Abstract
Several functional and structural imaging studies have investigated the neural basis of personality in healthy adults, but human lesions studies are scarce. Personality changes are a common symptom in patients with neurodegenerative diseases like frontotemporal dementia (FTD) and semantic dementia (SD), allowing a unique window into the neural basis of personality. In this study, we used the Interpersonal Adjective Scales to investigate the structural basis of eight interpersonal traits (dominance, arrogance, coldness, introversion, submissiveness, ingenuousness, warmth, and extraversion) in 257 subjects: 214 patients with neurodegenerative diseases such as FTD, SD, progressive non-fluent aphasia, Alzheimer’s disease, amnestic mild cognitive impairment, corticobasal degeneration, and progressive supranuclear palsy and 43 healthy elderly people. Measures of interpersonal traits were correlated with regional atrophy pattern using voxel-based morphometry (VBM) analysis of structural MR images. Interpersonal traits mapped onto distinct brain regions depending on the degree to which they involved agency and affiliation. Interpersonal traits high in agency related to left dorsolateral prefrontal and left lateral frontopolar regions, whereas interpersonal traits high in affiliation related to right ventromedial prefrontal and right anteromedial temporal regions. Consistent with the existing literature on neural networks underlying social cognition, these results indicate that brain regions related to externally-focused, executive control-related processes underlie agentic interpersonal traits such as dominance, whereas brain regions related to internally-focused, emotion- and reward-related processes underlie affiliative interpersonal traits such as warmth. In addition, these findings indicate that interpersonal traits are subserved by complex neural networks rather than discrete anatomic areas.
Keywords: neurodegenerative disease, personality, affiliation, agency, voxel-based morphometry
INTRODUCTION
Personality—an individual’s habitual pattern of cognition, emotion, and behaviour—is relatively stable and enduring (Endler, 2000). However, it is not entirely fixed throughout the lifespan, but is more likely to fluctuate during certain developmental phases such as early and late adulthood (Roberts, Walton, & Viechtbauer, 2006). These changes may be influenced in part by genetic predispositions (McCrae et al., 2000) or by environmental influences such as life experiences and age-related role expectations (Roberts & Caspi, 2003). Continuous changes in brain structure and function that occur throughout life also likely play a significant role. Accordingly, in people with brain injury or neurodegenerative disease, dramatic changes in personality can rapidly overcome established premorbid personality traits.
Studies measuring personality traits in a variety of neurodegenerative diseases such as frontotemporal dementia (FTD), Alzheimer’s disease (AD), and dementia with Lewy bodies (DLB) found that patients` social behaviours and personality traits change markedly relative to their premorbid state (Chatterjee, Strauss, Smyth, & Whitehouse, 1992; Galvin, Malcom, Johnson, & Morris, 2007; Rankin, Baldwin, Pace-Savitsky, Kramer, & Miller, 2005). FTD and AD patients demonstrated quantitative differences in degree and type of personality change (Chatterjee et al., 1992; Rankin, Kramer, Mychack, & Miller, 2003). The mildly increased introversion and submissiveness seen early in AD patients may be a realistic response to loss of cognitive capacity, rather than a direct effect of damage to social and emotional circuits in the brain, however, in FTD patients, personality changes relative to patients` premorbid state such as early decline in social interpersonal conduct and early impairment in regulation of personal conduct are core diagnostic features (Neary et al., 1998), and appear to directly result from brain damage. When FTD patients are grouped according to whether their atrophy is predominantly frontal or temporal, they show very different patterns of personality change (Rankin et al., 2003). The different patterns of personality change in different neurodegenerative diseases imply relationships between personality traits and brain structures.
Support for personality-brain structure relationships comes from a small study, which has linked FTD patients’ diminished agreeableness to volume loss in the right orbitofrontal cortex (Rankin et al., 2004). However, elucidation of the neural networks underlying different personality traits requires a more large-scale, detailed, whole-brain analysis in patients with divergent neurodegenerative diseases.
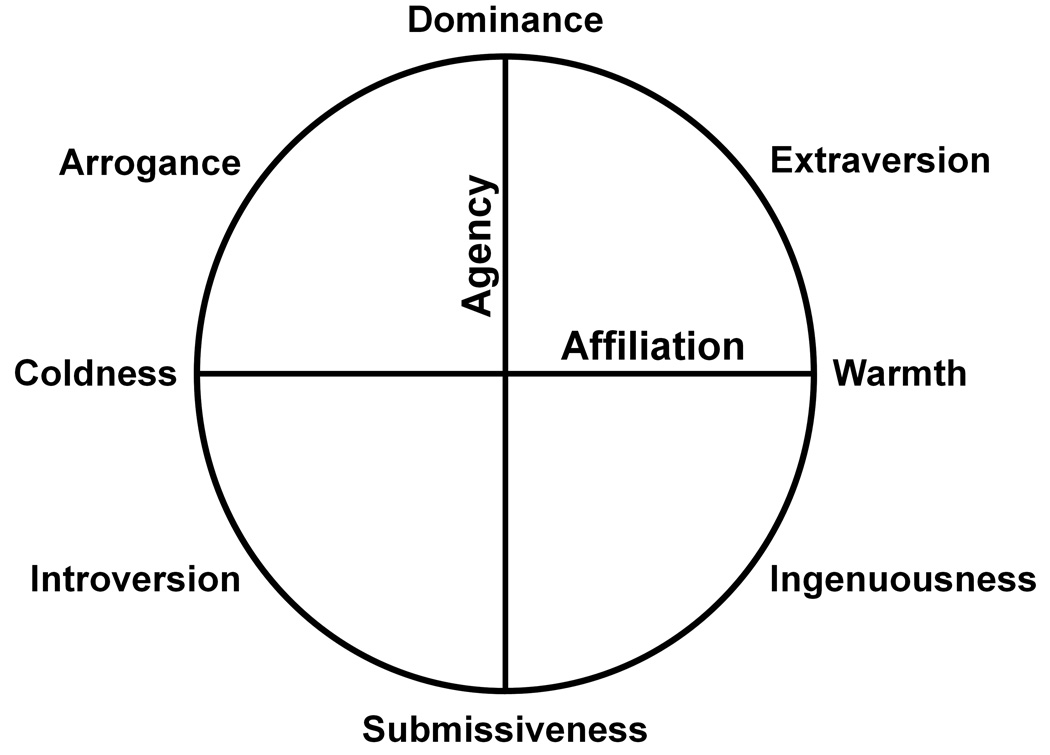
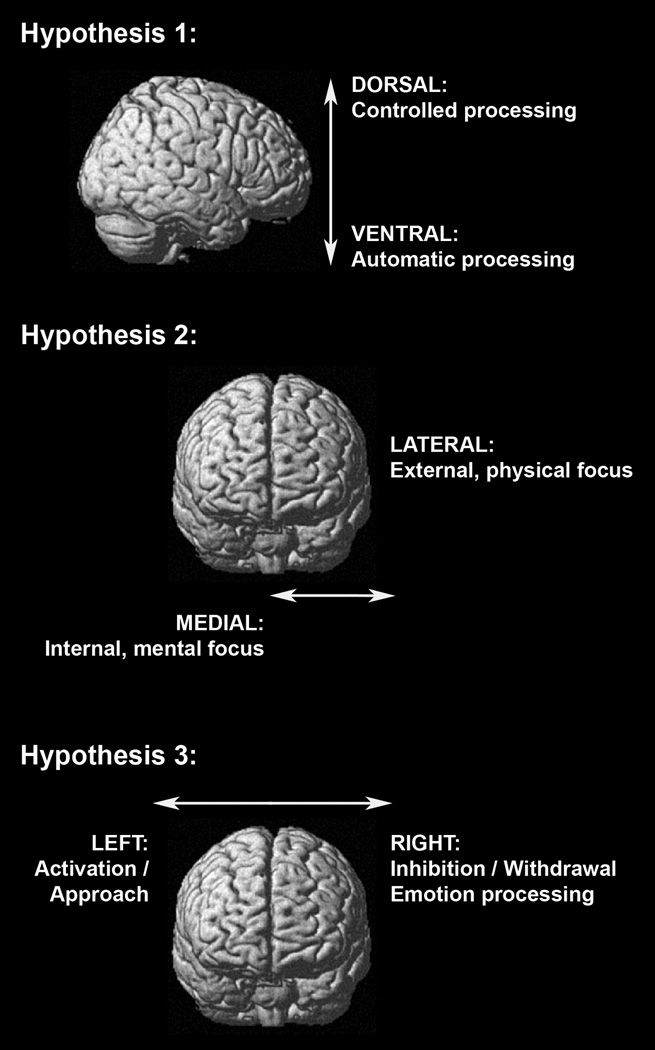
In this study, we hypothesized that specific interpersonal traits would correspond to anatomic changes in patients with various neurodegenerative diseases. To examine this hypothesis, we correlated quantitative measures of eight interpersonal traits using the Interpersonal Adjective Scales (IAS) (Fig. 1) with structural MR images using voxel-based morphometry (VBM) across the whole brain in a large cohort of subjects. The IAS circumplex, which is defined by the two dimensions of extraversion and agreeableness of the five-factor model (McCrae & Costa, 1989), was chosen, because we were most interested in the interpersonal and social dimensions of personality. We hypothesized that neural networks underlying interpersonal traits would be organized along three spatial gradients in the brain: a ventro-dorsal, a medio-lateral, and a right-left gradient (Fig. 2).
Figure 1.
The Interpersonal Circumplex of the Interpersonal Adjective Scales. Eight interpersonal traits, derived from two orthogonal dimensions, agency and affiliation, are evenly distributed around the circumference.
Figure 2.
Schematic anatomical organization of the three hypothesized spatial gradients of the brain: the ventro-dorsal (Hypothesis 1), the medio-lateral (Hypothesis 2), and the right-left (Hypothesis 3) gradient, displayed on a rendered standard brain from a single normal subject (MRIcron: ch2.nii.gz). The three hypotheses are based on existing models and meta-analyses of neuroimaging and electrophysiological studies of cognition, emotion and social behaviour (Amodio & Frith, 2006; Demaree et al., 2005; Koechlin et al., 2003; Lieberman, 2007; Northoff et al., 2006; Olsson & Ochsner, 2008; Petrides, 2005; Phillips et al., 2003).
These three spatial gradients are derived from existing models and meta-analyses of neuroimaging and electrophysiological studies of cognition, emotion, and social behaviour in humans and non-human primates. First, the ventro-dorsal gradient is clinically characterized by a continuum between more automatic, emotional, and stimulus-driven behaviour, mediated by more ventral prefrontal regions, versus more controlled, reflective behaviour, originating in more dorsal prefrontal regions (Amodio & Frith, 2006; Lieberman, 2007; Olsson & Ochsner, 2008; Phillips, Drevets, Rauch, & Lane, 2003). With respect to personality, therefore, we expected that traits involving emotionally affiliative behaviours such as warmth and ingenuousness (Fig. 1) would correlate predominantly with ventral prefrontal regions. Traits high in personal agency such as dominance and arrogance would correlate predominantly with dorsal prefrontal regions. Extraversion, a trait high in both emotional affiliation and personal agency, would probably have neural correlates in both ventral and dorsal prefrontal regions.
Second, the medio-lateral gradient is clinically characterized by a continuum between internally-focused processes, including emotions and thoughts, mediated by more medial fronto-parietal regions, versus more externally-focused, task-oriented, and controlled processes, originating in more lateral fronto-temporo-parietal regions (Koechlin, Ody, & Kouneiher, 2003; Lieberman, 2007; Northoff et al., 2006; Olsson & Ochsner, 2008; Tanji & Hoshi, 2008). Thus with regard to personality, we expected that traits involving higher levels of affiliation such as warmth or traits involving higher levels of internal orientation such as introversion would relate predominantly to midline fronto-parietal structures. Agentic traits involving higher levels of external orientation such as dominance, which reflects the tendency to negotiate with one’s environment to accomplish personal goals, and extraversion were expected to correlate with lateral fronto-temporo-parietal structures.
