Abstract
Modern cancer therapy combines recombinant viruses with traditional chemotherapeutic agents that are metabolized by hepatic cytochrome P450 3A4 (CYP3A4). A single dose of recombinant adenovirus (Ad) expressing beta-galactosidase (AdlacZ) significantly alters CYP3A2, the correlate of CYP3A4, in rats for 14 days. Recombinant adenovirus expressing human p53 (Adp53) also suppresses CYP3A2. Plasma clearance of docetaxel (DTX) in animals given AdlacZ (3.38 ± 0.22 L/h/kg) was significantly lower than that of those given DTX alone (6.41 ± 1.10 L/h/kg, p≤0.05). Area under the plasma concentration-time curve of DTX in rats given AdlacZ (2,987.37 ± 197.97 ng/ml/h) was significantly greater than those given drug alone (1,666.59 ± 317.04 ng/ml/h, p≤0.05). Both viruses prolonged DTX half-life (t1/2). Ad infection may cause significant variability in the pharmacokinetics and pharmacodynamics of anti-cancer agents and should be considered when designing therapeutic regimens for patients with viral infection and those enrolled in clinical trials employing recombinant viruses.
Introduction
Recombinant adenoviruses have been shown to be the most efficient vectors for gene delivery due to their ability to infect both dividing and quiescent cells with high efficiency.1 Adenovirus-based vectors are currently being developed as novel therapeutics for diverse applications including inherited genetic disorders, cancer, cardiovascular disease, neurodegenerative disorders and infectious disease.2 At present, cancer is the therapeutic target of most gene therapy clinical trials. One strategy of cancer gene therapy is the use of replication-deficient adenoviral vectors to transfer either immunostimulatory, anti-angiogenic, suicide or tumor suppressor genes alone or in combination to reduce tumor growth and spread.3 To date, adenovirus-mediated delivery of the tumor suppressor gene, p53, (Adp53) has made the most progress in the clinic. One form of this construct has been marketed as Gendicine in China since 2004, while others are currently undergoing late-stage clinical trials for a variety of malignancies in several other countries.4 Studies in both preclinical models of disease and human clinical trials have shown that Adp53, when used in combination with traditional anti-cancer therapeutic agents, can significantly enhance drug potency.5-9 In light of this synergistic or additive effect, recombinant adenoviruses are, therefore, likely to be given in conjunction with chemotherapeutic agents.
Docetaxel (DTX) is one of the most potent antineoplastic agents with a broad spectrum of antitumor activity. It has been used to treat various malignancies including breast, lung, ovarian, head and neck, and prostate cancer. It exhibits cytotoxicity by stabilizing microtubules and preventing depolymerization to free tubulin.10 DTX is predominantly metabolized by hepatic cytochrome P450 3A4 (CYP3A4) and eliminated through biliary secretion. To date, four major metabolites of this drug have been identified that possess less cytotoxic activity with respect to the parent compound.11 Several preclinical studies have described improvement in response to DTX when used in combination with Adp53.7, 8 A marked pharmacological advantage of this approach has been highlighted in a phase II clinical trial where the use of DTX and doxorubicin in combination with ADVEXIN®, another adenovirus-p53 construct, has shown a greater reduction in tumor size compared to that seen with chemotherapy alone.9 Although several other reports have supported this finding, the mechanism by which this occurs is unknown.
CYP3A4 is predominantly found in the human liver and is involved in the metabolism and clearance of more than 50% of currently marketed drugs. Given its broad range of substrates, CYP3A4 plays a significant role in a large number of clinically relevant drug interactions.12 We have previously found that systemic administration of a first-generation adenovirus expressing E. coli beta-galactosidase (AdlacZ) alters expression and function of rat hepatic CYP3A2, an isoform homologous to human CYP3A4.13 Additional studies indicate that the biology of the transgene product can significantly influence changes in CYP3A2 noted during treatment with recombinant adenoviruses.14 Based upon these results, we developed the hypothesis that changes in rat hepatic CYP3A2 following systemic administration of adenovirus could alter the pharmacokinetic profile of other CYP3A2 substrates given concomitantly either as part of a therapeutic regimen or for treatment of other underlying conditions. Therefore, the primary goal of this study was to determine how systemic administration of recombinant adenoviruses influences the pharmacokinetics and tissue distribution of DTX, a CYP3A2 substrate, in a rat model.
Results
Effect of systemic administration of recombinant adenoviruses on hepatic CYP3A2 expression and function prior to treatment with DTX
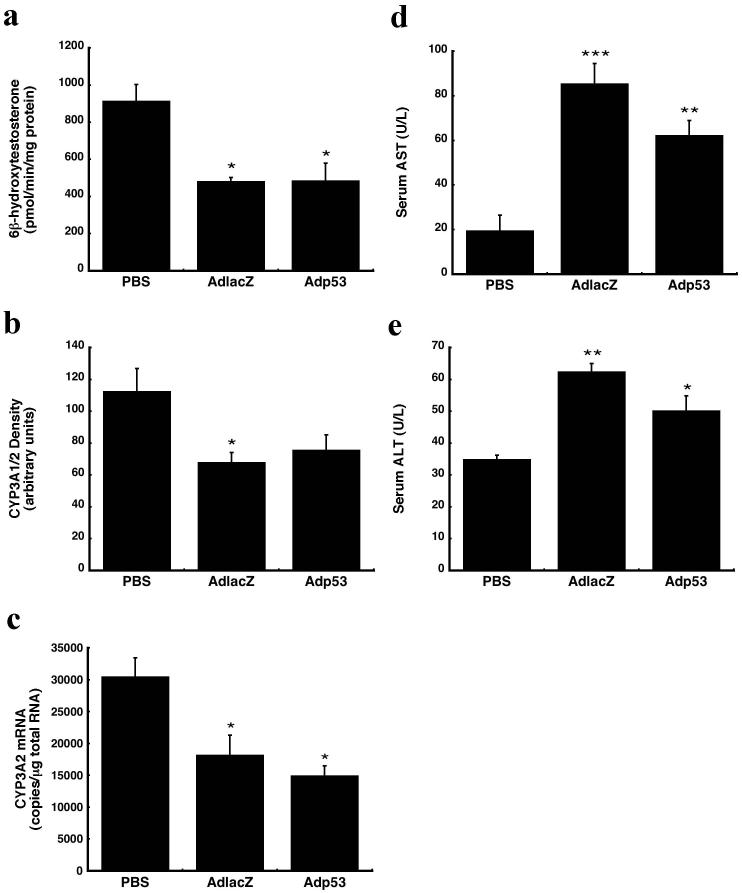
Rats were given a single dose (5.7 × 1011 vp/kg) of either Adp53 or AdlacZ through a catheter inserted in the jugular vein. AdlacZ, previously documented to suppress rat hepatic CYP3A213, was used as a positive control in these studies. Twenty-four hours after treatment with either virus, several animals from each group were sacrificed to characterize CYP activity and expression prior to DTX dosing. Remaining animals were given a single dose of DTX (10 mg/kg). Prior to DTX treatment, hepatic CYP3A2 catalytic activity was significantly reduced. The amount of 6β-hydroxytestosterone, the primary CYP3A2-specific metabolite of testosterone, generated in samples from animals treated with either virus was reduced by approximately 47% with respect to that found in samples from phosphate-buffered saline (PBS) treated animals (Figure1a, p≤0.05). CYP3A1/2 protein was also reduced by AdlacZ and Adp53 (40% and 33%, respectively, Figure 1b). Real time RT-PCR revealed that virus-induced inhibition of CYP3A2 occurs at the transcriptional level. CYP3A2 mRNA levels were 60% and 49% of control for animals treated with AdlacZ and Adp53, respectively (Figure 1c, p≤0.05). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured to assess liver toxicity. Both viruses significantly elevated serum AST, however, the most profound induction occurred in animals given AdlacZ. In this group, serum AST was four times above control values. A three-fold increase was seen in animals treated with Adp53 (Figure 1d, p≤0.01). A similar trend was observed in serum ALT (Figure 1e, p≤0.05).
