Abstract
Rationale
NAD+ acts not only as a co-factor for cellular respiration, but also as a substrate for NAD+-dependent enzymes, such as Sirt1. The cellular NAD+ synthesis is regulated by both the de novo and the salvage pathways. Nicotinamide phosphoribosyltransferase (Nampt) is a rate-limiting enzyme in the salvage pathway.
Objective
Here we investigated the role of Nampt in mediating NAD+ synthesis in cardiac myocytes and the function of Nampt in the heart in vivo.
Methods and Results
Expression of Nampt in the heart was significantly decreased by ischemia, ischemia/reperfusion and pressure overload. Upregulation of Nampt significantly increased NAD+ and ATP concentrations, while downregulation of Nampt significantly decreased them. Downregulation of Nampt increased caspase 3 cleavage, cytochrome c release, and TUNEL positive cells, which were inhibited in the presence of Bcl-xL, but did not increase hairpin 2 positive cells, suggesting that endogenous Nampt negatively regulates apoptosis but not necrosis. Downregulation of Nampt also impaired autophagic flux, suggesting that endogenous Nampt positively regulates autophagy. Cardiac specific overexpression of Nampt in transgenic mice increased NAD+ content in the heart, prevented downregulation of Nampt and reduced the size of myocardial infarction and apoptosis in response to prolonged ischemia and ischemia/reperfusion.
Conclusions
Nampt critically regulates NAD+ and ATP contents, thereby playing an essential role in mediating cell survival by inhibiting apoptosis and stimulating autophagic flux in cardiac myocytes. Preventing downregulation of Nampt inhibits myocardial injury in response to myocardial ischemia and reperfusion. These results suggest that Nampt is an essential gatekeeper of energy status and survival in cardiac myocytes.
Keywords: NAD+, Apoptosis, Myocardial ischemia
Introduction
Nicotinamide adenine dinucleotide (NAD+) participates in redox reactions as a transfer molecule for electrons. Due to its involvement in the mitochondrial TCA cycle and the electron transport chain, NAD+ acts as a key co-factor for energy production. NAD+ also serves as the substrate for various enzymes, including the nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1) 1, and the class III HDACs, i.e. the sirtuin family 2. Since the sirtuin family plays an essential role in mediating lifespan extension, stress resistance and regulation of metabolism 3, NAD+ may control the level of stress resistance in cells partly through regulation of sirtuins 4.
NAD+ can be freshly synthesized from amino acids, including tryptophan or aspartic acid, via the de novo pathway 5 or taken up efficiently from the extracellular space 6. Importantly, NAD+ can also be re-synthesized from NAD+ metabolites through the salvage pathway 5. In yeast, increased expression of pyrazinamidase/nicotinamidase 1 (PNC1), a nicotinamidase converting nicotinamide to nicotinic acid, is both necessary and sufficient for lifespan extension induced by calorie restriction and low-intensity stress, such as osmotic stress7. Nicotinamide phosphoribosyltransferase (Nampt) is a rate-limiting enzyme in the mammalian NAD+ salvage pathway, and has been proposed to be a functional equivalent of PNC1 in mammals 5. Upregulation of Nampt increases the cellular NAD+ level and enhances the transcriptional regulatory activity of the catalytic domain of Sirt1 in mouse fibroblasts 8. In HEK293 cells, Nampt is an essential component of the mitochondrial NAD+ salvage pathway and promotes cell survival through stimulation of mitochondrial sirtuins, including Sirt3 and Sirt4 4. However, since the cellular content of NAD+ may be regulated by multiple mechanisms 5 in a cell type-specific manner, it remains to be elucidated whether Nampt plays an essential role in regulating the cellular content of NAD+ in cardiac myocytes.
We have shown previously that Sirt1 protects cardiac myocytes from serum starvation-induced cell death in vitro 9, and that mild to modest overexpression of Sirt1 in the heart protects the heart from oxidative stress and retards the progression of aging-induced cardiomyopathy in vivo 10. Based upon these observations and according to the hormesis hypothesis 11, we have proposed that stimulation of the longevity mechanism may activate self-protective mechanisms against certain forms of stress in the heart 12. At present, however, how NAD+, an essential co-factor of Sirt1, is controlled in the heart remains unknown. We therefore investigated 1) whether expression of Nampt is affected by various pathological conditions in the heart and 2) whether endogenous Nampt regulates cellular NAD+ content, energy status and cell death/survival in cardiac myocytes. Since our initial results suggested that Nampt is downregulated by myocardial ischemia and reperfusion, we examined how supplementation of Nampt by transgenic overexpression of Nampt affects myocardial injury in response to ischemia and reperfusion in the mouse heart in vivo.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
NAD+and ATP measurements
NAD+ was measured using the EnzyChromTM NAD+/NADH Assay Kit according to the manufacturer's protocol (#ECND-100, Bioassay Systems, Hayward, CA).
Transgenic Mice
Nampt transgenic mice (Tg-Nampt) were generated using the α-myosin heavy chain promoter (courtesy of J. Robbins, University of Cincinnati, Cincinnati, Ohio, USA) to achieve cardiac-specific expression of the transgene on an FVB background. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey.
Statistics
Statistical analyses between groups were done by one-way ANOVA, and differences among group means were evaluated using Fisher’s project least significant difference post test procedure for group data with a P value less than 0.05 considered significant.
