Abstract
We tested the hypothesis that the Na+-component of dietary NaCl can have a pressor effect apart from its capacity to complement the extracellular osmotic activity of Cl− and thus expand plasma volume (PV). We studied 35 mostly normotensive blacks who ingested a low NaCl-diet, 30mmol/d, for three weeks, in the first and third of which Na+ was loaded orally with either NaHCO3 or NaCl, in random order, 250mmol/d. In subjects adjudged to be salt-sensitive (SS, n=18; ΔMAP ≥5mmHg with NaCl-load) but not in salt-resistant subjects (SR, n=17) loading with NaHCO3 also was pressor. The pressor effect of NaHCO3 was half that of NaCl: MAP (mmHg) increased significantly from 90 on low NaCl to 95 with NaHCO3 and to 101 with NaCl. The pressor effect of NaCl strongly predicted that of NaHCO3. As judged by hematocrit decrease, PV expansion with NaCl was the same in SR and SS and twice that with NaHCO3, irrespective of pressor effect. In SS, MAP varied directly with plasma Na+ concentration attained with all Na+-loading. In SS but not SR, NaHCO3 and NaCl induced decreases in renal blood flow (RBF) and increases in renal vascular resistance (RVR); changes in RBF were not different with the two salts. Responses of RBF and RVR to NaHCO3 were strongly predicted by those to NaCl. In establishing the fact of “sodium-selective” salt-sensitivity, the current observations demonstrate that the Na+-component of NaCl can have pressor and renal vasoconstrictive properties, apart from its capacity to complement Cl− in PV expansion.
Keywords: blood pressure physiopathology, sodium, chlorides, bicarbonates, renal circulation, African Americans
Introduction
Blood pressure (BP) is said to be “salt-sensitive” when it varies directly with the dietary intake of NaCl. Salt-sensitivity characterizes “essential” human hypertension in perhaps half of those affected, and confers its own cardiovascular risks, including that of the occurrence of hypertension.1 A pathophysiological mechanism for salt-sensitivity has not been defined, despite recent demonstrations that human salt-sensitive hypertension can be caused by single genetic alterations that abnormally enhance the renal tubular reclamation of Na+ and Cl−.2 These demonstrations comport with the formulation that an excessive renal retention of Na+ and Cl− initiates all salt-sensitivity, and does so only by these ions’ joint osmotic expansion of extracellular fluid and thereby of plasma volume (PV).2–5 Na+ and Cl− are the only physiologic ions whose osmotic activities distribute throughout, and near exclusively to, extracellular fluid. Accordingly, in men selected only for salt-sensitive hypertension and rendered normotensive by NaCl-restriction, NaCl-loading induced within days both hypertension and a substantial expansion of PV, whereas Na-citrate-loading induced neither.6 Such “selective” Na+-loading, i.e. without Cl−, has repeatedly failed to elicit a pressor effect in NaCl-sensitive hypertension.7 Allegedly, “only NaCl causes an expansion of PV and a rise in BP.”8 But selective Na+-loading appears not to have been investigated in salt-sensitive blacks. In them, compared to salt-sensitive whites, the pressor effect of dietary NaCl is on average greater,9 and hence the pressor agency of NaCl might involve more than its ions’ joint mediation of PV expansion. We find that in the more severely salt-sensitive blacks, selective Na+-loading (as NaHCO3) induces an increase in BP that cannot be ascribed only to the concomitant increase in PV.
Methods
Participants and Setting
We studied 35 healthy blacks, ages 35 to 56, with screening BP <160/100 mmHg and body weight (BW) within 30% of ideal BW, as inpatients at the General Clinical Research Center (GCRC), University of California, San Francisco (UCSF). The study was approved by and conducted according to the guidelines of the UCSF Committee on Human Research. All participants gave written informed consent.
Basal Diet
Throughout the study, participants ate a eucaloric basal metabolic diet providing, per 70 kg BW per day, 30 mmol Na+ and 45 mmol K+. They drank water, 20 g/kg BW per day during Na+-restriction and 35 g/kg BW during Na+-loading, respectively.
Intervention (Na+-Loading)
The study consisted of 3 consecutive 7-day periods: The 2 periods of oral Na+-loading were separated by a period of Na+-restriction. Na+, 250 mmol/70kg BW per day (but no more than 300mmol/d), was supplemented as NaCl during the 1st week and as NaHCO3 during the 3rd week or vice versa. All participants received placebo tablets during the 2nd (low-salt) week. Participants and nurses performing BP measurements were not informed about the content of the tablets.
Assessment of Na+-induced Pressor Effects
With an automated oscillometric device (Dinamap, Criticon Inc. Tampa, Florida) programmed to obtain 5 readings over a period of 5 minutes, BP was measured daily every 4 hours (between 6AM and 10PM) after 10 minutes of supine rest; an average daily BP was calculated. The first standardized BP measurements were obtained within about 2 hours of the subject’s arrival at the GCRC, at 2PM. The 2PM values of day 1 are reported as “initial” (baseline) BP in Table 1 and Figure 1. To determine the pressor effects of Na+ salts, the average mean arterial pressure (MAP) of days 5 and 6 during Na+-restriction, was subtracted from the average MAP of days 5 and 6 during loading of either NaCl or NaHCO3. Saltsensitivity (SS) was defined as a NaCl-induced increase in MAP of ≥5 mmHg, salt-resistance (SR) as an increase of <5 mmHg.
Table 1.
