Abstract
Gamma-amino-butyric acid (GABA) regulates the proliferation and migration of olfactory bulb (OB) interneuron progenitors derived from the subventricular zone (SVZ), but the role of GABA in the differentiation of these progenitors has been largely unexplored. This study examined the role of GABA in the differentiation of OB dopaminergic interneurons using neonatal forebrain organotypic slice cultures prepared from transgenic mice expressing GFP under the control of the tyrosine hydroxylase (Th) gene promoter (ThGFP). KCl-mediated depolarization of the slices induced ThGFP expression. The addition of GABA to the depolarized slices further increased GFP fluorescence by inducing ThGFP expression in an additional set of periglomerular cells. These findings showed that GABA promoted differentiation of SVZ-derived OB dopaminergic interneurons and suggested that GABA indirectly regulated Th expression and OB dopaminergic neuron differentiation through an acceleration of the maturation rate for the dopaminergic progenitors. Additional studies revealed that the effect of GABA on ThGFP expression required activation of L- and P/Q-type Ca+2 channels as well as GABAA and GABAB receptors. These voltage-gated Ca+2 channels and GABA receptors have previously been shown to be required for the co-expressed GABAergic phenotype in the OB interneurons. Together, these findings suggest that Th expression and the differentiation of OB dopaminergic interneurons are coupled to the co-expressed GABAergic phenotype, and demonstrate a novel role for GABA in neurogenesis.
Keywords: GABA, dopamine, tyrosine hydroxylase, olfactory bulb, differentiation, slice culture
Introduction
The glomerular and granule cell layer interneurons of the main olfactory bulb (OB) are derived primarily from the mammalian subventricular zone (SVZ), a proliferative region that generates neural progenitors and remains active throughout life (Alvarez-Buylla and Garcia-Verdugo 2002; Doetsch and Alvarez-Buylla 1996; Lois et al. 1996; Luskin 1993). Mature OB interneurons express a variety of neurotransmitter and neuroactive molecules, such as GABA and dopamine (DA) (Kosaka et al. 1998; Parrish-Aungst et al. 2007). The molecular mechanisms regulating the differentiation of OB DAergic interneurons, in particular, are not well-defined, but are clearly distinct from those in midbrain DAergic neurons (Cave and Baker 2008). OB DAergic interneurons are distributed primarily in the glomerular layer and can be readily identified by expression of tyrosine hydroxylase (Th), the first enzyme in the DA biosynthetic pathway. These interneurons receive excitatory glutamatergic input from both axo-dendritic synaptic contacts with olfactory receptor neurons (ORNs) and dendro-dendritic synapses with mitral and tufted (M/T) cells (Farbman 1992). Differentiation of OB DAergic neurons is activity-dependent since perturbations that prevent ORN synaptic activity produce a profound down regulation in DA production as well as the expression of both Th mRNA and protein (Baker et al. 1999; Baker et al. 1993). Studies with primary neonatal OB neuron cultures have shown that L-type Ca+2 channels are important for mediating the activity-dependent expression of TH (Cigola et al. 1998; McMillian et al. 1994; Puche and Shipley 1999). However, the mechanisms by which extrinsic signals and their associated membrane receptors in the OB glomerular layer provide positional information that initiates TH expression and terminal differentiation of OB DAergic neurons have not been elucidated.
GABA is expressed by a majority of OB interneurons, including the DAergic cells (De Marchis et al. 2007; De Marchis et al. 2004; Gutierrez-Mecinas et al. 2005; Kosaka et al. 1988; Kosaka et al. 1985; Parrish-Aungst et al. 2007; Puopolo and Belluzzi 1998). Release of both GABA and DA by OB periglomerular (PG) interneurons in response to excitatory input from either ORNs or M/T cells provides inhibitory feedback primarily through activation of D2 and GABAB receptors located on the ORN and M/T terminals in the glomeruli (Ennis et al. 2001; Keller et al. 1998). Release of GABA from PG interneurons can also inhibit neighboring PG neurons through dendro-dendritic synapses in the glomerular neuropil (Murphy et al. 2005; Smith and Jahr 2002).
In addition to its neurotransmitter role, GABA regulates the proliferation and migration rates of neuronal progenitors in the SVZ and RMS (Bolteus and Bordey 2004; Liu et al. 2005; Wang et al. 2003). In the OB granule cell layer, GABA also increases microtubule stability of interneuron progenitors which promotes the proper initiation, elongation and stabilization of dendrites (Gascon et al. 2006). Given these established developmental roles, a largely unexplored question is whether GABA can also modulate differentiation of specific interneuron phenotypes.
The present study reveals that GABA augments both Th promoter-driven GFP reporter gene (ThGFP) and TH protein expression in the glomerular layer of depolarized neonatal forebrain slice cultures. The findings suggest that differentiation of OB dopaminergic interneurons is regulated through a molecular pathway that is coupled to the co-expressed GABAergic phenotype.
Materials and Methods
Animals
ThGFP transgenic mice were obtained from Dr. Kazuto Kobayashi (Matsushita et al. 2002). Mice were housed in humidity-controlled cages at 22 °C under a 12:12 hour light:dark cycle and provided with food and water ad libitum. All procedures were carried out under protocols approved by the Cornell University Institutional Animal Care and Use Committee and conformed to NIH guidelines.
Slice Culture
Whole brains from mouse pups ranging in age from postnatal day 2 or 3 were dissected and placed into Petri dishes containing ice-cold dissection medium (Leibovitz’s L-15 media, pH 7.4, Life Technologies/Gibco BRL/Invitrogen, Carlsbad, CA). Brains were transected mid-sagittally and each hemisphere was placed with the medial face down into a small aluminum foil container and embedded in warm (37 oC) 3% low- melting-point agarose (Sigma, St. Louis, MO) in L-15 medium, then rapidly cooled on ice and cut into 200 μm sagittal slices with a vibratome (Model 1000; Ted Pella Inc., Redding, CA). Slices were cultured for 48–72 hours at 37°C and 5% CO2 in Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with B27 (1:50, Invitrogen), 0.5 mM L-glutamine (Invitrogen) and 25 μg/ml gentamycin (Sigma). Slices were either depolarized with 25 mM potassium chloride (KCl) or, as a control, treated with 25 mM sodium chloride (NaCl). The use of equimolar concentrations of either NaCl and KCl maintained a constant osmolarity and ionic strength of the slice culture media. The following pharmacological agents were used at the indicated final concentrations: GABA (Sigma) either 1, 10 or 100 μM; the L-type Ca+2 channel inhibitor, nifedipine (Sigma) at 10 μM; the P/Q inhibitor ω-agatoxin at 200 nM; the GABAA receptor antagonist bicuculline at 100 μM (Sigma); the GABAB antagonist CGP46381 at 100 μM (Tocris, Ellisville, MO); AMPA receptor antagonists, CNQX and DNQX at 20 μM and 10 μM, respectively (Sigma); the NMDA antagonist APV at 100 μM (Sigma) the mGluR antagonist, LY341495 at100 μM.