Results
Nampt is downregulated by pathological stimuli in vivo
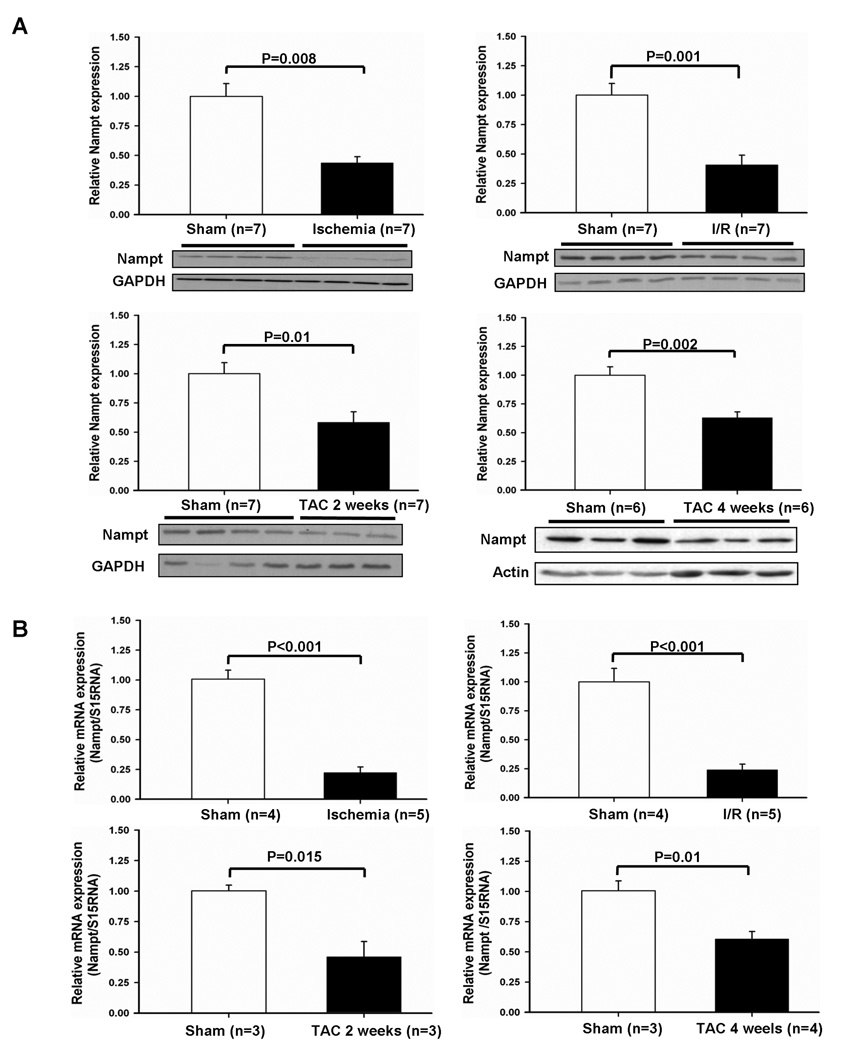
We examined how expression of Nampt is regulated in the heart under stress. Mice were subjected to clinically relevant forms of stress in the heart, including 24 hours of ischemia, 45 minutes of ischemia followed by 24 hours of reperfusion (I/R), and 2 or 4 weeks of pressure overload by thoracic aortic constriction (TAC). Mice develop compensated hypertrophy 2 weeks after TAC and heart failure 4 weeks after TAC 13. Protein expression of Nampt was significantly downregulated after ischemia, I/R, and 2 or 4 weeks of TAC (Fig. 1A). Nampt mRNA was also downregulated in response to these stimuli, as determined by RT- qPCR (Fig. 1B), suggesting that downregulation of Nampt protein expression is at least in part due to downregulation of Nampt mRNA. Downregulation of Nampt was observed within 6 hours of ischemia (Fig. S1A). Reduced Nampt levels during pressure overload reverted to the control level when pressure overload was removed (Fig. S1B). Downregulation of Nampt after I/R was confirmed by immunostaining at the level of individual myocytes in the heart (Fig. 1C). On the other hand, expression of Nampt in the heart increased during aging (6 fold at 1 year), suggesting that regulation of Nampt expression is stimulus-dependent (Fig. 1D).
Figure 1. Nampt is downregulated by pathological stimuli in vivo.
(A) Heart homogenates were prepared from mice subjected to ischemia for 24 hours, ischemia for 45 minutes and reperfusion for 24 hours, and transverse aortic constriction (TAC) for 2 or 4 weeks. Expression of Nampt, GAPDH and actin was evaluated by immunoblots. (B) The effect of pathological stress on the expression level of Nampt mRNA in the heart. Nampt mRNA level was determined by RT-qPCR and normalized by S15RNA. The expression level of sham operated mice is expressed as 1. (C) Representative Nampt stainings in heart sections from sham operation and ischemia/reperfusion are shown. (D) Heart homogenates were prepared from mice with indicated ages. Expression of Nampt and tubulin was evaluated by immunoblots.
Nampt critically regulates NAD+ and ATP levels in cardiac myocytes
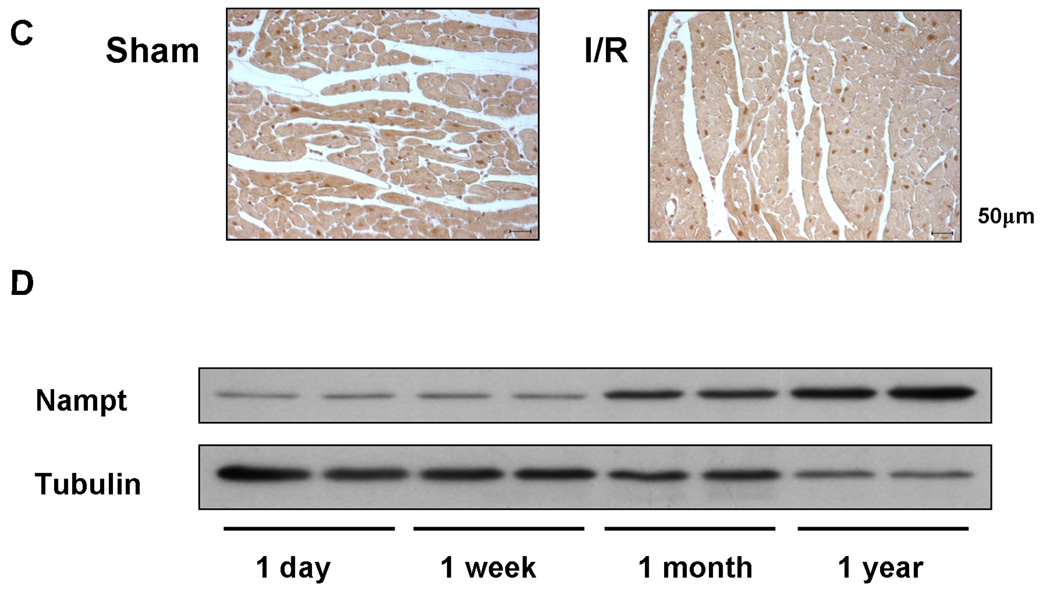
In order to elucidate the function of Nampt in cardiac myocytes, either Ad-Nampt or Ad-shRNA-Nampt was transduced into cultured neonatal rat cardiac myocytes. Transduction of Ad-Nampt dose-dependently increased expression of Nampt in cardiac myocytes (Fig. 2A). Upregulation of Nampt induced by Ad-Nampt (3 MOI) modestly but significantly increased the cellular level of NAD+ (1.32 ±0.15 fold, p<0.01) compared to Ad-LacZ (3 MOI) (Fig. 2B), accompanied by a significant increase in the cellular ATP content (1.19±0.13 fold, p<0.01) (Fig. 2C) in cardiac myocytes. The cellular level of NAD+ at baseline and in response to Nampt overexpression was greater when nicotinamide, a substrate of Nampt, was added to the culture medium. Conversely, transduction of Ad-shRNA-Nampt significantly decreased expression of Nampt in cardiac myocytes (Fig. 2D). Downregulation of Nampt induced by Ad-shRNA-Nampt significantly decreased the cellular level of NAD+ (0.67±0.36 fold, p<0.05), an effect which was reversed by supplementation of the culture media with NAD+ (500 µM), which is delivered into cells through transporter proteins, such as connexin 43 14 (Fig. 2E). Downregulation of Nampt also caused a significantly decrease in the cellular ATP content (0.84±0.15 fold, p<0.01) in cardiac myocytes, which was also reversed by addition of NAD+ (500 µM) to the culture media (Fig. 2F). As expected, glucose deprivation significantly reduced the ATP content, serving as a positive control of the assay.