Demographic characteristics of salt-resistant (SR) and salt-sensitive (SS) subjects
| Variable | SR | All SS | SS Subgroups | |
|---|---|---|---|---|
| sNaS | cSS | |||
| n (%) | 17 (49%)* | 18 (51%)* | 11 (61%)† | 7 (39%)† |
| Age, yrs | 45.2±2.7 | 49.6±1.8 § | 49.0±2.5 | 50.7±2.3 |
| BW, kg | 82.5±4.4 | 79.7±5.9 | 77.3±8.6 | 83.5±6.7 |
| BMI, kg/m2 | 27.2±1.4 | 24.8±1.3 ‡ | 24.5±2.7 | 25.3±2.1 |
| Initial SBP, mmHg | 120±8 | 133±6 ‡ | 133±7 | 134±11 |
| Initial DBP, mmHg | 68±4 | 80±4 ‡ | 82±4 | 77±6 |
| SBP≥140 or DBP≥90 mmHg, n (%) | 2 (12%) | 5 (28%) | 3 (27%) | 2 (29%) |
| Serum Creatinine, mg/100mL | 1.0±0.1 | 0.9±0.1 | 0.9±0.2 | 0.9±0.1 |
| Serum Na+, mmol/L | 137.9±0.9 | 139.6±1.1 ‡ | 139.5±2.1 | 139.6±2.8 |
| Serum K+, mmol/L | 4.2±0.1 | 4.2±0.1 | 4.3±0.3 | 4.2±0.2 |
| Serum Cl−, mmol/L | 103.1±1.3 | 103.2±1.4 | 102.9±1.8 | 103.6±2.0 |
| Total CO2, mmol/L | 26±2 | 28±1 | 28±2 | 29±3.5 |
Values are mean and 95% CI; sNas: “sodium-selective” salt-sensitive; cSS “classic” salt-sensitive;
Percent of total number of subjects enrolled;
Percent of all salt-sensitive subjects;
t-test: p<0.05. “Initial” SBP and DBP was obtained at 2PM on the 1st day of study, shortly after subjects were admitted, using the same standardized procedure as throughout the study.
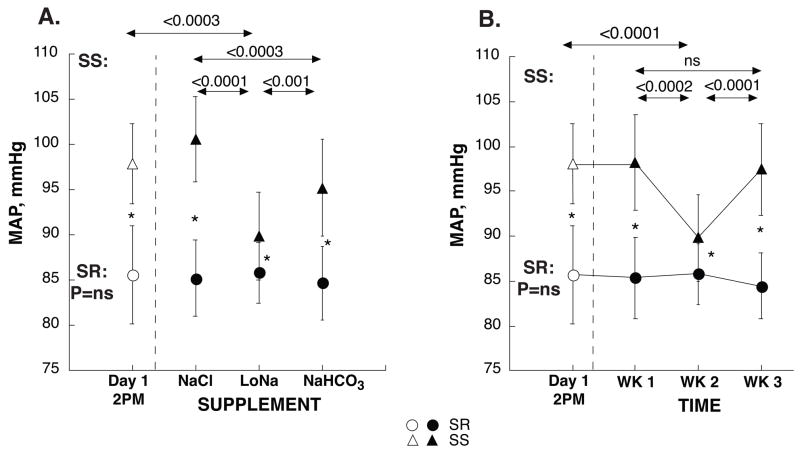
Figure 1. Initial MAP and MAP during oral loading of NaCl, low-Na+ and NaHCO3 in SS (▲) and SR (●) subjects, respectively.
Na+ was supplemented as NaCl during the 1st week and as Na-HCO3 during the 3rd week, or vice versa. About half of the subjects received NaCl first. For all participants, week 2 was the low-Na+ period. Panel A shows MAP values grouped by supplement; panel B shows the 3-week time course of MAP in the same subjects. “Initial” BP was obtained at 2PM on the 1st day of study, shortly after subjects were admitted, using the same standardized procedure as throughout the study. Other BP values are daily averages of days 5 and 6 of each of the 3 study periods. ★ SR subjects had significantly lower BP than SS, P<0.0001. In SR subjects MAP did not change with changing levels of dietary Na+. In SS subjects, MAP was significantly lower with low Na+ intake compared to values both on admission and during Na+-loading with either NaCl or NaHCO3. The sequence in which Na+-salts were loaded did not affect their pressor effects (panel B).
Metabolic Outcomes
We measured BW daily at 6AM. Spontaneously voided urine was collected daily over 24-hour periods and analyzed for Na+, Cl− and creatinine. During week 3 of the study we measured weekly cumulative Na+ excretion (UVNaCum), corrected for creatinine excretion and adjusted for 70 kg of BW. On the last day of each 7-day period, between 9AM and noon, blood samples were obtained with participants in supine position to determine levels of PRA, aldosterone, hematocrit, creatinine and serum electrolytes by standard techniques.
Renal Hemodynamics
PAH clearance studies were performed in 31 participants (16 SS and 15 SR) on the last day of each 7-day period using standard methods.
Data Analysis
We assessed the effects of Na+-loading as either NaCl or NaHCO3 on BP using repeated measures ANOVA, followed by Newman-Keuls test. To assess whether the order by which the supplements were administered had an effect on outcome we included “supplement order” as a between-group factor in the comparison. To rule out NaHCO3- or NaCl-induced effects on serum K+ 9 or hematocrit as a major source of between-group differences in pressor effects we repeated between-group comparisons of pressor effects using changes in and final concentrations of serum K+ and changes in hematocrit as covariates. We assessed the effects of NaCl or NaHCO3 on BW, hematocrit, electrolytes and renal hemodynamics and the pressor effect in salt-sensitive subgroups using paired and unpaired t-tests, respectively, for with-in group and between-group comparisons. Non-parametric tests were used to assess the effect on PRA and aldosterone as these variables were not normally distributed. Linear regression and Spearman rank correlation analyses were used to explore the relationship between variables. Data is presented as mean and 95% C.I. The null hypothesis was rejected at p<0.05.
Results
Salt-Sensitivity
18 of 35 participants (51%; 17 males, 1 female) were salt-sensitive (SS), average NaCl-induced ΔMAP 11±2 mmHg; 17 (49%; 15 males, 2 females) were salt-resistant (SR), average NaCl-induced ΔMAP −1±2 mmHg (Figure 1, Figure 2A). In SS, but not in SR, Na+-restriction during week 2 induced a significant hypotensive effect compared to initial BP.