For focal stimulation, slices were cultured in an RC-26G recording chamber (Warner Instruments, Hamden, CT) with a circulating bath of oxygenated Neurobasal medium (Invitrogen) supplemented with B27 (1:50, Invitrogen), 0.5 mM L-glutamine (Invitrogen) and 25 μg/ml gentamycin (Sigma). The media was maintained at 37 °C with a in-line solution heater (Warner) that was controlled by TC-324B single channel controller (Warner). The olfactory receptor nerve layer of cultured slices were stimulated with a 2-contact CE2C75 cluster electrode (FHC Inc., Bowdoin, ME) using 1 second pulses at 100 Hz and 7 V applied at 10 minute intervals for 7 hours.
Immunohistochemistry
Localization of single antigens was performed as previously published (Saino-Saito et al. 2007). Briefly, sections were fixed for 30 min with phosphate-buffered (pH 7.2), 4% formaldehyde and washed in phosphate-buffered saline (PBS) before being blocked with 1% bovine serum albumin in PBS and incubated overnight with primary antisera. Antigens were visualized by incubation with appropriate biotinylated secondary antiserum, the Vector Elite kit (Vector Laboratories; Burlingame, CA) and 3,3′-diaminobenzidine (DAB, 0.05%) as chromogen with hydrogen peroxide (0.003%). Slides were dehydrated through a graded series of alcohols and cover-slipped. For double label immunofluorescence, secondary antibodies conjugated to either Alexa 488 or Alexa 594 were used (Molecular Probes/Invitrogen, Carlsbad, CA). Slides were cover-slipped with Cytoseal (VWR, Westchester, PA). Primary antibodies used were rabbit anti-TH (lot 15-2, raised in our laboratory, 1:25,000 and 1:10,000, for single and double-labeling, respectively), chicken anti-GFP (1:5,000 and 1,000; AB16901, Chemicon, Temecula, CA), mouse anti-TH monoclonal (1:10,000, for double labeling; Roche, Nutley, NJ) and goat anti-olfactory marker protein (OMP, kindly provided by Frank Margolis 1:7,000).
Quantitative Analysis
GFP fluorescence intensities were analyzed using the MetaMorph Imaging System (Molecular Devices, Sunnyvale, CA). Images of all slices were acquired with a Zeiss Axiomat microscope (Carl Zeiss, Thornwood, NY) equipped with a digital camera and KS400 software using the same filter sets and exposure times. Images were inverted and converted to 8 bit black and white in Adobe Photoshop (Adobe Systems Inc., San Jose, CA). The integrated pixel density of the glomerular layer was measured. To correct for differences in diameter, thickness and initial fluorescence intensity, the data for each slice was expressed as a ratio of the fluorescence intensity after 48 hours in culture to that at time zero.
Stereological cell counts were performed using confocal stacks of 20 images encompassing a total depth of 20 μm and a 0.212 mm2 area from equivalent sections from matched slices. A minimum of three regions in the sagittal sections (dorsal, anterior and ventral) from three slices were used for cell counts under each culture condition. All analyses were conducted using a Zeiss Meta 510 scanning confocal microscope and Zeiss LSM Image Browser software.
For all quantitative analyses results are expressed as a mean ± standard deviation. Statistical significance was analyzed by two way ANOVA with appropriate post-hoc tests using StatView Software (Statview Software Inc, Cary, NC). Results were considered significant if p<0.05.
Results
Activity dependent OB ThGFP expression in the forebrain slice culture model system
To investigate the effect of GABA on activity-dependent OB TH expression, organotypic forebrain slice cultures were prepared from ThGFP transgenic mice containing a GFP reporter gene driven by the 9kb upstream Th gene promoter. Previous studies have demonstrated that this Th regulatory region is sufficient to mediate synaptic-activity dependent gene expression in the OB (Min et al. 1996; Saino-Saito et al. 2004). Although immunohistochemistry is sufficient both for labeling TH-expressing cells in tissue and observing qualitative changes in expression levels, this method cannot be used to quantify differences in TH expression levels between slices cultured under different conditions. By contrast, the ThGFP transgenic mice allow for quantitative analysis of Th promoter activity by fluorescence intensity measurements. In this study, ThGFP fluorescence intensities in living slices were measured at 0 and 48 hours in culture, and calculation of the fluorescence intensity ratio (48 hours relative to 0 hours) corrected for differences in diameter, thickness and initial fluorescence intensity of individual slices.
Addition of depolarizing concentrations of KCl to the culture media were used to simulate the afferent synaptic input necessary for induction of OB TH expression. The elevated KCl concentration (25mM) in this study is commonly used to induce neuronal depolarization in both primary and organotypic slice cultures (Cirrito et al. 2005; Li et al. 2001; Lohmann et al. 1998; Shahar et al. 2004; Shalizi et al. 2006; Suzuki et al. 2005). In KCl-depolarized forebrain slice cultures, there was almost total overlap in ThGFP and TH protein expression in the glomerular layer, whereas only ThGFP was expressed in the superficial granule and mitral cell layers (Fig. 1B). These expression patterns were nearly identical to those observed in vivo (cf. Fig. 1B vs. Fig. 1C) (Akiba et al. 2007; Saino-Saito et al. 2004).
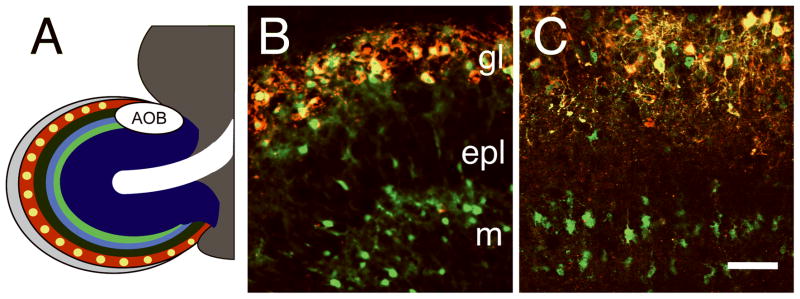
Figure 1.
Laminar expression pattern of TH and ThGFP in the OB. A, diagram of OB laminar organization: olfactory nerve layer (light gray), glomerular layer (red), glomeruli (yellow), external plexiform layer (dark green), mitral cell layer (light blue), internal plexiform layer (light green), granule cell layer (dark blue), rostral migratory stream (RMS, white) and accessory olfactory bulb (AOB). B and C, OB of a P5 transgenic mouse and depolarized forebrain slice culture, respectively, with TH (red) and ThGFP (green) expression shown. Overlapping regions of ThGFP and TH are yellow. Although TH expression is limited to the interneurons in the glomerular layer (gl), ThGFP is expressed in both the PG cells and granule cell interneurons in the mitral (m) cell layer as well as in scattered cells within the external plexiform (epl) layer. Bar = 50 μm.