Finally, our hypothesis that there would be a right-left gradient to some personality traits draws support from three complementary models of brain lateralization: the right-hemisphere model, the dominance/submission model, and the approach/withdrawal model (e. g., Demaree, Everhart, Youngstrom, & Harrison, 2005). The right-hemisphere model, which is mainly based on human lesion studies, posits that the right hemisphere is specialized for the perception and expression of emotions (Adolphs, 2002; Borod, Bloom, Brickman, Nakhutina, & Curko, 2002; Demaree et al., 2005). With respect to personality, therefore, we expected that emotionally affiliative traits would be more likely to lateralize to the right hemisphere. Taken together, the dominance/submission and approach/withdrawal models suggest a left-lateralization for interpersonal dominance and approach behaviours, and right-lateralization for interpersonal submissiveness and withdrawal behaviours. Thus we expected that assertive, approach-related traits such as dominance and extraversion predominantly would be mediated by the left forebrain, whereas more interpersonally passive traits such as submissiveness and ingenuousness would relate to the right forebrain. Because extraversion involves both agentic and affiliative aspects, these three models suggest it would draw upon circuitry in both the left (agentic) and right (affiliative) hemispheres.
MATERIAL AND METHODS
Subjects
A total of 214 patients diagnosed with one of seven neurodegenerative diseases were recruited into the study. Each disease was included because its primary cause of symptoms is understood to be damage to cortical and/or subcortical grey matter structures. The patient sample included 47 patients who met the research diagnostic criteria for the frontotemporal dementia (FTD) variant of frontotemporal lobar degeneration (FTLD), 41 with the semantic dementia (SD) variant of FTLD, and six with the progressive nonfluent aphasia (PNFA) variant of FTLD (Neary et al., 1998). In addition to the FTLD patients, 64 subjects had Alzheimer’s disease (AD) (diagnosed by NINDS-ADRDA criteria (McKhann et al., 1984), 18 had amnestic mild cognitive impairment (aMCI) (Petersen et al., 1999), 25 had corticobasal degeneration (CBD) (Boxer, Geschwind et al., 2006), and 13 had progressive supranuclear palsy (PSP) (Boxer, Geschwind et al., 2006; Litvan et al., 1996). Patients were recruited from a dementia specialty clinic after their diagnosis was derived by a multidisciplinary team consisting of neurologists, neuropsychologists, psychiatrists, and nurses, who performed extensive behavioural, neuropsychological, and neuroimaging assessments.
Forty-three elderly normal controls (NC) were recruited through advertisements in local newspapers and talks at local senior community centres. For inclusion, subjects had to have a normal neurologic exam, Clinical Dementia Rating Scale (CDR) = 0, Mini-Mental State Examination (MMSE) ≥ 28/30, and verbal and visuospatial delayed memory performance ≥ 25th percentile. They also had to have an informant to corroborate their daily functioning.
There were several reasons for including patients from different diagnostic groups as well as healthy elderly people into the study. First, greater variance of both interpersonal behaviour and grey matter volume increased the study’s statistical power to detect brain-behaviour relationships across the whole brain. Inclusion of healthy elderly ensured that the normal end of the regression line was represented in all analyses, regardless of the brain region or behaviour in question. Also, because we expected that interpersonal traits would be mediated by several brain structures, inclusion of subjects with different brain atrophy patterns but a similar trait expression maximized our ability to identify multiple parts of the brain circuit underlying that trait. Finally, by including such a large sample, we increased our statistical power to detect the small effect sizes we expected to see between interpersonal traits and brain structure.
Patients seen at the clinic represented a broad sample of the population in terms of gender, education level, and socioeconomic status (Table 1). Among the patients, mean age was 64.9 (SD=9.5), and they averaged 16.1 (SD=2.9) years of education. There were 123 males and 91 females, with a mean CDR score of 0.9 (SD=0.6) and mean MMSE of 22.1 (SD=7.0). Statistically significant differences were seen across groups in age, gender, and MMSE, so these variables were included as confounding covariates into all analyses.
Table 1.
Characteristics of participant sample classified by diagnostic group. F-statistic and p-values are for overall diagnostic group differences, identified using SAS proc GLM. Interpersonal trait scores were controlled for age, gender, and MMSE. FTD = Frontotemporal Dementia variant of Frontotemporal Lobar Degeneration; SD = Semantic Dementia variant of Frontotemporal Lobar Degeneration; PNFA = Progressive Nonfluent Aphasia variant of Frontotemporal Lobar Degeneration; AD = Alzheimer’s Disease; aMCI = Amnestic Mild Cognitive Impairment; CBD = Corticobasal Degeneration; PSP = Progressive Supranuclear Palsy; NC = Normal Controls. Values are listed as Mean (Standard Deviation).
| M (SD) | FTD n=47 |
SD n=41 |
PNFA n=6 |
AD n=64 |
aMCI n=18 |
CBD n=25 |
PSP n=13 |
NC n=43 |
F-statistic (df) |
P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 61.4 (7.8) | 63.8 (7.7) | 66.0 (9.8) | 66.6 (11.7) | 70.9 (9.4) | 64.0 (8.1) | 65.1 (5.9) | 67.6 (9.0) | 3.06 (7, 249) | .009 |
| Education | 16.4 (2.1) | 16.1 (3.0) | 15.3 (2.2) | 15.8 (3.3) | 17.5 (2.5) | 15.2 (2.6) | 16.7 (3.0) | 17.0 (2.7) | 1.79 (7, 239) | .09 |
| Gender (M/F) | 34/13 | 26/15 | 4/2 | 33/31 | 11/7 | 9/16 | 6/7 | 13/30 | χ2(7, N=257)=21.90 | .003 |
| MMSE | 23.0 (7.5) | 20.5 (8.1) | 18.0 (9.5) | 20.2 (5.7) | 28.3 (1.9) | 22.2 (6.8) | 26.2 (2.8) | 29.6 (0.6) | 13.59 (7, 249) | <.0001 |
| CDR | 1.2 (0.6) | 0.9 (0.5) | 1.0 (0.5) | 1.0 (0.5) | 0.5 (0) | 0.8 (0.7) | 0.8 (0.3) | 0 (0) | 22.36 (7, 226) | <.0001 |
| Assured/Dominant | 36.1 (14.7)† | 41.9 (14.1)* | 45.5 (16.7) | 35.6 (12.9)† | 48.1 (13.7) | 43.9 (14.0)* | 32.9 (14.8)† | 55.2 (10.8) | 7.49 (10, 246) | <.0001 |
| Unassured/Submissive | 55.8 (11.6)† | 52.7 (10.9)† | 49.5 (18.2) | 53.2 (12.3)† | 41.6 (10.6) | 48.9 (14.9) | 58.6 (13.9)† | 40.9 (10.2) | 6.72 (10, 246) | <.0001 |
| Gregarious/Extraverted | 34.8 (17.0)† | 39.7 (19.3)† | 44.2 (12.2) | 43.2 (14.2)* | 55.4 (11.2) | 40.1 (14.1)† | 29.4 (16.4)† | 55.4 (9.1) | 8.00 (10, 246) | <.0001 |
| Aloof/Introverted | 63.1 (14.3)† | 57.4 (16.4)† | 55.7 (12.9) | 52.6 (15.0)* | 41.5 (11.0) | 55.9 (11.6)† | 64.8 (18.2)† | 42.9 (7.9) | 8.48 (10, 246) | <.0001 |
| Warm/Agreeable | 31.3 (16.3)† | 38.4 (18.9)† | 52.8 (15.8) | 49.3 (15.1) | 53.6 (16.1) | 43.4 (15.8) | 49.0 (14.5) | 51.3 (10.2) | 8.58 (10, 246) | <.0001 |
| Coldhearted | 65.8 (16.6)† | 59.0 (16.6)† | 45.0 (8.8) | 48.1 (13.1) | 42.8 (12.5) | 54.2 (15.4) | 45.7 (8.6) | 44.7 (7.9) | 11.02 (10, 246) | <.0001 |
| Unassuming/Ingenuous | 53.5 (12.7) | 52.3 (13.4) | 64.5 (11.6) | 60.3 (12.0) | 55.5 (13.1) | 56.8 (13.6) | 63.2 (13.8) | 55.4 (14.9) | 2.48 (10, 246) | .02 |
| Arrogant/Calculating | 48.9 (13.9) | 45.5 (10.9) | 40.0 (12.1) | 40.5 (11.1) | 41.2 (10.1) | 42.5 (10.6) | 37.5 (8.6) | 43.0 (11.0) | 3.20 (10, 246) | .003 |
p<.05 vs. NCs based on post-hoc Dunnett-Hsu test controlling for age, gender and MMSE
p<.005 vs. NCs based on post-hoc Dunnett-Hsu test controlling for age, gender and MMSE
All subjects and their informants/caregivers signed an institutional review board-approved research consent form to participate in the study.
Interpersonal Adjective Scales (IAS)
The IAS is a well-validated self- or other-report questionnaire based on the circumplex model of personality, which aims to measure individual differences in interpersonal traits (Wiggins, 1995). In this model interpersonal traits are organized along two orthogonal dimensions: Agency or Power (characterized by the person’s assertiveness towards others) is represented by the vertical (Y) axis, while Affiliation or Love (characterized by social/emotional engagement) is represented by the horizontal (X) axis. Within this framework any specific social interaction, and by extension, any individual trait, can be represented as a `blend` of these two dimensions and described in terms of its two-dimensional coordinates. The questionnaire consists of 64 adjectives, each descriptive of an interpersonal behaviour (e.g., “self-assured”; “shy”; “iron-hearted”). These adjectives are rated for how accurately they describe a subject, on a 1 (very inaccurate) to 8 (very accurate) Likert-scale. They are summed into eight interpersonal traits that are evenly distributed around the circumference of the interpersonal circumplex. These traits are dominance, arrogance, coldness, introversion, submissiveness, ingenuousness, warmth, and extraversion (Fig. 1).
Loss of self-awareness is common in neurodegenerative diseases (Rankin et al., 2005). Thus, we assumed that not all patients in this study were capable of producing a valid self-assessment and used informant ratings to measure the interpersonal traits of all subjects. We considered informant ratings to be valid estimates of the subjects` interpersonal traits because behaviours described by the IAS all are observable, not only by the subject, but by people who frequently interact with them. Spouses, relatives or close friends were asked to fill out the personality questionnaire describing the subject’s current interpersonal characteristics. Though self and informant ratings of personality show unique variances in healthy adults, there is evidence for a high convergent validity between the two rating types (Connolly, Kavanagh, & Viswesvaran, 2007). In patients with dementia, collecting data from caregivers and others who know the patient well is an effective and reliable method for assessing personality traits (Siegler, Dawson, & Welsh, 1994; Strauss, Pasupathi, & Chatterjee, 1993), and informant ratings using the IAS in particular have excellent internal and temporal reliability (Kurtz, Lee, & Sherker, 1999). Questionnaires were scored using the IAS computer scoring program (Wiggins & Coutu, 1995), which generates T-scores by comparing subject scores to a gender-matched, community-based normative sample data set collected by the IAS developers (Wiggins, 1995). Questionnaires were completed within 6 months before or after the MRI scan for patients, and the average span of time between questionnaire and scan was 2.3 days (SD = 40.6 days).
Structural MRI
MRI scans were obtained on a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, N.J.) equipped with a standard quadrature head coil. A volumetric magnetization prepared rapid gradient echo MRI (MPRAGE, TR/TE/TI = 10/4/300 milliseconds) was used to obtain T1-weighted images of the entire brain, 15-degree flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness, with 154 – 174 coronal slices depending on patient head size and shape. Data for different subject groups were collected in an interleaved fashion between December 1998 and November 2007.
