Abstract
We tested the traditional hypothesis that an abnormally enhanced renal reclamation of dietary NaCl alone initiates its pressor effect (“salt-sensitivity”). Under metabolically controlled conditions, we grouped 23 normotensive African Americans as either salt-sensitive (SS) or salt-resistant (SR), depending on whether or not dietary NaCl-loading did or did not increase mean arterial blood pressure (MAP) by ≥ 5mmHg. We determined whether dietary NaCl-loading induces: a) greater increases in external Na+ balance, plasma volume and cardiac output (CO) in SS, compared to any in SR; and b) differential changes in systemic vascular resistance (SVR) that could account for the pressor differences between SS and SR. Using impedance cardiography, we measured CO and SVR daily at 4-hr intervals throughout the last 3 days of a 7-day period of low NaCl intake, 30mmol/d, and throughout a subsequent 7-day period of NaCl-loading, 250mmol/d. In the 11 SS, compared to the 12 SR, NaCl-loading induced no greater increases in Na+ balance, body weight, plasma volume and CO. Yet, from days 2 to 7 of NaCl-loading, changes of MAP in SS diverged progressively from those in SR. From days 2 to 4, progressive increases of MAP in SS reflected importantly impaired decreases of SVR, as judged from “normal” decreases of SVR in SR. In SS and SR combined, changes in both MAP and SVR on day 2 strongly predicted changes in MAP on day 7. In many normotensive African Americans, vascular dysfunction is critical to the initiation of a pressor response to dietary NaCl.
Keywords: blood pressure, sodium chloride, electrolyte balance, vascular resistance, cardiac output
Introduction
Salt-sensitivity, blood pressure (BP) that varies directly with dietary NaCl, characterizes much of human “essential” hypertension and increases the likelihood of the occurrence of hypertension, cardiovascular disease and death.1–3 It is widely formulated that dietary NaCl induces a persisting pressor effect only by an abnormal enhancement of its renal reclamation that entrains over days this physiologic sequence: positive Na+ balance, plasma volume expansion, a transient increase in cardiac output (CO) and a sustained increase in systemic vascular resistance (SVR). As formulated, the increase in CO peaks during the initial 3–4 day period of NaCl-loading, when it alone elicits the initial pressor effect of NaCl, and SVR remains “normal.” The pressor effect is sustained by the increase in SVR which occurs in normal autoregulatory response to the increase in CO.4–6 Although the restrictively renal dysfunction formulated accords with many observations,7, 8 recent observations in animal models of genetically determined salt-sensitive hypertension accord with the formulation that dietary NaCl-loading can induce a pressor effect that depends on a dysfunctional vascular response to dietary NaCl.9, 10 Neither formulation has been rigorously tested in humans. That would require examining the effect of NaCl-loading on BP, Na+ balance, plasma volume, CO and SVR over a closely observed, extended time course, both in those who are, and are not, salt-sensitive. In so examined normotensive African Americans, in whom BP is frequently salt-sensitive,11 we find that in many, vascular dysfunction is a major pathogenic factor in initiating the pressor effect of dietary NaCl.
Methods
Participants
We studied 23 healthy African Americans, ages 30 to 55 years with screening BP of 115–155/70–95 mmHg and without history or clinical evidence of renal disease, ischemic heart disease, stroke, or diabetes. An additional subject was excluded from analysis because she inadvertently received a non-steroidal anti-inflammatory drug during the study. One subject left on day 6 of NaCl-loading. Her data is included. The study was approved by the University of California San Francisco Committee on Human Research. All procedures followed were in accordance with institutional guidelines. All participants gave written informed consent.
Setting
Participants were admitted to the General Clinical Research Center at UCSF for a 2-week course of study. Throughout the study, participants ate a eucaloric metabolic diet, with calories derived from protein 16%, carbohydrates 50% and fat 34%, and drank deionized water. Per 70 kg of body weight (BW) the basal diet provided 30 mmol Na+ and 45 mmol K+. Physical activity was limited to daily walks on the center’s one floor.
Intervention (NaCl Load)
Week 1 served as baseline period. Throughout week 2, the basal diet was supplemented with NaCl, 220 mmol per 70 kg of BW per day, added to the diet as table salt and broth for a total daily NaCl intake of 250 mmol per 70 kg per day (but no more than 300 mmol per day).
Assessment of Salt-Sensitivity
With an automated oscillometric device (Dinamap, Criticon Inc. Tampa, Florida), programmed to obtain 5 readings within 5 minutes, BP was measured daily every 4 hours after 5 minutes of supine rest; an average daily BP was calculated. To determine salt-sensitivity the average mean arterial pressure (MAP) of days 5, 6 and 7 during NaCl-restriction, was subtracted from the average MAP of days 5, 6 and 7 during NaCl-loading. Salt-sensitivity was defined as a NaCl-induced increase in MAP of ≥5 mmHg, salt-resistance as an increase of <5 mmHg. This is a more rigorous criterion than that used in our previous study in which we compared the frequency of salt-sensitivity in normotensive Caucasians with that in African Americans.11
Hemodynamic Outcomes
Throughout the final three days of the low-NaCl period and throughout the 7-day period of NaCl-loading, CO was measured at 4-hour intervals (between 6AM and 10PM, immediately following BP measurements) using impedance cardiography (BioZ ICG monitor, Cardiodynamics, San Diego). ICG sensors were placed on the neck and chest according to the manufacturer’s instructions. Data were averaged over 30-beat intervals for 5 minutes. SVR was calculated as (MAP-CVP)/CO where CVP is the central venous pressure, assumed to be 6 mmHg, and MAP is the mean arterial pressure measured during acquisition of impedance data. Daily averages of CO and SVR were calculated.
A meta-analysis of 154 studies published between 1966 and 1997, comparing impedance cardiography with a variety of reference methods such as Fick and thermodilution showed an overall correlation of 0.82.12 Impedance cardiography has excellent intra-subject reproducibility.13 In the current study, during three days of baseline measurements, the mean within-subject coefficient of variation for average daily values of CO was 2.8%, of SVR 3.1%, of heart rate 2.3%, and of MAP 1.4%.
