Abstract
Objectives
To test the effect of bisphosphonate (BP) treatment for up to 3 years on bone necrosis and osteocyte death in the mandible using a canine model.
Materials and Methods
Dogs were treated with clinical doses of oral alendronate (ALN, 0.2 mg/kg/day or 1.0 mg/kg/day) for 1 or 3 years. In a separate study, dogs were treated with intravenous zoledronate (ZOL) at 0.06 mg/kg/day for 6 months. En bloc staining was used to identify necrotic areas in the mandible; viable osteocytes were identified using lacatate dehydrogenase.
Results
None of the treatments were associated with exposed bone, but 17–25% of dogs treated for one year and 25–33% of dogs treated for three years with ALN showed pockets of dead bone. Necrotic areas had no viable osteocytes and were void of patent canaliculi. No control animals demonstrated necrotic bone. ZOL treatment for 6 months was associated with osteocyte death greater than that seen in animals treated with ALN or saline. It is not clear whether osteocyte death occurs because of direct toxic effects of BPs, or because suppressed remodeling fails to renew areas that naturally undergo cell death. However, necrotic areas are associated with bone other than the mandible, e.g., the rib, which normally undergo high rates of remodeling.
Conclusions
Reduced remodeling rate using BPs may contribute to the pathogenesis for bone matrix necrosis. The development of an animal model that mimics important aspects of BP-related osteonecrosis of the jaw is important to understanding the pathogenesis of osteonecrosis.
Keywords: Osteonecrosis, bisphosphonates, osteocytes, mandible
Introduction
Osteonecrosis of the jaw (ONJ) was first identified as a serious potential health-related problem only five years ago (1–3). Since 2005, the number of publications describing the condition, its possible pathogenesis, prevention, treatment and the role that bisphosphonates may or may not play in the initiation or progression of the condition have multiplied by more than 3-fold to reach approximately 160 annually (Fig. 1). Most of these reports are retrospective studies of the epidemiology and risk factors associated with the condition (e.g., see Ref 4 for one of the better reports). These reports, while useful, will never be able address the root causes or pathogenesis of the condition, nor allow us to study any means by which the condition can be either prevented or treated.
Figure 1.
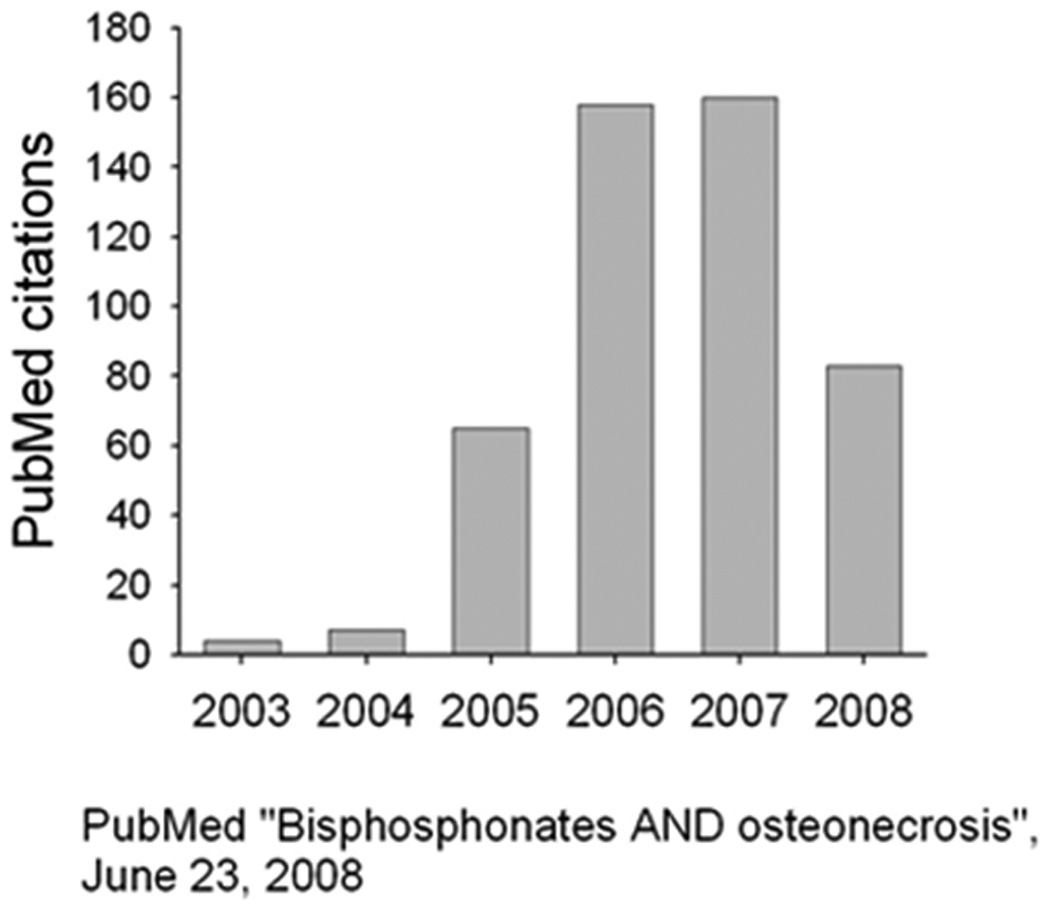
The number of citations in PubMed between 2003, when bisphosphonate-related osteonecrosis was first identified, and June of 2008. Following a search using the terms “Bisphosphonates and osteonecrosis”, citations specific to the jaw (e.g. excluding osteonecrosis of the femoral head) were identified.