Voxel-based Morphometry (VBM)
VBM pre-processing and analyses were performed using the SPM5 software package (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm) running on Matlab 7.1.0 (MathWorks, Natick, MA, USA). In all pre-processing steps, SPM5 default parameters were used, except the light clean-up procedure was used in the morphological filtering step. Default tissue probability priors (voxel size: 2×2×2mm) of the International Consortium for Brain Mapping (ICBM) were used, and spatially normalized, segmented, and modulated grey matter images were smoothed with a 12 mm isotropic Gaussian kernel.
VBM Analyses of IAS Interpersonal Traits
Covariates-only (multiple regression design) statistical analyses were used to show the relationship between interpersonal traits and grey matter volume. The significance of each effect was determined using the theory of Gaussian fields. Interpersonal traits were positively and negatively correlated with grey matter volume in all analyses. However, because of the highly inverse relationship between opposite traits, e.g., extraversion and introversion, similar neural correlates were expected for a positively correlated trait and its negatively correlated opposite trait. To prevent redundant results, we decided to focus on positive neural correlates, describing negative neural correlates only if those results diverged considerably from the positive correlation results. We focused on positive correlations because by default, brain damage results in loss of function, with the caveat that our secondary negative correlation analyses would identify any exceptions to this rule. By performing positive correlations, we assumed that decreased trait scores would be associated with decreased grey matter volume. Age, gender, MMSE (as a proxy for disease severity), and total intracranial volume were entered as nuisance covariates into all designs. To correct for non-uniform smoothness of grey matter, non-stationary cluster extent correction was performed within the framework of the Random Field Theory using the ns-toolbox (fmri.wfubmc.edu/cms/software#NS). Resulting SPM t-maps were superimposed on the MNI single subject brain using Automated Anatomical Labelling (AAL) (Tzourio-Mazoyer et al., 2002) and Brodmann’s atlases included in the MRIcron software package (http://www.sph.sc.edu/comd/rorden/mricro.html). Locations of clusters are reported in the MNI reference space.
Three different types of statistical analyses were performed:
1. Main Effect Analyses
T-scores of each interpersonal trait were separately correlated with grey matter volume to identify neural networks associated with each trait across all diagnostic groups, using a 1-tailed [1] t-contrast (with additional zeros for nuisance variables). The statistical threshold was set at p<.05 after whole-brain SPM family-wise error (FWE) correction.
2. Trait Component Analyses
Based on the circumplex theory underlying the IAS, each interpersonal trait is a blend of its adjacent traits (e. g., extraversion is a blend of dominance and warmth) (Fig. 1). Thus, one person with a high Extraversion score may show more agentic (oriented towards dominance) than affiliative (oriented towards warmth) interpersonal behaviour, whereas another person with an equally high Extraversion score may show more affiliative than agentic behaviour. To isolate the two components of each trait, we included an adjacent trait as a confounding variable into the main effect design matrix of the trait of interest, thereby removing the effect of the trait component lying next to that adjacent trait and isolating the neural correlates of the other trait component. Accordingly, two such analyses were performed for each trait. For example, to directly examine the neural correlates of the assertive, dominant component of extraversion, extraversion was analyzed controlling for warmth as a covariate by using a 1-tailed [1 0] t-contrast. Then, to isolate its affiliative component, extraversion was analyzed controlling for dominance. The statistical threshold was set at p<.05 after whole-brain FWE correction.
Because the interpersonal circumplex model on which the IAS is based assumes very high inter-correlations among all traits (approaching r=1.0 for opposite traits), we did not expect any meaningful independent neural correlates of a trait to remain significant if the covariance associated with all seven other traits was removed. Thus, we performed trait component analyses only with single adjacent traits, rather than attempted to partial out covariance associated with all other traits.
3. Checking for Co-Atrophy Effects in Main Effect and Trait Component Analyses
The main effect and trait component analyses do not rule out the possibility that significant findings hold true only in one diagnostic group and do not represent a generalizable brain-behaviour relationship. It is logically possible for this kind of illusory correlation to occur in any VBM analysis using patients from multiple neurodegenerative disease groups, because if disease group membership predicts a region of atrophy (G→A), and disease group membership also predicts altered behaviour (G→B), then that region of atrophy may appear to directly correlate with the behaviour (A↔B), when that correlation is actually spurious (A←/→B).
In order to investigate the main effect and trait component analyses for co-atrophy effects, we performed an error check on all neural correlates found significant in main effect or trait components analyses. We parameterized each diagnosis (0=no, 1=yes) and entered all 8 diagnostic groups into the design matrix as confounding covariates (using 7 dummy variables to represent the 8 groups). The results of this type of analysis show regions of atrophy significantly related to personality only if they appear in more than one diagnostic group. These results must be considered in light of the main effect results, however, because this method will improperly exclude any regions that are legitimately related to personality, but which only atrophy in a single diagnostic group. Also, statistical power will decrease in cases where inter-individual differences in brain-trait relationships within an affected disease group are more homogeneous. Due to the additional loss of power caused by adding 7 more variables to the regression model, we accepted a level of significance of p<.001 uncorrected for multiple comparisons within the brain areas of interest previously identified in main effect or trait component analyses, and p<.05 (FWE-corrected) for areas outside of these regions of interest.
RESULTS
Behavioural results
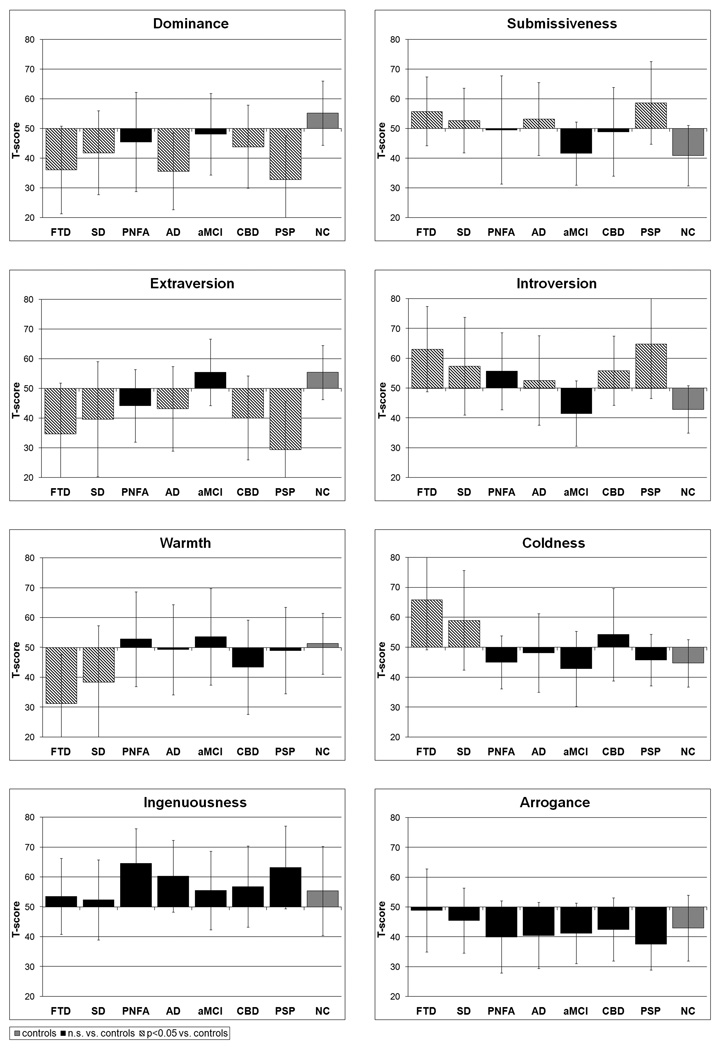
A general linear model procedure showed significant differences in six of the eight interpersonal traits across diagnostic groups, controlling for age, gender, and MMSE (Table 1, Fig. 3). Specifically, in the FTD, SD, AD, CBD, and PSP groups, Dominance and Extraversion scores were significantly lower than scores in elderly NCs, and the scores for their opposite traits, Submissiveness and Introversion, were significantly higher than NCs (p<.05 based on a post-hoc Dunnett-Hsu test). Warmth scores were significantly lower in the FTD and SD groups, and the scores for their opposite trait, Coldness, were significantly higher than in NCs (p<.005). Arrogance and Ingenuousness scores did not differ from NC scores in any patient group.
Figure 3.
Bar graphs of Dominance score, Submissiveness score, Extraversion Score, Introversion Score, Warmth Score, Coldness Score, Ingenuousness score, and Arrogance score classified by the 7 patient groups compared to a group of 43 healthy elderly (FTD = frontotemporal dementia; SD = semantic dementia; PNFA = progressive non-fluent aphasia; AD = Alzheimer’s disease; aMCI = amnestic mild cognitive impairment; CBD = corticobasal degeneration; PSP = progressive supranuclear palsy; NC = normal controls). Group comparisons were performed using a post-hoc Dunnett-Hsu test controlling for age, gender, and MMSE. Scores for NC comparison group are in grey, groups showing no significant statistical difference from NC subjects are in black, and groups with personality scores significantly different from NC subjects are in diagonal crosshatching.
Neuroimaging results
Dominance
Main Effect Analysis
Dominance score correlated significantly with the grey matter volume of the dorsolateral prefrontal cortex (DLPFC) (BA 9, 46, 9/46) (Petrides, 2005), including medial frontal gyri and medial orbital gyri (overlapping with the lateral frontopolar cortex), and with the grey matter volume of the left superior orbital gyrus (pFWE<.05) (Table 2). Based on the peak p-values and cluster sizes of these neural correlates, a slight left-lateralization bias was observed. Main Effect Co-Atrophy Check: When Dominance score was analyzed controlling for diagnostic group effects, only a left lateral prefrontal region, consisting of parts of the medial frontal gyrus (BA 46) and lateral frontopolar cortex (BA 10), remained significant (p<.001, uncorrected) (Table 2, Fig. 4). No region outside the significant regions previously identified in the main effect analysis emerged (pFWE<.05).
Table 2.
Main Effect and Trait Component Analyses of Dominance: Main Effects: Regions where Dominance score positively correlated with grey matter tissue density, across all groups, controlling for age, gender, MMSE and TIV. Trait Component Analyses: Dominance score was analyzed controlling for Extraversion score to isolate the non-affiliative component of dominance and analyzed controlling for Arrogance score to isolate the affiliative component of dominance. Results of both types of analyses are corrected for family-wise error (FWE) across the whole brain at a significance level of p<.05. In a second step, diagnostic groups were included as confounding variables into the design matrices of main effect and trait component analyses to control for potential co-atrophy effects (p<.001 uncorrected). Locations of clusters are reported in the MNI reference space.
| Anatomic region | BA | mm3 | x | y | z | T-score |
|---|---|---|---|---|---|---|
|
Dominance (Main Effects) | ||||||
| L medial frontal gyrus † | 10/46 | 912 | −32 | 56 | 14 | 5.53 |
| L medial orbital gyrus | 10/46 | “ | −38 | 58 | −2 | 4.60 |
| L superior orbital gyrus | 11 | “ | −28 | 64 | −6 | 4.46 |
| R medial frontal gyrus | 9/46 | 360 | 38 | 42 | 38 | 5.02 |
| R medial frontal gyrus | 9 | “ | 38 | 32 | 46 | 4.54 |
| R medial orbital gyrus | 46 | 144 | 44 | 56 | −4 | 4.66 |
| R medial frontal gyrus | 46 | 120 | 38 | 54 | 20 | 4.62 |
| L medial frontal gyrus | 9/46 | 112 | −36 | 38 | 40 | 4.57 |
| Dominance, non-affiliative part (Trait Component Analyses) | ||||||
| No significant suprathreshold clusters at FWE corrected level | ||||||
|
Dominance, affiliative part (Trait Component Analyses) | ||||||
| R medial frontal gyrus † | 9 | 22`399 | 38 | 42 | 38 | 5.97 |
| R medial orbital gyrus † | 46 | “ | 46 | 54 | −8 | 5.91 |
| R superior orbital gyrus † | 11 | “ | 14 | 68 | −14 | 5.74 |
| R superior frontal gyrus | 9 | “ | 16 | 36 | 54 | 5.60 |
| R inferior frontal gyrus, triangularis | 45 | “ | 52 | 36 | 18 | 5.55 |
| R inferior orbital gyrus | 47 | “ | 34 | 36 | −22 | 5.51 |
| L medial frontal gyrus † | 46/10 | 4`252 | −34 | 56 | 12 | 5.92 |
| L medial orbital gyrus | 11 | “ | −28 | 56 | −6 | 5.56 |
| L superior frontal gyrus | 9 | “ | −16 | 52 | 38 | 5.16 |
| L inferior orbital gyrus | 45/47 | “ | −52 | 38 | −8 | 4.85 |
| R anterior thalamus | − | 2`160 | 6 | −8 | 16 | 5.26 |
| R paracingulate gyrus | 32 | 400 | 4 | 42 | 18 | 4.92 |
| R medial insula | 48 | 77 | 46 | 4 | −2 | 4.53 |
p<0.001 (diagnostic groups included as confounding variables into design matrix)
Figure 4.