Figure 2. Nampt regulates NAD+ and ATP levels in cardiac myocytes.
Cardiac myocytes were transduced with Ad-LacZ, Ad-shRNA-scramble, Ad-Nampt or Ad-shRNA-Nampt at indicated MOIs. In A and D, cell lysates were subjected to immunoblot analyses with anti-Nampt, anti-GAPDH and anti-tubulin antibodies. In B, C, E and F, Cellular [NAD+] and [ATP] were measured using the EnzyChromTM NAD+/NADH Assay Kit and ATP Bioluminescent Assay Kit, respectively. In B, cells were cultured with either normal culture media or those supplemented with 20 mM nicotinamide for 16 hours. In C-F, the level of Nampt, [NAD+] and [ATP] in control virus transduced myocytes is expressed as 1. In E and F, cardiac myocytes were cultured with or without 500 µM NAD+ or glucose free medium for 24 hours. Data represent the mean of four experiments ± SEM.
Nampt positively regulates survival in cardiac myocytes
We investigated whether Nampt affects cell survival in cardiac myocytes. Cardiac myocytes were transduced with either Ad-LacZ or Ad-Nampt at 3 MOI. Forty-eight hours after transduction, myocytes were treated with methylmethane sulfonate (MMS), a DNA alkylating agent known to hyperactivate PARP-1 and induce necrotic cell death 15, or glucose deprivation for 4 hours. We then evaluated cell viability using the CellTiter-Blue assay. Myocytes transduced with Ad-Nampt were modestly but significantly more resistant to cell death than those with Ad-LacZ in the presence of MMS or glucose deprivation (Fig. S2A). In contrast, knockdown of Nampt with Ad- shRNA-Nampt significantly reduced cell survival even at baseline and in response to MMS or glucose starvation compared to treatment with control shRNA in cardiomyocytes (Fig. S2B)
Downregulation of Nampt stimulates apoptosis in cardiac myocytes
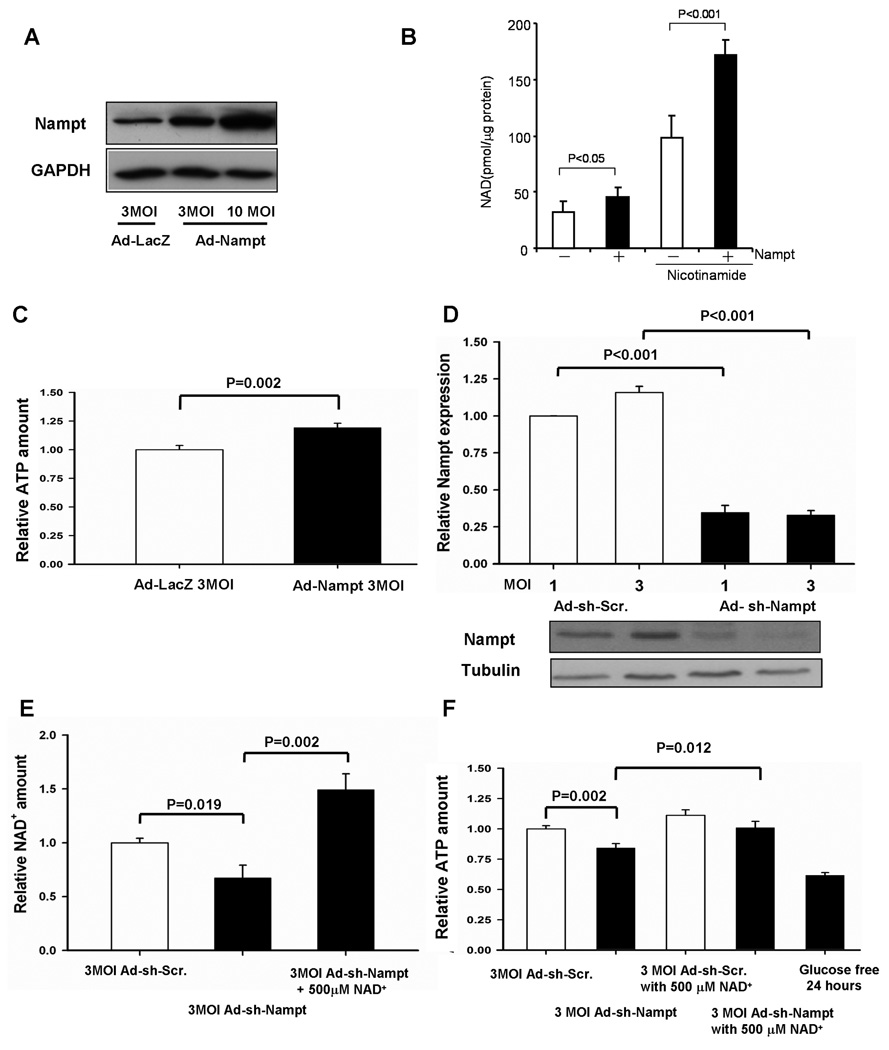
Since Nampt is downregulated by stress and downregulation of Nampt induces cell death, we examined how downregulation of Nampt affects apoptosis in cardiac myocytes. Compared to transduction of adenovirus harboring control shRNA (3 MOI), transduction of Ad-shRNA-Nampt significantly increased the number of TUNEL positive cardiac myocytes in vitro (Fig. 3A). Increases in TUNEL positive nuclei by Ad-shRNA-Nampt were abolished in the presence of adenovirus harboring Bcl-xL, an inhibitor of apoptosis (Fig. 3A). Ad-shRNA-Nampt induced cleavage of caspase-3, which was also abolished by Bcl-xL (Fig. 3B). The effect of chelerythrine upon cleavage of caspase-3 is shown as a positive control for apoptosis 16. Downregulation of Nampt induced cytochrome c release in cardiac myocytes, suggesting that the mitochondrial component of apoptosis is involved in cell death induced by Nampt downregulation (Fig. 3C). We have shown previously that inhibition of Sirt1, an NAD+-dependent enzyme, induces apoptosis in cardiac myocytes 9. Downregulation of both Nampt and Sirt1 failed to show additive effects upon increases in TUNEL positive myocytes (Fig. 3A), consistent with the notion that they may induce cardiac myocyte apoptosis via a common mechanism. Downregulation of Nampt did not significantly increase the number of necrotic myocytes, as evaluated by in situ ligation of hairpin probes with blunt ends (hairpin 2) 17 (Fig. 3D). Treatment of myocytes with 1.2 mM MMS, induced a significant increase in hairpin 2 labeled cardiac myocytes, thereby serving as a positive control. (Fig. 3D). Propidium iodide (PI) staining of unpermeabilized myocytes also showed that Nampt downregulation alone did not induce necrotic cell death (Fig. S3A). However, Nampt downregulation significantly increased PI positive myocytes in the presence of glucose deprivation (Fig. S3BC). Taken together, these results suggest that downregulation of Nampt induces apoptotic, but not necrotic, cell death at baseline, and could facilitate necrotic cell death under stress. Downregulation of Nampt slightly increased the activity of PARP, as evaluated by immunoblotting of proteins containing poly ADP-ribose polymer (Fig. S4), which would facilitate NAD+ depletion and cell death in cardiac myocytes.