In part for this reason, the American Society for Bone and Mineral Research (ASBMR) Task Force on Bisphosphonate-Associated Osteonecrosis of the Jaw recommended as necessary and essential “the development of an appropriate animal model” [emphasis ours] to determine the best management approach to those with ONJ. They state, “Such an animal model, once developed … would hopefully provide the rational/ethical basis on which human randomized clinical trial investigations could occur.” Bisphosphonate-associated (now termed bisphosphonate-related) osteonecrosis of the jaw (BRONJ) was defined by the ASBMR Task Force as “an area of exposed bone in the maxillofacial region that does not heal within 8 weeks after identification by a health care provider, in a patient who was receiving or had been exposed to a bisphosphonate and had not had radiation therapy to the craniofacial region (5). Thus, an animal model for BRONJ would have to be one treated with a bisphosphonate (BP) which developed exposed bone, perhaps in response to an infection or a dental procedure/trauma, which persisted for at least a period equivalent to the period of time required for normal healing in that animal species. In a dog, this would be equivalent to 4–6 weeks. No such model currently exists, and the issue of what an appropriate model might depend in part on the role that bone remodeling plays in the pathogenesis of BRONJ.
Clinical Relevance
Oral bisphosphonates are used to treat osteoporosis, and intravenous bisphosphonates at high doses are used to prevent skeletal metastasis in some cancers. Bisphosphonate use has been implicated in the pathogenesis of osteonecrosis of the jaw (BRONJ). Attempts to recapitulate BRONJ in an animal model have the potential to improve our understanding of the pathophysiology of BRONJ and provide a new model system to test preventative and therapeutic approaches for this condition.
Experimental animal studies of bisphosphonates and mandibular necrosis
We have been using oral bisphosphonates in a non-ovariectomized canine model to investigate the effect that bisphosphonates have on the mechanical and material properties of the bone tissue. As a serendipitous part of these studies, we collected mandibles from dogs that were treated with oral doses of alendronate (Fosamax) at doses used for the clinical treatment of osteoporosis (on a mg/kg basis), or at 5 times that dose. Treatment with alendronate occurred over 1, 2 or 3 years. The experimental design of this study, and data on bone properties not related to ONJ have been reported in several publications (6–11). Data on the changes to the mandible were reported earlier in 2008 as well (12).
En bloc basic fuchsin staining is a technique developed to identify the accumulation of microdamage in bone and to distinguish it from artifact caused by histological processing and sectioning (13–15). This process was also used by Frost (13) and Enlow (16) nearly 50 years ago to identify areas of bone (primarily rib) that were necrotic. Basic fuchsin works by passively diffusing into all voids in the bone tissue matrix (blood vessels, lacunae, canaliculi, and microcracks). Areas that do not stain lack a patent canalicular network that would allow diffusion of the stain into the empty spaces. Therefore, large regions void of stain can be easily identified using either standard transmission or confocal microscopy. Areas of necrotic bone can also be assessed using enzyme histochemistry for viable osteocytes. Our laboratory utilizes lactate dehydrogenase (LDH) to identify necrotic regions. Living, viable osteocytes produce LDH, and will stain, whereas LDH-positive osteocytes cannot be detected in areas of non-viable bone.