Results of the main effect analyses with co-atrophy check of (A) dominance, (B) extraversion, (C) warmth, (D) ingenuousness, and (E) coldness, superimposed on rendered, coronal, and axial slices of a standard brain from a single normal subject (MRIcron: ch2.nii.gz). Coloured regions are regions where brain atrophy significantly correlated with decreased trait scores, controlling for diagnostic group effects, age, gender, MMSE, and TIV. Results are uncorrected for multiple comparisons at a significance level of p<.001 using the T-score range.
Trait Component Analyses
When Dominance score was analyzed controlling for Extraversion score to isolate the non-affiliative component of dominance, no significant clusters were detected (pFWE<.05). This negative result implies that the neural correlates of dominance detected in the main effect analysis are related only to the affiliative, but not to the non-affiliative, component of dominance. Accordingly, when Dominance score was analyzed controlling for Arrogance score to highlight the affiliative component of dominance, correlations with brain regions detected in the main effect analysis increased, and additional regions emerged, including the right inferior frontal gyrus (pars triangularis), right rostral paracingulate gyrus, right anterior thalamus, right medial insula, and the left inferior orbital gyrus (Table 2). In contrast to the main effect analysis, the anatomy underlying the affiliative component of dominance was predominantly right-sided. Trait Component Co-Atrophy Check: When Dominance score was analyzed controlling for Arrogance score and diagnostic group effects, only lateral prefrontal regions, including predominantly right-sided regions of the DLPFC and the right lateral frontopolar cortex, remained significant (p<.001, uncorrected) (Table 2). No region outside the significant regions previously identified in the trait component analysis emerged (pFWE<.05).
Extraversion
Main Effect Analysis
Extraversion score correlated significantly with the grey matter volume of the DLPFC bilaterally, inferior orbital gyri, inferior frontal gyri (pars triangularis and opercularis), the right medial insula, right paracingulate gyrus, the left anterior thalamus, and left pre- and postcentral gyri (pFWE<.05) (Table 3). Main Effect Co-Atrophy Check: When Extraversion score was analyzed controlling for diagnostic group effects, bilateral regions of the DLPFC, inferior frontal gyri (pars triangularis and opercularis), the right paracingulate gyrus, the left inferior orbital gyrus, and left postcentral gyrus remained significant (p<.001, uncorrected) (Table 3, Fig. 4). No region outside the significant regions previously identified in the main effect analysis emerged (pFWE<.05).
Table 3.
Main Effect and Trait Component Analyses of Extraversion: Main Effects: Regions where Extraversion score positively correlated with grey matter tissue density, across all groups, controlling for age, gender, MMSE and TIV. Trait Component Analyses: Extraversion score was analyzed controlling for Warmth score to isolate the agentic component of extraversion and analyzed controlling for Dominance score to isolate the affiliative component of extraversion. Results of both types of analyses are corrected for family-wise error (FWE) across the whole brain at a significance level of p<.05. In a second step, diagnostic groups were included as confounding variables into the design matrices of main effect and trait component analyses to control for potential co-atrophy effects. Results of these additional analyses are presented at a significance level of p<.001 (uncorrected). Locations of clusters are reported in the MNI reference space.
| Anatomic region | BA | mm3 | x | y | z | T-score |
|---|---|---|---|---|---|---|
|
Extraversion (Main Effects) | ||||||
| R medial frontal gyrus † | 9 | 76`770 | 34 | 34 | 46 | 6.43 |
| L medial frontal gyrus * | 46 | “ | −30 | 54 | 18 | 6.38 |
| L medial frontal gyrus † | 9 | “ | −32 | 40 | 42 | 6.08 |
| L inferior orbital gyrus † | 47 | “ | −32 | 36 | −18 | 5.92 |
| R medial insula | 48 | “ | 40 | 6 | 6 | 5.87 |
| R inferior frontal gyrus, triangularis † | 45 | “ | 52 | 42 | 4 | 5.80 |
| R inferior frontal gyrus, opercularis † | 48 | “ | 46 | 16 | 24 | 5.79 |
| R medial frontal gyrus † | 46 | “ | 40 | 42 | 34 | 5.64 |
| L inferior frontal gyrus, opercularis † | 44/48 | “ | −48 | 12 | 26 | 5.62 |
| R inferior orbital gyrus | 47 | “ | 36 | 38 | −20 | 5.59 |
| R paracingulate gyrus † | 32 | “ | 8 | 16 | 42 | 5.56 |
| L postcentral gyrus † | 6 | 412 | −56 | −4 | 46 | 5.02 |
| R anterior thalamus | - | 168 | 8 | −12 | 18 | 4.92 |
| L precentral gyrus | 6 | 93 | −38 | −6 | 64 | 4.84 |
| Extraversion, agentic part (Trait Component Analyses) | ||||||
| L medial frontal gyrus † | 9 | 1`400 | −32 | 40 | 42 | 5.32 |
| L medial frontal gyrus † | 9/46 | “ | −42 | 30 | 42 | 4.65 |
| L medial frontal gyrus † | 46 | 560 | −30 | 54 | 18 | 5.19 |
| L medial frontal gyrus † | 46 | 144 | −40 | 56 | 2 | 4.62 |
| R medial frontal gyrus † | 9 | 48 | 38 | 32 | 46 | 4.57 |
| L postcentral gyrus † | 6 | 64 | −56 | −4 | 46 | 4.52 |
| L inferior frontal gyrus, opercularis † | 44/48 | 172 | −44 | 14 | 30 | 4.50 |
|
Extraversion, affiliative part (Trait Component Analyses) | ||||||
| R medial insula † | 48 | 7`625 | 38 | 6 | 6 | 5.68 |
| R anterior insula † | 47 | “ | 40 | 28 | −4 | 5.29 |
| R inferior frontal gyrus, triangularis † | 45 | “ | 54 | 38 | 6 | 4.77 |
| L inferior orbital gyrus † | 47 | 1`118 | −32 | 34 | −16 | 5.43 |
| Gyrus rectus † | 11 | 1`313 | 0 | 22 | −26 | 5.16 |
| R medial frontal gyrus † | 9 | 1`118 | 32 | 30 | 48 | 5.13 |
| L inferior frontal gyrus, opercularis † | 44 | 2`745 | −52 | 12 | 22 | 5.03 |
| L medial insula | 48 | “ | −40 | 12 | 6 | 4.95 |
| R inferior frontal gyrus, triangularis † | 48 | 1`702 | 46 | 18 | 22 | 4.91 |
| R paracingulate gyrus † | 32 | 3`702 | 8 | 16 | 42 | 4.86 |
| R paracingulate gyrus | 32 | “ | 6 | 32 | 34 | 4.78 |
| R supplementary motor area | 6 | “ | 4 | 16 | 58 | 4.70 |
| L medial frontal gyrus † | 46 | 263 | −28 | 54 | 18 | 4.86 |
pFWE<0.05 (diagnostic groups included as confounding variables into design matrix)
p<0.001 (diagnostic groups included as confounding variables into design matrix)
Trait Component Analyses
When Extraversion score was analyzed controlling for Warmth score to isolate the agentic component of extraversion, predominantly left lateral brain regions stayed in the analysis, including the medial frontal gyrus, inferior frontal gyrus (pars opercularis), and the postcentral gyrus (pFWE<.05) (Table 3). When Extraversion score was analyzed controlling for Dominance score to highlight the affiliative component of extraversion, correlations with the right anterior-medial insula increased compared to the main effect analysis, and additional correlations were seen in the gyrus rectus and the left insula. Simultaneously, the left postcentral gyrus dropped out of the analysis and correlations in the DLPFC were observed to be weaker (Table 3). The regions underlying the affiliative component of extraversion showed a right-lateralization bias. Trait Component Co-Atrophy Check: When Extraversion score was analyzed controlling for Warmth score and diagnostic group effects, all previously identified regions remained significant (p<.001, uncorrected) (Table 3). When Extraversion score was analyzed controlling for Dominance score and diagnostic group effects, the left insula and right supplementary motor area dropped out of the analysis (Table 3). No region outside the significant regions previously identified in the trait component analyses emerged (pFWE<.05).
Warmth
Main Effect Analysis
Warmth score correlated significantly with the grey matter volume in anterior brain regions, including the frontal lobes and the right temporal lobe (pFWE<.05) (Table 4). Among the primarily right prefrontal correlates of Warmth score, the core regions were the orbitofrontal cortex (OFC) (BA 10, 11, 13, 14, 47/12 (medial)) (Petrides & Pandya, 1994), predominantly its right posterior caudal areas, the right anterior and medial insula, the subgenual cingulate region (BA 25), the anterior medial prefrontal cortex (medial parts of BA 9, 10 and 32) (Petrides & Pandya, 1994), and the right caudate head (Table 4). Within the right anterior temporal regions, Warmth score correlated predominantly with limbic/paralimbic structures, involving the anterior parahippocampus (i. e., parts of the entorhinal, perirhinal and parahippocampal cortex), anterior hippocampus, amygdala, and superior temporal pole (Table 4). Main Effect Co-Atrophy Check: When Warmth score was analyzed controlling for diagnostic group effects, all left-hemispheric regions and the right caudate dropped out of the analysis, leaving neural correlates of warmth exclusively right-sided (p<.001, uncorrected) (Table 4, Fig. 4). No region outside the significant regions previously identified in the main effect analysis emerged (pFWE<.05).
Table 4.