Figure 1. Systemic administration of recombinant adenoviral vectors suppresses hepatic CYP3A2 24 hours after treatment.
(a) In vitro catalytic activity of CYP3A2 microsomal proteins measured by the production of testosterone metabolite, 6β-hydroxytestosterone. (b) Western blot analysis of hepatic CYP3A1/2 protein expression. (c) mRNA levels of CYP3A2, as determined by real time RT-PCR. (d) Effect of systemic administration of adenovirus on serum aspartate aminotransferase (AST). (e) Effect of systemic administration of adenovirus on serum alanine aminotransferase (ALT). Results are reported as the mean ± the standard error (SE) of the mean; n = 4-5 rats per treatment group. *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001 with respect to vehicle control (PBS).
Impact of systemic administration of recombinant adenoviruses on pharmacokinetics and tissue distribution of DTX
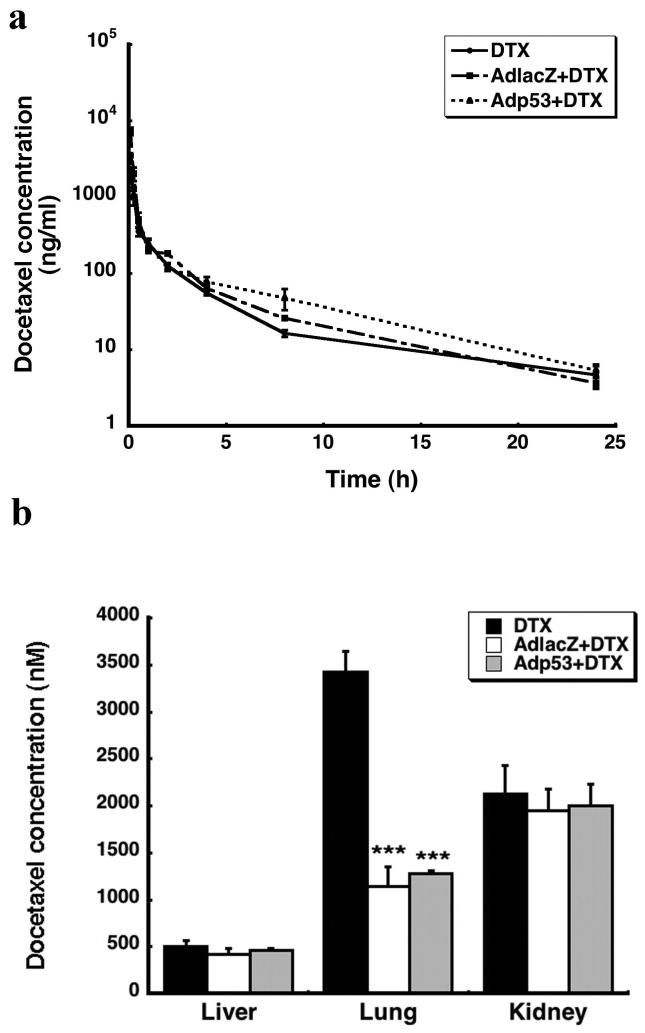
In order to determine how virus-induced changes in CYP3A2 affected the metabolism and clearance of DTX, pharmacokinetic parameters of the drug were measured 24 hours after virus administration. The presence of either virus markedly altered the pharmacokinetic profile of DTX (Figure 2a). The area under the plasma concentration time curve (AUC) for DTX was 2 and 1.4 fold greater in rats given AdlacZ and Adp53, respectively than that of animals receiving DTX alone (Table. 1). Administration of AdlacZ and Adp53 reduced DTX clearance by 47 % and 23 %, respectively. The terminal plasma half-life of DTX was prolonged 3 fold in the presence of either virus (Table 1, p≤0.05). Each virus significantly altered tissue distribution of DTX in the lung (Figure 2b). Samples from animals given AdlacZ contained about half the amount of drug found in tissue of animals given DTX alone. A similar result was seen with Adp53. There was no significant difference in the amount of DTX present in the liver and kidneys of animals treated with either virus and DTX or with the drug alone (Figure 2b, p=0.075).
Figure 2. Recombinant Adenoviruses Alter the Pharmacokinetic parameters of DTX.
(a) Observed plasma concentration-time curve after intravenous administration of DTX (10 mg/kg) to animals given the drug alone (DTX) and those treated with recombinant adenoviruses 24 hours earlier (AdlacZ + DTX, Adp53 + DTX). (b) Tissue distribution of DTX 24 hours after administration in the presence and absence of recombinant adenoviruses. Results are reported as the mean ± SE; n = 4-5 rats per treatment group. *** P ≤ 0.001 with respect to vehicle control (PBS).
Table 1. Calculated Pharmacokinetic Parameters of DTX (10 mg/kg) Alone or in Combination with Recombinant Adenovirus (5 × 1011 vp/kg)a.
| Parameter | DTX | DTX + AdlacZ | DTX + Adp53 |
|---|---|---|---|
| AUC (ng/ml/h) | 1,496.14 ± 281.62 | 2,987.37 ± 197.97* | 2,113.44 ± 197.01 |
| CL (L/h/kg) | 7.35 ± 1.22 | 3.38 ± 0.22* | 4.85 ± 0.43 |
| Vss (L/kg) | 17.43 ± 2.80 | 6.05 ± 0.67* | 17.68 ± 2.54 |
| t1/2 (h) | 1.98 ± 0.10 | 5.06 ± 0.37* | 5.55 ± 0.84* |
Data were obtained from plasma samples and reflect average values + standard errors of the means from 4-5 animals per treatment group.
p ≤ 0.05.