Basic fuchsin staining was applied to cross-sections taken from various locations through the mandibles of dogs that had been treated for one or three years with alendronate or vehicle (n = 12/dose or group). Sections were made through the level of the 2nd premolars, between the 3rd and 4th premolars, and through the 1st and 2nd molars (Fig. 2). These sections were subsequently stained en bloc with basic fuchsin, and viewed with a standard transmission microscope and with a confocal microscope. Areas devoid of stain that were at least 500 µm2 in area were considered necrotic. Based on this, we found 25% (n = 3) of the animals treated for three years with osteoporosis doses of alendronate, and 33% (n = 4) of those treated with a higher dose for three years, had regions of necrotic bone (Fig. 3). Three of the animals (n=2 treated with the lower dose and n=1 treated with the higher dose) had two separate regions of necrosis within the same tissue section. None of the 12 animals treated with saline vehicle for three years, demonstrated necrotic regions of bone. Those dogs treated for only one year with the lower dose of alendronate also demonstrated a 25% incidence of mandibular necrosis at the osteoporosis dose, and 17% (n = 2) incidence at the higher dose (Table 1). Again, no animals in the vehicle control group demonstrated any regions of necrotic bone in the mandible. Necrotic lesions in bisphosphonate-treated animals were primarily restricted to the alveolar bone region, an area of rapid bone turnover under normal conditions, although two animals treated with the higher dose of alendronate for 3 years had necrotic areas in the corpus or at the base of the mandible, where remodeling occurs at lower rates.
Figure 2.
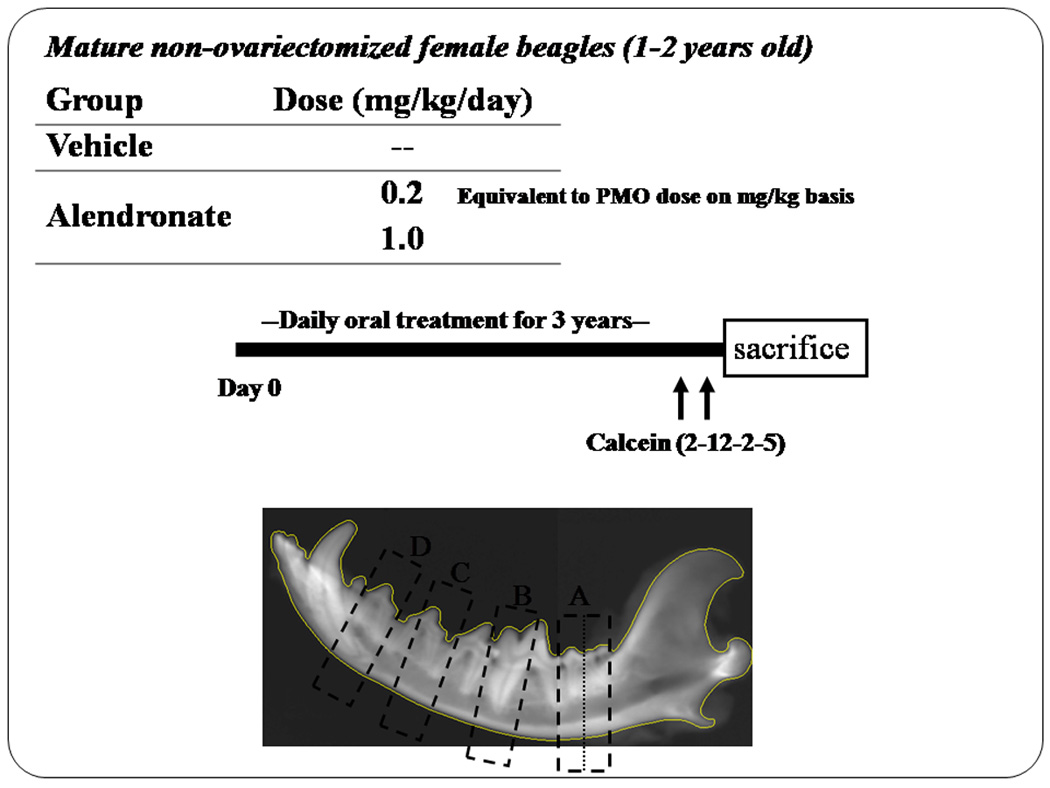
Experimental design for the initial experiments treating dogs for 3 years with two different doses of oral alendronate. The lower dose is the dose used to treat postmenopausal women with osteoporosis; the higher dose is used to treat Paget’s disease. Dogs were 1–2 years old at the initiation of treatment. Calcein labeling was initiated 3 weeks prior to sacrifice. Sections were taken at four locations through the mandible.