Main Effect and Trait Component Analyses of Warmth: Main Effects: Regions where Warmth score positively correlated with grey matter tissue density, across all groups, controlling for age, gender, MMSE and TIV. Trait Component Analyses: Warmth scorewas analyzed controlling for Ingenuousness score to isolate the agentic component of warmth and analyzed controlling for Extraversion score to isolate the non-agentic component of warmth. Results of both types of analyses are corrected for family-wise error (FWE) across the whole brain at a significance level of p<.05. In a second step, diagnostic groups were included as confounding variables into the design matrices of main effect and trait component analyses to control for potential co-atrophy effects. Results of these additional analyses are presented at a significance level of p<.001 (uncorrected). Locations of clusters are reported in the MNI reference space.
| Anatomic region | BA | mm3 | x | y | z | T-score |
|---|---|---|---|---|---|---|
|
Warmth (Main Effects) | ||||||
| R gyrus rectus * | 25 | 144’211 | 8 | 12 | −22 | 7.79 |
| R anterior insula † | 47/48 | “ | 32 | 16 | −12 | 7.35 |
| R medial insula † | 48 | “ | 38 | 8 | 2 | 7.34 |
| R inferior orbital gyrus † | 11 | “ | 20 | 16 | −22 | 7.27 |
| R paracingulate gyrus † | 32 | “ | 10 | 48 | 16 | 7.10 |
| R superior frontal gyrus † | 10 | “ | 8 | 50 | 2 | 6.77 |
| R paracingulate gyrus † | 32 | “ | 10 | 38 | 30 | 6.61 |
| R anterior parahippocampus * | 20 | “ | 30 | −14 | −20 | 6.36 |
| R head caudate | 25 | “ | 8 | 16 | 0 | 6.35 |
| R inferior temporal gyrus † | 20 | “ | 60 | −12 | −30 | 5.74 |
| L superior frontal gyrus | 10 | “ | −6 | 58 | 30 | 5.70 |
| L inferior orbital gyrus | 47 | “ | −32 | 34 | −18 | 5.67 |
| R medial temporal pole † | 36 | “ | 26 | 12 | −34 | 5.60 |
| L anterior insula | 48 | “ | −34 | 12 | −2 | 5.30 |
| R inferior frontal gyrus, triangularis | 48 | “ | 50 | 20 | 24 | 4.91 |
| L medial superior frontal gyrus | 32 | “ | −6 | 32 | 38 | 4.85 |
| Warmth, agentic part (Trait Component Analyses) | ||||||
| R gyrus rectus † | 25 | 107`006 | 6 | 12 | −24 | 7.09 |
| R paracingulate gyrus † | 32 | “ | 10 | 48 | 16 | 6.77 |
| R medial insula | 48 | “ | 38 | 8 | 2 | 6.44 |
| R inferior orbital gyrus † | 11 | “ | 20 | 16 | −22 | 6.16 |
| L inferior orbital gyrus | 47 | “ | −32 | 34 | −18 | 6.00 |
| R medial superior frontal gyrus | 8 | “ | 6 | 30 | 52 | 5.46 |
| R anterior parahippocampus † | 20 | “ | 30 | −16 | −18 | 5.10 |
| R medial temporal pole | 20 | “ | 46 | 12 | −38 | 4.99 |
| R inferior frontal gyrus, triangularis | 44/48 | “ | 50 | 24 | 28 | 4.85 |
| R head caudate | 25 | 2`648 | 8 | 14 | 2 | 6.24 |
| R head caudate | 25 | “ | 20 | 18 | 8 | 4.55 |
|
Warmth, submissive part (Trait Component Analyses) | ||||||
| R gyrus rectus † | 25 | 38`308 | 10 | 14 | −22 | 6.05 |
| R inferior orbital gyrus † | 11 | “ | 20 | 16 | −22 | 6.00 |
| R anterior insula † | 48 | “ | 32 | 14 | −14 | 5.89 |
| R superior temporal pole † | 38 | “ | 32 | 8 | −18 | 5.63 |
| R anterior parahippocampus † | 34 | “ | 22 | 10 | −22 | 5.59 |
| R medial temporal pole † | 20 | “ | 46 | 12 | −38 | 5.49 |
| R superior medial frontal gyrus † | 10 | “ | 12 | 52 | 6 | 5.47 |
| R inferior temporal gyrus † | 20 | “ | 56 | −12 | −32 | 5.17 |
| R paracingulate gyrus † | 32 | “ | 10 | 42 | 30 | 4.91 |
| R caudate head † | 25 | 1`399 | 8 | 16 | −2 | 5.11 |
pFWE<0.05 (diagnostic groups included as confounding variables into design matrix)
p<0.001 (diagnostic groups included as confounding variables into design matrix)
Trait Component Analyses
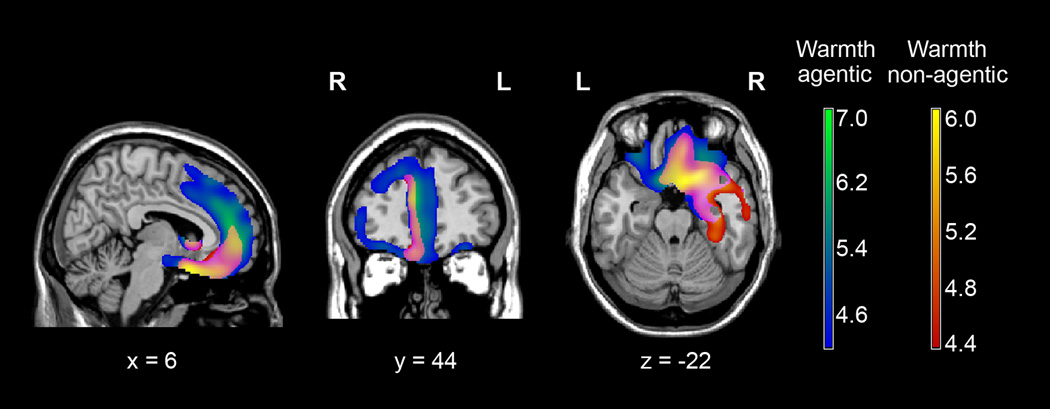
When Warmth score was analyzed controlling for Ingenuousness score to isolate the agentic component of warmth, correlations with the lateral prefrontal structures and the dorsal part of the anterior medial prefrontal cortex (BA 9, superior sections of BA 32) were strengthened in relation to right anterior temporal regions (pFWE<.05) (Table 4, Fig. 5). The right-lateralization bias slightly decreased. When Warmth score was analyzed controlling for Extraversion score to highlight the non-agentic component of warmth, the dorsal parts of the anterior medial prefrontal cortex, the right DLPFC, and right inferior frontal gyrus (pars triangularis) dropped out of the analysis, correlations with left hemispheric regions decreased considerably, and the right anterior temporal regions were strengthened in relation to prefrontal regions. Accordingly, the anatomy underlying the non-agentic component of warmth was almost completely right-sided (Table 4, Fig. 5). Trait Component Co-Atrophy Check: When Warmth score was analyzed controlling for Ingenuousness score and diagnostic group effects, the right inferior orbital gyrus, right anterior parahippocampus, and midline prefrontal structures such as the gyrus rectus and right paracingulate gyrus remained significant (p<.001, uncorrected) (Table 4). When Warmth score was analyzed controlling for Extraversion score and diagnostic group effects, all regions remained significant (Table 4). No region outside the significant regions previously identified in the trait component analyses emerged (pFWE<.05).
Figure 5.
Results of the trait component analyses of warmth, superimposed on sagittal (x=6), coronal (y=44), and axial (z=−22) slices of a standard brain from a single normal subject. Coloured regions ranging from blue to green are regions where brain atrophy significantly correlated with decreased scores of the agentic component of warmth. Coloured regions ranging from red to yellow are regions where brain atrophy significantly correlated with decreased scores of the non-agentic component of warmth. Note that common and distinct regions underlie the agentic and non-agentic components of warmth. Results are controlled for age, gender, MMSE, and TIV. Results are corrected for family-wise error across the whole brain at a significance level of p<.05 using the T-score range.
Ingenuousness
Main Effect Analysis
Ingenuousness score correlated significantly with the grey matter volume of the right anterior fusiform gyrus, right putamen, and the adjacent border of the right medial insula (pFWE<.05) (Table 5). Main Effect Co-Atrophy Check: When Ingenuousness score was analyzed controlling for diagnostic group effects, all brain regions remained significant (p<.001, uncorrected) (Table 5, Fig. 4). No region outside the significant regions previously identified in the main effect analysis emerged (pFWE<.05).
Table 5.
Main Effect and Trait Component Analyses of Ingenuousness: Main Effects: Regions where Ingenuousness score positively correlated with grey matter tissue density, across all groups, controlling for age, gender, MMSE and TIV. Trait Component Analyses: Ingenuousness score was analyzed controlling for Submissiveness score to isolate the affiliative component of ingenuousness and analyzed controlling for Warmth score to isolate the non-agentic component of ingenuousness. Results of both types of analyses are corrected for family-wise error (FWE) across the whole brain at a significance level of p<.05. In a second step, diagnostic groups were included as confounding variables into the design matrices of main effect and trait component analyses to control for potential co-atrophy effects. Results of these additional analyses are presented at a significance level of p<.001 (uncorrected). Locations of clusters are reported in the MNI reference space.
| Anatomic region | BA | mm3 | x | y | z | T-score |
|---|---|---|---|---|---|---|
|
Ingenuousness (Main Effects) | ||||||
| R anterior fusiform gyrus † | 20 | 523 | 38 | −14 | −26 | 4.69 |
| R putamen / medial insula † | 48 | 67 | 34 | −2 | 0 | 4.59 |
| Ingenuousness, affiliative part (Trait Component Analyses) | ||||||
| R anterior parahippocampus / anterior fusiform gyrus † | 20 | 18`698 | 38 | −18 | −22 | 5.43 |
| R amygdala † | 34 | “ | 22 | 6 | −18 | 5.38 |
| R anterior fusiform gyrus † | 20 | “ | 28 | −6 | −46 | 5.31 |
| R anterior hippocampus * | “ | 40 | −22 | −16 | 5.28 | |
| R putamen / medial insula † | 48 | “ | 36 | 2 | 0 | 5.14 |
| R medial temporal pole | 20 | “ | 44 | 14 | −38 | 5.05 |
| R gyrus rectus † | 11 | 619 | 8 | 44 | −22 | 4.61 |
|
Ingenuousness, submissive part (Trait Component Analyses) | ||||||
| No significant suprathreshold clusters at FWE corrected level | ||||||
pFWE<0.05 (diagnostic groups included as confounding variables into design matrix)
p<0.001 (diagnostic groups included as confounding variables into design matrix)
Trait Component Analyses
When Ingenuousness score was analyzed controlling for Submissiveness score to isolate the affiliative component of ingenuousness, correlations with all regions detected in the main effect analysis increased, especially with the right insula. Additional regions also emerged, including the right gyrus rectus and right anterior temporal regions, in particular the amygdala, anterior parahippocampus, and anterior hippocampus (pFWE<.05) (Table 5). When Ingenuousness score was analyzed controlling for Warmth score to isolate the non-agentic component of ingenuousness, no significant correlations remained at the FWE-corrected level. This negative result implies that the neural correlates of Ingenuousness score detected in the main effect analysis relate only to the affiliative, but not to the non-agentic, component of ingenuousness. Trait Component Co-Atrophy Check: When Ingenuousness score was analyzed controlling for Submissiveness score and diagnostic group effects, all regions, except the right medial temporal pole, remained significant (p<.001, uncorrected) (Table 5). No region outside the significant regions previously identified in the trait component analysis emerged (pFWE<.05).
Coldness
Main Effect Analysis
Coldness score correlated significantly with two left posterior brain regions (pFWE<.05) (Table 6). The larger cluster encompassed regions at the junction of the left inferior occipital gyrus, medial occipital gyrus, and fusiform gyrus. The smaller cluster mapped onto the border of the left angular and supramarginal gyri. Main Effect Co-Atrophy Check: When Coldness score was analyzed controlling for diagnostic group effects, the cluster encompassing regions at the junction of the left inferior occipital gyrus, medial occipital gyrus, and fusiform gyrus remained significant (p<.001, uncorrected) (Table 6, Fig. 4). No region outside the significant regions previously identified in the main effect analysis emerged (pFWE<.05).
Table 5.
