) and routine anticoagulation management (rate of 8.1 events/100 patient years managed).16
In comparison, the rate of major hemorrhagic events was not significantly different before and during AMS care. Interestingly, our baseline rate of these events was higher than that reported in the literature for other AMSs (4.6 events/100 patients year managed), while our rates fell within that reported for routine anticoagulation management (7.4–18.0 events/100 patient years managed).
Study limitations
There are a few limitations to this study. First, we used a before/after design for evaluation, as randomization was not feasible given the complicated nature of the cases referred to our specialty service, which was initiated as a pilot project. Second, our data are limited by the relatively small sample size: we applied strict inclusion criteria to comprehensively evaluate INR control and associated outcomes. However, as our sample demographic characteristics and INR control are similar to the broader clinic population, it is reasonable to assume that our data are reflective of our entire clinic population.
Conclusions
The findings of this study demonstrate that a pharmacist-managed anticoagulation clinic in a multidisciplinary setting offers not only safe and effective treatment, but is superior with respect to increased anticoagulantion control and decreased incidence of thromboembolic events, and shows a trend toward lower rates of hemorrhagic events. This is important in the Canadian health care context, with respect to the growing service needs of patients and physician shortages. The demonstrated improvement in care quality and cost savings justifies the development of further AMSs using this model.
Biographies
Tammy J. Bungard is an associate professor with the Division of Cardiology, Department of Medicine, University of Alberta, Edmonton, Alta.
Leslie Gardner is a consultant in Edmonton, Alta.
Stephen L. Archer is Harold Hines Jr. Professor and Director with the Section of Cardiology, Department of Medicine, University of Chicago, Chicago, Ill.
Peter Hamilton is a professor with the Division of General Internal Medicine, Department of Medicine, University of Alberta, Edmonton, Alta.
Bruce Ritchie is an associate professor with the Division of Hematology, Department of Medicine, University of Alberta, Edmonton, Alta.
Wayne Tymchak is a professor with the Division of Cardiology, Department of Medicine, University of Alberta, Edmonton, Alta.
Ross T. Tsuyuki is director of EPICORE Centre/COMPRIS and a professor of medicine, Division of Cardiology, Department of Medicine, University of Alberta, Edmonton, Alta.
Footnotes
Funding source: Funding for the AMS Program was provided by the Health Innovation Fund, Alberta Health and Wellness.
Competing interests: None declared.
Contributors: Substantial contributions to conception and design (TJB, LG, SLA, PH, BR, WT, RT), acquisition of data (TJB, LG, BR), or analysis and interpretation of data (TJB, LG, SLA, BR); drafting the article (TJB) and revising it critically for important intellectual content (LG, SLA, PH, BR, WT, RT); final approval of the version to be published: all authors.
References
- 1.Ansell Jack, Hirsh Jack, Poller Leon, Bussey Henry, Jacobson Alan, Hylek Elaine. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):S204–S233. doi: 10.1378/chest.126.3_suppl.204S. http://www.chestjournal.org/cgi/pmidlookup?view=long&pmid=15383473Erratum in: Chest. 2005 127(1):415–6. dosage error in text. [DOI] [PubMed] [Google Scholar]
- 2.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hylek E M, Skates S J, Sheehan M A, Singer D E. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335(8):540–546. doi: 10.1056/NEJM199608223350802. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=8678931&promo=ONFLNS19. [DOI] [PubMed] [Google Scholar]
- 4.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. http://archinte.ama-assn.org/cgi/doi/10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 5.Tsuyuki R T, Walters B, Yim R, Teo K K. Re: The Clinical Quality Improvement Network (CQIN) Investigators. Thromboembolic prophylaxis in 3,575 hospitalized patients with atrial fibrillation. 1998;14:695-702. Can J Cardiol. 2000;16(1):99. [PubMed] [Google Scholar]
- 6.Bungard Tammy J, Ackman Margaret L, Ho Geotham, Tsuyuki Ross T. Adequacy of anticoagulation in patients with atrial fibrillation coming to a hospital. Pharmacotherapy. 2000;20(9):1060–1065. doi: 10.1592/phco.20.13.1060.35038. http://www.atypon-link.com/PPI/doi/abs/10.1592/phco.20.13.1060.35038. [DOI] [PubMed] [Google Scholar]
- 7.Burkiewicz Jill S. Effect of access to anticoagulation management services on warfarin use in patients with atrial fibrillation. Pharmacotherapy. 2005;25(8):1062–1067. doi: 10.1592/phco.2005.25.8.1062. http://www.atypon-link.com/PPI/doi/abs/10.1592/phco.2005.25.8.1062. [DOI] [PubMed] [Google Scholar]
- 8.Charney R, Leddomado E, Rose D N, Fuster V. Anticoagulation clinics and the monitoring of anticoagulant therapy. Int J Cardiol. 1988;18(2):197–206. doi: 10.1016/0167-5273(88)90165-9. [DOI] [PubMed] [Google Scholar]
- 9.Conte R R, Kehoe W A, Nielson N, Lodhia H. Nine-year experience with a pharmacist-managed anticoagulation clinic. Am J Hosp Pharm. 1986;43(10):2460–2464. [PubMed] [Google Scholar]
- 10.Davis F B, Estruch M T, Samson-Corvera E B, Voigt G C, Tobin J D. Management of anticoagulation in outpatients: experience with an anticoagulation service in a municipal hospital setting. Arch Intern Med. 1977;137(2):197–202. [PubMed] [Google Scholar]
- 11.Errichetti A M, Holden A, Ansell J. Management of oral anticoagulant therapy. Experience with an anticoagulation clinic. Arch Intern Med. 1984;144(10):1966–1968. doi: 10.1001/archinte.144.10.1966. http://archinte.ama-assn.org/cgi/doi/10.1001/archinte.144.10.1966. [DOI] [PubMed] [Google Scholar]
- 12.Seabrook G R, Karp D, Schmitt D D, Bandyk D F, Towne J B. An outpatient anticoagulation protocol managed by a vascular nurse-clinician. Am J Surg. 1990;160(5):501–505. doi: 10.1016/S0002-9610(05)81015-3. http://linkinghub.elsevier.com/retrieve/pii/S0002961005810153. [DOI] [PubMed] [Google Scholar]
- 13.Bungard TJ, Archer SL, Hamilton P, Ritchie B, Tymchak W, Tsuyuki RT. Bringing the benefits of anticoagulation management services to the community: Alberta program may serve as a model of care. Can Pharm J. 2006;139(2):58–63. http://www.pharmacists.ca/content/cpjpdfs/mar_apr06/ClinicalReview-Bringingbenefits-Long.pdf. [Google Scholar]
- 14.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. [PubMed] [Google Scholar]
- 15.Hicks V, Fortin G, Ballinger G. Price indexes used in national health expenditures: feasibility study. Ottawa: Canadian Institute for Health Information; 2007. http://www.cihi.ca/cihiweb/en/downloads/spend_nhexenhance_e_PriceIndexes.pdf. [Google Scholar]
- 16.Ansell J E, Hughes R. Evolving models of warfarin management: anticoagulation clinics, patient self-monitoring, and patient self-management. Am Heart J. 1996;132(5):1095–1100. doi: 10.1016/S0002-8703(96)90040-X. http://linkinghub.elsevier.com/retrieve/pii/S000287039690040X. [DOI] [PubMed] [Google Scholar]