Figure 3.
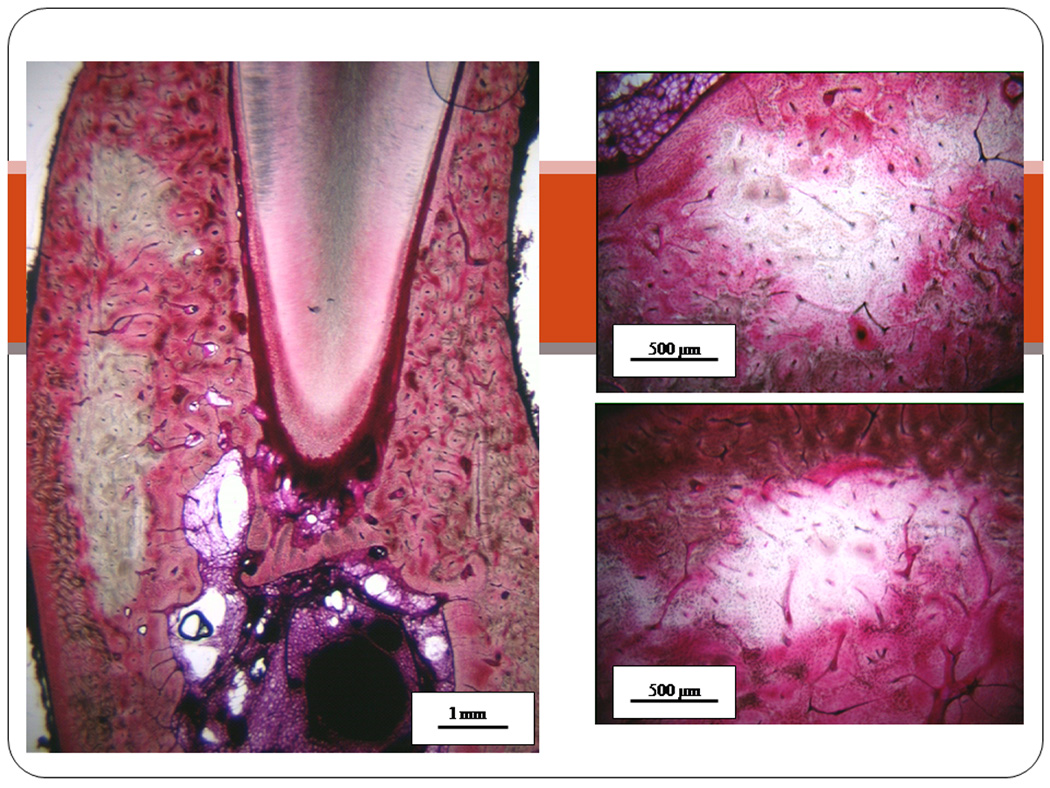
Photomicrographs of mandible bone matrix necrosis in beagle dogs treated with bisphosphonate. Following 1 or 3 years of oral alendronate treatment, mandibles were stained en bloc with basic fucshin which allows identification of necrotic bone matrix due to its lack of stain uptake compared to viable regions of bone. Images are from three different animals treated with oral alendronate for 1 or 3 years.
Table 1.
Mandible necrosis summary
| 1 year treatment | ||||
|---|---|---|---|---|
| Treatment | Dose (mg/kg/day) |
Animals | Sites per animal |
Animals w/ Necrosis |
| Vehicle | 12 | 1 | 0 | |
| Alendronate |
0.2 1.0 |
12 12 |
3 3 |
3 (25%) 2 (17%) |
| Raloxifene | 0.5 | 12 | 1 | 0 |
| 3 year treatment | ||||
|---|---|---|---|---|
| Treatment | Dose (mg/kg/day) |
Animals | Sites per animal |
Animals w/ Necrosis |
| Vehicle | 12 | 4 | 0 | |
| Alendronate |
0.2 1.0 |
12 12 |
4 4 |
3 (25%) 4 (33%) |
It is important to stress that treatment with oral BPs, even at high doses for 3 years in these dogs, did not create BRONJ, as defined above, in any of the dogs. Nevertheless, treatment was associated with necrotic bone in that osteocytes in significant pockets of bone tissue were dead. We suggest that this may be an indication of pre-ONJ that does not progress to exposed bone unless there are other precipitating factors (eg trauma, infection, compromise of the immune-system, or some combination of these well-defined risk factors).