Metabolic Outcomes
BW was measured daily at 6AM. Spontaneously voided urine was collected daily over 24-hour periods and analyzed for Na+ and creatinine. Net external balance of Na+ was calculated from its dietary intake and urinary output. On day 7 of the baseline period, and on days 2 and 7 of the NaCl-loading period, blood samples were obtained at 9AM to determine levels of serum electrolytes, creatinine, total protein, hematocrit, plasma renin activity (PRA) and aldosterone by standard techniques. Change in plasma volume was estimated from NaCl-induced changes in both serum protein concentration and hematocrit values.
Data Analysis
Effects of NaCl-loading in SS and SR subjects, respectively, were calculated as % change from baseline values where baseline value is defined as the average value of days 5, 6 and 7 of the low-NaCl period. NaCl-induced hemodynamic and metabolic changes on days 2 and 7 of NaCl-loading are predetermined primary outcomes. Unpaired and paired t-tests, respectively, were used for between-group (SS vs SR) and within-group (NaCl effect) comparisons. Non-parametric tests were used when variances were unequal. Relationships between variables were explored using regression analysis. Data is presented as mean and 95% C.I. The null hypothesis was rejected at P<0.05. P values were adjusted for multiple comparisons using the Bonferroni method. Analyses were carried out using “Statistica” (Statsoft Inc., Tulsa, OK).
Results
Eleven of 23 subjects (48%) were salt-sensitive (SS), mean NaCl-induced ΔMAP 10.4 (6.9/14) mmHg; 12 (52%) were salt-resistant (SR), mean ΔMAP 0.2 (−1.2/1.6) mmHg. SS subjects were slightly older than SR but SS and SR did not differ from each other with respect to admission BP, serum electrolytes and creatinine (Table 1). All subjects had a BMI of less than 31 and, using the MDRD study equation,14 a calculated GFR of >60mL/min per 1.73m2. At the end of the low-NaCl period SS and SR were not different with respect to MAP (SS: 85 (80/90) mmHg, SR: 90 (84/96) mmHg); CO (SS: 5.3 (4.9/5.8) L/min, SR: 5.5 (4.9/6.1) L/min); heart rate (HR; SS: 65 (58/71) bpm, SR 72 (67/78) bpm); stroke volume (SV; SS: 84 (75/93) mL, SR: 78 (65/92) mL) and SVR (SS: 15.0 (13.7/16.3) mmHg per L/min, SR: 16.0 (14.3/17.7) mmHg per L/min).
Table 1.
Demographic characteristics of salt-sensitive (SS) and salt-resistant (SR) subjects
| Variable | SS | SR | Total N or average of SS and SR combined |
|---|---|---|---|
| N | 11 | 12 | 23 |
| Gender | 2F | 2F | 4F |
| Age (years) * | 49 (45/52) | 43 (38/48) | 46 |
| BMI | 25 (23/27) | 26 (24/28) | 26 |
| SBP (mmHg) † | 122 (113/131) | 128 (121/135) | 125 |
| DBP (mmHg) † | 78 (72/85) | 74 (67/80) | 76 |
| MAP (mmHg) † | 93 (86/100) | 92 (85/98) | 92 |
| Heart rate (bpm) † | 66 (61/71) | 71(61/81) | 69 |
| Serum Creatinine (mg/dL) | 1.0 (0.9/1.1) | 1.0 (0.9/1/1) | 1.0 |
| cGFR (mL/min/1.73m2) ‡ | 85 (80/91) | 92 (77/107) | 89 |
| Serum Na+ (mmol/L) | 140 (139/141) | 141 (139/143) | 141 |
| Serum K+ (mmol/L) | 4.3 (4.1/4.4) | 4.2 (4.1/4.3) | 4.2 |
| Serum Cl− (mmol/L) | 106 (104/106) | 107 (105/109) | 106 |
| Hematocrit (%) | 43.5 (41/46) | 42.4 (41/44) | 43.0 |
Data is presented as mean and 95%CI.
SS subjects are slightly older than SR subjects, P<0.05. Otherwise, SS and SR subjects are very similar. Metabolic data was collected during screening visits, in general about two weeks prior to admission.