TH expression in OB DAergic neurons requires glutamatergic input from either axo-dendritic synapses with olfactory receptor neurons (ORN) or dendro-dendritic synapses with M/T cells (Fig. 2A). Although the M/T dendritic projections remain largely intact during slice culture preparation, the ORN axonal projections are severed from their respective soma. Despite being severed, focal stimulation of the ORN axons induced ThGFP expression and showed that the OB microcircuits necessary for activity-dependent induction of OB TH expression are preserved in the slice culture model system (Fig. 2B and 2C). To confirm that glutamatergic pathways modulated ThGFP expression in the slice culture model system, KCl-depolarized slices were treated with selective glutamate receptor antagonists (Fig. 2D). In these experiments, a similar and significant reduction in ThGFP expression was observed following either individual or combined treatment with antagonists for AMPA receptors, NMDA receptors and metabotropic glutamate receptors (DNQX, APV and LY341495, respectively). Consistent with a role for activation of excitatory microcircuits, ThGFP expression was also significantly reduced in depolarized slice cultures treated with tetrodotoxin (TTX), an inhibitor of TTX-sensitive Na+ channels (Fig. 2E).
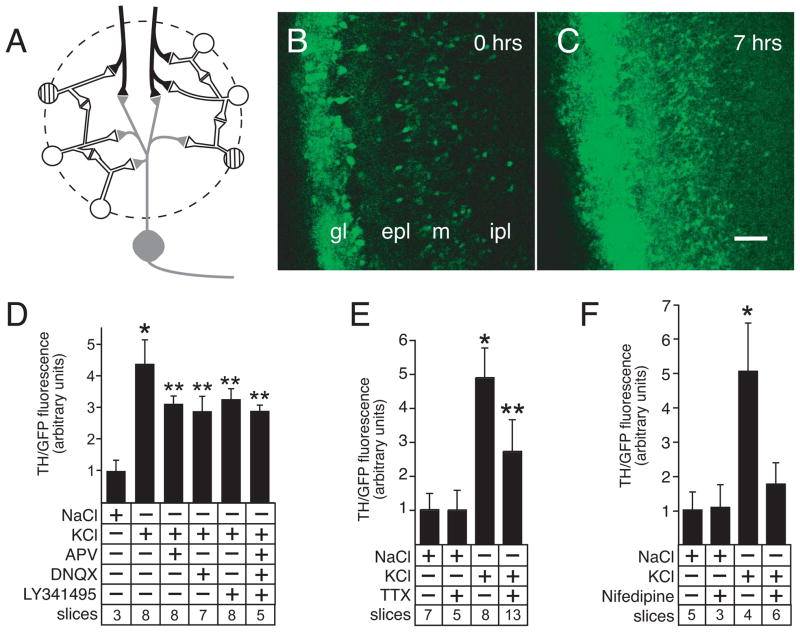
Figure 2.
Induction of ThGFP expression in forebrain slice cultures. A, diagram of the synaptic connections in a typical glomerulus. Olfactory receptor neuron axon terminals (black) form axo-dendro-dendritic synapses with mitral/tufted projection neurons (gray) and PG interneurons (white and striped). PG interneurons also make dendro-dendritic connections with both mitral/tufted cells and other PG interneurons. Dopaminergic interneurons expressing TH (striped cells) are only a subset of the total PG interneuron population. B and C, focal stimulation of the olfactory receptor nerve layer in forebrain slice cultures at 0 and 7 hours, respectively. Consistent with expression patterns observed both in vivo and in depolarized slice cultures, focal stimulation induced ThGFP expression in the periglomerular cells within the glomerular layer (gl) as well as in granule cells located in the external plexiform (epl) and mitral cell (m) layers. By contrast, expression is minimal in the internal plexiform layer (ipl). These findings confirm functionality of both the axon terminals in the forebrain slice cultures and the synaptic connections that are necessary for activity-dependent expression of TH. This induction of ThGFP by focal stimulation of olfactory receptor axon terminals confirms integrity of glomerular layer microcircuits in forebrain slice cultures. Bar = 50 μm. D, ThGFP expression induced by depolarization is partially blocked by treatment with antagonists to glutamatergic receptors (NMDA receptor, AMPA receptor and metabotropic receptor are blocked with APV, DNQX and LY341495, respectively), either individually or in combination. E, treatment with TTX (a sodium channel blocker) also partially reduces ThGFP expression induced by depolarization. F, treatment with the L-type Ca+2 channel blocker, nifedipine, prevents induction of ThGFP expression above baseline levels (control NaCl-treated). Data are expressed as mean ± standard deviation. Single asterisk indicates values that are significantly greater than cultures treated with NaCl. Double asterisk indicate values significantly greater than NaCl treated cultures, but significantly less than cultures treated with only KCl. For the graphs shown in D – F, “slices” indicates the number of cultured slices used to calculate the mean and standard deviation shown for condition tested.
Synaptic activity-dependent gene expression is mediated by Ca+2 influx and activation of second messenger signal transduction pathways in several neuronal systems (Chawla 2002; West et al. 2001). Previous studies have also shown that L-type Ca+2 channels are necessary for depolarization-induced TH expression in neonatal primary OB neuron cultures (Cigola et al. 1998; McMillian et al. 1994; Puche and Shipley 1999). Consistent with these previous findings, ThGFP expression was reduced to basal control levels in depolarized slice cultures treated with nifedipine, an L-type Ca+2 channel blocker (Fig. 2F). These findings indicated that induction of ThGFP expression in depolarized slice cultures was dependent on L-type Ca+2 channels.
GABA enhances activity-dependent ThGFP expression in the glomerular layer
Previous reports have shown that GABA can depolarize OB glomerular interneurons (Murphy et al. 2005; Smith and Jahr 2002). However, GABA was not sufficient to induce ThGFP expression by itself in the slice cultures (Fig. 3A). By contrast, GABA enhanced ThGFP expression levels in a dose-dependent manner when the slice cultures were simultaneously depolarized with KCl (Fig. 3A, 3B and 3D–H). The addition of 10 μM GABA was the lowest concentration in these dose-response studies that generated a significant increase in ThGFP expression levels relative to depolarized slices not treated with GABA. For this reason, 10μM GABA was used for all subsequent experiments involving the addition of GABA. Also, GABA did not induce transgene expression in depolarized slices co-treated with either the L-type Ca+2 channel blocker nifedipine (Fig. 3C) or glutamate receptor antagonists (Fig. 3I). This finding was consistent with synaptic activity being prerequisite for GABA to enhance ThGFP expression levels.