Main Effect and Trait Component Analyses of Coldness: Main Effects: Regions where Coldness score positively correlated with grey matter tissue density, across all groups, controlling for age, gender, MMSE and TIV. Trait Component Analyses: Coldness score was analyzed controlling for Introversion score to isolate the agentic component of coldness and analyzed controlling for Arrogance score to isolate the non-agentic component of coldness. Results of both types of analyses are corrected for family-wise error (FWE) across the whole brain at a significance level of p<.05. In a second step, diagnostic groups were included as confounding variables into the design matrices of main effect and trait component analyses to control for potential co-atrophy effects. Results of these additional analyses are presented at a significance level of p<.001 (uncorrected). Locations of clusters are reported in the MNI reference space.
| Anatomic region | BA | mm3 | x | y | z | T-score |
|---|---|---|---|---|---|---|
|
Coldness (Main Effects) | ||||||
| L inferior occipital gyrus † | 37/19 | 4’810 | −34 | −66 | −4 | 5.99 |
| L supramarginal / angular gyrus | 41 | 342 | −40 | −46 | 28 | 4.57 |
|
Coldness, agentic part (Trait Component Analyses) | ||||||
| L inferior occipital gyrus † | 37/19 | 101 | −34 | −66 | −4 | 4.54 |
|
Coldness, submissive part (Trait Component Analyses) | ||||||
| L inferior occipital gyrus * | 37 | 6`688 | −34 | −64 | −4 | 6.33 |
| R calcarine gyrus | 18 | 160 | 22 | −84 | 2 | 4.61 |
pFWE<0.05 (diagnostic groups included as confounding variables into design matrix)
p<0.001 (diagnostic groups included as confounding variables into design matrix)
Trait Component Analyses
When Coldness score was analyzed controlling for Introversion score to isolate the agentic component of coldness, the left angular, supramarginal, and medial occipital gyri dropped out of the analysis, and correlations with the left inferior occipital and fusiform gyri decreased (pFWE<.05) (Table 6). When Coldness score was analyzed controlling for Arrogance score to isolate the non-agentic component of coldness, correlations with the left inferior and medial occipital gyri, fusiform gyrus, angular gyrus, and supramarginal gyrus increased. In addition, a small cluster mapped onto the right lateral calcarine gyrus, adjacent to the lingual gyrus (Table 6). The overlapping locations of the neural correlates of coldness and its agentic and non-agentic components suggest that in terms of their clinical characteristics, there is respectively little difference between the agentic and non-agentic components of coldness. Trait Component Co-Atrophy Check: When Coldness score was analyzed controlling for Introversion score and diagnostic group effects, the left inferior occipital gyrus remained significant (p<.001, uncorrected) (Table 6). When Coldness score was analyzed controlling for Arrogance score and diagnostic group effects, also the left interior occipital gyrus remained significant (Table 6). No region outside the significant regions previously identified in the trait component analyses emerged (pFWE<.05).
Arrogance, Introversion, Submissiveness
No significant correlations at the FWE-corrected level were observed between Arrogance score, Introversion score, or Submissiveness score and grey matter volume in main effect and trait component analyses.
DISCUSSION
The current neuroimaging results demonstrate that specific cortical and subcortical regions of the brain are associated with interpersonal traits in patients with different brain atrophy patterns. More importantly, interpersonal traits characterized by different degrees of agency and affiliation mapped onto distinct grey matter regions along the three hypothesized spatial gradients. Dominance, the primary agentic interpersonal trait, as well as the agentic component of extraversion, related predominantly to left dorsolateral prefrontal and left lateral frontopolar regions. In contrast, warmth, the primary affiliative trait, as well as the affiliative components of ingenuousness and extraversion, related predominantly to right midline prefrontal regions (subgenual-orbitofrontal regions, paracingulate gyrus, and ventral striatum), the right insula, and right anteromedial temporal regions. These anatomical dissociations, in which left dorsolateral prefrontal and left lateral frontopolar structures underlie agentic traits, while right ventromedial prefrontal and right anteromedial temporal structures underlie affiliative traits, highlight the complexity of interpersonal behaviours. Consistent with the existing literature on neural networks underlying social cognition, these findings indicate that brain regions related to externally-focused, executive control-related processes underlie agentic interpersonal traits (Koechlin et al., 2003; Lieberman, 2007; Tanji & Hoshi, 2008), whereas brain regions related to internally-focused, emotion- and reward-related processes underlie affiliative interpersonal traits (Adolphs, 2002; Kringelbach & Rolls, 2004; Lieberman, 2007; O'Doherty, 2004; Olsson & Ochsner, 2008; Phillips et al., 2003).
Agentic Interpersonal Traits (Dominance, Extraversion) and Externally-Focused, Controlled Executive Behaviour
Interpersonal agency can be conceptualized as the tendency to actively negotiate with one’s social environment in order to achieve personal goals. Two of the three high-agency traits of the IAS circumplex, dominance and extraversion, related to common brain regions. Dominance mapped exclusively onto a left lateral prefrontal region, consisting of parts of the medial frontal gyrus (BA 46) and lateral frontopolar cortex (BA 10). Extraversion related predominantly to lateral prefrontal structures, with a clear left-lateralization bias when its dominant, agentic elements were isolated. This correlation pattern is compatible with the three hypothesized spatial gradients, which suggest predominantly dorsal substrates for controlled processes like those required for social negotiations, lateral substrates for processes with an external, task-oriented rather than introspective focus, and left-lateralized substrates for the active, approach behaviours that characterize agentic traits like dominance and extraversion (Fig. 2) (Amodio & Frith, 2006; Demaree et al., 2005; Koechlin et al., 2003; Lieberman, 2007; Olsson & Ochsner, 2008; Phillips et al., 2003; Tanji & Hoshi, 2008).
Comparisons with previous structural and functional neuroimaging findings of personality are difficult, because of differences in study samples (young, healthy people in almost all previous studies), in personality assessment tools, and in neuroimaging techniques. Especially, in light of the considerable impact of aging on neural correlates of personality traits (Wright, Feczko, Dickerson, & Williams, 2007), comparisons with neuroimaging findings of personality traits in young, healthy adults seem of little use. Using the five-factor model (FFM) (McCrae & John, 1992) Wright and colleagues showed in a sample of healthy elderly that extraversion scores correlate with cortical thickness of specific lateral PFC regions (Wright et al., 2007). Their findings are comparable to our neuroimaging results, given the fact that FFM extraversion corresponds quite well to IAS dominance and IAS extraversion (McCrae & Costa, 1989).
Lateral prefrontal structures, consisting of the DLPFC, ventrolateral prefrontal cortex (VLPFC) (BA 45, 47/12 (lateral)) (Petrides, 2005), and the lateral frontopolar cortex, are critically involved in higher-order control processes, which exert top-down regulation of cognition and, probably to a lesser extent, of emotion (Koechlin et al., 2003; Olsson & Ochsner, 2008; Tanji & Hoshi, 2008). Cognitive control, which is the ability to coordinate thoughts and actions, subserves executive cognitive functions such as selective attention, task management, and action planning. That ability is required for flexible, goal-directed behaviour, which is typically observed in highly agentic individuals. Dominance and the agentic component of extraversion were associated with lateral rostral prefrontal regions (BA 10, 46), which are at the highest level of cognitive control (Koechlin et al., 2003; Petrides, 2005; Ramnani & Owen, 2004). These areas, especially the lateral frontopolar cortex (BA 10), process stimulus-independent abstract information (Christoff, Ream, Geddes, & Gabrieli, 2003) and are implicated in the integration of multiple cognitive operations in the pursuit of behavioural goals (Koechlin et al., 2003; Ramnani & Owen, 2004). These functions are involved in planning and reasoning, which are of fundamental importance for the complex processing involved in interpersonal negotiations. More posterior and caudal lateral prefrontal areas (BA 9, 44, 45), which related to the agentic component of extraversion, represent a lower level of executive control. These regions are involved in first-order executive processes, such as action selection based on the immediate context of external signals (Koechlin et al., 2003; Petrides, 2005). Dominance mapped onto a more rostral prefrontal region (BA10/46) than the agentic component of extraversion (BA 6, 9, 44, 46), suggesting that dominant/assertive interpersonal behaviour may be based on higher level of cognitive control than extraverted behaviour.
Lateral prefrontal structures are also implicated in self-regulation processes such as impulse control and self-control of emotional experience and responses (Lieberman, 2007), abilities critical for achievement of social goals. While emotion regulation processes utilizing an `internal-focused` (personal relevance) strategy recruit medial prefrontal regions, an `external-focused` regulation strategy in which emotions are reinterpreted in their situational context recruits lateral prefrontal structures (Ochsner, Knierim et al., 2004; Ochsner, Ray et al., 2004). `External-focused` regulation strategy fits well to the external orientation of dominant/assertive and extraverted individuals in social interactions. Moreover, the DLPFC and the lateral frontopolar cortex are involved in the integration of emotion and cognition (Gray, Braver, & Raichle, 2002) and the integration of motivation (reward expectancy) and cognition (Pochon et al., 2002). The integrative functions of these brain regions may support rational decision-making and self-controlled behaviour in emotional contexts, skills critical for successfully navigating complex interpersonal situations.
Affiliative Interpersonal Traits (Warmth, Extraversion, Ingenuousness) and Internally-Focused, Emotional, and Reward-Related Behaviour
Individuals with affiliative traits tend to seek out social contact and emotional connection with others, and show a greater receptivity to the personal, emotional features of their social exchanges. The neural correlates of warmth, as well as those of the affiliative components of extraversion and ingenuousness, overlapped extensively with the circuitry of reward and emotion processing (Adolphs, 2002; Kringelbach & Rolls, 2004; O'Doherty, 2004; Phillips et al., 2003), including right insular cortex, right caudo-medial OFC, and dorsal midline prefrontal structures.
Previous neuroimaging studies investigating neural correlates of highly affiliative personality traits comparable to the IAS affiliative traits are scarce. Consistent with our findings Rankin and colleagues linked FTD patients’ diminished FFM agreeableness, which corresponds quite well to IAS warmth and IAS ingenuousness (McCrae & Costa, 1989), to volume loss of the right OFC (Rankin et al., 2004). In contrast, Wright and colleagues found no significant correlations between FFM agreeableness and regional cortical thickness in healthy elderly (Wright et al., 2007).
The right anterior and medial insular cortex correlated significantly with a number of traits and trait components, including warmth, the non-agentic component of warmth, ingenuousness, the affiliative component of ingenuousness, and the affiliative component of extraversion. Neurophysiological studies in animals and humans indicate that, together with midbrain and brain stem regions, the insula integrates visceral, autonomic and somatosensory sensations, enabling the interoceptive representation of the physiological condition of the body (Craig, 2002). In humans, this information is accessible to conscious awareness (interoceptive awareness) via the right insula (Craig, 2002; Critchley, 2005), and influences emotional feelings (emotional awareness) (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004). It seems likely that higher interoceptive and emotional awareness in interpersonal relationships motivates affiliative social behaviours. Anterior insular cortices are also sites of projection of unmyelinated tactile afferent fibers, which mediate affective aspects of a pleasant touch, a relevant affiliative stimulus (Olausson et al., 2002).
Right caudo-medial regions (BA 11) of the orbitofrontal cortex (OFC) emerged in the analyses of warmth, its agentic and non-agentic components, and the affiliative components of extraversion and ingenuousness. The OFC receives and integrates inputs from all sensory modalities (Carmichael & Price, 1995b), though at a higher level of sensory processing than the insula (Kringelbach & Rolls, 2004; Mesulam & Mufson, 1982). The OFC, especially its caudal part, connects extensively with the limbic/paralimbic system, including the amygdala, the anterior cingulate, and the hippocampus, and with interoceptive regions such as the insula and hypothalamic and brain stem autonomic centres (Carmichael & Price, 1995a; Mesulam & Mufson, 1982; Ongur, An, & Price, 1998; Rempel-Clower, 2007). This connectivity pattern suggests that in particular the caudal OFC plays a central role in emotion experience and regulation by the integration of exteroceptive (e.g. visual stimuli) and interoceptive stimuli. This functional role is crucial for affiliative behaviour, which is guided by exteroception and interoception of emotional stimuli during social interactions (Depue & Morrone-Strupinsky, 2005). Indeed, patients with circumscribed bilateral lesions of the orbitofrontal cortex are known to show aberrant emotion processing, including impaired emotion recognition and emotional empathy (Hornak et al., 2003).