AUC: area under plasma concentration-time curve extrapolated to infinity, CL: systemic clearance, Vss: volume of distribution at steady state, t1/2: terminal elimination half-life.
Effect of systemic administration of DTX on hepatic CYP3A2 expression and function 24 hours following adenovirus infection
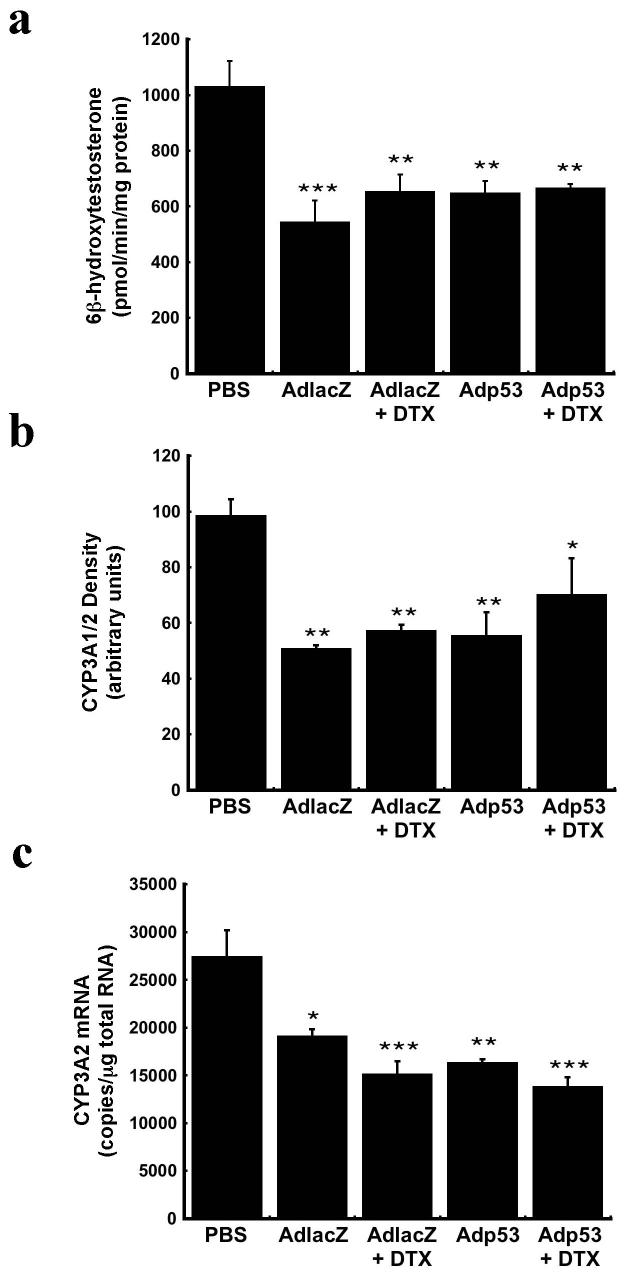
DTX itself can induce CYP3A4 gene expression in certain cell types such as peripheral mononuclear cells from those with lung cancer15 while leaving hepatic CYP3A4 unaffected.16 Taking this into consideration, CYP3A2 expression and function was assessed after DTX administration in order to confirm our hypothesis that the synergistic effect between the drug and the adenovirus observed in the clinic was due to virus-induced changes in CYP. Twenty-four hours after administration of DTX, testosterone hydroxylation assays revealed no further changes in CYP3A2 activity with respect to saline treated animals. Hepatic CYP3A2 activity was still suppressed by approximately 35% in animals given either virus (Figure 3a). At this time, 48 hours after virus administration, hepatic CYP3A2 activity was still significantly compromised in animals receiving AdlacZ alone (p≤0.001) while it began to recover to baseline levels in animals given Adp53. DTX also did not affect CYP3A1/2 protein expression. Western blot analysis revealed that the significant reduction in CYP3A1/2 protein in animals treated with either virus alone remained at the same level when DTX was given (Figure 3b, p≤0.05). CYP3A2 mRNA was approximately 50% of control in all treatment groups except AdlacZ treated animals (Figure 3c, p≤0.01). For this treatment group, CYP3A2 mRNA began to recover to 70% of that seen in samples from saline-treated rats.
Figure 3. DTX Does not Alter the Pattern of Virus-Induced Changes in Hepatic CYP3A2 Expression and Function.
(a) In vitro catalytic activity of CYP3A2 microsomal proteins measured 48 hours after administration of either adenovirus alone or in combination with DTX. (b) Western blot analysis of liver microsomal proteins for CYP3A1/2. (c) Gene expression of CYP3A2, as determined by real time RT-PCR. Results are reported as the mean ± SE; n = 4-5 rats per treatment group. *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001 with respect to vehicle control (PBS).
Serum transaminase levels 24 hours following administration of DTX in the presence of recombinant adenovirus
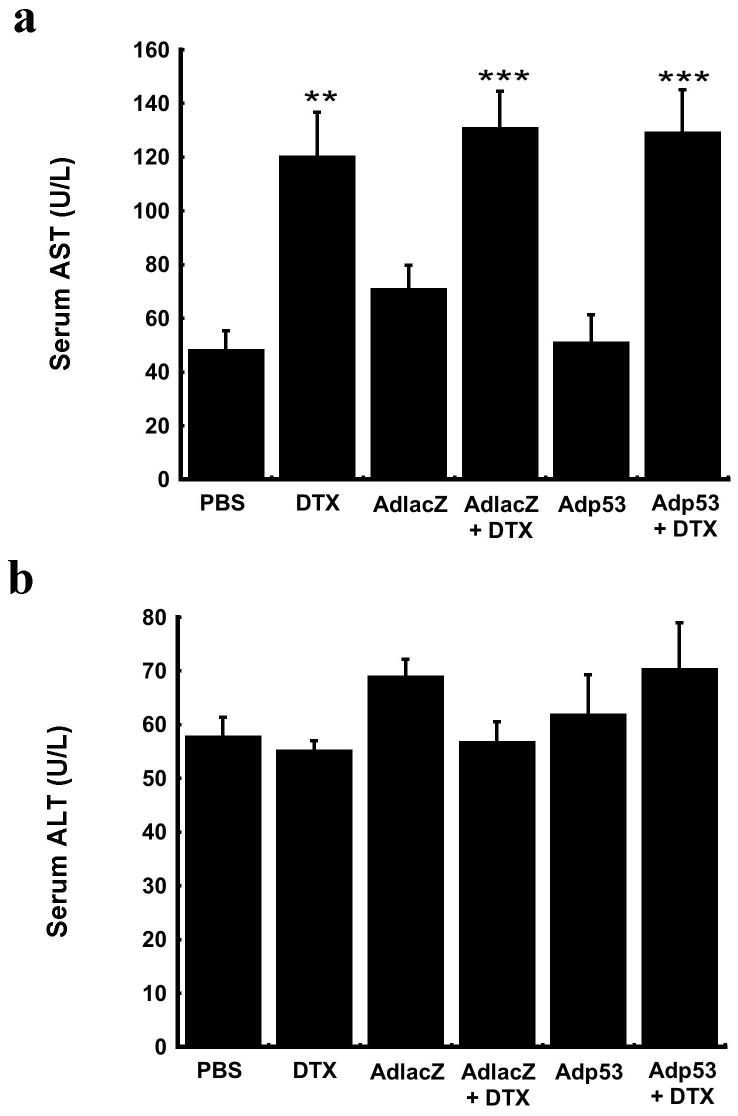
Serum AST and ALT were measured to assess the degree of liver toxicity associated with systemic administration of DTX when given 24 hours after adenovirus. At this time point, 48 hours after administration of virus, these enzymes were not significantly elevated above baseline values in animals treated with either virus alone. Despite this, serum AST was significantly elevated by a factor of 3 in all animals receiving DTX (Figure 4a, p≤0.01). ALT levels were unaffected by the drug (Figure 4b).