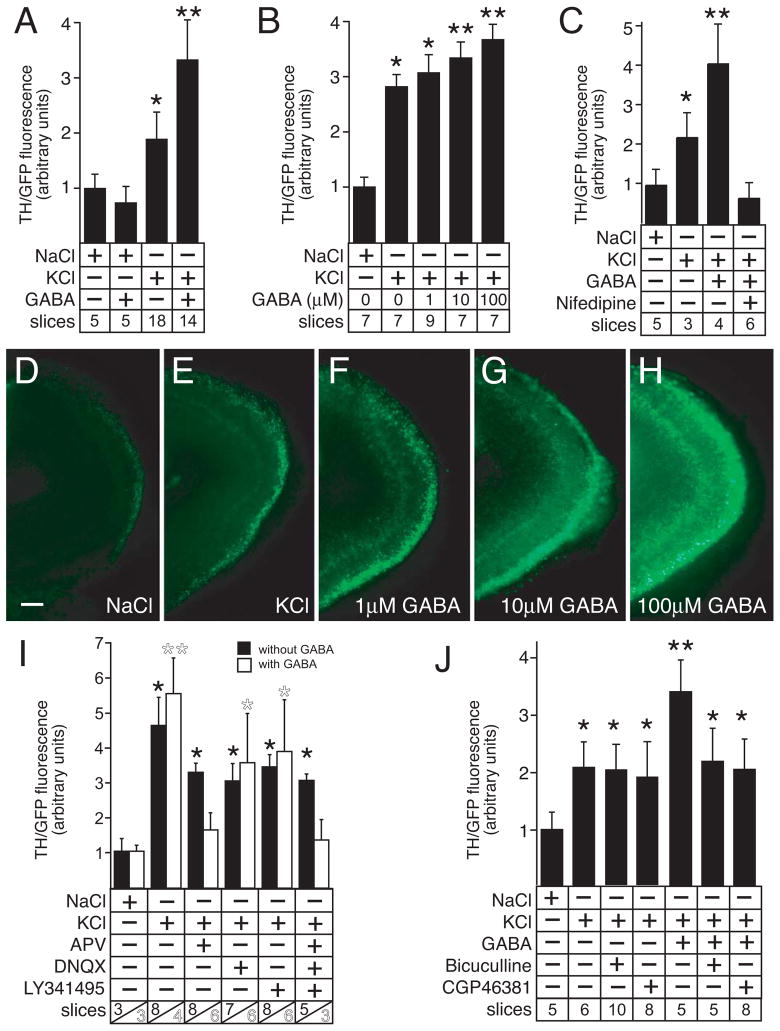
Figure 3.
GABA increases ThGFP expression in depolarized slice cultures. A, GABA increases ThGFP expression in slice cultures that are simultaneously depolarized with KCl, but GABA does not induce ThGFP expression in control NaCl-treated slices. B, GABA produces a dose-dependent increase of ThGFP in depolarized slices. C, GABA does not induce ThGFP expression in depolarized slice cultures treated with nifedipine, a L-type calcium channel blocker. D – H, ThGFP expression in representative slice cultures treated with either NaCl (D), KCl (E) KCl with 1 μM GABA (F), KCl with 10 μM GABA (G), or KCl with 100 μM GABA (H). Together, these slices show the dose-dependent increase of GFP expression in the glomerular layer of depolarized slices treated with GABA. Bar = 50 μm. I, antagonists of either ionotropic AMPA and NMDA glutamate receptors (DNQX and APV, respectively) or metabotropic glutamate receptors (LY341495) prevent GABA from inducing ThGFP expression above levels observed without GABA. The data shown for culture conditions without GABA is the same as shown in Fig. 2D. J, the GABAA or GABAB receptor antagonists bicuculline and CGP46381, respectively, can block induction of ThGFP expression by exogenous application of GABA, and neither antagonist effects ThGFP expression in the absence of exogenous GABA. These findings suggest that endogenous levels of GABA do not alter ThGFP expression induced by KCl-mediated depolarization. Single asterisk indicates values that are significantly greater than cultures treated with NaCl. Double asterisk indicate values significantly greater than cultures treated with only KCl. For the graphs shown in A – C, I and J, “slices” indicates the number of cultured slices used to calculate the mean and standard deviation shown for condition tested. For I, the number of slices cultured with or without GABA are shown in white and black numbers, respectively.
GABAA and GABAB receptors are co-expressed with TH in the OB (Bonino et al. 1999; Panzanelli et al. 2005). To identify the specific receptors necessary for the GABA-mediated increase in ThGFP expression, KCl-depolarized slice cultures were treated with GABA and either the GABAA or GABAB receptor antagonists, bicuculline and CGP46381, respectively. Application of either antagonist blocked the enhancement of ThGFP expression induced by GABA, but neither antagonist had an effect on transgene expression in the absence of exogenous GABA (Fig. 3J). Prolonged excitation (≥15 minutes) of OB PG interneurons has been reported to produce a gradual rundown in the ability of these cells to release GABA (Smith and Jahr 2002). Since the slice cultures in this study were examined 48 hours after depolarization, the endogenous GABA levels were likely insufficient to generate a measurable effect on ThGFP expression and, therefore, an exogenous source of GABA was required to modify transgene expression.
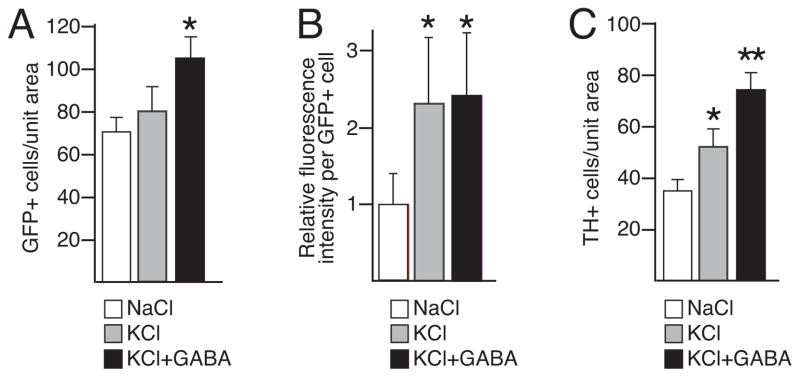
The GABA-mediated enhancement in ThGFP expression in the glomerular layer could be produced by either an increase in the number of cells expressing ThGFP or by an increase in the transgene expression levels per cell. In equivalent regions of matched slices, stereological cell counts revealed that depolarization conditions in the absence of GABA did not significantly change the total number of GFP+ cells (Fig. 4A, 4B and 5A). By contrast, the fluorescence intensity per GFP+ cell substantially increased under these conditions, notably in the processes of the PG cells (Fig. 4B). Together, these results indicated that the increase in GFP fluorescence induced by KCl-mediated depolarization resulted from an up-regulation of GFP expression in cells with already detectable levels of GFP at the time of slice culture preparation.
