Abstract
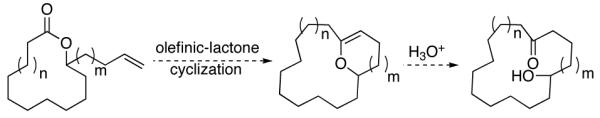
Olefinic-lactone cyclization reactions that result in the generation of macrocycles are described.
Macrocyclic motifs have drawn the attention of synthetic, biological, and medicinal chemists due to their presence in a number of biologically active small molecules that include both natural and non-natural products.1,2 For those scientists that target their synthesis, when the macrocycle of interest contains a lactone or lactam, a lactone or lactam-forming reaction has been a generally reliable method.3,4 However, when the target lacks these functionalities, synthetic chemists have been forced to turn to other, oftentimes less dependable methods to generate the macrocycle.5 From an awareness of the difficulties associated with the synthesis of non-lactone macrocycles, we became interested in using lactone formation to generate non-lactone macrocycles. As outlined in Scheme 1, central to the success of this idea would be a novel olefinic-lactone ring-closing metathesis reaction to give macrocyclic enol ethers (Scheme 1). To the best of our knowledge, similar cyclizations had not been demonstrated prior to this work.
Scheme 1.

The Use of Lactones to Generate non-Lactone Macrocycles.
To test the viability of the proposed strategy, we examined the olefinic-lactone cyclization of readily available 13-membered lactone 5 (eq 1). When 5 was subjected to our previously disclosed reduced Ti ethylidene conditions for olefinic-ester cyclizations we were pleased to isolate dihydropyran 6 in quantitative yield.6
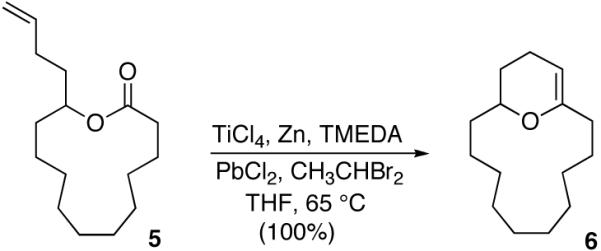 |
(1) |
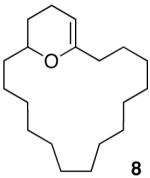
Having demonstrated the cyclization of 5, we decided to examine the scope of the reaction. The reaction to generate a dihydropyran was successful with several other substrates including 16- and 17-membered lactones 7 and 9, respectively (Table 1, entries 2 and 3).7 We were also pleased to find that the cyclization was not limited to the generation of 6-membered enol ethers as 7-membered ring macrocycle 12 was also prepared from 17-membered lactone 11 (entry 4).
Table 1.
Olefinic lactone cyclizations
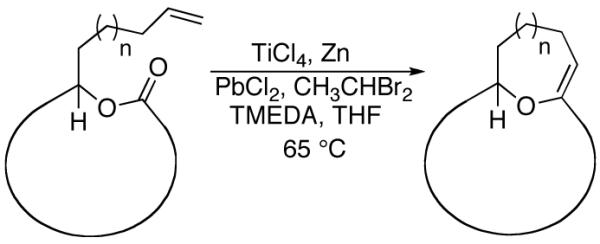 | |||
|---|---|---|---|
| entry | lactone | cyclic enol ether | yield |
| 1 | 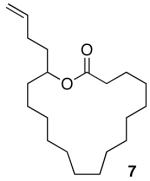 |
 |
87% |
| 2 | 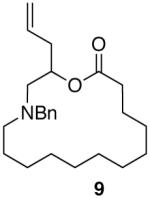 |
 |
69% |
| 3 | 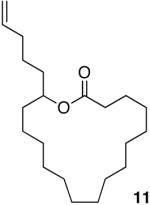 |
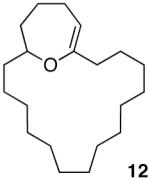 |
78% |
We next decided to demonstrate the utility of the products from the cyclization reactions. To this goal, we converted cyclic enol ether 8 into macrocyclic ketone 14, macrocyclic ketal 13, and macrocyclic pyran 15 as illustrated in Scheme 2. Exposure of 8 to m-CPBA in MeOH gave 13 in 68% yield.8 When 8 was sequentially hydrolyzed with SiO2 and treated with benzoic anhydride we isolated ketoester 14 in 63% overall yield from lactone 7. Finally, the reduction of 8 using Et3SiH and TFA gave pyran 15 as a single diastereomer in 87% yield.