Figure 3. Knockdown of Nampt causes apoptotic cell death in cardiac myocytes.
Cardiac myocytes were transduced with Ad-shRNA-scramble, Ad-shRNA-Nampt, or Ad-shRNA-Sirt1 alone or in combination. In some cases, Ad-LacZ or Ad-Bcl-xL was co-transduced. Myocytes were then cultured for 96 hours. (A) (upper) TUNEL staining of myocytes. Nuclei were counterstained with DAPI. (lower) Relative TUNEL-positive nuclei among different groups. The value in control myocytes was designated as 1. (B) Cell lysates were subjected to immunoblot analyses with anti-Nampt, anti-Bcl-xL, anti-cleaved caspase-3 (C. Caspase-3), and anti-tubulin antibodies. Myocytes treated with chelerythrine (CE, 10 µM for 2 hours) were used as a positive control for apoptosis. (C) Cardiac myocytes were transduced with Ad-shRNA-scramble (Scr) or Ad-snRNA-Nampt (Nampt) (3 MOI) and cultured for 5 days. The cytosolic fraction was subjected to immunoblot analyses with anti-cytochrome c (Cyto c), anti-cytochrome c oxidase I (Cox) and anti-tubulin antibodies. The mitochondrial fraction (Mito) was included as a positive control for Cox. (D) Nuclei were stained using the Texas Red labeled blunt end probe synthesized with pfu. Myocytes treated with 1.2 mM methylmethane sulfonate (MMS) for three hours were used as a positive control for necrotic cell death. (left) Representative images of DAPI and hairpin 2 staining and merged images. (right) Quantitative analyses of hairpin 2 positive cardiac myocytes. The value in control myocytes was designated as 1. * p<0.05 vs Ad-shRNA-scramble. The results are mean of 4 experiments.
Knockdown of Nampt inhibits autophagic flux
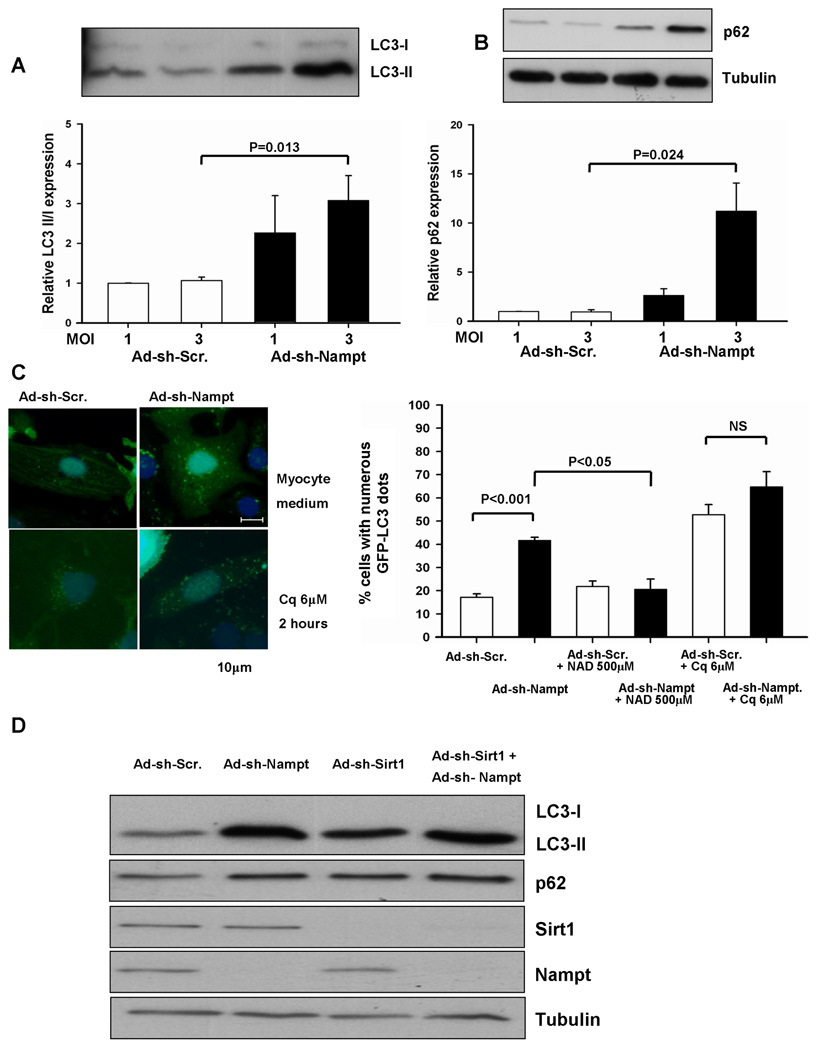
FK866, a potent inhibitor of Nampt, induces autophagy, but not apoptosis, in SH-SY5Y neuroblastoma cells 6. Since downregulation of Nampt decreases the ATP content in cardiac myocytes, we examined whether downregulation of Nampt induces autophagy, a compensatory mechanism which preserves cellular ATP levels 18. Knockdown of Nampt by Ad-shRNA-Nampt increased LC3-II, a lipidated form of LC3 that is an indicator of autophagosome accumulation, in a dose-dependent manner (Fig. 4A). Importantly, however, knockdown of Nampt also induced a significant increase in p62 (SQSTM1/sequestosome 1), a protein known to be degraded by autophagy 19–21 (Fig. 4B), suggesting that Nampt downregulation inhibits autophagic flux. Nampt knockdown did not affect mRNA expression of p62 (Fig. S5A), suggesting that increased p62 expression is unlikely to be due to increased production. In order to further examine whether downregulation of Nampt affects autophagic flux, we evaluated the effect of chloroquine, which inhibits autophagosome-lysosome fusion, upon accumulation of autophagosomes 22. Chloroquine treatment alone increased LC3-II and Ad-shRNA-Nampt failed to further increase LC3-II, suggesting that downregulation of Nampt does not increase autophagosome formation (Fig. S5B). Accumulation of autophagosomes was also evaluated by observation of GFP-LC3 dots. Downregulation of Nampt induced accumulation of GFP-LC3 dotes in cardiac myocytes, consistent with the results of the LC3-II immunoblot analysis. Accumulation of GFP-LC3 dots was not due to adenovirus transduction because it was not observed when control adenovirus was used. As expected, accumulation of GFP-LC3 dots was induced by chloroquine due to suppression of autophagosome-lysosome fusion. As with the LC3-II immunoblot analysis, downregulation of Nampt in the presence of chloroquine did not further increase autophagosome accumulation, again suggesting that downregulation of Nampt does not stimulate autophagic flux, but rather inhibits either autophagosome-lysosomal fusion or lysosomal degradation of autophagosome cargos (Fig. 4C).