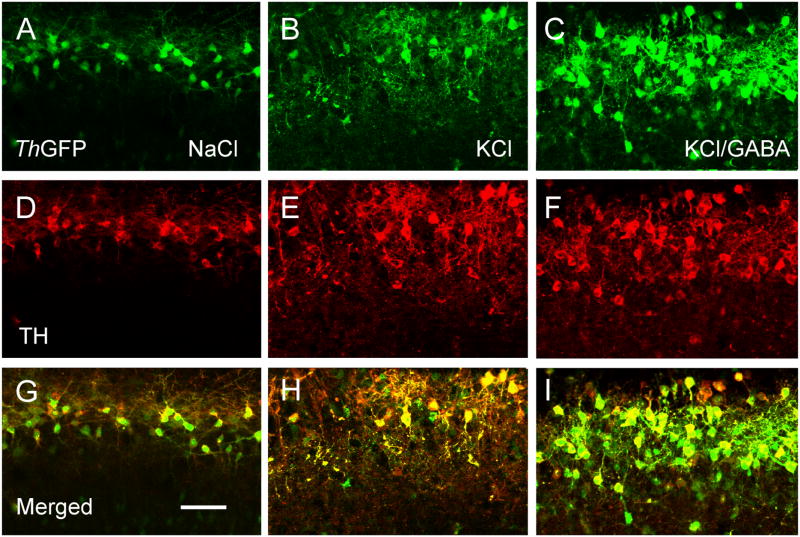
Figure 4.
GABA increases co-expression of TH and ThGFP in depolarized slice cultures. Expression of ThGFP (green, A – C) and TH (red, D – F) in slices treated with NaCl, KCl and KCl with GABA. The merged images are shown in G – I. KCl increases the number of TH cells relative to NaCl-treated cultures, but there is no change in the number of cells expressing ThGFP. By contrast, the number cells expressing TH and ThGFP increase in cultures treated with GABA and KCl as compared to cultures treated with only KCl. Bar = 50 μm.
Figure 5.
The number of GFP+ and TH+ cells relative to fluorescence intensity per GFP+ cell in slices cultures. A, number of GFP+ cells in equivalent regions of matched slices treated with either NaCl, KCl, or KCl with GABA. B, relative fluorescence intensity per GFP+ cell in slices cultures treated with either NaCl, KCl, or KCl with GABA. C, number of TH+ cells in equivalent regions of matched slices treated with either NaCl, KCl, or KCl with GABA. Single asterisk indicates values that are significantly greater than from cultures treated with NaCl. Double asterisk indicate values significantly greater than with cultures treated with only KCl.
An equivalent analysis of depolarized slice cultures treated with GABA, revealed that the total number of GFP+ cells increased relative to cultures treated with only KCl (Fig. 4B, 4C) without changing the GFP fluorescence intensity per GFP+ cell (Fig. 5B). These findings revealed that the GABA-mediated enhancement of GFP fluorescence in the glomerular layer resulted from an increased number of cells expressing the transgene. Stereological cell counts performed on the same sections used for the ThGFP analysis showed that GABA also increased the number of PG cells expressing the endogenous TH protein (Fig. 4D–F and Fig. 5C).
In contrast to the lack of change in the number of ThGFP cells, KCl-mediated depolarization in the absence of GABA significantly increased the number of TH+ cells relative to NaCl controls (Fig. 4D, 4E and Fig. 5C). These differences may be attributed to the detection sensitivity of the GFP protein as compared to the endogenous TH protein. Under the non-depolarizing conditions, the low KCl concentration (5 mM) inherent to the culture media was likely sufficient to induce weak levels of Th promoter activity. However, there are an estimated 20 copies of the ThGFP transgene in the diploid transgenic mouse genome, as compared to the endogenous 2 copies of Th (Matsushita et al. 2002). This large difference in copy number likely results in greater expression levels of the GFP protein relative to the endogenous TH protein, which results in a greater detection sensitivity for the GFP protein.
P/Q-type Ca+2 channels are necessary for GABA to enhance ThGFP expression
Strong activation of L-type Ca+2 channels has previously been reported to stimulate opening of P/Q-type Ca+2 channels in OB interneurons (Murphy et al. 2005). To ascertain if P/Q type channels were also involved in the L-type Ca+2 channel-mediated induction of ThGFP expression, depolarized slice cultures were treated with ω–agatoxin, a P/Q-type Ca+2 channel-specific antagonist. In these studies, ω–agatoxin specifically blocked the GABA-mediated increase of ThGFP expression without diminishing the KCl-induced response (Fig. 6). Since activation of P/Q Ca+2 channels is critical for triggering dendritic release of GABA in OB PG interneurons (Murphy et al. 2005), these observations indicated that both GABA release and the enhancement of TH expression by GABA in glomerular interneurons were dependent on activation of both L- and P/Q-type Ca+2 channels in response to excitatory input.
Figure 6.
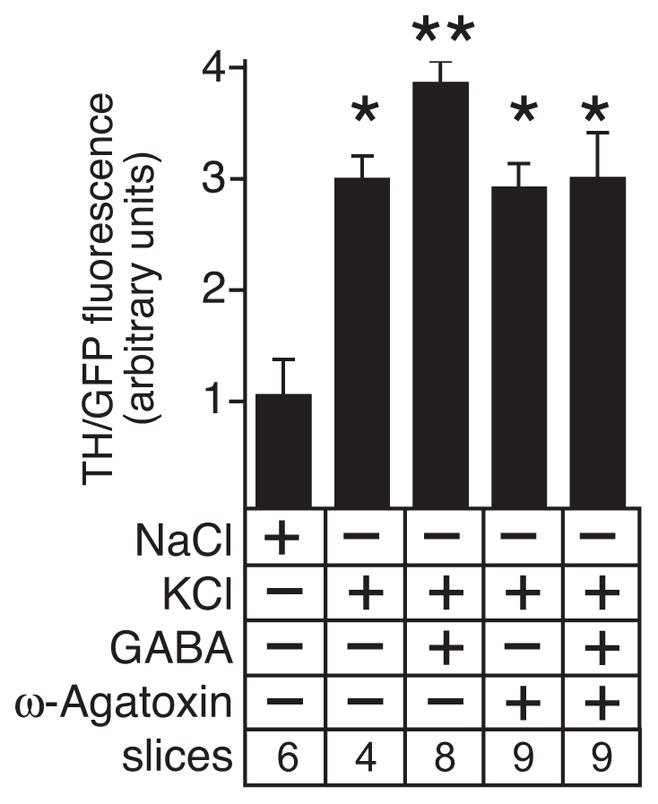
The P/Q-type Ca+2 channel blocker, ω-agatoxin, prevents the GABA-mediated increase of ThGFP expression in depolarized slice cultures without diminishing the ThGFP expression levels induced by depolarization. Single asterisk indicates values that are significantly greater than cultures treated with NaCl. Double asterisk indicate values significantly greater than cultures treated with either only KCl or NaCl. “Slices” indicates the number of cultured slices used to calculate the mean and standard deviation shown for condition tested.