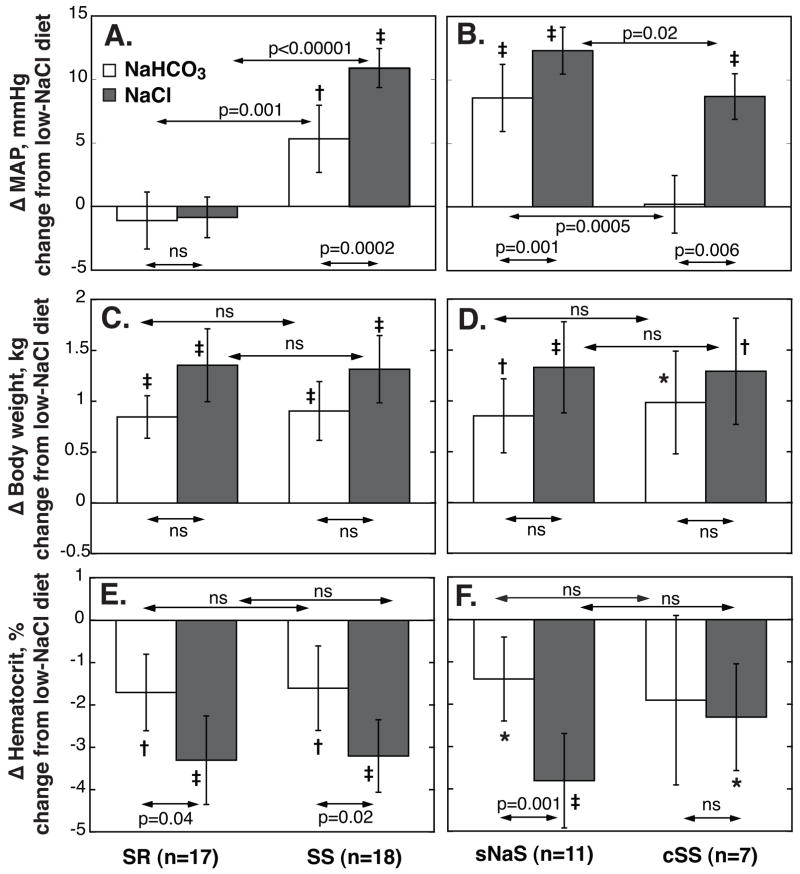
Figure 2. NaHCO3- (□) and NaCl-induced (
 ) changes in MAP, body weight (BW) and hematocrit in salt-resistant (SR) and salt-sensitive (SS) subjects, left panels, and in selective sodium-sensitive (sNaS) and classic salt-sensitive (cSS) subgroups, right panels.
) changes in MAP, body weight (BW) and hematocrit in salt-resistant (SR) and salt-sensitive (SS) subjects, left panels, and in selective sodium-sensitive (sNaS) and classic salt-sensitive (cSS) subgroups, right panels.
Values are average changes from baseline (average of days 5 and 6 of low-NaCl) on days 5 and 6 of Na+-loading periods. Values are means, error bars are 95% C.I. P values for within-group comparisons of Na+-loading vs low-NaCl period: ★P<0.05; †, P<0.01; and ‡ P<0.001; respectively. NaHCO3-loading induced a significant pressor response in SS but not in SR, panel A. In 11 of 18 SS the NaHCO3-induced pressor effects was ≥5 mmHg (sNaS), in 7 SS it was <5 mmHg (cSS), panel B. Responses of BW (panels C and D) and hematocrit (panels E and F) to Na+-loading did not differ between SR and SS and between sNaS and cSS, respectively.
Demographic Characteristics
SS subjects were slightly older than SR and had a significantly higher initial BP, higher serum Na+ concentration and lower body mass index (BMI), Table 1. After adjusting for age and BMI, the difference remained significant for DBP and MAP but not for SBP.
Pressor Effects of NaHCO3
In SS but not in SR, NaHCO3-loading, compared to Na+-restriction, induced a significant pressor effect (Figures 1, Figure 2A, Table 2). However, this pressor effect was significantly less than that of NaCl. The sequence in which Na+-salts were administered did not affect their pressor effect. ANOVA, summary of effects: SS vs SR (group), P=0.004; NaCl vs low-NaCl vs NaHCO3 (intervention), P<0.0001; sequence (of Na+-salt), P=0.65; interaction of intervention by group, P<0.00001; interaction of intervention by sequence, P=0.58; interaction of sequence by group, P=0.27.
Table 2.
Blood pressure, pulse pressure (PP), body weight (BW), hematocrit, aldosterone and PRA during low NaCl intake (lowNa) and during oral Na+-loading with NaCl and NaHCO3, respectively, in salt-resistant (SR) and salt-sensitive (SS) subjects and in SS sub-groups (sodium-selective salt-sensitive, sNaS, and classic salt-sensitive, cSS)
| SR (n=17) | All SS (n=18) | SS Subgroups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sNaS (n=11) | cSS (n=7) | ||||||||||||
| Variable | Diet | mean | 95% CI | p* | mean | 95% CI | p* | mean | 95% CI | p* | mean | 95% CI | p* |
| MAP, mmHg | NaHCO3 | 84.7 | 4.1 | ns | 95.2 | 5.4 | <0.001 | 99.9 | 7.4 | <0.001 | 87.7 | 3.1 | ns |
| Low Na | 85.8 | 3.4 | 89.9 | 4.8 | 91.4 | 7.5 | 87.4 | 3.8 | |||||
| NaCl | 84.9 | 4.2 | ns | 100.7 | 4.7 | <0.001 | 103.7 | 6.9 | <0.001 | 96.1 | 3.3 | <0.001 | |
| SBP, mmHg | NaHCO3 | 117.5 | 5.2 | ns | 128.9 | 6.8 | <0.001 | 135.4 | 8.5 | <0.001 | 118.7 | 6.3 | ns |
| Low Na | 117.1 | 3.7 | 120.2 | 5.6 | 122.3 | 8.7 | 117.0 | 4.5 | |||||