Scheme 2.
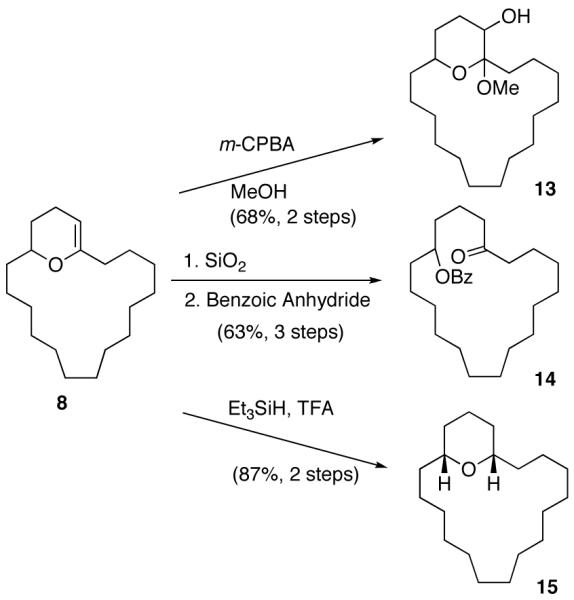
The Ring-Expansion, Reduction, and Oxidation of 8
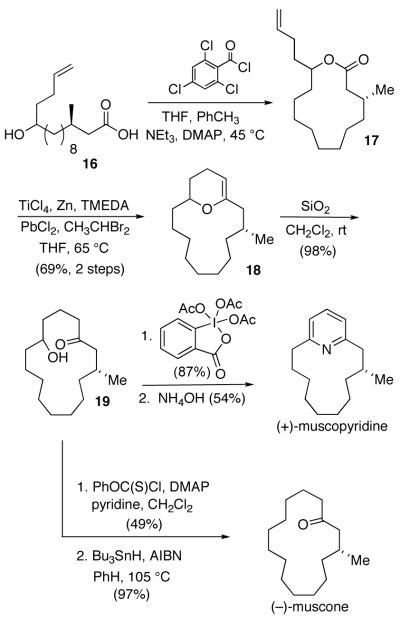
As a further illustration of its utility, we applied the lactone cyclization, ring expansion sequence to the synthesis of the natural products (R)-(-)-muscone and (R)-(+)-muscopyridine (Scheme 3).9,10,11,12 Our synthesis of both substrates began with seco-acid 16.13 Yamaguchi macrolactonization of 16 gave 13-membered cyclization precursor 17. Olefinic-lactone cyclization resulted in the generation of macrocyclic dihydropyran 18. Silica gel hydrolysis of the enol ether gave hydroxyketone 19, which served as a precursor to both muscone and muscopyridine. (R)-(+)-Muscopyridine resulted from the oxidation of the 2° alcohol in 19 followed by pyridine formation.14 (R)-(-)-Muscone came from the deoxygenation of 19 using Barton-McCombie conditions.15
Scheme 3.
Olefinic-Lactone Cyclizations to (-)-Muscone and (+)-Muscopyridine
In summary, we have described a unique and efficient approach to macrocycles that utilizes an olefinic-lactone cyclization reaction in the key step. We continue in our study of the scope and utility of this reaction sequence.
Supplementary Material
Acknowledgment
We are grateful to the National Institutes of Health, General Medical Sciences (GM56677) for support of this work. We would like to thank the support staff at the University of Utah and especially Dr. Charles Mayne (NMR) and Dr. Jim Muller (mass spectrometry) for help in obtaining data.
References
- 1.For examples of natural products that contain macrocycles see references 9,11 andBlunt JW, Copp BR, Hu W-P, Munro MHG, Northcote PT, Prinsep MR. Nat. Prod. Rep. 2008;25:35. doi: 10.1039/b701534h.Ishibashi M. In: Macrolide Antibiotics. Omura S, editor. Academic Press; 2002. pp. 57–98.
- 2.For recent examples of bioactive non-natural products that contain macrocycles seeShan D, Chen S, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, Huang X-Y. Proc. Nat. Acad. Sci. 2005;102:3772. doi: 10.1073/pnas.0500658102.Metaferia BB, Chen L, Baker HL, Huang X-Y, Bewley CA. J. Am. Chem. Soc. 2007;129:2434. doi: 10.1021/ja068538d.Anquetin G, Horgan G, Rawe S, Murray D, Madden A, MacMathuna P, Doran P, Murphy PV. Eur. J. Org. Chem. 2008:1953.