Discussion
Modulation of OB dopaminergic interneuron differentiation by GABA
In the current study, GABA induced ThGFP expression in a subset of depolarized glomerular layer cells through mechanisms that required L- and P/Q-type Ca+2 channels as well as GABAA and GABAB receptors. This GABA-mediated induction of ThGFP expression required depolarization as GABA, alone, was not sufficient. These results indicated that the number of depolarized OB glomerular cells expressing ThGFP was proportional to the external GABA concentration. Consistent with this finding, the increase in ThGFP expression levels mediated by GABA was dose-dependent. GABA also induced TH protein expression in the ThGFP-containing glomerular interneurons indicating that these cells were likely dopaminergic. Since the presence of TH is considered a marker of terminal differentiation in OB DAergic neurons, these findings suggest that GABA modulates maturation of the OB DAergic phenotype.
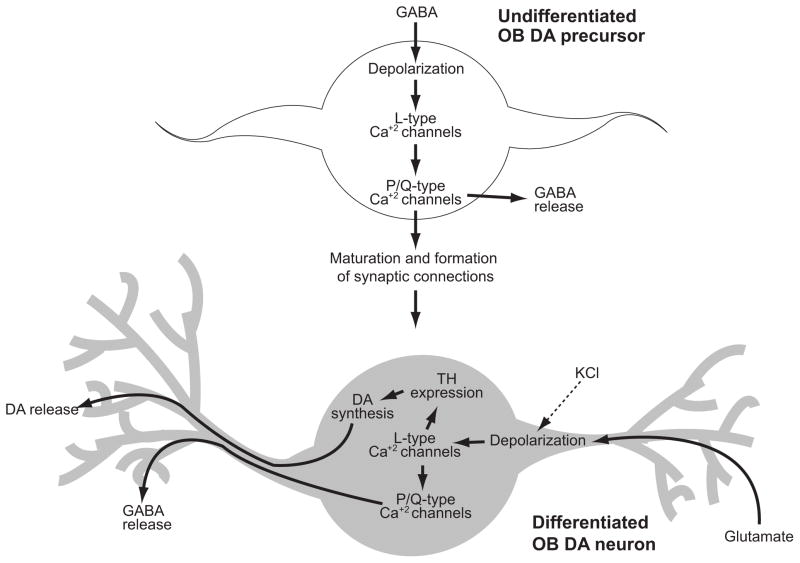
OB interneurons, including the glomerular DAergic cells, are generated throughout life, which results in a continuous ensemble of undifferentiated precursors among the differentiated neurons in the OB. The finding that GABA did not increase ThGFP levels per cell suggests that GABA does not directly modulate TH expression in differentiated OB DAergic neurons. Rather, the data support a model in which GABA indirectly regulates TH expression in OB DAergic neurons by modulating the maturation rate and formation of synaptic connections necessary for TH expression (Fig. 7). Consistent with this interpretation, previous studies have shown that GABA is crucial for synaptogenesis and integration of neuronal progenitors into developing neural circuits (reviewed in Akerman and Cline 2007; Ge et al. 2007). In the OB granule cell layer, GABA modulates maturation of interneuron progenitors by regulating the initiation, elongation and stabilization of dendrites (Gascon et al. 2006). In the present context, GABA may also promote both the development of dendritic projections and establishment of synaptic connections in OB DAergic progenitor cells, which enables the progenitors to receive the synaptic input necessary to induce TH expression (Fig. 7). Once synaptic connections are formed and glutamatergic input is received, TH expression is induced and differentiation is completed. Although the results of the GABA titration experiments indicate that the maturation rate is proportional to the ambient levels of GABA, PG interneurons in vivo typically require approximately four weeks to fully develop their dendritic and axonal morphologies (Belluzzi et al. 2003). Consistent with the model proposed here, these previous studies also indicated that GABAergic input precedes glutamatergic input in the maturation process of these interneurons (Belluzzi et al. 2003; Carleton et al. 2003).
Figure 7.
Mechanistic model for differentiation of OB DAergic neurons. In the glomerular layer, undifferentiated OB DAergic precursors are depolarized by ambient GABA. This depolarization sequentially activates L- and P/Q-type Ca+2 channels which stimulates GABA release (Murphy et al. 2005) as well as dendritic outgrowth and synaptogenesis (reviewed in Akerman and Cline 2007; Ge et al. 2007). Establishment of synaptic connections with olfactor receptor neurons and mitral/tufted cells provides glutamatergic input that results in depolarization and activation of L-type Ca+2 channels which both promotes TH expression and GABA release. In the slice culture model system, membrane potentials are partially depolarized by bath application of KCl, which activates L-type Ca+2 channels and up-regulates TH expression independently of glutamatergic input. The present study suggests that maturation rate and differentiation of OB DAergic precursors (as determined by the ability to express TH) is proportional to levels of ambient GABA. Although GABA can depolarize and activate L-type channels in precursors cells, GABA by itself is not sufficient induce TH expression. These finding suggests that additional mechanisms activated by either KCl-mediated depolarization or glutamatergic input are necessary for activity-dependent induction of TH expression in OB DAergic neurons.
An alternative mechanism by which GABA could mediate an increase in the number of ThGFP and TH expressing cells in the glomerular layer is through an increase in either the proliferation or migration rates of OB DAergic progenitors. However, this seems unlikely since the concentration of GABA (10 μM) used in the studies to induce TH expression substantially reduced progenitor migration rates in the RMS (Bolteus and Bordey 2004). In addition, the depolarization conditions used in the current study (25 mM KCl) also increase the ambient levels of GABA in the RMS, which would further reduce progenitor migration (Bolteus and Bordey 2004). Similarly, GABA decreases progenitor proliferation rates in the RMS and SVZ (Liu et al. 2005), which are already low (Lois and Alvarez-Buylla 1994).
A striking finding in this study was the molecular inter-relationship between the co-expressed OB GABAergic and DAergic phenotypes. Although the expression of the GABA synthesizing enzymes (GAD 1/2) in the OB are not activity-dependent (Baker et al. 1988), the release of GABA from PG interneurons is activity-dependent and requires L- and P/Q-type Ca+2 channels (Murphy et al. 2005). Our results indicate that both of these Ca+2 channels are also required for the GABA-mediated induction of TH expression, suggesting that these Ca+2 channels are also required for the maturation of OB DAergic precursor cells. The current studies also indicated that L-type channels remain critical for TH expression in differentiated OB DAergic neurons. Thus, the co-expressed DAergic and GABAergic phenotypes are coupled by a common subset of voltage gated Ca+2 channels.
Although GABA can depolarize and activate L-type channels in OB DAergic precursors cells, the current study showed that GABA by itself is not sufficient to induce TH expression. This unexpected finding suggests that L-type Ca+2 channels are necessary, but not sufficient for activity-dependent reduction of TH expression. Thus, additional membrane receptors/channels and their cognate signaling pathways activated by either KCl-mediated depolarization or glutamatergic input are also necessary for TH expression in OB DAergic neurons. Consistent with this possibility, preliminary slice culture studies indicate that the activity-dependent synaptic release of the neurotrophin, BDNF, can also enhance depolarization-induced ThGFP expression (unpublished observation; YA, HB and JWC).