| NaCl | 118.5 | 4.5 | ns | 137.4 | 6.0 | <0.001 | 141.3 | 8.4 | <0.001 | 131.3 | 6.2 | <0.001 | |
| DBP, mmHg | NaHCO3 | 68.3 | 3.7 | ns | 78.4 | 4.9 | <0.01 | 82.3 | 7.0 | <0.001 | 72.2 | 2.6 | ns |
| Low Na | 70.1 | 3.4 | 74.7 | 4.5 | 76.0 | 7.1 | 72.7 | 3.7 | |||||
| NaCl | 68.2 | 4.1 | <0.05 | 82.4 | 4.3 | <0.001 | 84.9 | 6.4 | <0.001 | 78.5 | 3.0 | <0.001 | |
| PP, mmHg | NaHCO3 | 49.2 | 2.8 | <0.01 | 50.5 | 3.4 | <0.001 | 53.1 | 3.3 | <0.001 | 46.5 | 6.1 | ns |
| Low Na | 47.0 | 1.9 | 45.5 | 2.1 | 46.3 | 3.0 | 44.3 | 2.4 | |||||
| NaCl | 50.3 | 1.8 | <0.001 | 55.0 | 3.2 | <0.001 | 56.5 | 3.6 | <0.001 | 52.7 | 5.9 | <0.01 | |
| BW, kg | NaHCO3 | 83.1 | 4.5 | <0.001 | 79.4 | 6.1 | <0.001 | 77.5 | 9.0 | <0.01 | 82.5 | 6.7 | <0.05 |
| Low Na | 82.3 | 4.5 | 78.6 | 6.0 | 76.7 | 9.0 | 81.5 | 6.4 | |||||
| NaCl | 83.7 | 4.6 | <0.001 | 79.9 | 6.1 | <0.001 | 78.0 | 9.1 | <0.001 | 82.8 | 6.7 | <0.01 | |
| Hct, % | NaHCO3 | 41.5 | 2.2 | <0.01 | 42.6 | 1.4 | <0.01 | 42.9 | 2.0 | <0.05 | 42.1 | 1.7 | ns |
| Low Na | 43.2 | 2.2 | 44.2 | 1.7 | 44.4 | 2.3 | 44.0 | 2.6 | |||||
| NaCl | 39.8 | 1.7 | <0.001 | 41.0 | 1.6 | <0.001 | 40.5 | 1.6 | <0.001 | 41.7 | 3.1 | <0.01 | |
| Aldo,¶ ng/100mL | NaHCO3 | 4.0 | 2.0/7.0 | <0.01 | 3.0 | 2.0/4.0 | <0.001 | 3.0 | 2.0/4.0 | <0.05 | 3.0 | 2.0/4.0 | <0.05 |
| Low Na | 18.0 | 13.0/31 | 9.0 | 6.0/11.0 | 9.0 | 7.0/10.5 | 11.0 | 6.0/23.0 | |||||
| NaCl | 4.0 | 2.0/6.0 | <0.05 | 3.0 | 2.0/5.0 | <0.01 | 3.0 | 2.0/5.0 | <0.05 | 3.5 | 3.0/5 | <0.05 | |
| PRA,¶ ng/mL/hr | NaHCO3 | 1.1 | 0.5/1.9 | <0.001 | 0.4 | 0.2/0.7 | <0.001 | 0.3 | 0.3/0.7 | <0.01 | 0.6 | 0.1/2.1 | <0.05 |
| Low Na | 3.0 | 2.6/7.9 | 2.3 | 0.8/4.9 | 2.2 | 1.3/3.9 | 2.4 | 0.7/11.0 | |||||
| NaCl | 0.4 | 0.2/0.3 | <0.001 | 0.3 | 0.1/0.5 | <0.001 | 0.3 | 0.2/0.4 | <0.05 | 0.3 | 0.1/0.9 | <0.05 | |
compared to low Na+ diet;
values are median and 25th/75th percentile
In 11 of 18 the SS the NaHCO3-induced increase in MAP was ≥5 mmHg. In these 11 “sodium-selective” salt-sensitive subjects (sNaS), the mean NaHCO3-induced increase in MAP was two thirds that induced by equimolar amounts of NaCl (Figure 2B, Table 2). In 7 of 18 the SS the NaHCO3-induced increase in MAP was <5 mmHg. In these “classic” salt-sensitive subjects (cSS) the mean NaCl-induced increase in MAP was significantly lower than that in sNaS. In fact, the pressor effect of NaHCO3 in sNaS was similar to that of NaCl in cSS (Figure 2B, Table 2). For all SS and SR subjects combined, changes in MAP induced by NaCl strongly predicted those induced by NaHCO3 (Figure 3A).
Figure 3. Relationship between NaCl- and NaHCO3-induced changes in MAP, renal blood flow (RBF) and renal vascular resistance (RVR) in all subjects combined.
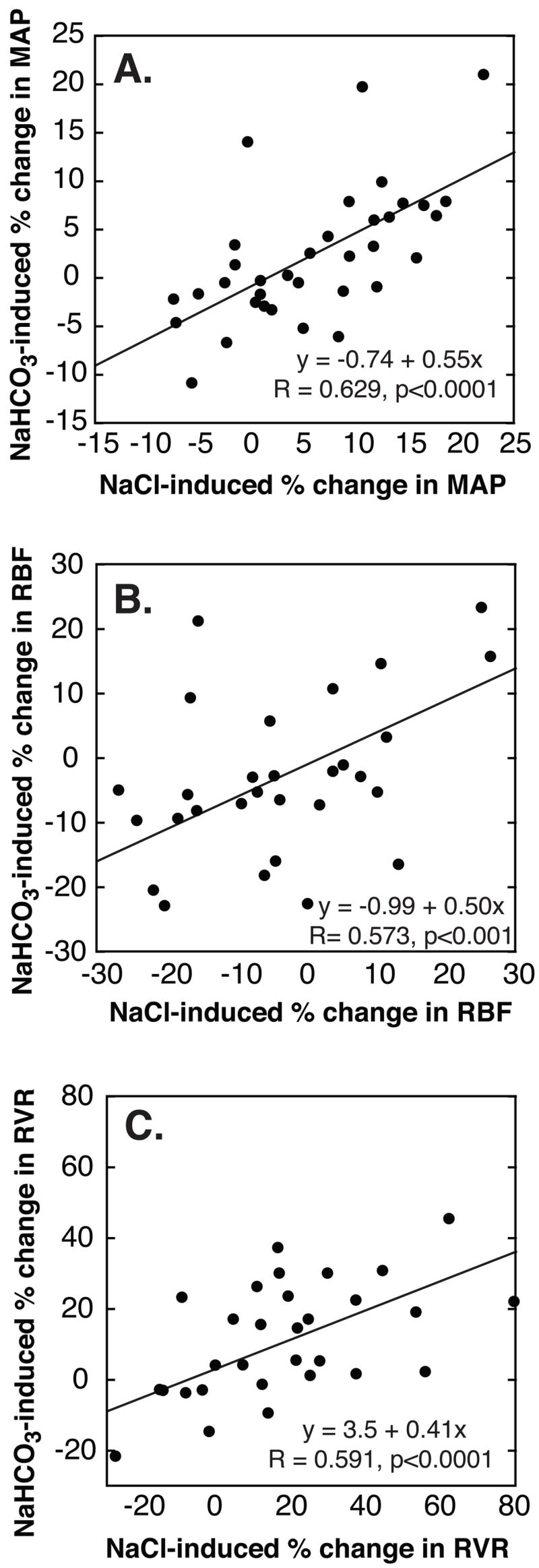
NaCl-induced changes in MAP (A), RBF (B) and RVR (C) are highly predictive of NaHCO3-induced changes. For all subjects combined, the magnitude of the NaHCO3-induced changes amounts to about half that induced by NaCl. NaCl- and NaHCO3-induced effects are presented as % change from low-NaCl baseline.