Figure 4. Systemic administration of DTX can increase serum transaminases 24 hours after treatment.
Serum (a) AST and (b) ALT levels. Results are reported as the mean ± SE; n = 4-5 rats per treatment group. **P ≤ 0.01, *** P ≤ 0.001 with respect to vehicle control (PBS).
Effect of systemic administration of DTX on hepatic transgene expression and virus distribution
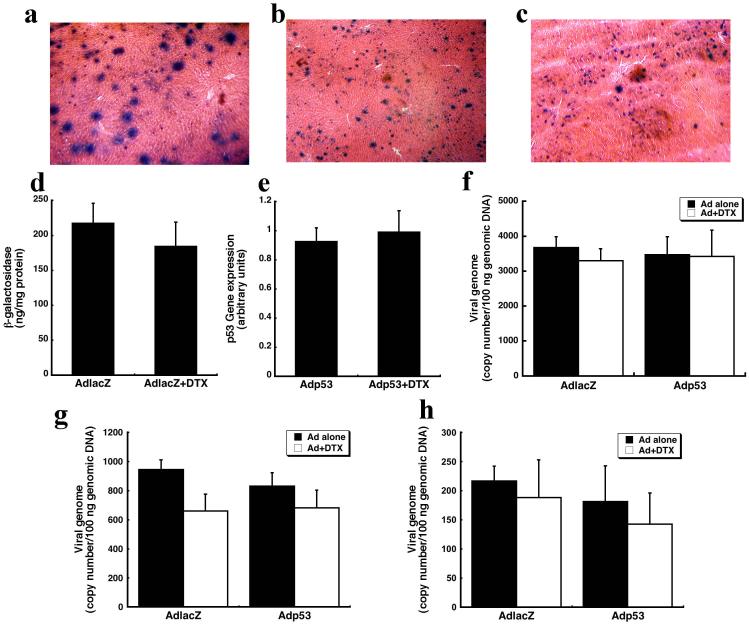
Hepatic tissue sections were stained histochemically to evaluate the degree of transgene expression after a single dose of AdlacZ. A dose of 5.7 × 1011 vp/kg transduced approximately 30% of hepatocytes 24 hours after administration (Figure 5a). There were no significant differences in the number of beta-galactosidase-positive cells present in the liver of animals treated with AdlacZ either in the presence or absence of DTX twenty-four hours later (Figures 5b and 5c). A quantitative ELISA confirmed that the amount of beta-galactosidase in the liver of animals treated with AdlacZ alone (217 ± 57 ng beta-galactosidase/mg protein) was similar to that of animals treated with AdlacZ and DTX (184 ± 70 ng beta-galactosidase /mg protein, Figure 5d). There was also no statistical difference in the amount of p53 mRNA in the liver, measured by RT-PCR, in samples from animals treated with Adp53 alone and in conjunction with DTX (Figure 5e). Together these results indicate that DTX does not alter transgene expression patterns when given 24 hours after the virus.
Figure 5. DTX does not effect hepatic transgene expression and virus distribution.
(a) Representative hepatic tissue section 24 hours after intravenous administration of AdlacZ. (b) Representative tissue section 48 hours after intravenous administration of the same virus. (c) Representative tissue section from an animal treated with AdlacZ and then DTX 24 hours later (sacrificed 48 hours after administration of virus). (d) Beta-galactosidase expression, as measured by ELISA, in the liver 48 hours after administration of either AdlacZ alone or in combination with DTX. (e) p53 expression, as determined by RT-PCR. (f) Distribution of viral genomes in the liver following systemic administration of either adenovirus alone or in combination with DTX. (g) Distribution of virus in the lung after administration of either virus alone or in combination with DTX. (h) Distribution of viral genomes in the kidney following systemic administration of either adenovirus alone or in combination with DTX. Results are reported as the mean ± SE; n = 4-5 rats per treatment group.
There was also no statistical difference in the number of viral genomes in liver samples taken from animals given AdlacZ (4,253 ± 313 virus genomes(VG)/100 ng genomic DNA) and Adp53 (3,635 ± 573 VG/100 ng genomic DNA) alone (Figure 5f). The number of viral genomes present in the lung and the kidney of animals treated with AdlacZ were also approximately the same as those given Adp53 (Figures 5g and 5h). Pulmonary tissues of animals receiving AdlacZ and Adp53 contained 842 ± 175 and 774 ± 99 VG/100 ng genomic DNA respectively. Renal samples obtained from animals given AdlacZ contained 291 ± 67 VG in 100 ng of renal genomic DNA. Those given Adp53 contained 218 ± 109 VG/100 ng genomic DNA (Figure 5h). DTX did not significantly affect the distribution patterns of either virus. The number of viral genomes in the liver at this time were 3,676 ± 316 (VG)/100 ng genomic DNA (AdlacZ) and 3,476 ± 514 (VG)/100 ng genomic DNA (AdlacZ + DTX, Figure 5f). Similarly, 3,301 ± 343 (VG)/100 ng genomic DNA and 3,423 ± 756 VG/100 ng genomic DNA were found in hepatic isolates of animals receiving Adp53 alone and with DTX respectively (Figure 5f). There were also no statistical differences in the number of viral genomes present in the lung or the kidney between any of the treatment groups (Figures 5g and 5h).