on admission;
cGFR denotes calculated GFR using the MDRD study equation.14
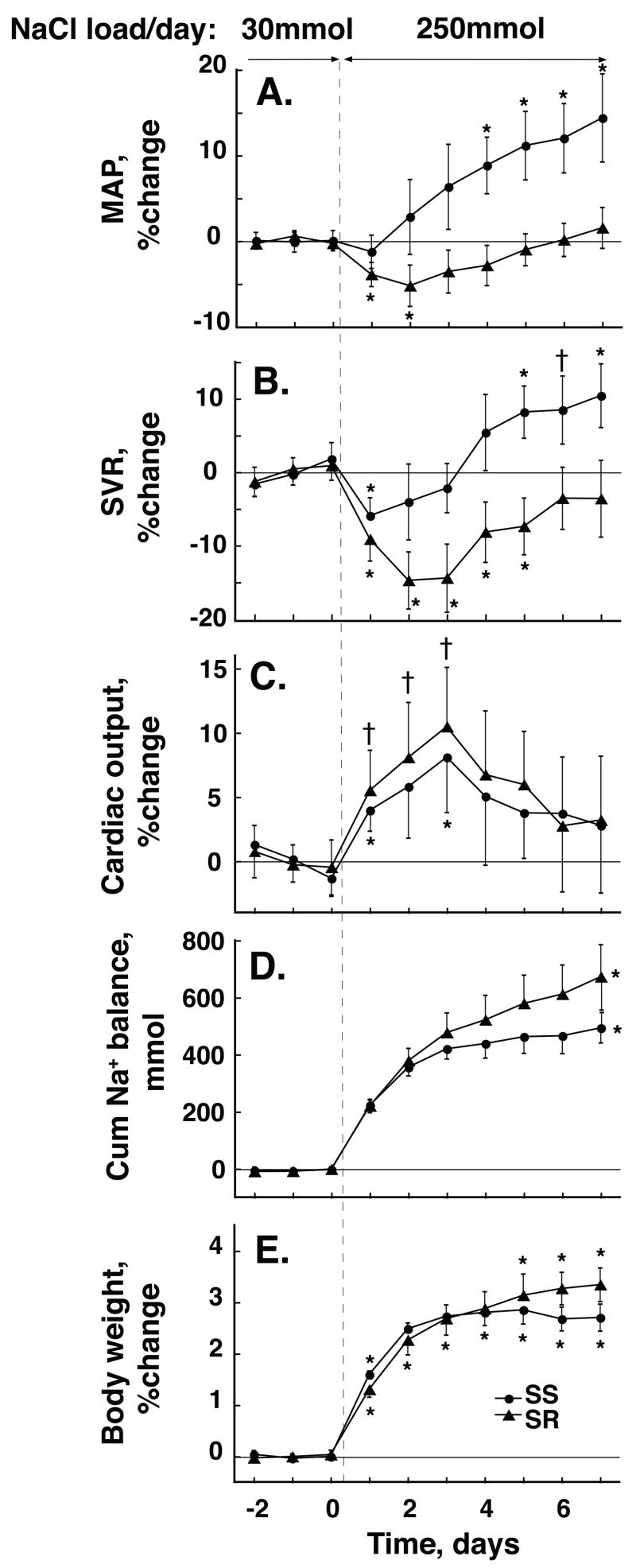
In SR NaCl-loading induced a transient but significant decrease in MAP during the first two days of its in initiation. MAP then increased progressively to values not different from those at baseline. In contrast, in SS, MAP increased progressively from day 2 of NaCl-loading (Figure 1A). ΔMAP on day 2 was −4.7 mmHg in SR and +2.4 mmHg in SS, P<0.01. ΔMAP on day 7 was +1.4 mmHg in SR and +14.4 mmHg in SS, P<0.001 (Table 2).
Figure 1. Time course of NaCl-induced changes in mean arterial pressure, MAP, systemic vascular resistance, SVR, cardiac output, CO, cumulative Na+ balance and change in body weight, BW, in salt-resistant (SR,▲) and salt-sensitive (SS, ●) subjects.
Values are shown as percent change from baseline (average of day 5 through 7 of low-salt) except for Na+ balance which is measured in mmol. Values are means and 95% C.I. ⋆ P<0.01 and † P<0.05, respectively, compared to low-salt period. Responses of MAP and SVR to NaCl-loading differ significantly in SS vs SR from day 2. Responses of CO and BW do not differ between groups. Net cumulative Na+ balance is slightly but significantly more positive in SR than in SS by day 7.
Table 2.
Effect of dietary NaCl on hemodynamics, body weight, net external Na+ balance, hematocrit, and serum protein and electrolyte concentrations during 7 consecutive days of NaCl-loading: Difference between salt-sensitive (SS) and salt-resistant (SR) subjects.
| Day | ΔMAP (mmHg) | ΔSVR (mmHg/L/min) | ΔCardiac Output (L/min) | ΔHeart Rate (bpm) | ΔStroke Volume (mL) | ΔBody Weight (kg) | ΔCumulative Na+ Balance (mmol) |
|---|---|---|---|---|---|---|---|
| 1 | 2.4 (0.53/4.3) | 42 (−7/91) | −0.01 (−0.28/0.10) | −0.4 (−2.8/2.1) | 0 (−3.6/3.6) | 0.21 (−0.08/0.49) | 2 (−26/31) |
| 2 * | 7.1 (3.2/11.0) † | 137 (60/214) † | −0.11 (−0.43/0.20) | 1.3 (−3.0/5.6) | −2.8 (−9.2/3.7) | 0.16 (−0.35/0.66) | −23 (−74/28) |
| 3 | 8.6 (4.3/13.0) † | 156 (83/228) † | −0.11 (−0.42/0.19) | 1.2 (−4.3/6.6) | −3.1 (−9.5/3.3) | 0.06 (−0.48/0.59) | −57 (−132/19) |
| 4 | 10.1 (6.9/13.3) ‡ | 170 (93/248) † | −0.07 (−0.43/0.29) | 2.7 (−2.0/7.5) | −3.4 (−10/3.4) | −0.05 (−0.74/0.64) | −83 (−180/13) |
| 5 | 10.4 (7.1/13.8) ‡ | 194 (132/257) ‡ | −0.09 (−0.37/0.18) | 2.1 (−3.1/7.3) | −2.6 (−8.8/3.7) | −0.25 (−1.09/0.58) | −117 (−228/−6) |
| 6 | 10.2 (7.0/13.5) ‡ | 149 (81/217) † | 0.09 (−0.27/0.45) | 2.9 (−2.6/8.5) | −1.2 (−8.7/6.3) | −0.50 (−1.17/0.16) | −146 (−261/−30) |
| 7 * | 10.9 (6.8/15.0) ‡ | 168 (86/250) † | 0.04 (−0.34/0.42) | 4.2 (−1.7/10) | −3.0 (−10/4.4) | −0.55 (−1.26/0.16) | −179 (−300/−59) § |
| Day | ΔSerum Protein (g/dL) | ΔHematocrit (%) | ΔSerum Na+ (mmol/L) | ΔSerum Cl− (mmol/L) | ΔSerum K+ (mmol/L) | ||
| 2 * | −0.055 (−0.44/0.33) | −0.40 (−1.9/1.1) | −1.2 (−3.5/1.1) | −3.1 (−5.8/−0.4) | −0.26(−0.41/−0.10) † | ||
| 7 * | 0.008 (−0.36/0.37) | 0.84 (−1.2/2.9) | −1.9 (−4.1/0.3) | −2.7 (−5.0/−0.4) † | −0.11(−0.31/0.08) | ||
Data is displayed as difference of differences, ȳSS − ȳSR ||, whereby ȳSS and ȳSR denote the mean NaCl-induced difference from baseline (i.e. average of the last 3 days of low-NaCl diet) in SS and SR subjects, respectively, on days 1 through 7 of NaCl-loading. 95% lower and upper confidence limits, shown in parenthesis, were calculated from the pooled SD. MAP, mean arterial pressure; SVR, systemic vascular resistance.