Mean values of ΔMAP adjusted for changes in serum K+ or hematocrit were not different from unadjusted means.
Effects of NaHCO3 and NaCl on Renal Hemodynamics
In SS but not SR, both NaHCO3 and NaCl induced significant decreases in RBF and increases in RVR (Figure 4). Changes in RBF did not differ significantly between SS subgroups. But average changes tended to be greater in (the more salt-sensitive) sNaS than in cSS. Since this study was designed to compare 2 groups only, SR and SS, subgroup analysis was not expected to achieve sufficient power to detect relevant differences. For all subjects combined, NaHCO3-induced changes in RBF and RVR, respectively, were strongly predicted by NaCl-induced changes (Figure 3B and C).
Figure 4. NaHCO3- (□) and NaCl-induced (
 ) changes in renal blood flow, panel A, and renal vascular resistance (RVR), panel B, in salt-resistant (SR) and salt-sensitive (SS) subjects.
) changes in renal blood flow, panel A, and renal vascular resistance (RVR), panel B, in salt-resistant (SR) and salt-sensitive (SS) subjects.
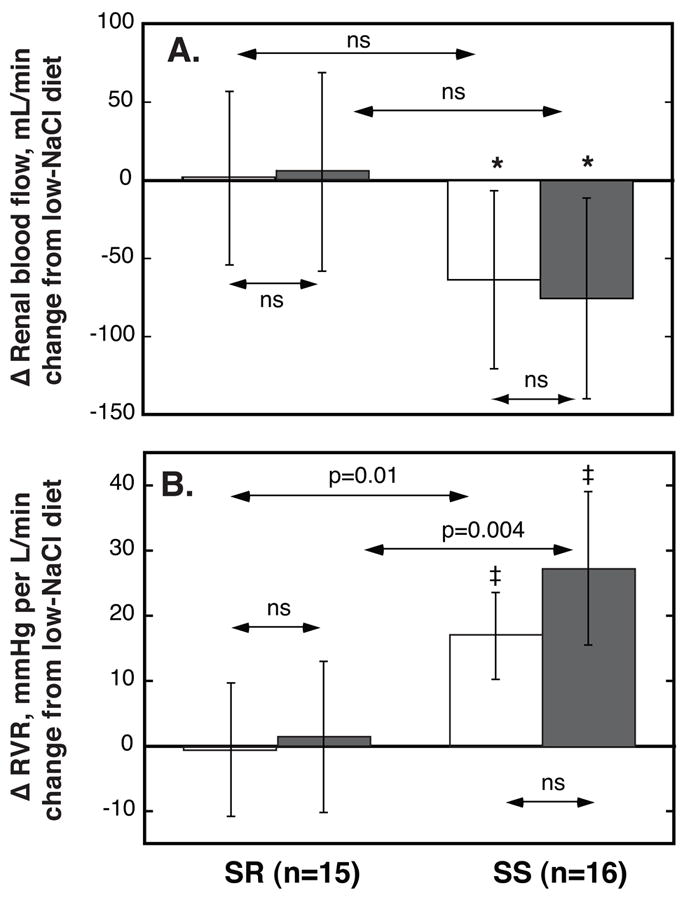
Values are average changes from day 7 of low-NaCl to day 7 of Na+-loading periods. Values are means, error bars are 95% C.I. P values for within-group comparisons of Na+-loading vs low-NaCl period: ★P<0.05; †, P<0.01; and ‡ P<0.001; respectively. In SS but not in SR NaHCO3 and NaCl induced decreases in RBF and increases in RVR. The changes in both variables were similar with the two salts.
Metabolic Effects of NaHCO3 and NaCl
Both NaCl and NaHCO3 induced significant increases in BW (Figure 2C and D and Table 2). Increases were similar in SR and SS as well as in SS sub-groups and tended to be larger with NaCl than with NaHCO3. Both NaCl and NaHCO3 induced significant decreases in hematocrit values. Decreases were similar in SS and SR but were significantly larger with NaCl than with NaHCO3 in both groups (Figures 2E and F and Table 2). Changes in BW and hematocrit were not predictive of pressor effects for either Na+-salt.
UVNaCum during loading of either NaCl or NaHCO3 did not differ between SS and SR. For all subjects combined, UVNaCum was 17% greater with NaHCO3 than with NaCl, P=0.07. During NaCl-loading UVNaCum was, mmol/mg creatinine per 70kg BW, 0.63±0.11 in SS (n=8) and 0.63±0.10 in SR (n=8) and during NaHCO3-loading it was 0.78±0.16 (n=9) and 0.69±0.08 (n=8) in SS and SR, respectively.
Changes in plasma aldosterone and PRA were similar with NaCl and NaHCO3 and significant in both SR and SS (Table 2). The NaCl-induced decrease in PRA but not in aldosterone was slightly but significantly greater in SR than in SS. NaHCO3-induced changes did not differ between SS and SR.
Both Na+-salts induced significant increases in serum levels of Na+ in SS and SR. In both groups, the increase was slightly larger with NaCl than with NaHCO3, the difference being significant in SS (Table 3). As expected, NaHCO3-loading induced a small but significant decrease in serum K+ in both SS and SR (Table 3). Serum K+ levels remained within the normal range in all but one SR subject who developed mild hypochloremic alkalosis with NaHCO3-loading. The NaHCO3-induced decrease was slightly but significantly larger in SS than in SR. NaCl-loading induced a significant increase and NaHCO3-loading a significant decrease in serum Cl− in both SS and SR (Table 3).
Table 3.