Discussion
The combination of virotherapy and chemotherapy has become a promising strategy for cancer treatment. Many reports have also illustrated that this combination affords superior clinical efficacy with respect to single-agent regimens for some cancers.17, 18 We have previously found that a single systemic dose of recombinant adenovirus significantly suppresses expression and function of hepatic CYP3A2 in the rat13, which is 90% structurally and functionally equivalent to human CYP3A4. Based on these results, if adenovirus is given to a patient in combination with other drugs primarily metabolized by this enzyme, it can promote and prolong the pharmacological actions of them by preventing their clearance and extending their time in the systemic circulation. However, most antineoplastic agents are potent, cytotoxic agents with very narrow therapeutic indices. Thus, increasing their plasma concentration could potentially cause serious adverse consequences.19
To further investigate potential changes in drug metabolism and distribution profiles during virus infection, we chose DTX, a CYP3A2 substrate used in combination with virotherapy, as a model drug. DTX was given 24 hours after adenovirus administration. This dosing regimen was selected based upon the fact that CYP3A2 would be significantly reduced13 and to prevent compounded changes in CYP and the associated toxicity that might occur had the two agents been given simultaneously. Total CYP3A activity is a strong predictor of DTX clearance (CL) and is responsible for inter-patient variability of DTX metabolism.20, 21 As shown in Table 1, treatment with either virus increased the AUC and reduced plasma CL. The half-life of DTX was also extended in animals given virus. Despite variable reports in the literature describing the effect of DTX on CYP15, 16, 22, results outlined in this report suggest that DTX alone does not affect hepatic CYP3A expression and function. Given this information, we believe that the observed changes in the pharmacokinetic profiles of DTX are largely due to virus-mediated inhibition of CYP3A2.
Although intravenous administration, as performed in our studies, is not the most common method for delivering recombinant viruses for cancer and other clinical therapies utilizing these vectors; several studies have revealed that up to 90% of a single dose of adenovirus can disseminate to the liver within 10 minutes after localized, tissue-specific infusion.23, 24 Thus, the improved efficacy of DTX when given with adenovirus in several clinical trials is still most likely due to virus-mediated changes in CYP in the liver and other target tissues. We also realize that although CYP enzymes responsible for drug metabolism and clearance are highly concentrated in the liver, local CYP expression patterns in other organs associated with drug metabolism and clearance such as the kidney and intestine25 as well as specific cancer cells also play a critical role in modulating the pharmacokinetic and pharmacodynamic profiles of anticancer agents.26-28 Thus, intratumoral infusion of recombinant adenovirus type 5 or administration of other viruses that are detargeted from the liver to reduce associated hepatotoxicity29, 30 may also alter local CYP expression patterns and dictate how certain cancers respond to a given therapeutic regimen. Studies to illustrate this effect in tumor cell lines and other organs associated with drug metabolism and clearance in preclinical models of cancer are currently underway in our laboratory.
It has been reported that DTX can change adenovirus transduction efficiency and transgene expression in vitro by altering virus transport patterns via microtubule networks.8, 31, 32 In contrast, we found no significant difference in transgene expression in the liver of animals receiving adenovirus alone or in combination with the drug. A similar result was seen with respect to the number of viral genomes distributed to target organs. One of the most surprising findings of this study was that recombinant adenoviruses alter tissue distribution of DTX in the lung but not other organs like the liver and kidney. The mechanism for this effect is not clear, however, virus-induced changes in tissue and plasma protein binding and blood flow may be responsible for this effect. An increase in plasma protein binding often correlates with a decrease in the free fraction of drug and reduces tissue penetration.33 Many plasma components, including α1-acid glycoprotein (AAG), lipoproteins and albumin bind DTX with high affinity.10 In vitro studies have shown that DTX is extensively bound to AAG, an acute phase protein that is often elevated in response to inflammation.34 Expression of this protein is stimulated by interleukin-1 (IL-1) and interleukin-6 (IL-6)35, cytokines commonly produced in response to systemic administration of recombinant adenoviruses in the rat36. Although, we did not assess AAG levels in this study, we believe that this phenomenon may have occurred in animals given the AdlacZ vector because a significant reduction in volume of distribution of DTX was found. This could also explain the greater changes in AUC and CL of DTX in animals given AdlacZ with respect to those given Adp53. We have previously found that recombinant adenovirus at the dose employed in these studies, suppresses several CYP isoforms responsible for the metabolism and clearance of compounds involved in regulation of renal blood flow.37 Imaoka et al. have recently reported that similar CYP isoforms such as CYP2B1, responsible for production of epoxyeicosatrienoic acids (EETs) and endothelium vasodilators, are present in the lung.38 It is possible that CYP2B1 may be suppressed during adenovirus infection, reducing pulmonary blood flow and limiting drug distribution to the lung. Additional studies to determine how systemic administration of recombinant adenovirus affects pulmonary CYP2B1 are warranted.
The effect of microbial infection and inflammation on CYP expression and function has been extensively studied in vitro and in several animal models. Although the mechanism by which CYP is suppressed is not fully understood, cytokines and nitric oxide are thought to mediate this effect initially.39, 40 Along these lines, we have found that the immunogenic and biological nature of the transgene cassette can dictate virus-mediated changes in CYP3A2 for a period of 14 days14, beyond the timeframe associated with the acute phase of the innate immune response. In the present study, serum transaminases were markedly elevated by both viruses twenty-four hours after treatment (Figure 1), suggesting that adenovirus-mediated hepatotoxicity might initially contribute to repression of CYP3A2 activity. However, CYP3A2 remained suppressed in all animals 48 hours after treatment despite the fact that serum ALT, a strong indicator of liver damage41, returned to baseline levels (Figure 4b) while AST, a strong indicator of toxicity in tissues other than the liver42 was elevated in only those animals given DTX (Figure 4a). Thus, we believe that short lived inflammatory mediators may indeed play a role in adenovirus-induced changes in CYP expression and function, but other factors associated with certain cell signal transduction pathways initiated during infection in the liver may be responsible for long-term suppression of CYP.43 Additional studies to elucidate specific mechanisms by which adenovirus infection modulates CYP expression and function are currently under active investigation in our laboratories.
In summary, we have shown that a single systemic dose of recombinant adenovirus expressing the tumor suppressor gene, p53, significantly compromises hepatic CYP3A2 expression and catalytic activity. This effect altered the pharmacokinetic profile of DTX, a CYP3A2 substrate and these results may be extrapolated to other drugs metabolized by this enzyme. The information described here is not only useful in the context that recombinant adenoviruses are currently used in the clinic for gene therapy and vaccine protocols but that this ubiquitous virus is capable of causing significant illness and high mortality in specialized patient populations. 44-46 Thus, it seems reasonable to conclude that care should be taken when designing therapeutic regimens that include drugs metabolized primarily by the CYP3A enzyme family for those treated with recombinant viruses and patients susceptible to adenovirus infection.