Hemodynamic and metabolic differences on days 2 and 7 of NaCl-loading were predetermined as primary outcomes;
SS vs SR, P<0.01;
SS vs SR, P<0.001; and
SS vs SR, P<0.05, whereby P values are adjusted according to Bonferroni;
ȳSS − ȳSR = (SSHiNa-SSLowNa) − (SRHiNa-SRLowNa).
In SR NaCl-loading induced a highly significant decrease in SVR that reached its nadir, 14.7% below baseline, on day 2. In SS, NaCl-loading induced a much smaller although significant, decrease in SVR that reached its nadir, −5.9%, on day 1 (Figure 1B). On day 2 in SS, the difference from baseline, −4%, was not significant. In SR, SVR returned to levels not different from baseline by day 6 of the NaCl load, whereas in SS, SVR rose significantly, to 8.2% above baseline, by day 5 and remained significantly increased thereafter. On all days but on day 1, changes in SVR differed highly significantly between SR and SS; P<0.01 on day 2 and 7, respectively (Table 2). On each day from day 2 to 7, the average increase in SVR in SS, 3.1% per day (1.6/4.5), closely approximated that in SR, 2.6% per day (1.5/3.6), indicating that the slopes of changes in SVR were parallel in the two groups.
In both SS and SR subjects, NaCl-loading induced near immediate, similar, transient increases in CO (Figure 1C, Table 2). In most subjects the maximum increase occurred on day 2 or 3 and amounted in SS to 10.5% (6.6/15.2) above baseline, and in SR to 11.6% (6.8/16.3), P=ns. In both SS and SR, values of CO on day 7 of NaCl-loading were significantly lower than those on day 3 and not significantly different from baseline.
In both SS and SR, the increase in CO resulted from an increase in SV which, in both groups, increased progressively during the initial 3-day period of NaCl-loading and thereafter remained increased above baseline by 10% (6/14) in SS and by 15% (7/24) in SR; SS vs. SR, P=ns. With NaCl-loading in both groups, HR decreased progressively over time by 6% (2/10) in SS and by 9% (4/15) in SR; SR vs. SS, P=ns (Table 2).
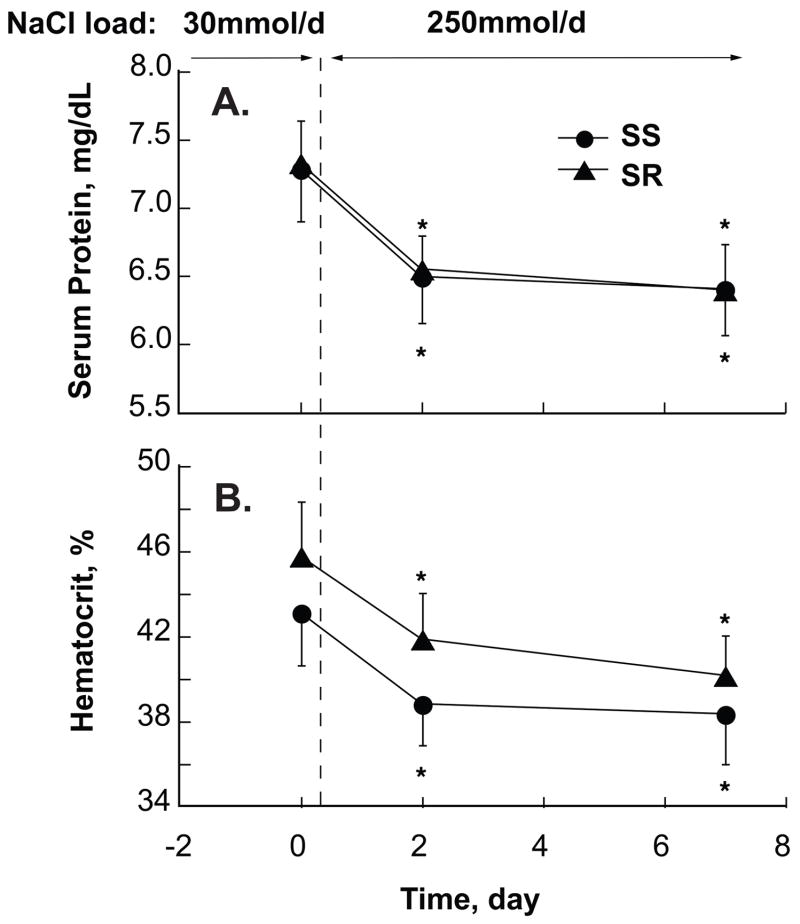
The 7-day net cumulative Na+ balance was slightly but significantly greater in SR, 673 (560/785) mmol, than SS, 493 (441/545) mmol, P<0.05 (Figure 1D). The NaCl-induced increase in BW was slightly, although not significantly, greater in SR than SS (Figure 1E). In both groups changes in BW as well as Na+ balance were largest in the first two days of, and persisted throughout, the NaCl-loading period (Table 2). By day 2 of NaCl-loading, plasma protein concentration had decreased highly significantly from its baseline value in both SR, −11% (−13/−8) and SS, −11% (−15/−8); SR vs SS P=ns (Figure 2A). This decrease persisted unchanged on day 7 in both SR, −12.5% (−17/−8), and SS −11% (−14/−8), indicating a similarly substantial and persisting expansion of plasma volume in both groups. In both groups, NaCl-loading induced a decrease in hematocrit values of about 12% on both days 2 and 7 (Figure 2B).