The medial posterior orbitofrontal and subgenual areas (BA 11, 25) were the core regions revealed in the warmth analysis. Based on evidence from a meta-analysis of functional neuroimaging analyses in the OFC, these posterior regions likely code the reward value of simple sensory stimuli such as interoceptive sensations or another’s friendly touch (Kringelbach & Rolls, 2004). Damage to other parts of the reward circuit (O'Doherty, 2004) such as the right caudate head, right putamen, anterior medial prefrontal cortex, and the right amygdala also corresponded with lower levels of affiliative traits. The association of these regions with reward is consistent with the affiliation trait model, which proposes that affiliative behaviour is a highly rewarding experience (Depue & Morrone-Strupinsky, 2005). Our results, which suggest that these regions mediate affiliative traits, are also compatible with functional neuroimaging studies of social cooperation (Rilling et al., 2002), maternal and romantic love (Bartels & Zeki, 2004; Noriuchi, Kikuchi, & Senoo, 2008) and charitable donations (Moll et al., 2006). Furthermore, the adjacent subgenual cingulate region (BA 25) functions abnormally in depression (Seminowicz et al., 2004), a disorder involving disruption of affiliative, prosocial behaviour.
Warmth and, to a small extent, extraversion, also related to the anterior medial prefrontal cortex, a more dorsal prefrontal midline structure. According to the medio-lateral hypothesis of social cognition (Lieberman, 2007), these medial regions are key to processing internal aspects of self and other, including emotions and thoughts. Interestingly, warmth related predominantly to the ventro-anterior medial prefrontal cortex, whereas extraversion related to the dorso-anterior medial prefrontal cortex. The ventro-anterior medial prefrontal cortex is implicated in the evaluation of the self-referential and emotional-value of stimuli (Amodio & Frith, 2006; Northoff et al., 2006; Olsson & Ochsner, 2008; Phillips et al., 2003). In contrast, the dorso-anterior medial prefrontal cortex is characterized by processes involving self-reflection and internal monitoring, including monitoring and regulating one’s own emotion (Ochsner, Knierim et al., 2004; Ochsner, Ray et al., 2004) and internal monitoring of actions (Botvinick, Cohen, & Carter, 2004). The functional dissociation of these regions is reflected by their divergent neuroanatomical connectivity patterns. The ventro-anterior medial prefrontal cortex is densely connected with limbic regions, which process and integrate sensory modalities (Ongur & Price, 2000). In contrast, the dorso-anterior medial prefrontal cortex is extensively connected with the lateral prefrontal cortex (Ongur & Price, 2000), which is critically implicated in processes of higher cognitive functions (Koechlin et al., 2003; Tanji & Hoshi, 2008). The functional and anatomical dissociation of these regions is consistent with the hypothesized ventro-dorsal spatial gradient (automatic, emotional versus reflective, controlled behaviour) and the characteristics of warmth and extraversion.
The right amygdala, adjacent parahippocampus, and hippocampus were core regions in the neural correlates of warmth, its non-agentic component, and the affiliative component of ingenuousness. The amygdala links emotional and motivational context to the perception of sensory signals across modalities (Costafreda, Brammer, David, & Fu, 2008; Mesulam, 1998). Accordingly, individuals with damage to the amygdala might not perceive social cues signalling affection due to the failure to integrate emotion with perceptual inputs. Moreover, given that the amygdala is part of the reward circuit of the brain, these individuals might not perceive the rewarding aspects of affiliative relationships anymore, which is fundamental for social affiliation (Depue & Morrone-Strupinsky, 2005). Lesion and functional neuroimaging studies in humans converge to indicate that the amygdala together with the adjacent parahippocampal and hippocampal regions critically contribute to the encoding and retrieval of autobiographical memory and its emotional valence (Dolcos, LaBar, & Cabeza, 2004; Mesulam, 1998; Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003). In social encounters with a closely familiar person, the retrieval of autobiographical memories is essential to evoke the appropriate emotional feelings for that person, facilitating social affiliation (Depue & Morrone-Strupinsky, 2005).
The association of affiliative traits with these emotion and reward-related brain regions implies both that affiliative interpersonal behaviour relies heavily on emotion processing and that it is a socially rewarding process. This is consistent with the neurobehavioral affiliation trait model (Depue & Morrone-Strupinsky, 2005), which posits that the affiliative trait is `at its core characterized by emotional feelings of warmth and affection`, which reflects the `capacity to experience reward elicited by affiliative stimuli` such as a friendly-comforting words, a smile, or a pleasant touch.
Interpersonal Traits Sharing Agentic and Affiliative Components
According to the circumplex model, each trait is a blend of the orthogonal dimensions of agency and affiliation (Wiggins, 1995), thus combinations of agency-related and affiliation-related brain regions were expected for some traits. Gradations of the degree to which either interpersonal agency or affiliation is involved in the trait were expected to mirror the relative presence, or absence, of correlations with the neural networks underlying agency or affiliation.
Extraversion, a trait high in both agency and affiliation, showed correlations with both networks. Analysis of its neural correlates revealed predominantly dorsolateral prefrontal structures, but also midline prefrontal and lateral orbitofrontal regions, areas implicated in internal focus and emotion processing (Adolphs, 2002; Kringelbach & Rolls, 2004; Lieberman, 2007). In contrast to our analyses of pure dominance, VLPFC regions appeared in the analyses of extraversion and the affiliative component of extraversion, suggesting a relationship between VLPFC and interpersonal traits that are both highly agentic and highly affiliative. Accordingly, the left VLPFC is involved in verbal processing and production (Bookheimer, 2002; Hillis et al., 2004), skills underpinning successful communication in social settings, and the right posterior VLPFC is involved in processing one’s own or other’s physical features and actions (Gallese, Keysers, & Rizzolatti, 2004; Lieberman, 2007). Communicating verbally with others, and understanding others actions, are both externally-oriented processes typical of high-agency/high-affiliation interpersonal traits like extraversion.
Ingenuousness, a blend of warmth (high affiliation) and submissiveness (low agency), did not correlate with the cortical midline structures seen in extraversion and warmth, nor the dorsolateral frontal regions underlying agentic traits, but correlated with parts of the network underlying warmth, including the right anterior fusiform gyrus, right putamen, and adjacent border of the medial insula. Ingenuous individuals, who are described as gentle and mild in social interactions (Wiggins, 1995), tend to cooperate with the needs and wishes of others rather than asserting their own goals. Accordingly, brain regions associated with the perception of social and emotional cues were expected to correlate with ingenuousness. In fact neural correlates of ingenuousness and in particular its affiliative component encompassed primarily right anteromedial temporal regions, which is consistent with these expectations, given the critical role of these regions in linking perceptive and mnemonic information with its emotional context (Mesulam, 1998). Moreover, these regions overlapped completely with the neural correlates of warmth. Notably, the association of warmth with right temporal regions was stronger when the agentic component was removed from warmth (Fig. 5), underlining the relationship between the submissive, non-agentic aspects of affiliative traits and right temporal regions. Support for this notion comes from the neuroimaging results of extraversion, a blend of high affiliation and high agency, which showed no correlations with right temporal regions.
Coldness, Arrogance, Introversion, Submissiveness
A region at the junction of the left inferior occipital gyrus, medial occipital gyrus, and fusiform gyrus correlated with coldness, suggesting that persons who had the lowest levels of coldness (greatest warmth) had the most damage to this region. Persons scoring high in coldness tend to emphasize autonomy from others and are unaffectionate when such behaviours would be appropriate (Wiggins, 1995). If this result is taken to be meaningful, one interpretation is based on the function of the occipital-fusiform junction as visual association cortex (Mesulam, 1998) which contributes to the perception of complex social scenes (Geday, Gjedde, Boldsen, & Kupers, 2003). The corresponding region on the right side is involved in the perception of biologically relevant visual cues such as face and human body perception (Peelen & Downing, 2005). Assuming that social perception in posterior brain regions arises from a balanced inhibitory circuit between bilateral structures (Kapur, 1996), we could speculate that damage to the left occipital-fusiform junction might lead to paradoxical functional facilitation of its homologous structure on the right, increasing sensitivity to visual social cues. This greater social attentiveness might lead to higher levels of affiliative behaviour. However, this interpretation is speculative, and it remains possible that this result was an artefact of our study design and sample. Notably, only a few AD, aMCI, and NC subjects had low ratings of coldness, suggesting that statistical restriction of range for Coldness score could be the source of these unexpected, though highly statistically significant results.
Three interpersonal traits, arrogance, introversion, and submissiveness, showed no neural correlates at the FWE-corrected level of significance. Similar to the analysis of coldness, these negative results may have occurred based on the nature of the data and the type of correlation analyses performed. In this study we focused on positive correlation analyses, assuming that decreased trait scores would be associated with decreased grey matter volume. However, the scores of arrogance, introversion, and submissiveness were higher in most diagnostic groups compared to NCs. Thus, positive brain-behaviour correlations with these traits would be somewhat paradoxical, given that affected individuals are expected to have increased behaviour scores as a result of loss of tissue. In contrast, when we performed negative correlations with these traits, significant neural correlates emerged that overlapped largely with the positive neural correlates of their corresponding opposite traits (i. e., ingenuousness, extraversion, and dominance). Alternatively, when performing negative correlations with ingenuousness, extraversion, and dominance, no neural correlates emerged at the FWE-corrected level of significance.
This suggests that the structure of the IAS, with the possibility of eight separate interpersonal trait scores, may not be consistent with the actual functioning of neural circuits underlying personality, and this instrument may have better psychometric validity if only four trait scores are derived based on four continuums of behaviour: dominance-submissiveness, extraversion-introversion, warmth-coldness, and ingenuousness-arrogance. Similarly to the neuroimaging results of the eight interpersonal traits, the results of the four continuums of behaviour showed the strongest neuroanatomic support for the warmth-coldness and extraversion-introversion continuums, followed by ingenuousness-arrogance, and finally by dominance-submissiveness. Ingenuousness-arrogance and dominance-submissiveness demonstrated no unique neural signatures apart from the other continuums in this sample, and thus may not be as psychometrically valid, at least as measured by the IAS.
Hemispheric Laterality vis-à-vis Existing Laterality Theories
Neural correlates of agentic traits, which encompassed lateral prefrontal regions, showed a left lateralization-bias. In contrast, neural correlates of affiliative traits, which encompassed medial prefrontal and temporal regions, showed a right lateralization-bias. These results can be interpreted in light of the hypothesized right-left spatial gradient, which is based on three complementary hemispheric laterality hypotheses, i.e., 1) the right-hemisphere model of emotion processing, 2) the dominance/submission model, and 3) the approach/withdrawal model (e. g., Demaree et al., 2005). Agentic traits were conceptualized as assertive and approaching behaviours, thus, a left-lateralization bias was expected based on the dominance/submission and the approach/withdrawal models. In contrast, affiliative traits were conceptualized as emotion-driven behaviours, thus, a right-lateralization bias was expected based on the right-hemisphere model. However, if warmth is conceptualized as an approaching behaviour, a left-lateralization bias would be expected based on the approach/withdrawal model. The fact that neural correlates of warmth were exclusively right-lateralized and overlapped extensively with regions involved in emotional processing, suggests that warmth is more characterized by emotional (affiliative) than approaching (agentic) aspects. Moreover, warmth can also be conceptualized as an internalizing behaviour, which is associated with low-dominance feelings (Demaree et al., 2005). Then, the observed right-lateralization of the neural correlates of warmth is also in line with the dominance/submission model.
Based on the dominance/submission and the approach/withdrawal models a right lateralization-bias for non-agentic traits such as submissiveness was expected (Demaree et al., 2005). This expectation could not be directly tested due to the missing neural correlates of submissiveness. However, indirect evidence for a right lateralization-bias comes from the distinct neural correlates of extraversion and ingenuousness. Extraversion, a blend of high affiliation and high agency, mapped quite equally onto both prefrontal hemispheres, whereas ingenuousness, a blend of high affiliation and low agency, mapped only onto right-hemispheric, primarily temporal regions, demonstrating a left-right (and fronto-temporal) gradient along the agency dimension.