Most instances of BRONJ have involved intravenous administration of zoledronate (ZOL) or pamidronate in patients with cancer who are generally immune-suppressed, and often at doses 10x those used to treat postmenopausal women for osteoporosis. Therefore, our laboratory undertook a short term study in dogs to compare the effects of oral alendronate at the osteoporosis dose (0.2 mg/kg/day) for 3 or 6 months with intravenous zoledronate given at the dose used in cancer patients (0.06 mg/kg/month) [unpublished studies]. Sections from the mandible were stained using LDH histochemistry and basic fuchsin to assess osteocyte viability and matrix necrosis. In this study, no animals showed evidence of necrosis using basic fuchsin staining. However, using LDH histochemistry there was clear evidence of regions with non-viable osteocytes. The areas of non-viable osteocytes in the alveolar bone were 3–4 times greater in animals treated for 6 months with i.v. ZOL compared to animals treated either with alendronate or with saline vehicle (Fig. 4). This shows that, even short term treatment with high doses of ZOL in a healthy dog can lead to significant osteocyte death and necrosis of alveolar bone. Again, however, no areas of exposed bone were visible in the oral cavity of any of these dogs.
Figure 4.
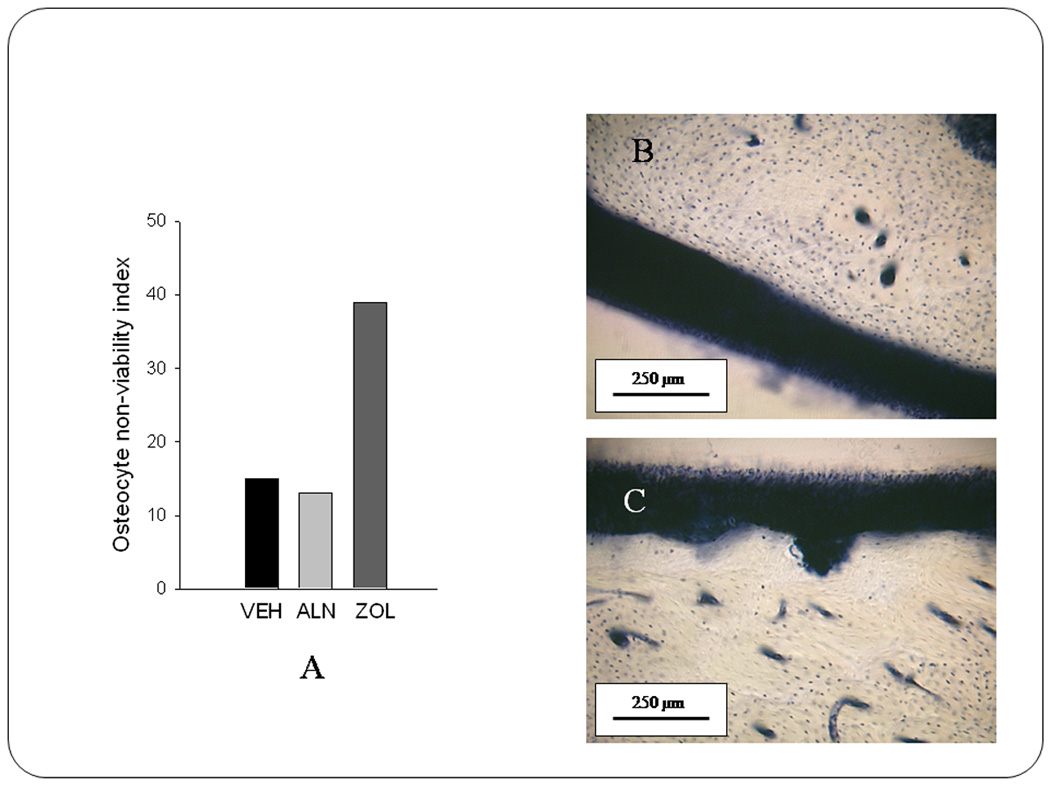
Accumulation of regions with non-viable osteocytes in the mandible is prominent with high dose zoledronate treatment. Following 6 months of treatment with either saline (VEH), oral alendronate (ALN), or intravenous zoledronate (ZOL) mandible tissue was assessed for osteocyte viability using lactate dehydrogenase staining, which results in viable cells staining blue. Using an ordinal scale classification, it can be shown that regions of non-viable osteocytes were more prominent in ZOL-treated animals (panel A). Although both VEH and ALN-treated animals had regions of non-viable osteocytes, there tended to be very few regions, and these were small (panel B). Conversely, some of the regions of non-viable osteocytes in ZOL-treated animals were significant in size (panel C).