Figure 2. NaCl-induced changes in serum protein concentration (A) and hematocrit values (B).
In salt-resistant (SR, ▲) and salt-sensitive (SS, ●) subjects serum protein concentration and hematocrit values are significantly and similarly reduced by day 2 of NaCl-loading and remain so by day 7, indicating a persistent significant and similar NaCl-induced increase in plasma volume in both groups. ⋆ P<0.05, respectively, compared to low-salt period.
During NaCl-restriction but not during NaCl-loading, serum levels of Na+ and Cl− were significantly lower in SR than in SS. In SR, NaCl-loading induced significant increases in Na+ and Cl− on both day 2 and 7. In SS, NaCl-loading induced a significant increase in Cl− but not in Na+ on day 7. During NaCl-restriction levels of serum K+ were similar in SS and SR. In SS but not in SR, NaCl-loading induced a significant decrease in serum K+. Baseline values and NaCl-induced changes in PRA and aldosterone on days 2 and 7 of NaCl-loading did not differ between groups.
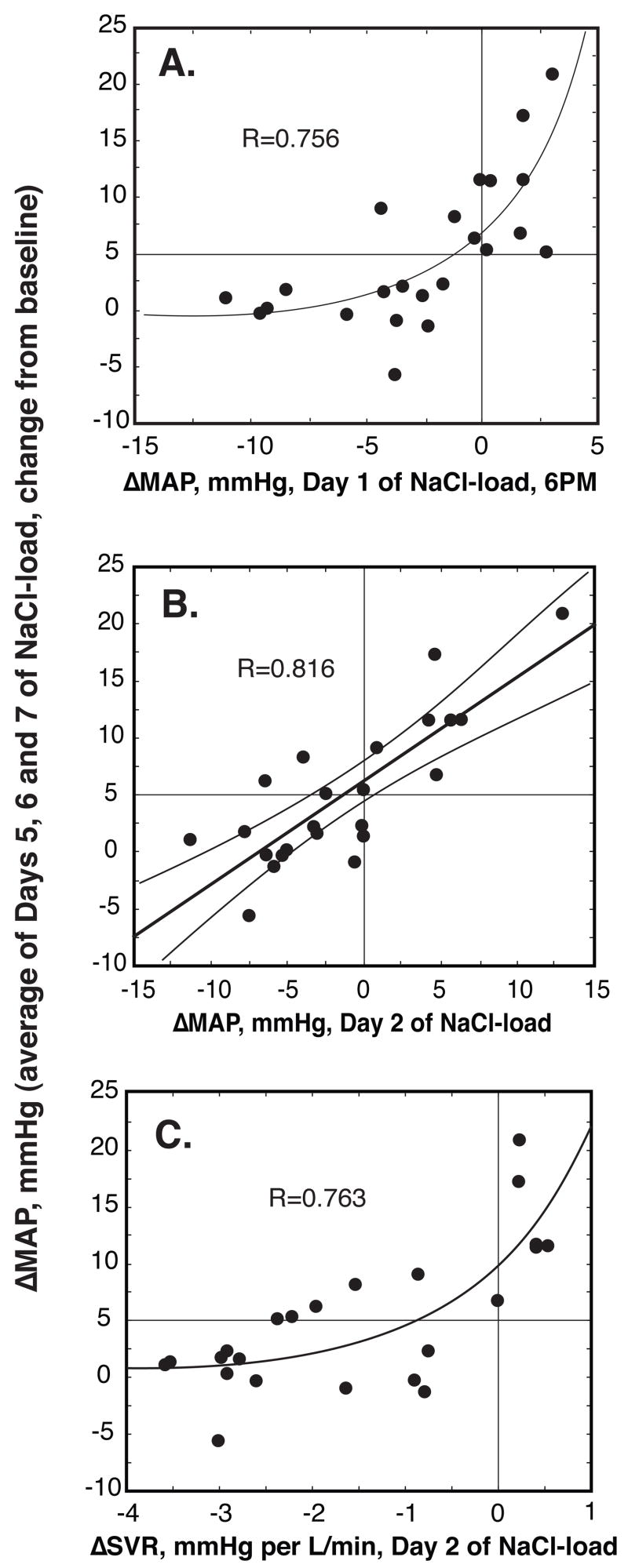
In all subjects combined, changes in MAP on day 2 were strongly predictive of those on day 7, R=0.756, P<0.001, as well as of the average pressor response of days 5, 6 and 7 (Figure 3A). In fact, the pressor response at 6PM on day 1, i.e. nine hours after initiating the NaCl load, when subjects had ingested only about 150 mmol of NaCl per 70 kg of BW, was strongly predictive of the pressor response on day 7, R=0.726, P<0.001, as well as of the average pressor response of days 5, 6 and 7 (Figure 3B). Similarly, the NaCl-induced changes in SVR on day 2 were highly predictive of the pressor response on day 7, R=0.713, P<0.001 as well as of the average pressor response of days 5, 6 and 7 (Figure 3C). Changes in CO were not predictive of changes in MAP.
Figure 3. Relationship between NaCl-induced changes in average MAP of days 5, 6 and 7 of NaCl-loading and initial NaCl-induced changes in MAP and SVR.
(A) ΔMAP, day 1 at 6PM; (B) ΔMAP, day 2, 24-hr average; (C) ΔSVR, day 2, 24-hr average. The initial NaCl-induced changes in MAP and SVR are highly predictive of NaCl-induced changes in average MAP of days 5, 6 and 7. Changes in MAP at 6PM in panel (A) are relative to the average values measured at this time during the 3-day period preceding NaCl-loading; changes in MAP and SVR in panels (B) and (C) are relative to the 24-hr mean values measured during the 3-day period preceding NaCl-loading.