Figure 4. Knockdown of Nampt affects autophagic flux, in a manner similar to chloroquine treatment.
(A and B) Myocytes were transduced with Ad-shRNA-scramble (Ad-sh-Scr.) or Ad-shRNA-Nampt (Ad-sh-Nampt) at indicated MOIs and cultured for 96 hours. Cell lysates were subjected to immunoblot analyses with anti-LC3 (A), anti-p62 (B), and anti-tubulin (B) antibodies. The values of LC3-II/LC3-I and p62 expression in myocytes transduced with Ad-shRNA-scramble are designated as 1. Experiments were conducted 3 times. (C) Myocytes were transduced with Ad-GFP-LC3 (10 MOI) and either Ad-shRNA-scramble or Ad-shRNA-Nampt at 3 MOI and cultured for 96 hours. Some myocytes were treated with chloroquine (Cq, 6 µM) for 2 hours or NAD+ (500 µM) for 24 hours. (left) Representative images of GFP-LC3 staining are shown. (right) The percentage of cells with punctate GFP-LC3 staining is shown. N=4. (D) Myocytes were transduced with Ad-shRNA-scramble, Ad-shRNA-Nampt, or Ad-shRNA-Sirt1 alone or in combination and cultured for 96 hours. Cell lysates were subjected to immunoblot analyses with anti-LC3, anti-p62, anti-Sirt1, anti-Nampt, and anti-tubulin antibodies. The results shown are representative of three experiments.
Expression and/or activation of several proteins (mTOR, AMPK, p70S6K, 4E–BP1 and Beclin-1) associated with autophagy were not significantly affected by knockdown of Nampt (Fig. S6). Exogenously applied NAD+ significantly reversed the accumulation of autophagosomes induced by knockdown of Nampt (Fig. 4C), suggesting that the effect of Nampt downregulation was mediated through decreases in NAD+.
The level of proteins containing acetyl-lysine was significantly increased after knockdown of Nampt in cardiac myocytes. The level of acetyl-lysine in Sirt1 substrates, including Histone H3 and H4, was also increased by Nampt knockdown (Fig. S7). These results are consistent with the notion that the downregulation of Nampt inhibits the activity of Sirt1. Since Sirt1 stimulates autophagy in HeLa and MEF cells 23, we examined the effect of Sirt1 knockdown upon autophagy in cardiac myocytes. Downregulation of Sirt1 by adenovirus harboring Ad- shRNA-Sirt1 caused accumulation of LC3-II and p62 (Fig. 4D), suggesting that, like downregulation of Nampt, downregulation of Sirt1 inhibits autophagic flux. Furthermore, Ad-shRNA-Nampt and Ad-shRNA-Sirt1 did not show additive effects upon accumulation of LC3-II and p62 (Fig. 4D). These results are consistent with the notion that downregulation of Nampt inhibits autophagic flux through suppression of Sirt1.
Generation of transgenic mice with cardiac specific overexpression of Nampt
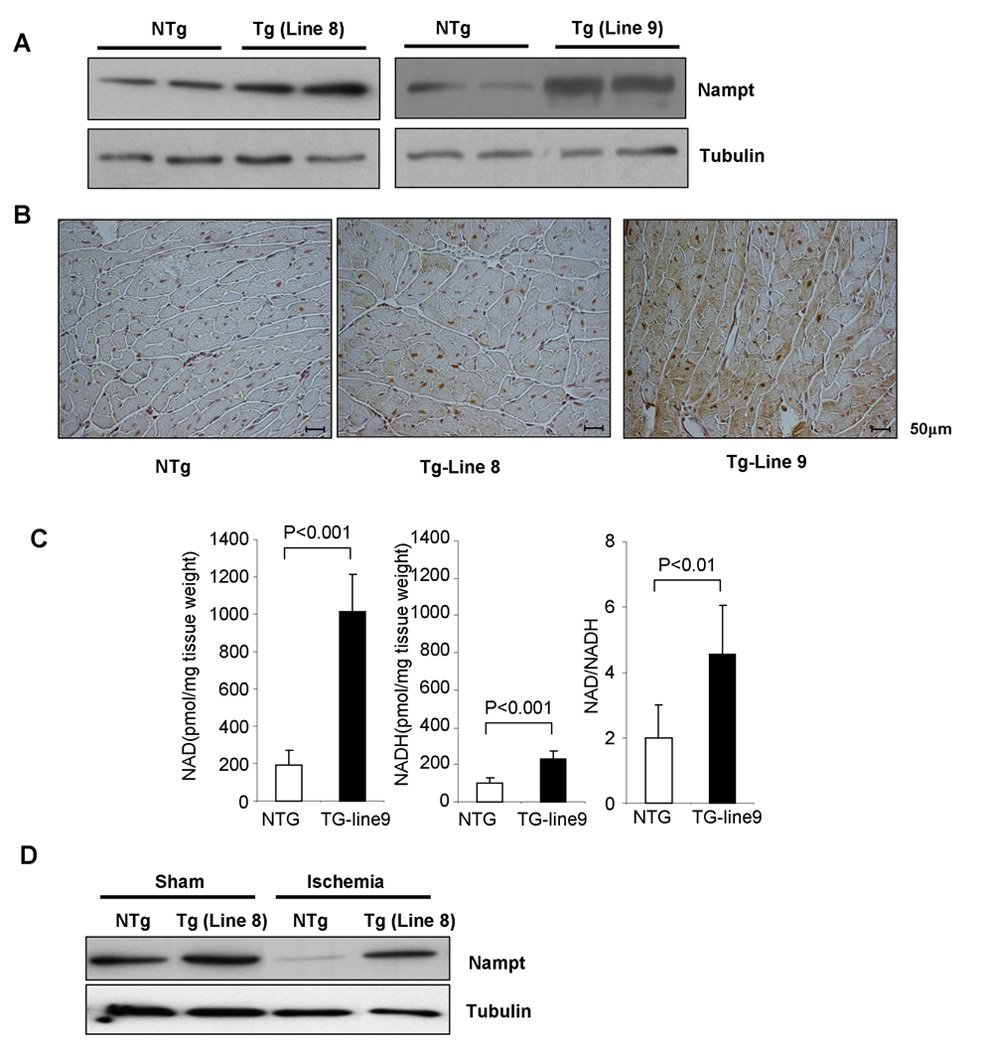
In order to examine the in vivo function of Nampt, transgenic mice with cardiac specific overexpression of Nampt (Tg-Nampt) were generated. Lines #8 and #9 expressed Nampt 2 fold and 3.5 fold in the heart, respectively, compared to respective control non-transgenic (NTg) mice (Fig. 5A). Immunostaining showed that expression of Nampt in Tg-Nampt mice was diffusely elevated in the cytosol of cardiac myocytes (lines #8 and #9) (Fig. 5B). Tg-Nampt mice exhibited a normal baseline cardiac phenotype: organ weight, cardiac chamber size, and left ventricular function in Tg-Nampt were not significantly different from those in NTg mice (Table S1 and S2). The level of NAD+, NADH, and NAD/NADH in the heart was significantly greater in Tg-Nampt than in NTg (Fig. 5C). Overexpression of Nampt in the heart in vivo induced greater increases in NAD+ content than that in cardiac myocytes in vitro (Fig. 2B). Although endogenous Nampt in the heart is downregulated in response to ischemia, the level of Nampt in Tg-Nampt mice after ischemia was equivalent to the basal level of Nampt in NTg (Fig. 5D).