The pathophysiology of mandibular necrosis
It is not clear whether osteocyte death subsequent to treatment with bisphosphonates in the canine model (or in human BRONJ) occurs through direct toxic effects of bisphosphonates on bone cells (17), or whether it occurs through reduced remodeling of regions which normally undergo cell death (Fig. 5). Bisphosphonates, at doses consistent with those used for osteoporosis, are known to prevent osteocyte apoptosis in vivo (18,19). There is no direct evidence of the in vivo effects of bisphosphonates on osteocytes at higher doses, although pamidronate and alendronate have been shown to inhibit protein prenylation in osteoblasts and to negatively affect osteoblast differentiation in vitro (17). Alendronate (20) and pamidronate (21) have been shown to suppress the activity of osteoblasts in vivo, and Idris et al. (17) recently showed that calvarial osteoblasts are capable of ingesting sufficient amounts of alendronate in vivo to inhibit protein prenylation. Therefore, it is possible that high doses of potent BPs delivered over a short period of time, or longer term administration of less potent BPs, which may nevertheless have a high binding affinity (such as alendronate) and remain in the bone for long periods of time (22), may accumulate in the skeleton to levels that become toxic to osteocytes. The alternative hypothesis is that the death of osteocytes and the development of some necrotic bone matrix is normal and it accumulates with bisphosphonate treatment because remodeling is suppressed. Cell death is a physiological process that occurs in living bone all the time. Usually dead cells are removed by normal processes of remodeling, but in cases where remodeling is suppressed, these cells may not be removed and may accumulate in pockets of necrotic bone. Which of these two hypotheses is correct – if either is – remains an open question, and one that can probably only be answered using an animal model in which necrosis can be created. In either case, this osteocyte death without replacement inevitably leads to tissue necrosis, which, when combined with a dental extraction or oral infection, especially in those who are immune-compromised, could progress to frank jaw osteonecrosis.
Figure 5.
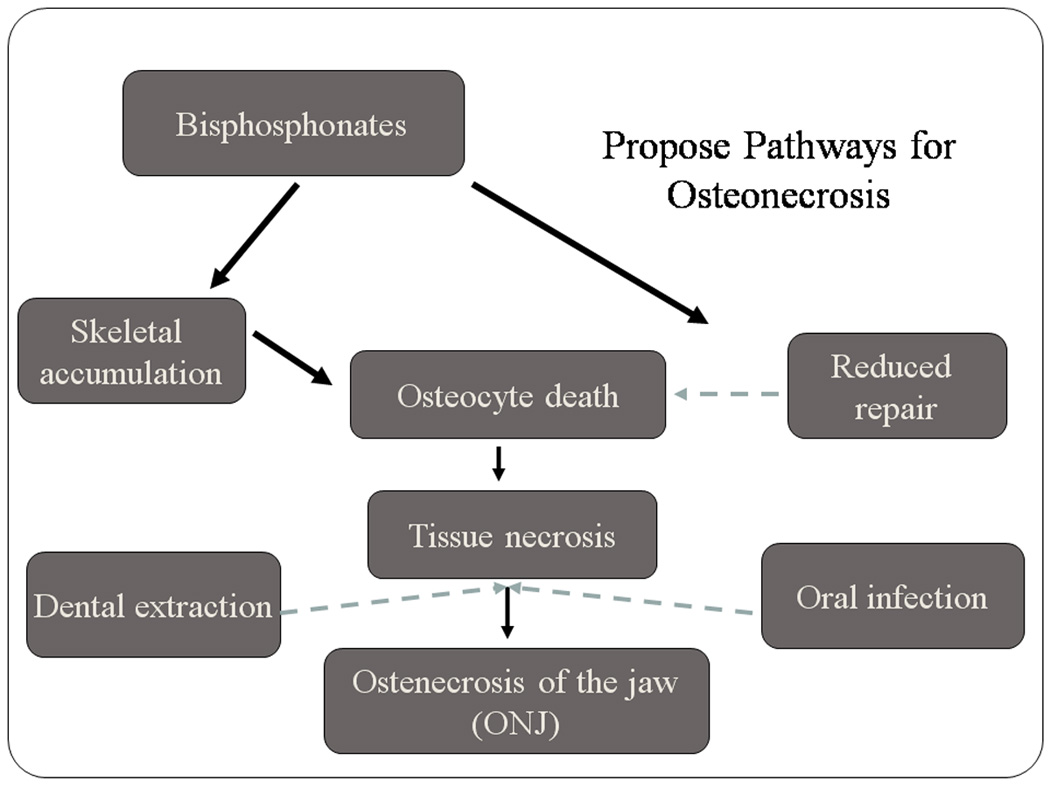
Flow chart showing two possible pathways for the accumulation of necrotic bone with non-viable osteocytes. In one situation (left side), bisphosphonates accumulate in the skeletal tissue to levels that become toxic to osteocytes, causing their death, and subsequent matrix necrosis. A second pathway (right side) is through reduced remodeling caused by bisphosphonates which would inhibit the repair of osteocytes that die as a consequence of normal physiological processes associated with tissue renewal. Failure to replace the dead osteocytes would then lead to larger regions of necrosis without viable osteocytes.