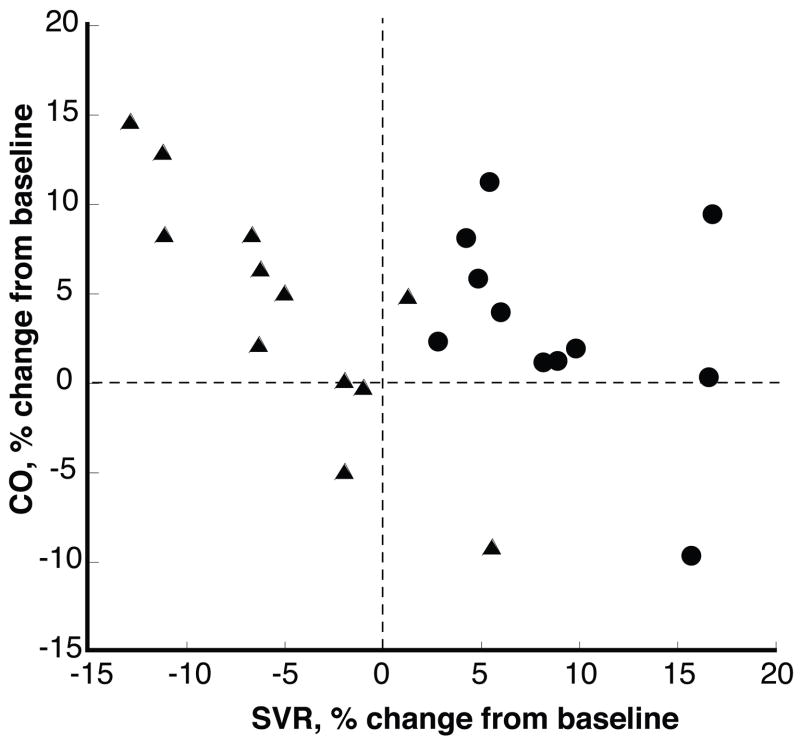
During the last three days of NaCl-loading SVR was increased in all 11 SS. In two of them the increase in SVR was accompanied by a slightly greater increase in CO and in two SS SVR and CO were increased similarly (Figure 4). In all but two SR, SVR was decreased. In SR, but not in SS, there was a strong inverse relationship between the changes in CO and SVR, R=0.873.
Figure 4. Relationship between NaCl-induced changes in CO and SVR in SR and SS.
Values are averages of days 5, 6 and 7 of NaCl-loading expressed as % change form baseline. SVR was above baseline in all SS (●) and below baseline in most SR (▲). In two of the SS the increase in SVR was accompanied by a slightly greater increase in CO and in another two SVR and CO were increased similarly. In SR, but not in SS, changes in CO were inversely related to changes in SVR.
Discussion
According to the now classic “nephrogenic” formulation, the effect of dietary NaCl on BP in those grouped as SS would differ from that in those grouped as SR only because the renal reclamation of NaCl would be greater in the SS, and hence evoking of greater sequential increases in external Na+ balance, plasma volume and CO.4, 5, 15 But with NaCl-loading in the current study, the increases in these variables in the SS did not exceed those in the SR, and hence could not alone have dictated the pressor effect in the SS, nor could a greater renal reclamation of NaCl.
However, over the initial 3-day period of NaCl-loading in the current study, SVR decreased sharply in the SR, but changed little in the SS. Indeed, a decrease in MAP attended the initial decrease in SVR in SR. Similarly discordant effects on SVR and MAP16–18 have also been noted in the first 3-day period of NaCl-loading in animal models of salt-sensitive hypertension and their salt-resistant controls. That SVR might be an important determinant of the initial pressor effect of dietary NaCl does not comport with the traditional “nephrogenic” formulation of salt-sensitivity.4, 5 This formulation holds that during the initial several days of NaCl loading, SVR remains “normal” i.e. little changed from baseline, hence not determining of the initial pressor effect of dietary NaCl, sharp increases in CO fully accounting for that effect.4, 5 The main observations cited in support of this formulation are those of Guyton and his colleagues in their classic studies of the 70% nephrectomized dog in which NaCl-loading in an amount “about five times normal” induced immediate increases in MAP and CO several times those of the current study.19 Over the first several days in these studies, SVR changed relatively little, decreasing slightly, compared to the 40 % increase in CO, which fully accounted for a similar increase in MAP during this initial period of NaCl-loading. Subsequently, CO progressively decreased to values approaching those at baseline, while a reciprocally increasing SVR became the dominant determinant of the sustained pressor effect of dietary NaCl. The increase in SVR is formulated to be the normal autoregulatory response to an increase in CO that excessively perfuses tissue. This increase in SVR is the only way formulated that SVR can be a determinant of the pressor effect of NaCl.
But in the studies of the 70% nephrectomized dog, control observations were not reported. In later studies of two non-surgical animal models of salt-sensitive hypertension, the angiotensin II-infused dog16 and the Dahl salt-sensitive (DS) rat,17 Cowley and his colleagues observed that in both the normal canine control and the Dahl salt-resistant (DR) rat, there occurred NaCl-induced increases in CO similar to those induced in the salt-sensitive animals. In further accord with the current observations, in both salt-resistant control animals, the increases in CO were offset by a “reciprocal reduction” in SVR which accounted for the nonoccurrence of a NaCl-induced pressor effect in the normal dog,16 and a transient decrease in BP in the DR rat.17 Ganguli et al made similar observations in Dahl rats.18 In the salt-resistant animals, the observation that the NaCl-induced increase in CO did not evoke an increase in SVR would “appear to contradict the theory of autoregulation.”16 If it is assumed that the “reciprocal reduction” of SVR observed in the salt-resistant animals represents a normal vasodilatory response to dietary NaCl, the observations taken as a whole permit the inference that in the salt-sensitive animals, an impaired vasodilatory response to NaCl-loading can be a major determinant initiating the pressor effect of dietary NaCl.20
The current studies are the first comparing salt-sensitive and salt-resistant normotensive (or hypertensive) humans with respect to the effect of NaCl-loading on the time courses of changes in BP, CO and SVR. Thus, given that SVR decreased sharply during the early course of NaCl-loading in the SR – as might define a normal vasodilatory response in this population of study subjects, under the conditions of this study – the observation in the SS that SVR changed little during this period does not indicate that SVR remained “normal”, but suggests instead a systemic vascular dysfunction that is expressed as an impaired vasodilatory response to dietary NaCl-loading.