Clinical Implications
In this study, interpersonal traits associated with antisocial behaviour, such as increased coldness and arrogance, were predicted by cortical atrophy of right ventromedial prefrontal and right anterior temporal regions. These findings support the growing evidence that damage to these brain regions are associated with disturbed emotional and social behaviours (Edwards-Lee et al., 1997; Hornak et al., 2003; Mychack, Kramer, Boone, & Miller, 2001; Rosen et al., 2005). Two diagnostic groups of our sample are associated with early damage to these regions: FTD patients, showing frontal and right temporal damage (Seeley et al., 2008), and SD patients, who show asymmetric damage to anterior temporal, insular, and medial frontal cortex (Rosen et al., 2002). Accordingly, warmth, the most emotion-based interpersonal trait, was significantly decreased only in these two groups compared to NCs. These results raise the question of whether particular regions within the anatomy of warmth are more likely to contribute to personality change in FTD compared to SD. However, single diagnostic group analyses of behavioural correlates using VBM is methodologically unsound, because severe restriction of range in both anatomic and behavioural diversity results in inadequate power for unbiased whole-brain analyses. Because of this, neural correlates of personality within single diagnostic groups could not be directly examined in this study, however, given that the left temporal lobe was not significantly associated with any interpersonal trait, these findings support the clinical observation that predominantly left-sided SD patients have near-normal social behaviour, while their behaviour becomes increasingly disordered with the advent of right-sided involvement (Edwards-Lee et al., 1997).
In contrast to warmth, which was significantly decreased only in FTD and SD patients, dominance and extraversion were significantly decreased in FTD, SD, AD, CBD, and PSP patients compared to NCs. The fact that diagnostic groups with different brain atrophy patterns showed similar scores of dominance and extraversion implies that a network of brain regions underlies these traits, and damage to different components of the network may result in similar personality changes. Thus, these traits may have decreased in FTD and AD patients because of damage to dorsolateral frontal structures involved in the agentic circuit. In addition to this frontal cortical damage, PSP and CBD patients also show extensive subcortical grey and white matter atrophy (Josephs et al., 2008), which may have contributed to frontal-subcortical dysfunction affecting behaviour and personality.
In contrast to the other diagnostic groups, personality traits were not significantly altered in PNFA and aMCI patients compared to NCs. The aMCI patients were selected specifically for their primarily amnestic clinical presentation, because these patients have more likely underlying AD-pathology than another neuropathology (Petersen et al., 2006). Given that multiple studies of personality in AD suggest early decreases in extraversion and dominance (Chatterjee et al., 1992; Rankin et al., 2005), the negative result in aMCI patients was hence surprising. However, in these earlier studies the patients were either at a later stage of the disease (Chatterjee et al., 1992) than our patients, or the study analyzed degree of personality change from baseline (Rankin et al., 2005), while this study examined current personality compared to controls, in which any effect is likely to be more subtle. Moreover, none of the core brain regions found to be associated with personality change in this study are typically damaged in aMCI patients (Bell-McGinty et al., 2005). This raises the question of whether personality changes in aMCI patients and even early AD patients may be more of a psychological response to loss of cognitive capacity, rather than a direct result of damage to neural networks mediating personality traits.
The negative findings in the PNFA patients were more expected, as these patients never have been demonstrated to undergo personality changes. However, the missing association between PNFA patients and altered personality might be also due to the low number of patients (n=6), especially given the fact that the personality traits showed high variability within patient groups (Fig. 3). Thus, characterization of subtle personality changes in larger groups of PNFA patients remains an important line of future investigation.
Limitations
Voxel-based morphometry allows an unprecedented degree of quantitative specificity in the analysis of structural MRI scans across large groups of patients with focal atrophy; however, this technique is not without limitations. First, the localisation of clusters on brain structures is accomplished via inter-subject spatial normalisation, which attempts to align corresponding regions of subjects’ brains by correcting for inter-individual differences in global brain shape. However, because of the variability in brain anatomy across subjects perfect overlapping of corresponding brain regions is hardly achievable, causing the anatomical localisations of the neuroimaging results to be probabilistic (Brett, Johnsrude, & Owen, 2002).
Second, because the VBM method is essentially based on an atrophy model that relies on the use of a clinically-defined sample of subjects with diverse atrophy patterns, the extent to which results can be generalized beyond a study’s population of interest is widely debated. Others have effectively used this method to accurately localize cognitive functions to brain areas previously identified in healthy controls using other techniques, suggesting generalization is often both possible and appropriate (e. g., Amici et al., 2007; Boxer, Garbutt et al., 2006). However, the influence of disease-specific patterns of co-atrophy remains a potential confound. To address this issue, we controlled for diagnostic group effects in our VBM analyses, highlighting brain-behaviour relationships that held true in more than one disease group. This increases the likelihood that our results are not restricted to our study sample, but may be generalizable to normal brain function. Future refinements to VBM analytic technique may help clarify the degree to which normal aging (Wright et al., 2007) and neural plasticity in the context of disease may limit such generalization.
Finally, the degree to which structural VBM is truly a whole-brain analysis is limited by the particular composition of the subject sample. Our study intentionally included a large sample of patients with a diverse selection of diseases known collectively to affect nearly all cortical structures, in order to maximize sample-wide variability in both brain atrophy and behaviour. However, some brain regions likely suffered from restriction of range and a corresponding loss of power to detect brain-behaviour relationships, particularly in cases where only small numbers of subjects had atrophy to an important region. Thus, unlike the results of a functional study, our structural VBM results do not necessarily represent the complete neural circuit of any interpersonal trait, but depict clearly only the parts of it that were best represented by our particular sample.
Conclusions
The neural correlates of five interpersonal traits were delineated in a group comprised of patients with diverse patterns of cortical brain atrophy due to neurodegenerative diseases, as well as healthy elderly people. These neural correlates showed anatomical dissociations within the frontotemporal lobes depending on the degree to which the associated interpersonal traits involved agency and affiliation. Cortical atrophy in left dorsolateral prefrontal and left lateral frontopolar structures was predicted by decreased agentic interpersonal traits such as dominance, whereas cortical atrophy in right ventromedial prefrontal and right anteromedial temporal structures was predicted by decreased affiliative interpersonal traits such as warmth. These results support the hypotheses that brain regions related to externally-focused, executive control-related processes underlie agentic interpersonal traits, whereas brain regions related to internally-focused, emotion- and reward-related processes underlie affiliative interpersonal traits. In addition, these findings indicate that interpersonal traits are subserved by complex neural networks rather than discrete anatomic areas.
ACKNOWLEDGEMENTS
This research was supported in part by the National Institute on Aging (NIA) [5-K23- AG021606-02, PPG P01-AG1972403, and AG19724-01A1]; the State of California, Alzheimer’s Disease Research Center of California (ARCC) [01-154-20]; the Larry L. Hillblom Foundation, Inc., [2002/2J]; UCSF [GCRC-M01-RR00079]; the Swiss National Science Foundation (SNF) [PBBEB-113383], and the Scientific Society Basle.
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion of Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL, Miller BL, et al. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. Journal of Neuroscience. 2007;27:6282–6290. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, Dekosky ST, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Archives of Neurology. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Borod JC, Bloom RL, Brickman AM, Nakhutina L, Curko EA. Emotional processing deficits in individuals with unilateral brain damage. Applied Neuropsychology. 2002;9:23–36. doi: 10.1207/S15324826AN0901_4. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. Journal of Neuroscience. 2006;26:6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Archives of Neurology. 2006;63:81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nature Reviews Neuroscience. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995a;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology. 1995b;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Strauss ME, Smyth KA, Whitehouse PJ. Personality changes in Alzheimer's disease. Archives of Neurology. 1992;49:486–491. doi: 10.1001/archneur.1992.00530290070014. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behavioral Neuroscience. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Connolly JJ, Kavanagh EJ, Viswesvaran C. The convergent validity between self and observer ratings of personality: A meta-analytic review. International Journal of Selection and Assessment. 2007;15:110–117. [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW. Brain lateralization of emotional processing: historical roots and a future incorporating "dominance". Behavioral and Cognitive Neuroscience Reviews. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–350. doi: 10.1017/S0140525X05000063. discussion 350–395. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Endler N. The Interface between Personality and Cognition. European Journal of Personality. 2000;14:377–389. [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Malcom H, Johnson D, Morris JC. Personality traits distinguishing dementia with Lewy bodies from Alzheimer disease. Neurology. 2007;68:1895–1901. doi: 10.1212/01.wnl.0000263131.80945.ad. [DOI] [PubMed] [Google Scholar]
- Geday J, Gjedde A, Boldsen AS, Kupers R. Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage. 2003;18:675–684. doi: 10.1016/s1053-8119(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Reexamining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiology of Aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119:1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kurtz JE, Lee PA, Sherker JL. Internal and temporal reliability estimates for informant ratings of personality using the NEO-PI-R and IAS. Assessment. 1999;6:103–113. doi: 10.1177/107319119900600201. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr The structure of interpersonal traits: Wiggins's circumplex and the five-factor model. Journal of Personality and Social Psychology. 1989;56:586–595. doi: 10.1037//0022-3514.56.4.586. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT, Jr, Ostendorf F, Angleitner A, Hrebickova M, Avia MD, et al. Nature over nurture: temperament, personality, and life span development. Journal of Personality and Social Psychology. 2000;78:173–186. doi: 10.1037//0022-3514.78.1.173. [DOI] [PubMed] [Google Scholar]
- McCrae RR, John OP. An introduction to the five-factor model and its applications. Journal of Personality Special Issue: The Five-Factor Model: Issues and Applications. 1992;60:175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychack P, Kramer JH, Boone KB, Miller BL. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56:S11–S15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biological Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. Journal of Comparative Neurology. 1998;401:480–505. [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Downing PE. Selectivity for the human body in the fusiform gyrus. Journal of Neurophysiology. 2005;93:603–608. doi: 10.1152/jn.00513.2004. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Archives of Neurology. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. New York: Elsevier; 1994. pp. 17–57. [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B. The neural system that bridges reward and cognition in humans: an fMRI study. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL, et al. Self awareness and personality change in dementia. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:632–639. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Mychack P, Miller BL. Double dissociation of social functioning in frontotemporal dementia. Neurology. 2003;60:266–271. doi: 10.1212/01.wnl.0000041497.07694.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Rosen HJ, Kramer JH, Schauer GF, Weiner MW, Schuff N, et al. Right and left medial orbitofrontal volumes show an opposite relationship to agreeableness in FTD. Dementia and Geriatric Cognitive Disorders. 2004;17:328–332. doi: 10.1159/000077165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL. Role of orbitofrontal cortex connections in emotion. Annals of the New York Academy of Sciences. 2007;1121:72–86. doi: 10.1196/annals.1401.026. [DOI] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Caspi A. The cumulative continuity model of personality development: Striking a balance between continuity and change in personality traits across the life course. In: Staudinger U, Lindenberger U, editors. Understanding human development: Lifespan psychology in exchange with other disciplines. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2003. pp. 183–224. [Google Scholar]
- Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132:1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Siegler IC, Dawson DV, Welsh KA. Caregiver ratings of personality change in Alzheimer’s disease patients: a replication. Psychology and Aging. 1994;9:464–466. doi: 10.1037//0882-7974.9.3.464. [DOI] [PubMed] [Google Scholar]
- Strauss ME, Pasupathi M, Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer’s disease. Psychology and Aging. 1993;8:475–480. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiological Reviews. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wiggins JS. Interpersonal Adjective Scales: Professional Manual. Odessa FL: Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- Wiggins JS, Coutu J. Odessa FL: Psychological Assessment Resources, Inc; 1995. Interpersonal Adjective Scales (IAS) Scoring Program. [Google Scholar]
- Wright CI, Feczko E, Dickerson B, Williams D. Neuroanatomical correlates of personality in the elderly. Neuroimage. 2007;35:263–272. doi: 10.1016/j.neuroimage.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]