To begin to understand whether the mechanism underlying the accumulation of necrotic bone matrix is specific to bisphosphonates, we have assessed mandible tissue from non-ovariectomized beagle dogs that were treated for 1 year with a different anti-remodeling agent, the selective estrogen receptor modulator (SERM) raloxifene (Evista). These animals, treated daily with the clinical dose of oral raloxifene, were part of the same study described above for alendronate. To date we have assessed two different regions of the mandible in these raloxifene-treated animals and have not identified any regions of matrix necrosis (using basic fuchsin staining). Based on analyses of the vertebrae from these raloxifene-treated animals compared to those treated with alendronate for 1 year, we know that the turnover suppression is not equivalent (−20% in raloxifene and −70% in alendronate compared to vehicle) (6,23). Therefore it remains unclear if remodeling suppression to a similar degree as that which occurs with bisphosphonates would similarly increase the amount of necrotic bone matrix or whether this effect (accumulation of necrotic bone) is specific to the bisphosphonates.
The alveolar process of the mandible normally is subject to a high rate of bone turnover. The cause for this is not known, although it may be related to the large bite forces engendered in this region, or to the need to clear bacterial agents present in the relatively “dirty” oral environment. Whatever the reason, it has been shown that bone turnover in the alveolar region of the adult canine mandible is 2–4x greater than in the basal portion of the mandible (12,24), and 15–20x higher than in bones from the appendicular skeleton (24,25). It is possible that when this high rate of turnover is artificially suppressed with bisphosphonates, areas which normally undergo cellular necrosis are not remodeled, but accumulate, leading after a period of remodeling suppression to large areas devoid of viable cells. It is possible to test whether a high rate of turnover is associated with osteocyte necrosis by comparing the alveolar process to the basal region of the mandibular corpus (Fig. 6), a site of very low turnover (24), and to the rib, a site of high turnover (~20%/yr, approximately half that in the alveolar process of the mandible, but 10x higher than in other sites in the appendicular skeleton) (25). If suppression of remodeling at sites of normally high turnover is related to the development of large areas of cellular necrosis, then we might expect to see necrotic bone in the rib, but not in the basal portion of the mandible, even in those samples in which necrotic bone is found in the alveolus. In fact, this is exactly what we observe. We detected few instances of osteocyte necrosis 500 µm2 or larger at the base of the mandible. However, we found a 20% incidence of necrotic bone in the rib in dogs treated with alendronate at the osteoporosis dose, and a 40% incidence with the high dose. Thus, we hypothesize that areas of bone that are normally subject to high rates of turnover, and in which remodeling is artificially suppressed by as much as 85%, are subject to develop necrotic bone. Patches of necrotic bone in non-dental tissues may never represent a serious health problem because they are less subject to trauma in which bone is exposed, are not adjacent to epithelial tissues, and not likely to be exposed to bacterial agents that cause infection or inflammatory processes. It is still not clear, however, whether the regions of necrosis themselves – at either site – are caused by a normally high rate of osteocyte death in these regions that simply can no longer be cleared because remodeling is suppressed, or whether the rate of vascular flow in these regions allows more bisphosphonate to enter these areas, either permitting greater access to bone extracellular fluids around the osteocytes, or permitting greater accumulation in the skeleton, with direct toxic consequences to the osteocytes.