At pointed odds with the traditional formulation of salt-sensitivity, the initial pressor effect of dietary NaCl depends on such a vascular dysfunction. Only in combination with it could the NaCl-induced transient increase in CO have induced an initial pressor effect in the SS. Since dietary NaCl induced similar transient increases in CO in the SS and SR, dietary NaCl could not have induced its initial pressor effect had it also induced a decrease in SVR like that it induced in the SR. In single-point echocardio-graphically derived measurements of SVR made on the last day of a 4-day period of NaCl-loading, Sullivan et al. observed this variable to decrease in SR, and to change relatively little in SS.21 Since at that time, as in the current study, CO was similarly moderately increased in SR and SS, the authors concluded the pressor effect of NaCl in SS reflected impairment of a normal NaCl-induced decrease in SVR. Indeed, in the currently studied SR significant decreases in SVR persisted on the 4th and 5th day of NaCl-loading but not thereafter, when SVR was substantially increased in the SS and that increase was on average the major determinant of the pressor effect of NaCl. It might be argued that in the SR, a NaCl-induced decrease in SVR caused the increase in CO by entraining renal retention of NaCl that expands plasma volume. However, in the normal dog16 and in the salt-resistant humans studied by Sullivan,21 as in the currently studied SR, HR did not increase with NaCl-loading when SVR decreased, as such a mechanism would predict, at least early in the course of NaCl-loading. Indeed, in the currently studied SR, as in the SS, HR decreased with NaCl-loading.
By the end of the NaCl-loading period, SVR was increased in all 11 SS, but in two of these, a persisting increase in CO was the dominant determinant of the increase in MAP. In another two SS, the increase in MAP appeared to be determined as much by an increase in CO as by that in SVR. Thus, within the SS group, there may be subgroups which differ qualitatively or temporally from each other with respect to hemodynamic response to NaCl-loading, much as proposed by Sullivan who suggested that some SS are “output responders” whereas others are “resistance responders.”21
Yet, in most of the currently studied subjects, a major vascular determinacy of the pressor effect of dietary NaCl-loading would seem to operate from its outset. In all subjects combined, the changes induced in SVR, and in MAP, on day 2 of NaCl-loading are highly predictive of the changes induced in MAP on day 7. Indeed, on the first day of NaCl-loading in all subjects, the change in MAP occurring at 6PM, relative to the MAP occurring at this time during the preceding 3-day period of NaCl-restriction, are also highly predictive of the change in MAP induced by NaCl-loading on day 7. In the SR, NaCl-loading induced over the initial 2-day period a progressive decrease in both SVR and MAP indicating the operation of a robust anti-pressor systemic vasodilatation in response to an increasing plasma volume and CO. By contrast, by day 2 of NaCl-loading in the SS, SVR and MAP were not significantly changed from baseline despite similar increases in plasma volume and CO. Yet, from day 2 to 7 of NaCl-loading, SVR increased in parallel in SR and SS, which suggests that by day 2, the pressor effect of dietary NaCl in the SS is modulated by, and importantly determined by, a strong opposing vasodilatory effect of dietary NaCl. The current observations provide evidence that in many healthy normotensive African Americans, an impaired vasodilatory response to dietary NaCl can be a major pathogenic factor in the pressor effect of dietary NaCl. A “vasogenic” initiation of salt’s pressor effect might in some instances have a sympathetic or “hormonal-sympathetic” basis, several possible mechanisms of which have been described.10, 20, 22–24
That Na+ balance in the SS was not greater than that in the SR might seem inconsistent with the capacity of an increase in renal perfusion pressure to effect an increase in urinary Na+ excretion. This capacity assumes a relatively unhindered transmission of the perfusion pressure. It has been reported that in African Americans with either salt-sensitive hypertension25 or normotensive salt-sensitivity,8 but not in those without salt-sensitivity, NaCl-loading over a week induces a decrease in renal blood flow, and hence an increase in renal vascular resistance. In the salt-sensitive normotensive subjects, the reported extent of the decrease in renal blood flow and the increase in renal vascular resistance varied directly with that of the pressor effect of NaCl. Thus, with such a NaCl-induced renal impairment in the current study, pressure natriuresis might be quite subtle, and take the form of a less positive Na+ balance than that occurring without the pressor effect in SS subjects, as the current data might suggest. Such a renal impairment could well be part of a more general vascular dysfunctional response to dietary NaCl-loading in the salt-sensitive state.
Limitations
The current studies were performed in a selected group of people, normotensive middle-aged African Americans, and thus the results may not apply to other populations or instances of salt-sensitivity. However, for all animal models of salt-sensitivity in which a salt-resistant control group was studied along with the salt-sensitive group results similar to the current ones were reported.16–18
We calculated SVR from measured values of MAP and CO and an arbitrary constant value of CVP as is commonly done, particularly in healthy individuals in whom a relatively modest increase in circulatory volume over several days is unlikely to induce a substantial increase in CVP. In calculating SVR other investigators have also made the tacit assumption that CVP or right atrial pressure does not change with NaCl-loading.16–18 Yet, in DS but not in DR rats right atrial pressure increased slightly but significantly by 2.8 mmHg when when NaCl-intake was increased 16-fold for five days and by 1.5 mmHg when it was increased 7-fold, 26 as in the current study. Such a small NaCl-induced increase of CVP in SS but not in SR would have resulted in smaller NaCl-induced group differences in SVR than those reported. However, negation of NaCl-induced group differences in SVR would require substantially greater increases in CVP, given the fact that group differences in NaCl-induced changes of BP were >10 mmHg.