Serum electrolyte and creatinine concentrations during oral Na+-loading with NaCl and NaHCO3, respectively, in salt-resistant (SR) and salt-sensitive (SS) subjects and in SS subgroups (sodium-selective salt-sensitive, sNaS, and classic salt-sensitive, cSS)
| SR (n=17) | All SS (n=18) | SS Subgroups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sNaS (n=11) | cSS (n=7) | ||||||||||||
| Variable | Diet | mean | 95% CI | p* | mean | 95% CI | p* | mean | 95% CI | p* | mean | 95% CI | p* |
| Na+, mmol/L | NaHCO3 | 136.3 | 1.43 | <0.001 | 137.8 | 1.14 | <0.001 | 137.6 | 1.56 | 0.0015 | 138.1 | 1.77 | <0.01 |
| Low Na | 133.0 | 1.42 | 135.1 | 1.20 | 134.8 | 1.75 | 135.6 | 1.58 | |||||
| NaCl | 137.4 | 1.33 | <0.001 | 138.6 | 1.14 | <0.001 | 138.5 | 1.73 | <0.001 | 138.6 | 1.40 | <0.01 | |
| K+, mmol/L | NaHCO3 | 3.8 | 0.15 | <0.01 | 3.6 | 0.11 | <0.001 | 3.7 | 0.16 | <0.001 | 3.6 | 0.14 | <0.05 |
| Low Na | 4.1 | 0.15 | 4.1 | 0.12 | 4.2 | 0.12 | 4.0 | 0.23 | |||||
| NaCl | 4.0 | 0.09 | <0.05 | 3.9 | 0.14 | <0.001 | 3.9 | 0.20 | <0.01 | 3.8 | 0.17 | ns | |
| Cl−, mmol/L | NaHCO3 | 97.6 | 1.71 | <0.01 | 97.8 | 0.91 | <0.001 | 97.9 | 1.26 | <0.001 | 97.6 | 1.40 | <0.01 |
| Low Na | 99.5 | 1.15 | 100.9 | 0.81 | 101.1 | 1.10 | 100.6 | 1.23 | |||||
| NaCl | 105.4 | 1.20 | <0.001 | 105.3 | 0.77 | <0.001 | 105.6 | 0.96 | <0.001 | 104.8 | 1.26 | <0.01 | |
| HCO3−, mmol/L | NaHCO3 | 30.8 | 1.31 | <0.001 | 33.0 | 0.77 | <0.001 | 33.2 | 1.24 | <0.001 | 32.7 | 0.83 | <0.001 |
| Low Na | 25.9 | 1.18 | 27.0 | 0.73 | 26.8 | 1.00 | 27.3 | 1.10 | |||||
| NaCl | 25.9 | 1.33 | ns | 26.7 | 0.90 | ns | 26.5 | 1.36 | ns | 26.9 | 1.10 | ns | |
| Creatinine, mg/100mL | NaHCO3 | 1.03 | 0.08 | ns | 0.99 | 0.10 | ns | 1.00 | 0.15 | ns | 0.96 | 0.12 | ns |
| Low Na | 1.00 | 0.07 | 0.97 | 0.09 | 1.00 | 0.14 | 0.93 | 0.08 | |||||
| NaCl | 0.95 | 0.09 | <0.05 | 0.93 | 0.10 | ns | 0.98 | 0.16 | ns | 0.87 | 0.05 | <0.05 | |
compared to low Na diet
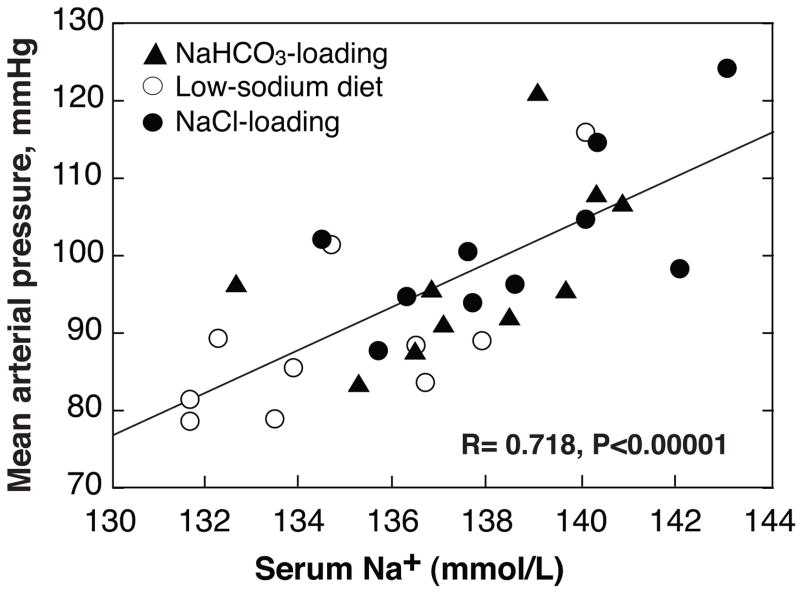
In SS, but not in SR, BP varied directly and highly significantly with the serum concentration of Na+ (R=0.541, P<0.0001). When sNaS only were included in the analysis the relationship was stronger than in all SS combined (Figure 5).
Figure 5. Relationship between serum Na+-concentration and mean arterial pressure in sodium-selective salt-sensitive (sNaS) subjects at the ends of low-NaCl diet (○) and Na+-loading with either NaCl (●) or NaHCO3 (▲).
In sNaS plasma Na+ with and without Na+-loading is highly predictive of BP.
For SS and SR combined the responses to NaHCO3 of serum Na+, aldosterone and PRA but not those of hematocrit or K+ were strongly predicted by the responses to NaCl.
Discussion
In the currently studied blacks, half were judged to be salt-sensitive (SS), half salt-resistant (SR). In at least two thirds of the SS, selective dietary Na+-loading with NaHCO3 induced a pressor effect of at least 5 mmHg, despite a sustained restriction of dietary NaCl. Thus, salt-sensitivity occurs not only frequently in blacks, but in many it is “sodium-selective” (sNaS) in that Na+-loading is pressor without concomitant Cl−-loading. In sNaS, the mean NaHCO3-induced increase in MAP was fully two thirds that induced by equimolar NaCl-loading. To our knowledge, these observations provide the first demonstrations both that a non-Cl− salt of Na+ can induce a pressor effect; and that any salt other then NaCl can induce a pressor effect in humans.
In one third of the currently studied SS, selective Na+-loading induced little or no pressor effect. For such salt-sensitivity that is induced only by Na+- and Cl−-loading combined6, 10–12 we assign the modifier, “classic” (cSS). In sNaS, NaHCO3 induced a pressor effect similar to that induced by NaCl in cSS. For all subjects combined the pressor response to NaHCO3-loading varied directly and highly significantly with the pressor response to NaCl-loading. The pressor effect of NaHCO3-loading amounted to half that of NaCl-loading. These observations would suggest that in many salt-sensitive blacks, a selective pressor effect of dietary Na+ is a major component of the pressor effect of dietary NaCl, and the magnitude of the selective pressor effect of Na+ a major determinant of the severity of NaCl-sensitivity.