Methods
Adenovirus Vector Production
First-generation adenoviral vectors containing either wild-type p53 cDNA (Adp53) or E. coli beta-galactosidase (AdlacZ) under the control of a cytomegalovirus (CMV) promoter were amplified in human embryonic kidney (HEK) 293 cells and purified by two rounds of cesium chloride gradient ultracentrifugation. Virus concentration was calculated using the method of Maizel et al.47: virus particle/ml = (absorbance at 260 nm) × (dilution factor) × 1.1 × 1012.
Administration of Adenoviral Vectors and DTX
All animal procedures were performed in accordance with the guidelines established by the National Institutes of Heath for humane treatment of animals and approved by the Institutional Animal Care and Use Committee of The University of Texas at Austin. Adult male Sprague-Dawley rats (7 - 8 weeks old; Charles River Laboratories, Wilmington, MA) underwent jugular cannulation as previously described.48 Twenty four hours later, a single dose of 5.7 × 1011 vp/kg of either AdlacZ, Adp53 or PBS (vehicle) was administered in a volume of 500 μl followed by an equal volume of saline to ensure that virus was completely flushed from the catheter. Twenty-four hours after virus administration, 4-5 animals were sacrificed from each group to assess the effect of virus on CYP3A2. Remaining animals received 500 μl of either 10 mg/kg docetaxel (DTX, Sanofi-Aventis Pharmaceuticals, Bridgewater, NJ), docetaxel vehicle [13% ethanol, 25% polysorbate 80 (v/v), 62% saline (0.9%)] or PBS followed by 500 μl of saline through the jugular cannula. Blood was collected via the cannula immediately prior to docetaxel administration and at 5, 15, 30, 60, 120, 240 and 480 min after dosing. Samples were processed and plasma collected by centrifugation at 3,000 × g for 10 minutes at 4°C and stored at -80°C for docetaxel analysis. Animals were sacrificed 24 hours after docetaxel administration. At this time, blood was collected and major organs (liver, lungs, kidneys) were harvested.
Testosterone Hydroxylation Assay
Hepatic microsomal proteins were prepared according to established methods.49 In order to assess hepatic CYP3A2 activity, samples were incubated with testosterone and formation of the enzyme specific metabolite, 6β-hydroxytestosterone, quantified by high-performance liquid chromatography as described.50
Western Blot analysis
Microsomal proteins (20 μg) and a CYP3A2 protein standard (XenoTech, LLC, Lenexa, KS) were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis as described.13 Immunoblotting for CYP3A2 protein expression was performed using a ployclonal rabbit anti-rat CYP3A2 antibody (1:3000 dilution, BD Gentest, Woburn, MA). CYP3A1 and CYP3A2 co-migrate during electrophoresis. The antibody used to detect CYP3A2 was polyclonal with cross reactivity to CYP3A1, therefore all protein levels for CYP3A2 are reported as CYP3A1/2.
Real-time RT-PCR
Hepatic RNA was isolated from tissue snap frozen in liquid nitrogen using TRIzol according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). RNA was reversed transcribed with random nanomers using a RETROscript reverse transcription kit (Ambion, Austin, TX). Real-time PCR was carried out using the Power SYBR Green PCR Master Mix kit (Applied Biosystems, Foster City, CA) with the following primers specific for CYP3A2: 5′-TTG ATC CGT TGT TCT TGT CA-3′ (forward) and 5′-GGC CAG GAA ATA CAA GAC AA-3′ (reverse). DNA amplifications were carried out using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) with the following cycling conditions: 50°C for 2 minutes, 95°C for 10 minutes, 95°C for 15 seconds, and 62°C for 1 minute for a total of 40 cycles. Copy number was calculated from standard curves obtained using a cloned CYP3A2 template (plasmid DNA).
Real-time PCR
Genomic DNA was extracted from frozen hepatic tissues using a QIAamp DNA mini kit (Qiagen Valencia, CA), according to the manufacturer’s instructions. Real-time PCR of adenovirus hexon protein was carried out according to an established method using the Taqman Universal PCR Master Mix kit (Applied Biosystems) and the following primers: 5′-ACT ATA TGG ACA ACG ACG TCA ACC CAT T-3′ (forward) and 5′-ACC TTC TGA GGC ACC TGG ATG T-3′ (reverse).14 The internal probe sequence, tagged with 6FAM fluorescence dye at the 5′end and TAMRA quencher at the 3′ end, was 5′-ACC ACC GCA ATG CTG GCC TGC-3′.
Evaluation of Beta-Galactosidase Expression
The amount of beta-galactosidase in tissue samples was quantified by an enzyme-linked immunosorbent assay (ELISA, Roche Applied Science, Indianapolis, IN) and visually localized by histochemical staining as described previously.51
Evaluation of p53 Expression
The amount of p53 mRNA in hepatic tissue was assessed by a semi-quantitative RT-PCR method using the following primers: 5′-AAG CAG TCA CAG CAC ATG ACG GAG-3′ (forward) and 5′-GAG TCT TCC AGT GAG ATG ATG GT-3′ (reverse) as previously described.52 Values were normalized to 18S ribosomal mRNA.
Analysis of Serum Transaminases
Serum aspartate aminotransferase (AST) levels and alanine aminotransferase (ALT) levels were determined using VITROS AST/SGOT and ALT/SGPT DT slides on a VITROS DT60 AutoAnalyzer (Ortho-Clinical Diagnostics).
Docetaxel Assay
Tissue samples were processed by a standard protein precipitation technique. DTX was isolated from these samples and plasma using a solid phase extraction technique as described.28 DTX concentrations were determined using a dual liquid chromatography-mass spectrometry assay according to an established protocol.53
Pharmacokinetic Analysis
Pharmacokinetic parameters (plasma clearance (CL), the terminal elimination half life (t1/2), and the volume of distribution at steady state (Vss)) were calculated by a non-compartmental fit analysis using WinNonlin 4.0 (Pharsight Corporation, Mountain View, CA). The area under the plasma concentration-time curve (AUC) was calculated using the linear trapezoidal method with extrapolation of the terminal concentration to infinity.
Statistical Analysis
Statistical analysis of data was performed using SigmaStat (Systat Software, Inc., San Jose, CA). An unpaired two-tailed Student t test was used to compare differences in pharmacokinetic parameters, transgene expression and virus distribution. Differences in all other parameters were evaluated by a one-way analysis of variance (ANOVA) followed by a Bonferroni/Dunn post-hoc test. For each of these tests, differences were determined to be significant when the probability of chance explaining the results was reduced to less than 5% (p<0.05).