Perspectives
That the initiation of some human salt-sensitivity associates more with diminished (initial) systemic vasodilation in response to dietary NaCl-loading than with an abnormally enhanced renal reclamation of Na+ and Cl− expands the scope of potential mechanisms of human salt-sensitivity and thereby the range of potential preventive and therapeutic approaches to much of human hypertension.
Acknowledgments
The authors would like to thank the nursing, kitchen and laboratory staff of the General Clinical Research Center, University of California, San Francisco, for their excellent assistance in conducting these studies and the study participants for their invaluable commitment of time and their willingness to follow a very demanding study protocol.
Sources of Support
Studies were carried out in the General Clinical Research Center, Moffitt/MZ Hospital, University of California, San Francisco, with funds provided by the National Center for Research Resources, M0 RR-00079, U.S. Public Health Service. This research was supported in addition by NIH/NHLBI Grant RO1-HL64230 and gifts from the Emil Mosbacher, Jr., Foundation.
Abbreviations
- SS
salt-sensitive
- SR
salt-resistant
- MAP
mean arterial pressure
- CO
cardiac output
- SVR
systemic vascular resistance
- BW
body weight
- ICG
impedance cardiography
- BP
blood pressure
Footnotes
Conflicts of Interest
None
References
- 1.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure: age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt Sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 4.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 5.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 6.Meneton P, Jeunemaitre X, De Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz TW, Al-Bander HA, Morris RC., Jr “Salt-sensitive” essential hypertension in men” Is the sodium ion alone important? N Engl J Med. 1987;317:1043–1048. doi: 10.1056/NEJM198710223171702. [DOI] [PubMed] [Google Scholar]
- 8.Schmidlin O, Forman A, Tanaka M, Sebastian A, Morris RC., Jr NaCl-induced renal vasoconstriction in salt-sensitive African-Americans: Antipressor and hemodynamic effects of potassium bicarbonate. Hypertension. 1999;33:633–639. doi: 10.1161/01.hyp.33.2.633. [DOI] [PubMed] [Google Scholar]
- 9.Schmidlin O, Tanaka M, Bollen AW, Yi SL, Morris RC., Jr Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension. 2005;45:867–873. doi: 10.1161/01.HYP.0000164628.46415.66. [DOI] [PubMed] [Google Scholar]
- 10.Brooks VL, Osborn JW. Hormonal-sympathetic interactions in long-term regulation of arterial pressure: an hypothesis. Am J Physiol. 1995;268:R1343–R1358. doi: 10.1152/ajpregu.1995.268.6.R1343. [DOI] [PubMed] [Google Scholar]
- 11.Morris RC, Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt-sensitivity: Effects of race and dietary potassium. Hypertension. 1999;33:18–23. doi: 10.1161/01.hyp.33.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Raaijmakers E, Faes TJ, Scholten RJ, Goovaerts HG, Heethaar RM. A meta-analysis of three decades of validating thoracic impedance cardiography. Crit Care Med. 1999;27:1203–1213. doi: 10.1097/00003246-199906000-00053. [DOI] [PubMed] [Google Scholar]
- 13.Van De Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance cardiography: the next vital sign technology? Chest. 2003;123:2028–2033. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Cruz DN, Simon DB, Nelson-Williams C, Farhi A, Finberg K, Burleson L, Gill JR, Lifton RP. Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension. 2001;37:1458–1464. doi: 10.1161/01.hyp.37.6.1458. [DOI] [PubMed] [Google Scholar]
- 16.Krieger JE, Liard J-F, Cowley AW., Jr Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am J Physiol. 1990;259:H1629–H1636. doi: 10.1152/ajpheart.1990.259.6.H1629. [DOI] [PubMed] [Google Scholar]
- 17.Greene AS, Yu ZY, Roman RJ, Cowley AW., Jr Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol. 1990;258:H508–H514. doi: 10.1152/ajpheart.1990.258.2.H508. [DOI] [PubMed] [Google Scholar]
- 18.Ganguli M, Tobian L, Iwai J. Cardiac output and peripheral resistance in strains of rats sensitive and resistant to NaCl hypertension. Hypertension. 1979;1:3–7. doi: 10.1161/01.hyp.1.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Guyton AC, Granger HJ, Coleman TG. Autoregulation of the total systemic circulation and its relation to control of cardiac output and arterial pressure. Circ Res. 1971;XXVII, XXIX(Supp 1):I-93–I-97. [PubMed] [Google Scholar]
- 20.Fine DM, Ariza-Nieto P, Osborn JW. Does whole body autoregulation mediate the hemodynamic responses to increased dietary salt in rats with clamped ANG II? Am J Physiol. 2003;285:H2670–H2678. doi: 10.1152/ajpheart.00395.2003. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, Connor MJ. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension. 1987;9:398–406. doi: 10.1161/01.hyp.9.4.398. [DOI] [PubMed] [Google Scholar]
- 22.Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol. 2005;32:426–432. doi: 10.1111/j.1440-1681.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 23.Qi N, Rapp JP, Brand PH, Metting PJ, Britton SL. Body fluid expansion is not essential for salt-induced hypertension in SS/Jr rats. Am J Physiol. 1999;277:R1392–R1400. doi: 10.1152/ajpregu.1999.277.5.R1392. [DOI] [PubMed] [Google Scholar]
- 24.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 25.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension. 1991;18:805–812. doi: 10.1161/01.hyp.18.6.805. [DOI] [PubMed] [Google Scholar]
- 26.Reddy RS, Baylis C, Kotchen TA. Hemodynamic responses to acute volume expansion in Dahl salt-sensitive rats. Am J Physiol. 1991;260:R32–38. doi: 10.1152/ajpregu.1991.260.1.R32. [DOI] [PubMed] [Google Scholar]