- 3.For a review on macrolactonization seeParenty A, Moreau X, Capagne M-M. Chem. Rev. 2006;106:911. doi: 10.1021/cr0301402.
- 4.For recent examples of macrolactamization seeTan L, Ma D. Angew. Chem. Int. Ed. 2008;47:3614. doi: 10.1002/anie.200800397.Qin H-L, Panek JS. Org. Lett. 2008;10:2477. doi: 10.1021/ol800749w.Nicolaou KC, Dethe DH, Leung GYC, Zou B, Chen DY-K. Chem. Asian J. 2008;3:413. doi: 10.1002/asia.200700361.Komano K, Shimamura S, Inoue M, Hirama M. J. Am. Chem. Soc. 2007;129:14184. doi: 10.1021/ja076671f.
- 5.(a) Prunet J. Angew. Chem. Int. Ed. 2003;42:2826. doi: 10.1002/anie.200301628. [DOI] [PubMed] [Google Scholar]; (b) Gradillas A, Perez-Castells J. Angew. Chem. Int. Ed. 2006;45:6086. doi: 10.1002/anie.200600641. [DOI] [PubMed] [Google Scholar]
- 6.Iyer K, Rainier JD. J. Am. Chem. Soc. 2007;129:12604. doi: 10.1021/ja073880r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In addition to the 3° amine in 9, acetals, ethers, and internal olefins are also amenable to the cyclization conditions. See reference 6 andJohnson HWB, Majumder U, Rainier JD. J. Am. Chem. Soc. 2005;127:848. doi: 10.1021/ja043396d.
- 8.Rainier JD, Allwein SP, Cox JM. J. Org. Chem. 2001;66:1380. doi: 10.1021/jo001514j. [DOI] [PubMed] [Google Scholar]
- 9.Muscopyridine isolation:Schinz H, Ruzicka L, Geyer U, Prelog V. Helv. Chim. Acta. 1946;29:1524.
- 10.For synthetic work to muscopyridine see:Suwa K, Morie Y, Suzuki Y, Ikeda K, Sato M. Tetrahedron Lett. 2008;49:1510.Furstner A, Leitner A. Angew. Chem. Int. Ed. 2003;42:308. doi: 10.1002/anie.200390103.Hagiwara H, Katsumi T, Kamat VP, Hoshi T, Suzuki T, Ando M. J. Org. Chem. 2000;65:7231. doi: 10.1021/jo000785r.Hadj-Abo F, Hesse M. Helv. Chim. Acta. 1992;75:1834.Sakane S, Matsumura Y, Yamamura Y, Ishida Y, Maruoka K, Yamamoto H. J. Am. Chem. Soc. 1983;105:672.Utimoto K, Kato S, Tanaka M, Hoshino Y, Fujikura S, Nozaki H. Heterocycles. 1982;18:149.Saimoto H, Hiyama T, Nozaki H. Tetrahedron Lett. 1980;21:3897.Biemann K, Buchi G, Walker BH. J. Am. Chem. Soc. 1957;79:5558.
- 11.Muscone isolation:Walbaum HJ. J. Prakt. Chem. 1906;73:488.Ruzicka L. Helv. Chem. Acta. 1926;9:715.
- 12.For synthetic work to muscone seeEden I, Cao W, Price M, Colton M. Tetrahedron. 2008;64:5497. doi: 10.1016/j.tet.2008.03.109.Knopff O, Kuhne J, Fehr C. Angew. Chem. Int. Ed. 2007;46:1307. doi: 10.1002/anie.200604518.Bulic B, Lucking U, Pfaltz A. Synlett. 2006:1031.and references contained therein.
- 13.Available in 8 steps from 10-(tert-butyldimethylsilyloxy)decanal. See supporting information andNagano H, Tada A, Isobe Y, Yajima T. Synlett. 2000:1193.Li G, Patel D, Hruby V. Tetrahedron: Asymmetry. 1993;4:2315.Yokokawa F, Asano T, Okino T, Gerwick WH, Shioiri T. Tetrahedron. 2004;60:6859.
- 14.Hagiwara H, Katsumi T, Kamat VP, Hoshi T, Suzuki T, Ando M. J. Org. Chem. 2000;65:7231. doi: 10.1021/jo000785r. [DOI] [PubMed] [Google Scholar]
- 15.Barton DHR, McCombie SW. J. Chem. Soc., Perkin Trans. 1. 1975:1574. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.