Acknowledgments
The authors would like to thank Blake Roessler at the University of Michigan for kindly providing the Adp53 construct and Tim Madden at the M.D. Anderson Cancer Center (Houston, TX) for assistance with analysis of DTX in plasma samples. We also thank Michael Boquet and Courtney Clemens for expert technical assistance. This work was supported by research grant R21GM69870 from the National Institutes of Health (M.A.C.).
Footnotes
Competing Interests Statement
The authors declare no competing financial interests.
References
- 1.Ritter T, Lehmann M, Volk HD. Improvements in gene therapy: averting the immune response to adenoviral vectors. BioDrugs. 2002;16(1):3–10. doi: 10.2165/00063030-200216010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Cao H, Koehler DR, Hu J. Adenoviral vectors for gene replacement therapy. Viral Immunol. 2004;17(3):327–333. doi: 10.1089/vim.2004.17.327. [DOI] [PubMed] [Google Scholar]
- 3.Vattemi E, Claudio PP. Adenoviral gene therapy in head and neck cancer. Drug News Perspect. 2006 Jul-Aug;19(6):329–337. doi: 10.1358/dnp.2006.19.6.1015352. [DOI] [PubMed] [Google Scholar]
- 4.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005 Sep;16(9):1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 5.Gurnani M, Lipari P, Dell J, Shi B, Nielsen LL. Adenovirus-mediated p53 gene therapy has greater efficacy when combined with chemotherapy against human head and neck, ovarian, prostate, and breast cancer. Cancer Chemother Pharmacol. 1999;44(2):143–151. doi: 10.1007/s002800050959. [DOI] [PubMed] [Google Scholar]
- 6.Nemunaitis J, Swisher SG, Timmons T, et al. Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancer. J Clin Oncol. 2000 Feb;18(3):609–622. doi: 10.1200/JCO.2000.18.3.609. [DOI] [PubMed] [Google Scholar]
- 7.Inoue A, Narumi K, Matsubara N, et al. Administration of wild-type p53 adenoviral vector synergistically enhances the cytotoxicity of anti-cancer drugs in human lung cancer cells irrespective of the status of p53 gene. Cancer Lett. 2000 Aug 31;157(1):105–12. doi: 10.1016/s0304-3835(00)00480-8. [DOI] [PubMed] [Google Scholar]
- 8.Yoo GH, Piechocki MP, Oliver J, et al. Enhancement of Ad-p53 therapy with docetaxel in head and neck cancer. Laryngoscope. 2004 Nov;114(11):1871–1879. doi: 10.1097/01.mlg.0000147914.51239.ed. [DOI] [PubMed] [Google Scholar]
- 9.Cristofanilli M, Krishnamurthy S, Guerra L, et al. A nonreplicating adenoviral vector that contains the wild-type p53 transgene combined with chemotherapy for primary breast cancer: safety, efficacy, and biologic activity of a novel gene-therapy approach. Cancer. 2006 Sep 1;107(5):935–944. doi: 10.1002/cncr.22080. [DOI] [PubMed] [Google Scholar]
- 10.Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999 Feb;36(2):99–114. doi: 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R. Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res. 1996 Mar 15;56(6):1296–1302. [PubMed] [Google Scholar]
- 12.Fujita K. Cytochrome P450 and anticancer drugs. Curr Drug Metab. 2006 Jan;7(1):23–37. doi: 10.2174/138920006774832587. [DOI] [PubMed] [Google Scholar]
- 13.Callahan SM, Ming X, Lu SK, Brunner LJ, Croyle MA. Considerations for use of recombinant adenoviral vectors: dose effect on hepatic cytochromes P450. J Pharmacol Exp Ther. 2005 Feb;312(2):492–501. doi: 10.1124/jpet.104.075374. [DOI] [PubMed] [Google Scholar]
- 14.Callahan SM, Boquet MP, Ming X, Brunner LJ, Croyle MA. Impact of transgene expression on drug metabolism following systemic adenoviral vector administration. J Gene Med. 2006 May;8(5):566–576. doi: 10.1002/jgm.884. [DOI] [PubMed] [Google Scholar]
- 15.Fujitaka K, Oguri T, Isobe T, Fujiwara Y, Kohno N. Induction of cytochrome P450 3A4 by docetaxel in peripheral mononuclear cells and its expression in lung cancer. Cancer Chemother Pharmacol. 2001 Jul;48(1):42–46. doi: 10.1007/s002800100291. [DOI] [PubMed] [Google Scholar]
- 16.Nallani SC, Goodwin B, Buckley AR, Buckley DJ, Desai PB. Differences in the induction of cytochrome P450 3A4 by taxane anticancer drugs, docetaxel and paclitaxel, assessed employing primary human hepatocytes. Cancer Chemother Pharmacol. 2004 Sep;54(3):219–229. doi: 10.1007/s00280-004-0799-9. [DOI] [PubMed] [Google Scholar]
- 17.Kruyt FA, Curiel DT. Toward a new generation of conditionally replicating adenoviruses: pairing tumor selectivity with maximal oncolysis. Hum Gene Ther. 2002 Mar 1;13(4):485–495. doi: 10.1089/10430340252809784. [DOI] [PubMed] [Google Scholar]
- 18.Gabrilovich DI. INGN 201 (Advexin): adenoviral p53 gene therapy for cancer. Expert Opin Biol Ther. 2006 Aug;6(8):823–832. doi: 10.1517/14712598.6.8.823. [DOI] [PubMed] [Google Scholar]
- 19.Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer. 2006 Jul;6(7):546–558. doi: 10.1038/nrc1887. [DOI] [PubMed] [Google Scholar]
- 20.Goh BC, Lee SC, Wang LZ, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002 Sep 1;20(17):3683–3690. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Alexandre J, Rey E, Girre V, et al. Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Oncol. 2007 Jan;18(1):168–172. doi: 10.1093/annonc/mdl321. [DOI] [PubMed] [Google Scholar]
- 22.Nallani SC, Genter MB, Desai PB. Increased activity of CYP3A enzyme in primary cultures of rat hepatocytes treated with docetaxel: comparative evaluation with paclitaxel. Cancer Chemother Pharmacol. 2001 Aug;48(2):115–122. doi: 10.1007/s002800100283. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Hu JK, Krol A, Li YP, Li CY, Yuan F. Systemic dissemination of viral vectors during intratumoral injection. Mol Cancer Ther. 2003 Nov;2(11):1233–1242. [PubMed] [Google Scholar]
- 24.Wang Y, Yang Z, Liu S, et al. Characterisation of systemic dissemination of nonreplicating adenoviral vectors from tumours in local gene delivery. Br J Cancer. 2005 Apr 25;92(8):1414–1420. doi: 10.1038/sj.bjc.6602494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavek P, Dvorak Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr. Drug Metab. 2008;9(2):129–143. doi: 10.2174/138920008783571774. [DOI] [PubMed] [Google Scholar]