Activity-dependent TH expression in a forebrain slice culture model system
The current study demonstrated that KCl-depolarized slice cultures are an effective model system to study activity-dependent TH expression in the OB. Although bath application of KCl broadly depolarizes neurons, the ability of inhibitors for glutamate receptors and TTX-sensitive channels to reduce ThGFP expression levels in depolarized slices indicated that several key components of physiologically-relevant microcircuits necessary for induction of TH expression were functional in this model system. Some variability in the induced levels of ThGFP expression relative to non-depolarized (NaCl-treated) slices was also observed, but this likely resulted from fluctuations in basal ThGFP expression levels. Since the ThGFP expression levels were expressed as a ratio of fluorescence intensity at 48 hours relative to 0 hours, variation in basal expression levels measured at 0 hours affect the magnitude of the relative ratio. Despite this variability, the qualitative response to KCl and GABA were consistent throughout the study.
The current slice culture studies also show that L-type Ca+2 channels are essential for activity-dependent TH expression. These findings are consistent with both primary culture studies that suggested the importance of L-type channels (Cigola et al. 1998; McMillian et al. 1994; Puche and Shipley 1999) and electrophysiology studies that identified L-type channels as the major carrier of Ca+2 current in OB DAergic neurons (Pignatelli et al. 2005). In other neuronal systems, L-type Ca+2 channels modulate gene expression through activation of transcription factors, including CREB and AP-1 (Chawla 2002; West et al. 2001). The TH gene proximal promoter contains evolutionarily conserved binding sites for CREB and AP-1, both of which are necessary for TH expression in the OB (Baker et al. 2001; Liu et al. 1999; Trocme et al. 1998). Together, these data suggest that odor mediated activation of L-type Ca+2 channels induces TH expression in DAergic PG cells through the activity of CREB, AP-1 and related transcription factors.
Consistent with the critical role of L-type Ca+2 channels for the induction of TH expression, the L-type channel blocker, nifedipine, reduced ThGFP expression to basal levels. The residual ThGFP expression following treatment with either TTX or glutamate receptor antagonists was likely produced by direct KCl-mediated activation of L-type Ca+2 channels in the OB DAergic neurons. The CNS expresses two major subclasses of L-type channels, CaV1.2 and CaV1.3, that are distinguished by the presence or absence of α1C and α1D subunits, respectively (Lipscombe et al. 2004). Both subclasses are expressed in the OB glomerular layer (Ludwig et al. 1997; Tanaka et al. 1995). Although L-type channels are generally activated at high voltages, there are significant functional differences between the subclasses, including activation voltage thresholds (Lipscombe et al. 2004). Previous reports have shown that activation of CaV1.3 channels occurs at potentials as low as −55 mV, with half activation potentials of −40 mV (Xu and Lipscombe 2001). These voltages are approximately 20–25 mV more hyperpolarized than corresponding potentials observed for CaV1.2 channels under the same conditions. This lower activation threshold of CaV1.3 channels is likely to be significant since Ca+2 channels in OB DAergic neurons have been reported to activate at membrane potentials between −50 and −60 mV (Pignatelli et al. 2005; Puopolo et al. 2005). Under the 25 mM KCl-mediated depolarizing conditions used in the current study, Goldman-Hodgkin-Katz calculations predict a neuronal membrane potential of approximately −47 mV (see Supplemental Fig. 3), which is sufficient to partially activate CaV1.3 L-type Ca+2 channels.
Conclusions
The studies reported here indicate that GABA can modulate differentiation of OB DAergic neurons. This novel finding suggests that the regulation of dendritic outgrowth and stability as well as synaptogenesis by GABA also controls the number of OB glomerular interneurons expressing activity-dependent phenotypes, such as the DAergic phenotype. In the adult brain, the OB and hippocampus are the two principal sites of neurogenesis and GABA also regulates progenitor proliferation, migration, dendritic outgrowth and synaptogenesis in the hippocampus. However, unlike the current study in the OB, the ability of GABA to modulate differentiation of specific hippocampal interneuron phenotypes has not been reported.
A second novel finding in the current study is the molecular inter-relationship between the co-expressed GABAergic and DAergic phenotypes in OB interneurons. Since a majority of OB glomerular layer interneurons express GABA (Kosaka et al. 1998; Parrish-Aungst et al. 2007), determining whether other neuronal phenotypes are also coupled to the co-expressed GABAergic phenotype is of considerable significance. In view of established findings indicating that adult-derived OB interneurons are essential for new odor memory consolidation (reviewed in Lledo et al. 2006), the results of this current study make it tempting to speculate that GABA can modulate neural network plasticity by regulating differentiation of specific molecular phenotypes of OB interneurons.
Supplementary Material
Acknowledgments
Grant information: supported by NIH R01DC008955
References
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30(8):382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Sasaki H, Saino-Saito S, Baker H. Temporal and spatial disparity in cFOS expression and dopamine phenotypic differentiation in the neonatal mouse olfactory bulb. Neurochem Res. 2007;32(4–5):625–634. doi: 10.1007/s11064-006-9134-7. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Cummings DM, Munger SD, Margolis JW, Franzen L, Reed RR, Margolis FL. Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): Biochemical and morphological consequences in adult mice. J Neurosci. 1999;19:9313–9321. doi: 10.1523/JNEUROSCI.19-21-09313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Liu N, Chun HS, Saino S, Berlin R, Volpe B, Son JH. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci. 2001;21(21):8505–8513. doi: 10.1523/JNEUROSCI.21-21-08505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- Baker H, Towle AC, Margolis FL. Differential afferent regulation of dopaminergic and GABAergic neurons in the mouse main olfactory bulb. Brain Res. 1988;450(1–2):69–80. doi: 10.1016/0006-8993(88)91545-4. [DOI] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23(32):10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24(35):7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonino M, Cantino D, Sassoe-Pognetto M. Cellular and subcellular localization of gamma-aminobutyric acidB receptors in the rat olfactory bulb. Neurosci Lett. 1999;274(3):195–198. doi: 10.1016/s0304-3940(99)00697-7. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6(5):507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cave JW, Baker H. Dopamine systems in the forebrain. In: Pasterkamp RJ, Smidt MP, Burbach JPH, editors. Development and Engineering of Dopamine Neurons. Austin: Landes BioScience; 2008. [Google Scholar]
- Chawla S. Regulation of gene expression by Ca2+ signals in neuronal cells. Eur J Pharmacol. 2002;447(2–3):131–140. doi: 10.1016/s0014-2999(02)01837-x. [DOI] [PubMed] [Google Scholar]