Figure 5. Generation of Tg-Nampt mice.
(A) Representative immunoblots of heart homogenates obtained from Tg-Nampt and NTg mice with anti-Nampt and anti-tubulin antibodies. (B) Representative immunostaining of Nampt in left ventricular myocardial sections. (C) The level of [NAD+], [NADH] and [NAD+]/[NADH] in NTg and Tg-Nampt (line #9) was evaluated. Data represent the mean of 5–6 experiments ± SEM. (D) Tg-Nampt (line 8) or NTg control mice were subjected to ischemia or sham operation for 24 hours. Expression of Nampt and tubulin was determined by immunoblot analyses. The results shown are representative of three experiments.
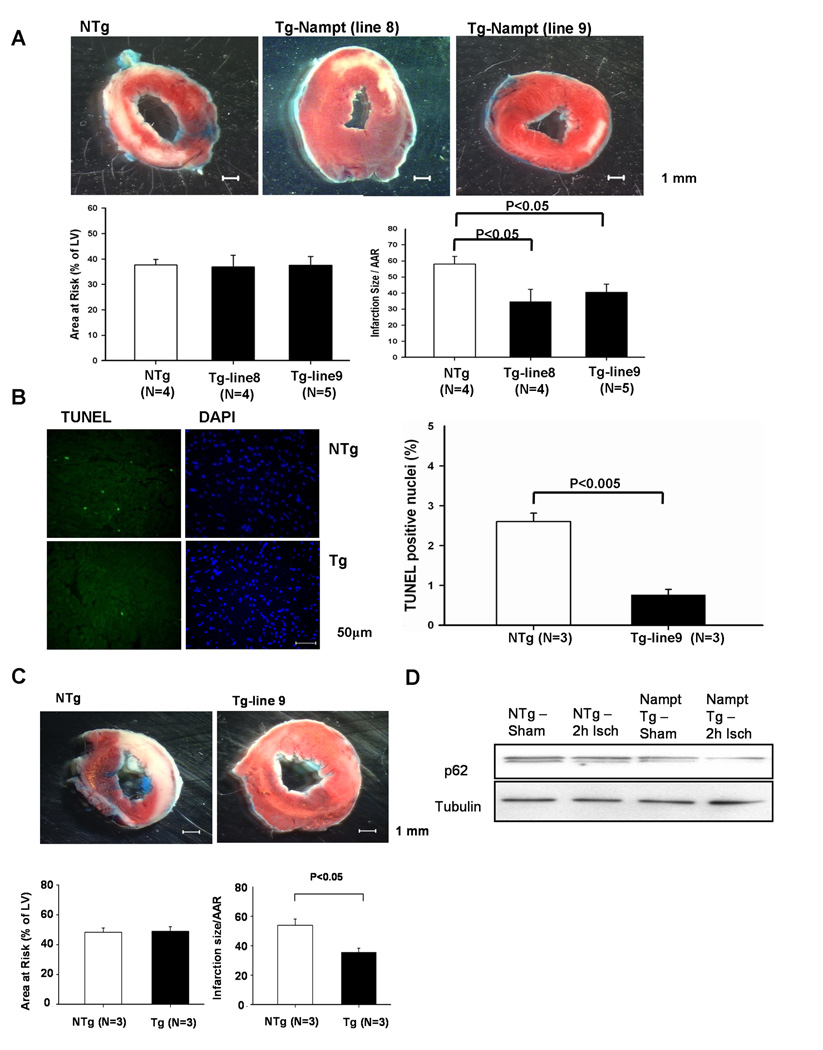
To examine the effect of Nampt upon myocardial injury due to I/R, Tg-Nampt and NTg mice were subjected to 45 minute of ischemia and twenty-four hours of reperfusion, and the size of myocardial infarction was evaluated by TTC staining (Fig. 6A). The size of the area at risk was not significantly different between Tg-Nampt and NTg mice (Fig. 6A). However, the size of myocardial infarction/area at risk after I/R was significantly smaller in Tg-Nampt mice than in NTg mice (Fig. 6A), and the number of TUNEL positive cells in the ischemic border zone was also smaller in Tg-Nampt mice than in WT mice (Fig. 6B). Protection against I/R injury in Tg-Nampt was observed in both lines #8 and #9. These results suggest that Nampt plays a protective role against I/R and that downregulation of Nampt may play an important role in mediating myocardial injury in response to ischemia and I/R.
Figure 6. I/R injury is attenuated in Tg-Nampt mice.
(A) Tg-Nampt and NTg mice were subjected to 45 minutes of ischemia and 24 hours of reperfusion. (upper) Gross appearance of LV myocardial sections after Alcian blue and triphenyltetrazolium chloride (TTC) staining. (lower left) The area at risk (AAR) (% of LV) was comparable between NTg and Tg-Nampt. (lower right) The infarction area/AAR was significantly smaller in Tg-Nampt than in NTg mice. (B) (left) LV myocardial sections were subjected to TUNEL and DAPI staining. Representative images of the staining in the border zone are shown. (right) The number of TUNEL-positive myocytes is expressed as a percentage of total nuclei detected by DAPI staining. (C and D) Tg-Nampt and NTg mice were subjected to 2 hours of ischemia. (C) (upper) Gross appearance of LV myocardial sections after Alcian blue and TTC staining. (lower left) The area at risk (AAR) (% of LV) was comparable between NTg and Tg-Nampt. (lower right) The infarction area/AAR was significantly smaller in Tg-Nampt than in NTg. (D) Expression of p62 and tubulin at the baseline and after 2 hours of ischemia was determined by immunoblot analyses. The results shown are representative of five experiments.
Since downregulation of Nampt inhibits autophagy, we hypothesized that increased expression of Nampt during ischemia may protect the heart by stimulating autophagy. The size of myocardial infarction/area at risk after 2 hours of ischemia was significantly smaller in Tg-Nampt than in NTg (Fig. 6C). Furthermore, the level of autophagic flux after 2 hours of ischemia was greater in Tg-Nampt than in NTg, as indicated by less accumulation of p62 in Tg-Nampt (Fig. 6D). Taken together, these results are consistent with the notion that downregulation of Nampt during ischemia may be detrimental, in part due to inhibition of autophagic flux.