In previous studies of mainly white hypertensive subjects,6, 11 and of animal models of salt-sensitive hypertension13–15 in which selective dietary loading of Na+ failed to elicit a pressor effect, the failure might be inferred to reflect a failure to expand PV to some critical extent.5, 7 As judged by the currently observed changes in hematocrit, it appears that both NaHCO3 and NaCl induced expansion of PV. In accord with previous observations,6 the current observations would indicate that PV expansion with NaHCO3 was about half that with NaCl, not only in SS but also in SR.
NaCl-induced PV expansion is formulated to be a critical event in the pathogenesis of salt-sensitive blood pressure by entraining a transient increase in cardiac output (CO) that elicits a pressor effect in two phases and ways: 1) an immediate direct pressor effect; and several days later, 2) an indirect, sustained pressor effect that is mediated by an increase in systemic vascular resistance (SVR) occurring only in autoregulatory response to the increase in CO.2, 3 However, in NaCl-loaded salt-sensitive subjects,16–19 the apparent extent of neither PV expansion16–19 nor increase in CO20 induced by NaCl has predicted the extent of salt-sensitivity. In a recent study comparing normotensive salt-sensitive and salt-resistant blacks with respect to the time courses of their hemodynamic and metabolic responses to NaCl-loading, we observed similar increases in Na+ balance, PV and CO. By contrast, in the 2 groups, the divergent pressor responses induced by dietary NaCl-loading were attended from their outset by divergent vascular responses, SVR decreasing sharply and immediately in the salt-resistant, but changing little in the salt-sensitive subjects.20 In all subjects combined, the changes in SVR induced by NaCl on the 2nd day of its loading were strongly predictive of the changes induced by NaCl on MAP on the 7th day of its loading; NaCl-induced changes in PV and CO were not predictive. These observations indicate that in the SS subjects studied, an impaired systemic vasodilatory response to NaCl-loading, but not an abnormally increased PV or CO, is critical to the pathogenesis of salt’s pressor effect.
In keeping with these observations, in the current study, the extent to which loading with either NaCl or NaHCO3 induced PV expansion does not account for the extent of either salt’s pressor effect. As judged by the decrease in hematocrit, the increase in PV induced by NaCl-loading in the SS is indistinguishable from that induced in SR. Indeed, while inducing a pressor effect only slightly less than that induced by NaCl, NaHCO3 induced in the sNaS a decrease in hematocrit only one-third that induced by NaCl in the SR. In all SS combined, as well as in sNaS and cSS subgroups, the Na+-induced mean changes in MAP adjusted for changes in hematocrit were not different from unadjusted mean changes in MAP. These observations and considerations suggest that in most salt-sensitive blacks, the pressor effect of dietary NaCl involves something more than its capacity to mediate osmotic expansion of PV, the only capacity of NaCl called for in the traditionally formulated mechanism of NaCl’s pressor effect.2, 3, 5
In the SS, and particularly in the sNaS subgroup, BP varied directly and highly significantly with the plasma concentration of Na+, with and without Na+-loading, but not with changes induced in BW or hematocrit. This observation suggests that in some salt-sensitive humans, the plasma concentration of Na+ attained with Na+-loading may critically determine the extent of the pressor effect of dietary NaCl. The observation accords with observations Qi et al. reported in the NaCl-loaded Dahl SS/Jr rat, in which BP varied directly and highly significantly with plasma Na+ concentration, irrespective of the hydration state orally imposed.21 Qi et al. proposed, as have others,4, 22, 23 that “directly mediated increases in plasma Na+ can increase arterial BP in rats by mechanisms that are apparently not related to fluid volume changes.” An increase in plasma Na+ concentration may elicit a pressor effect by increasing CSF Na+ concentration24 and by thereby activating central sympatho-excitatory mechanisms that give rise to increased sympathetic outflow.21, 25–27
In SS but not in SR, NaHCO3-, as well as NaCl-loading, induced a clear-cut reduction in RBF and hence, a robust increase in renal vascular resistance, a phenomenon previously described only with NaCl-loading in salt-sensitive humans28, 29 including normotensive blacks.19 Clearly, the Cl−-component of NaCl is not required to elicit this dysfunctional renal hemodynamic response to Na+-loading. It has been proposed that a similar renal hemodynamic response is critically involved in the salt-sensitivity of patients with “non-modulating” essential hypertension.30 The extent of Na+-induced renal vasoconstrictive changes in those with salt-sensitivity has been related to the extent of their pressor response.19, 31
Perspectives
In establishing the fact of “sodium-selective” salt-sensitivity, and its occurrence in many blacks who are salt-sensitive, the current observations extend to human relevance the consideration of ion-selective pressor phenomena, previously observed only as Cl−-sensitive hypertension in the spontaneously hypertensive rat,32 and in the stroke-prone spontaneously hypertensive rat.33, 34 In some instances of salt-sensitivity, initiation of the pressor effect of dietary NaCl would appear to require something beyond, or other than, an abnormally enhanced renal retention of its ionic components and their complimentary osmotic capacities to expand PV,20, 33, 34 possibly an increase in plasma Na+ that elicits an increased CNS sympathetic outflow.4, 21–23, 25 Accordingly, the current observations expand the scope of possible pathogenic mechanisms of human hypertension and hence the scope of its potential treatment and prevention.
Acknowledgments
The authors would like to thank the nurses, dieticians and laboratory technicians of the GCRC for their excellent assistance in conducting these studies and the study participants for their invaluable commitment of time and their willingness to follow a very demanding study protocol.
Sources of Support
This research was supported by NIH/NHLBI Grant RO1-HL64230 and gifts from the Emil Mosbacher, Jr., Foundation. Studies were carried out at the GCRC, Moffitt/MZ Hospital, University of California, San Francisco, with funds provided by the National Center for Research Resources, M0 RR-00079, U.S. Public Health Service.