- Cigola E, Volpe BT, Lee JW, Franzen L, Baker H. Tyrosine hydroxylase expression in primary cultures of olfactory bulb: role of L-type calcium channels. J Neurosci. 1998;18:7638–7649. doi: 10.1523/JNEUROSCI.18-19-07638.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Bovetti S, Carletti B, Hsieh YC, Garzotto D, Peretto P, Fasolo A, Puche AC, Rossi F. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci. 2007;27(3):657–664. doi: 10.1523/JNEUROSCI.2870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchis S, Temoney S, Erdelyi F, Bovetti S, Bovolin P, Szabo G, Puche AC. GABAergic phenotypic differentiation of a subpopulation of subventricular derived migrating progenitors. Eur J Neurosci. 2004;20(5):1307–1317. doi: 10.1111/j.1460-9568.2004.03584.x. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93(25):14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 2001;86(6):2986–2997. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell Biology of Olfaction. New York: Cambridge University Press; 1992. [Google Scholar]
- Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, De Roo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J Neurosci. 2006;26(50):12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30(1):1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Crespo C, Blasco-Ibanez JM, Gracia-Llanes FJ, Marques-Mari AI, Nacher J, Varea E, Martinez-Guijarro FJ. Distribution of D2 dopamine receptor in the olfactory glomeruli of the rat olfactory bulb. Eur J Neurosci. 2005;22(6):1357–1367. doi: 10.1111/j.1460-9568.2005.04328.x. [DOI] [PubMed] [Google Scholar]
- Keller A, Yagodin S, Aroniadou-Anderjaska V, Zimmer LA, Ennis M, Sheppard NF, Jr, Shipley MT. Functional organization of rat olfactory bulb glomeruli revealed by optical imaging. J Neurosci. 1998;18(7):2602–2612. doi: 10.1523/JNEUROSCI.18-07-02602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Hama K, Nagatsu I, Wu JY, Kosaka T. Possible coexistence of amino acid (γ-aminobutyric acid), amine (dopamine) and peptide (substance P); neurons containing immunoreactivities for glutamic acid decarboxylase, tyrosine hydroxylase and substance P in the hamster main olfactory bulb. Exp Brain Res. 1988;71:633–642. doi: 10.1007/BF00248757. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res. 1998;30(2):101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Hataguchi T, Hama T, Nagatsu I, Wu J-Y. Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gamma-aminobutyric acid. Brain Res. 1985;343:166–171. doi: 10.1016/0006-8993(85)91172-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ. Rapid translocation of Zn(2+) from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J Neurophysiol. 2001;86(5):2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92(5):2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Liu N, Cigola E, Tinti C, Jin BK, Conti B, Volpe BT, Baker H. Unique regulation of immediate early gene and tyrosine hydroxylase expression in the odor-deprived mouse olfactory bulb. J Biol Chem. 1999;274:3042–3047. doi: 10.1074/jbc.274.5.3042. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8(9):1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Ilic V, Friauf E. Development of a topographically organized auditory network in slice culture is calcium dependent. J Neurobiol. 1998;34(2):97–112. doi: 10.1002/(sici)1097-4695(19980205)34:2<97::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursor. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17(4):1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Matsushita N, Okada H, Yasoshima Y, Takahashi K, Kiuchi K, Kobayashi K. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem. 2002;82(2):295–304. doi: 10.1046/j.1471-4159.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- McMillian MK, Mullis SB, Wu GC, Hudson PM, Pennypacker KR, Hong JS. Regulation of tyrosine hydroxylase in olfactory bulb cultures: selective inhibition of depolarization-induced increase by endogenous opioids. Brain Res. 1994;658(1–2):105–111. doi: 10.1016/s0006-8993(09)90015-4. [DOI] [PubMed] [Google Scholar]
- Min N, Joh TH, Corp ES, Baker H, Cubells JF, Son JH. A transgenic mouse model to study transsynaptic regulation of tyrosine hydroxylase gene expression. J Neurochem. 1996;67(1):11–18. doi: 10.1046/j.1471-4159.1996.67010011.x. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat Neurosci. 2005;8(3):354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Perazzini AZ, Fritschy JM, Sassoe-Pognetto M. Heterogeneity of gamma-aminobutyric acid type A receptors in mitral and tufted cells of the rat main olfactory bulb. J Comp Neurol. 2005;484(1):121–131. doi: 10.1002/cne.20440. [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol. 2007;501(6):825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Pignatelli A, Kobayashi K, Okano H, Belluzzi O. Functional properties of dopaminergic neurones in the mouse olfactory bulb. J Physiol. 2005;564(Pt 2):501–514. doi: 10.1113/jphysiol.2005.084632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puche AC, Shipley MT. Odor-induced, activity-dependent transneuronal gene induction in vitro: mediation by NMDA receptors. J Neurosci. 1999;19(4):1359–1370. doi: 10.1523/JNEUROSCI.19-04-01359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M, Bean BP, Raviola E. Spontaneous activity of isolated dopaminergic periglomerular cells of the main olfactory bulb. J Neurophysiol. 2005;94(5):3618–3627. doi: 10.1152/jn.00225.2005. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Belluzzi O. Functional heterogeneity of periglomerular cells in the rat olfactory bulb. Eur J Neurosci. 1998;10(3):1073–1083. doi: 10.1046/j.1460-9568.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- Saino-Saito S, Cave JW, Akiba Y, Sasaki H, Goto K, Kobayashi K, Berlin R, Baker H. Er81 and CaMKIV identify anatomically and phenotypically defined subsets of mouse olfactory bulb interneurons. J Comp Neurol. 2007:502. doi: 10.1002/cne.21293. [DOI] [PubMed] [Google Scholar]
- Saino-Saito S, Sasaki H, Volpe BT, Kobayashi K, Berlin R, Baker H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J Comp Neurol. 2004;479(4):389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- Shahar T, House SB, Gainer H. Neural activity protects hypothalamic magnocellular neurons against axotomy-induced programmed cell death. J Neurosci. 2004;24(29):6553–6562. doi: 10.1523/JNEUROSCI.0886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311(5763):1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Smith TC, Jahr CE. Self-inhibition of olfactory bulb neurons. Nat Neurosci. 2002;5(8):760–766. doi: 10.1038/nn882. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sato M, Morishima Y, Nakanishi S. Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J Neurosci. 2005;25(41):9535–9543. doi: 10.1523/JNEUROSCI.2191-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka O, Sakagami H, Kondo H. Localization of mRNAs of voltage-dependent Ca(2+)-channels: four subtypes of alpha 1- and beta-subunits in developing and mature rat brain. Brain Res Mol Brain Res. 1995;30(1):1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- Trocme C, Sarkis C, Hermel JM, Duchateau R, Harrison S, Simonneau M, Al-Shawi R, Mallet J. CRE and TRE sequences of the rat tyrosine hydroxylase promoter are required for TH basal expression in adult mice but not in the embryo. Eur J Neurosci. 1998;10(2):508–521. doi: 10.1046/j.1460-9568.1998.00059.x. [DOI] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550(Pt 3):785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98(20):11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21(16):5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.