Discussion
Expression of Nampt and its contribution to NAD+ synthesis are cell type-dependent. Our results clearly suggest that Nampt is both necessary and sufficient for the regulation of the intracellular level of NAD+ in cardiac myocytes. It should be noted, however, that a 75% reduction of Nampt expression by shRNA-induced knockdown causes only a 30% reduction in intracellular NAD+ content, suggesting that the NAD+ may also be synthesized from alternative sources, including nicotinic acid and tryptophan, in cardiac myocytes 5. Alternatively, only a small amount of Nampt may be sufficient to maintain the cellular level of NAD+ in cardiac myocytes. It should be noted that Nampt overexpression induced greater increases in NAD+ contents in the heart in vivo than in cardiac myocytes in vitro. We speculate that the availability of NAD+ precursors, which could be greater in the heart in vivo than in cardiac myocytes kept in culture medium in vitro, may affect the relative importance of Nampt mediated NAD+ production among the various mechanisms of NAD+ synthesis.
Expression of Nampt is significantly downregulated under stress in the heart in vivo, at both protein and mRNA levels. Downregulation of Nampt may contribute to decreases in NAD+ during pathological conditions in the heart. During heart failure, enzymes consuming NAD+, such as PARP 24 and Sirt1, are upregulated 10, whereas Nampt is downregulated. Nampt downregulation also stimulates PARP (Fig. S4). This would induce conditions in which the cellular level of NAD+ is decreased. It has been shown that expression of Nampt is upregulated by hypoxia in cultured cardiac myocytes in vitro 4. However, upregulation was not observed in vivo at either the mRNA or protein level. Interestingly, expression of Nampt was significantly upregulated during aging in the mouse heart in vivo, although the level of NAD+ was similar between 1–2 months and 12–14 months of age (Fig. S8). Upregulation of Nampt during aging may be a compensatory mechanism to maintain the cardiac level of NAD+. Thus, expression of Nampt is regulated in a stimulus-dependent manner. The molecular mechanism regulating expression of Nampt remains to be elucidated.
One of the most prominent actions of Nampt in cardiac myocytes is to regulate the cellular ATP level. A positive correlation was observed between the cellular Nampt level and both NAD+ and ATP. Decreases in the cellular ATP content due to knockdown of Nampt were normalized by exogenously supplied NAD+. Transfer of electrons is one the most important functions of NAD+ as a co-enzyme, and the energy produced by glycolysis and the citric acid cycle is transferred to NAD+ by its reduction to NADH. Our results suggest that Nampt could be an important target for modulation of the energy status in cardiac myocytes.
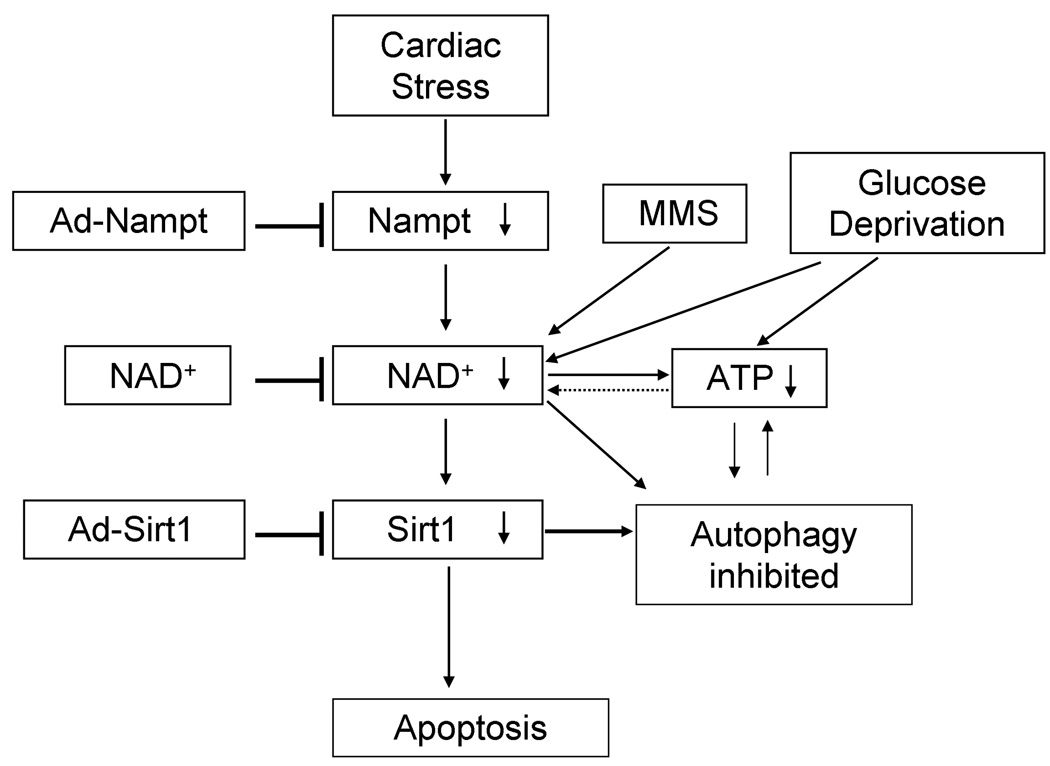
Our results suggest that the downregulation of Nampt causes increases in cell death in cardiac myocytes (Fig. 7). The cell death induced by Nampt knockdown has characteristic features of apoptosis mediated through a mitochondrium-dependent mechanism, but not necrosis or autophagic cell death because 1) the cell death was accompanied by cytochrome c release and inhibited by Bcl-xL, 2) there was no increase in propidium iodide positive nuclei or hairpin 2 ligation positive cells 17, and 3) the cell death was not accompanied by increases in autophagy. The fact that downregulation of Nampt induces apoptosis, which is an energy consuming process, despite the fact that Nampt knockdown decreases the cellular ATP content, is intriguing. It is possible that decreases in cellular ATP content may not be severe enough to induce either autophagy or necrosis. In fact, decreases in the cellular ATP content due to Nampt knockdown were not as severe as those due to glucose deprivation. Downregulation of Nampt does increase necrotic cell death during glucose starvation, suggesting that more severe ATP depletion causes necrosis. One mechanism mediating apoptosis induced by downregulation of Nampt is suppression of Sirt1, an NAD+-dependent enzyme. Although Nampt downregulation causes only a partial reduction in NAD+, Nampt may directly channel NAD+ into the active site of Sirt1 25. We have shown previously that downregulation of Sirt1 induces apoptosis in cardiac myocytes 9. Activation of PARP-1, an NAD+-consuming enzyme, induces death of cardiac myocytes through suppression of Sirt1 24. The fact that downregulation of both Nampt and Sirt1 failed to show an additive effect upon myocyte death is consistent with the notion that these two molecules are in the same signaling pathway inducing death of cardiac myocytes.
Figure 7. Current hypothesis.