Footnotes
Conflicts of Interest
None
References
- 1.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt Sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 2.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 3.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990;259:R865–877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 4.Meneton P, Jeunemaitre X, De Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 5.Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol. 2006;290:R514–R523. doi: 10.1152/ajpregu.00819.2005. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz TW, Al-Bander HA, Morris RC., Jr “Salt-sensitive” essential hypertension in men: Is the sodium ion alone important? N Engl J Med. 1987;317:1043–1048. doi: 10.1056/NEJM198710223171702. [DOI] [PubMed] [Google Scholar]
- 7.Kotchen TA. To salt, or not to salt? Am J Physiol. 1999;276:H1807–H1810. doi: 10.1152/ajpheart.1999.276.5.H1807. [DOI] [PubMed] [Google Scholar]
- 8.O’Shaughnessy KM, Karet FE. Salt handling and hypertension. J Clin Invest. 2004;113:1075–1081. doi: 10.1172/JCI21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris RC, Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt-sensitivity: Effects of race and dietary potassium. Hypertension. 1999;33:18–23. doi: 10.1161/01.hyp.33.1.18. [DOI] [PubMed] [Google Scholar]
- 10.Sharma AM, Schattenfroh S, Thiede H-M, Oelkers W, Distler A. Effects of sodium salts on pressor reactivity in salt-sensitive men. Hypertension. 1992;19:541–548. doi: 10.1161/01.hyp.19.6.541. [DOI] [PubMed] [Google Scholar]
- 11.Shore AC, Markandu ND, MacGregor GA. A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J Hypertens. 1988;6:613–617. doi: 10.1097/00004872-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens. 1990;8:663–670. doi: 10.1097/00004872-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz TW, Morris RC., Jr Dietary chloride as a determinant of “sodium-dependent” hypertension. Science. 1983;222:1139–1141. doi: 10.1126/science.6648527. [DOI] [PubMed] [Google Scholar]
- 14.Whitescarver SA, Ott CE, Jackson BA, Guthrie GP, Jr, Kotchen TA. Salt-sensitive hypertension: contribution of chloride. Science. 1984;223:1430–1432. doi: 10.1126/science.6322303. [DOI] [PubMed] [Google Scholar]
- 15.Luft FC, Steinberg H, Ganten U, Meyer D, Gless KH, Lang RE, Fineberg NS, Rascher W, Unger T, Ganten D. Effect of sodium chloride and sodium bicarbonate on blood pressure in stroke-prone spontaneously hypertensive rats. Clin Sci. 1988;74:577–585. doi: 10.1042/cs0740577. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–198. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 17.Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 18.Dustan HP, Valdes G, Bravo EL, Tarazi RC. Excessive sodium retention as a characteristic of salt-sensitive hypertension. Am J Med Sci. 1986;292:67–74. doi: 10.1097/00000441-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Schmidlin O, Forman A, Tanaka M, Sebastian A, Morris RC., Jr NaCl-induced renal vaso-constriction in salt-sensitive African-Americans: Antipressor and hemodynamic effects of potassium bicarbonate. Hypertension. 1999;33:633–639. doi: 10.1161/01.hyp.33.2.633. [DOI] [PubMed] [Google Scholar]
- 20.Schmidlin O, Forman A, Sebastian AR, Curtis Morris J. What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007;49:1032–1039. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi N, Rapp JP, Brand PH, Metting PJ, Britton SL. Body fluid expansion is not essential for salt-induced hypertension in SS/Jr rats. Am J Physiol. 1999;277:R1392–R1400. doi: 10.1152/ajpregu.1999.277.5.R1392. [DOI] [PubMed] [Google Scholar]
- 22.Friedman SM, McIndoe RA, Tanaka M. The relation of blood sodium concentration to blood pressure in the rat. J Hypertens. 1990;8:61–66. doi: 10.1097/00004872-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol. 2004;287:H1160–H1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kawano Y, Yoshida K, Kawamura M, Yoshimi H, Ashida T, Abe H, Imanishi M, Kimura G, Kojima S, Kuramochi M, Omae T. Sodium and noradrenaline in cerebrospinal fluid and blood in salt- sensitive and non-salt-sensitive essential hypertension. Clin Exp Pharmacol Physiol. 1992;19:235–241. doi: 10.1111/j.1440-1681.1992.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 25.Bunag RD, Miyajima E. Sympathetic hyperactivity elevates blood pressure during acute cerebroventricular infusions of hypertonic salt in rats. J Cardiovasc Pharmacol. 1984;6:844–851. doi: 10.1097/00005344-198409000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Cowley AW., Jr Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension. 1989;13:243–249. doi: 10.1161/01.hyp.13.3.243. [DOI] [PubMed] [Google Scholar]
- 27.Huang BS, Amin MS, Leenen FH. The central role of the brain in salt-sensitive hypertension. CurrOpinCardiol. 2006;21:295–304. doi: 10.1097/01.hco.0000231398.64362.94. [DOI] [PubMed] [Google Scholar]
- 28.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension. 1991;18:805–812. doi: 10.1161/01.hyp.18.6.805. [DOI] [PubMed] [Google Scholar]
- 29.Sanai T, Kimura G. Renal function reserve and sodium sensitivity in essential hypertension. J Lab Clin Med. 1996;128:89–97. doi: 10.1016/s0022-2143(96)90117-1. [DOI] [PubMed] [Google Scholar]
- 30.Hollenberg NK, Williams GH. Abnormal renal function, sodium-volume homeostasis and renin-system behavior in normal-renin hypertension: the evolution of the non-modulator concept. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. 2. New York: Raven Press Ltd; 1995. pp. 1837–1856. [Google Scholar]
- 31.van Paasen P, De Zeeuw D, Navis G, De Jong PE. Does the renin-angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension? Hypertension. 1996;27:202–208. doi: 10.1161/01.hyp.27.2.202. [DOI] [PubMed] [Google Scholar]
- 32.Wyss JM, Liumsiricharoen M, Sripairojthikoon W, Brown D, Gist R, Oparil S. Exacerbation of hypertension by high chloride, moderate sodium diet in the salt-sensitive spontaneously hypertensive rat. Hypertension. 1987;9:III/171–III/175. doi: 10.1161/01.hyp.9.6_pt_2.iii171. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Schmidlin O, Yi S-L, Bollen AW, Morris RC., Jr Genetically determined chloride-sensitive hypertension and stroke. Proc Natl Acad Sci. 1997;94:14748–14752. doi: 10.1073/pnas.94.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidlin O, Tanaka M, Bollen AW, Yi SL, Morris RC., Jr Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension. 2005;45:867–873. doi: 10.1161/01.HYP.0000164628.46415.66. [DOI] [PubMed] [Google Scholar]