- 26.Martinez C, Garcia-Martin E, Pizarro RM, Garcia-Gamito FJ, Agundez JA. Expression of paclitaxel-inactivating CYP3A activity in human colorectal cancer: implications for drug therapy. Br J Cancer. 2002 Sep 9;87(6):681–686. doi: 10.1038/sj.bjc.6600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLoia JA, Zamboni WC, Jones JM, Strychor S, Kelley JL, Gallion HH. Expression and activity of taxane-metabolizing enzymes in ovarian tumors. Gynecol Oncol. 2008 Feb;108(2):355–360. doi: 10.1016/j.ygyno.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Zamboni WC, Strychor S, Joseph E, Parise RA, Egorin MJ, Eiseman JL. Tumor, tissue, and plasma pharmacokinetic studies and antitumor response studies of docetaxel in combination with 9-nitrocamptothecin in mice bearing SKOV-3 human ovarian xenografts. Cancer Chemother Pharmacol. 2008 Aug;62(3):417–426. doi: 10.1007/s00280-007-0620-7. [DOI] [PubMed] [Google Scholar]
- 29.Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, Opolon P, Tordjmann T, Vigne E, Perricaudet M, Benihoud K. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol. Ther. 2008;16(8):1474–1480. doi: 10.1038/mt.2008.132. [DOI] [PubMed] [Google Scholar]
- 30.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132(3):397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Okegawa T, Lombardi DP, Frenkel EP, Hsieh JT. Enhanced transgene expression in androgen independent prostate cancer gene therapy by taxane chemotherapeutic agents. J Urol. 2002 Jan;167(1):339–346. [PubMed] [Google Scholar]
- 32.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007 Mar;7(2):141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 33.Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res. 2007 May;24(5):918–933. doi: 10.1007/s11095-006-9210-3. [DOI] [PubMed] [Google Scholar]
- 34.Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel : recent developments. Clin Pharmacokinet. 2006;45(3):235–252. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- 35.Won KA, Baumann H. The cytokine response element of the rat alpha 1-acid glycoprotein gene is a complex of several interacting regulatory sequences. Mol Cell Biol. 1990 Aug;10(8):3965–3978. doi: 10.1128/mcb.10.8.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JS, Tian J, Lozier JN, Byrnes AP. Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Mol Ther. 2004 Jun;9(6):932–941. doi: 10.1016/j.ymthe.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Le HT, Boquet MP, Clark EA, Callahan SM, Croyle MA. Renal pathophysiology after systemic administration of recombinant adenovirus: changes in renal cytochromes P450 based on vector dose. Hum Gene Ther. 2006 Nov;17(11):1095–1111. doi: 10.1089/hum.2006.17.1095. [DOI] [PubMed] [Google Scholar]
- 38.Imaoka S, Hashizume T, Funae Y. Localization of rat cytochrome P450 in various tissues and comparison of arachidonic acid metabolism by rat P450 with that by human P450 orthologs. Drug Metab Pharmacokinet. 2005 Dec;20(6):478–484. doi: 10.2133/dmpk.20.478. [DOI] [PubMed] [Google Scholar]
- 39.Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu. Rev. Pharmacol. Toxicol. 2006;46:123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 40.Renton KW. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin Drug Metab Toxicol. 2005 Dec;1(4):629–640. doi: 10.1517/17425255.1.4.629. [DOI] [PubMed] [Google Scholar]
- 41.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul. Toxicol. Pharmacol. 1998;27(2):119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- 43.Callahan SM, Wonganan P, Croyle MA. Molecular and Macromolecular Alterations of Recombinant Adenoviral Vectors Do Not Resolve Changes in Hepatic Drug Metabolism During Infection. Virology Journal. 2008 doi: 10.1186/1743-422X-5-111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaller M, Hogaboam CM, Lukacs N, Kunkel SL. Respiratory viral infections drive chemokine expression and exacerbate the asthmatic response. J Allergy Clin Immunol. 2006 Aug;118(2):295–302. doi: 10.1016/j.jaci.2006.05.025. quiz 303-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seemungal TA, Wedzicha JA. Viral infections in obstructive airway diseases. Curr Opin Pulm Med. 2003 Mar;9(2):111–116. doi: 10.1097/00063198-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Shirali GS, Ni J, Chinnock RE, Johnston JK, Rosenthal GL, Bowles NE, Towbin JA. Association of viral genome with graft loss in children after cardiac transplantation. N. Engl. J. Med. 2001;344(20):1498–1503. doi: 10.1056/NEJM200105173442002. [DOI] [PubMed] [Google Scholar]
- 47.Maizel JV, Jr., White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 48.Boquet MP, Wonganan P, Dekker JD, Croyle MA. Impact of Route of Systemic Administration of Adenovirus on Virus-Mediated Toxicity: Focus on Mortality, Virus Distribution, and Drug Metabolism. J. Pharmacol. Toxicol. Methods. 2008 doi: 10.1016/j.vascn.2008.07.003. e-pub Aug 3, 2008:doi:10.1016/j.vascn.2008.1007.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coon MJ, van der Hoeven TA, Dahl SB, Haugen DA. Two forms of liver microsomal cytochrome P-450, P-450lm2 and P-450LM4 (rabbit liver) Methods Enzymol. 1978;52:109–117. doi: 10.1016/s0076-6879(78)52012-0. [DOI] [PubMed] [Google Scholar]
- 50.van der Hoeven T. Assay of Hepatic Microsomal Testosterone Hydroxylases by High-Performance Liquid Chromatography. Anal. Biochem. 1984;138(1):57–65. doi: 10.1016/0003-2697(84)90768-1. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Raper SE, Cohn JA, Engelhardt JF, Wilson JM. An approach for treating the hepatobiliary disease of cystic fibrosis by somatic gene transfer. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4601–4605. doi: 10.1073/pnas.90.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn WS, Bae SM, Lee JM, Namkoong SE, Yoo JY, Seo YS, Nam SL, Cho YL, Nam KH, Kim CK, Kim YW. Anti-cancer effect of adenovirus p53 on human cervical cancer cell growth in vitro and in vivo. Int. J. Gynecol. Cancer. 2004;14(2):322–332. doi: 10.1111/j.1048-891x.2004.014217.x. [DOI] [PubMed] [Google Scholar]
- 53.Parise RA, Ramanathan RK, Zamboni WC, Egorin MJ. Sensitive liquid chromatography-mass spectrometry assay for quantitation of docetaxel and paclitaxel in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jan 5;783(1):231–236. doi: 10.1016/s1570-0232(02)00659-1. [DOI] [PubMed] [Google Scholar]