The proposed Nampt pathway in cardiac myocytes under stresses
Our results suggest that downregulation of Nampt inhibits autophagic flux in cardiac myocytes. Since downregulation of Nampt potently induces accumulation of p62 and mimics the action of chloroquine, a chemical inhibitor of autophagosome lysosome fusion 22, we speculate that downregulation of Nampt inhibits either autophagosome-lysosomal fusion or degradation of the cargos in the lysosome, which would explain the accumulation of LC3-II in the presence of Nampt downregulation. Since inhibition of autophagic flux is normalized by addition of NAD+, basal autophagy in cardiac myocytes must be NAD+-dependent, which is intriguing because autophagy is inhibited even in the presence of mild energy starvation, a condition which favors autophagy. Downregulation of Nampt during myocardial ischemia may possibly exacerbate energy deprivation through suppression of autophagy. Consistent with this notion, inhibition of Nampt downregulation reduced the size of myocardial infarction after prolonged ischemia.
FK866, an inhibitor of Nampt, induces autophagy in neuroblastoma cells 6. Although FK866 induced increases in LC3 positive vesicles in neuroblastoma cells, whether or not FK866 also stimulates lysosomal degradation of autophagosome cargo has not been tested. FK866 treatment did not induce apoptosis in neuroblastoma cells, whereas downregulation of Nampt induces apoptosis in cardiac myocytes. We speculate that the role of NAD+ in mediating different forms of cell death may be cell type-dependent. Alternatively, inhibition of Nampt may affect the cellular NAD+ level differently in different cell types.
Since the histone deacetylase activity of Sirt1 is NAD+-dependent, downregulation of Nampt causes suppression of Sirt1 activity. Recent evidence suggests that Sirt1 stimulates autophagy through deacetylation of Atgs in cancer cells 23. Downregulation of Sirt1 increased accumulation of p62, suggesting that it suppresses autophagic flux, thereby mimicking the effect of Nampt downregulation in cardiac myocytes. Furthermore, downregulation of both Nampt and Sirt1 did not show additive effects upon inhibition of autophagic flux, consistent with the notion that Nampt may inhibit autophagic flux through suppression of Sirt1. Further investigation is required to elucidate the role of Sirt1 in mediating the effect of Nampt downregulation upon autophagy in cardiac myocytes. An electron transport chain present in lysosomes maintains a proton gradient at the expense of NADH 26. Decreases in NAD+ may directly inhibit the function of lysosomes, which may also contribute to the suppression of autophagy induced by downregulation of Nampt.
Nampt can act as a secreted hormone, termed visfatin, and may exert insulin-mimetic effects 27. However, overexpression of Nampt in cardiac myocytes stimulates neither Akt/p70S6K signaling in vitro nor cardiac hypertrophy in vivo, suggesting that the action of Nampt as an extracellular ligand may be negligible at a level of up to 4 fold overexpression in the heart.
Nampt expression in the heart significantly reduced the size of myocardial infarction after ischemia and I/R, suggesting that Nampt has a protective function in the heart in vivo. Since downregulation of endogenous Nampt after ischemia and I/R is normalized in Tg-Nampt, the result suggests that downregulation of Nampt has a causative role in mediating myocardial injury during ischemia or I/R. Since downregulation of Nampt decreases cellular ATP content, increases apoptosis and inhibits autophagy, we speculate that all of these contribute to myocardial injury after I/R. Reperfusion after prolonged ischemia causes release of NAD+ from mitochondria through mPTP opening and subsequent downregulation of NAD+ 28, which would exacerbate myocardial damage by I/R. Importantly, since the detrimental effects of Nampt downregulation in cardiac myocytes appear to be rescued by exogenously applied NAD+, increasing the cellular level of NAD+ by targeting Nampt may be a promising strategy to reduce injury from I/R. Increased Nampt provides protection against cell death and requires an intact mitochondrial NAD+ salvage pathway, as well as the mitochondrial NAD+-dependent deacetylases SIRT3 and SIRT4, in vitro. 4 Whether or not this holds true for the in vivo heart has not been tested. Further studies should elucidate the downstream molecular mechanism by which NAD+ protects the heart from ischemic injury.
Supplementary Material
Acknowledgement
The authors thank Daniela Zablocki for critical reading of the manuscript.
Sources of funding
This work was in part supported by U.S. Public Health Service Grants HL 059139, HL067724, HL069020, AG023039, AG027211, and HL 91469.
Non-standard Abbreviations and Acronyms
- Ad
adenovirus
- I/R
ischemia/reperfusion
- MMS
methylmethane sulfonate
- Nampt
nicotinamide phosphoribosyltransferase
- NTg
non-transgenic
- PARP
poly(ADP-ribose) polymerase
- PI
propidium iodide
- PNC
pyrazinamidase/nicotinamidase
- TAC
transverse aortic constriction
- Tg
transgenic
Footnotes
Subject codes: 131, 151
Disclosures
None.
References
- 1.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 3.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- 6.Billington RA, Genazzani AA, Travelli C, Condorelli F. NAD depletion by FK866 induces autophagy. Autophagy. 2008;4:385–387. doi: 10.4161/auto.5635. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 9.Alcendor RR, Kirshenbaum LA, Imai S, Sadoshima J. Sir2α, a longevity factor and a class III histone decetylase, is an essential endogenous inhibitor of apoptosis in cardiac myocytes. Circ Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- 10.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 11.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CP, Odewale I, Alcendor RR, Sadoshima J. Sirt1 protects the heart from aging and stress. Biol Chem. 2008;389:221–231. doi: 10.1515/BC.2008.032. [DOI] [PubMed] [Google Scholar]
- 13.Sadoshima J, Montagne O, Wang QM, Yang GP, Warden J, Liu J, Takagi G, Karoor V, Hong C, Johnson GL, Vatner DE, Vatnerin SF. The MEKKI-JNK pathway plays a protective role in pressure overload, but does not mediate cardiac hypertrophy. J. Clin. Invest. 2002;110:271–279. doi: 10.1172/JCI14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. Faseb J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 15.Horton JK, Stefanick DF, Wilson SH. Involvement of poly(ADP-ribose) polymerase activity in regulating Chk1-dependent apoptotic cell death. DNA Repair (Amst) 2005;4:1111–1120. doi: 10.1016/j.dnarep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto S, Seta K, Morisco C, Vatner SF, Sadoshima J. Chelerythrine Rapidly Induces Apoptosis through Generation of Reactive Oxygen Species in Cardiac Myocytes. J Mol Cell Cardiol. 2001;33:1829–1848. doi: 10.1006/jmcc.2001.1446. [DOI] [PubMed] [Google Scholar]
- 17.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 19.Bjorkoy G, Lamark T, Johansen T. p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy. 2006;2:138–139. doi: 10.4161/auto.2.2.2405. [DOI] [PubMed] [Google Scholar]
- 20.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 21.Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4 doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 25.Denu JM. Vitamins and aging: pathways to NAD+ synthesis. Cell. 2007;129:453–454. doi: 10.1016/j.cell.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Gille L, Nohl H. The existence of a lysosomal redox chain and the role of ubiquinone. Arch Biochem Biophys. 2000;375:347–354. doi: 10.1006/abbi.1999.1649. [DOI] [PubMed] [Google Scholar]
- 27.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 28.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.