Figure 6.
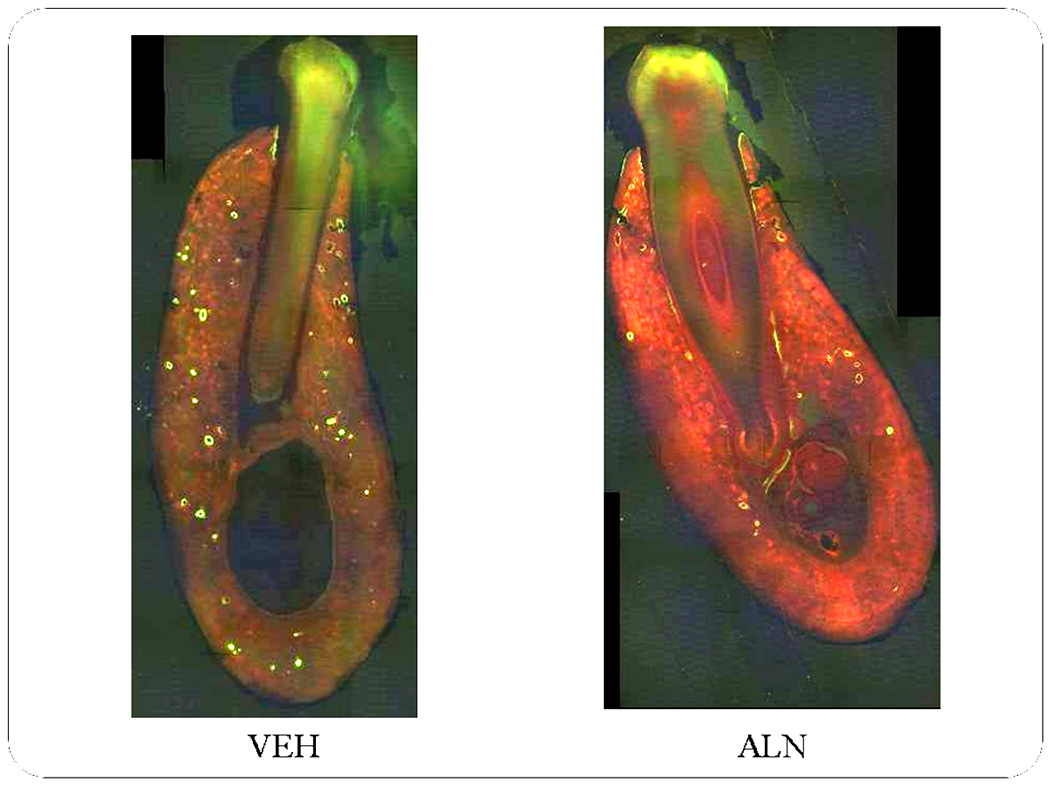
Photomicrographs of mandible cross-sections from animals treated for 3 years with vehicle (VEH) or alendronate (ALN). Through administration of fluorochrome labels at the end of the experiment, the level of bone remodeling can be determined using ultraviolet fluorescent microscopy. Based on the number of calcein-labeled osteons it is clear that there is a high level of turnover in VEH animals, particularly in the alveolar portion of the bone. Conversely, far fewer osteons are labeled in the ALN-treated animal, indicative of a reduced remodeling rate.
If a high rate of intracortical remodeling is a necessary condition for the pathogenesis of BRONJ, then this reflects on the future development of animal models. Mice and rats may not provide good models for BRONJ because neither of these species, under normal circumstances, has intracortical remodeling in the mandible. Although some have suggested that mandibular remodeling occurs to some degree in C3H/HeJ and C57BL/6J mice, it is not common, nor is it consistent from one mouse to another (27). The marsh rice rat (28) may represent a potential animal model in which mandibular bone can become necrotic. Gotcher and Jee showed that treatment with clodronate at doses between 0.1 and 10.0 mg/kg/day for 6–18 weeks led to necrotic and exposed alveolar bone, especially at the high doses and longest period of exposure. Although rabbits have extensive intracortical remodeling in their long bones, an examination of the adult rabbit mandible suggests that remodeling is not very common in it either. Rabbits injected 3x/week for 8 months with 2.4 mg/kg of alendronate or risedronate did not demonstrate any areas of necrotic tissue within the mandible upon sacrifice. Limited osteonal remodeling was found in these rabbit mandibles (Fig. 7).
Figure 7.
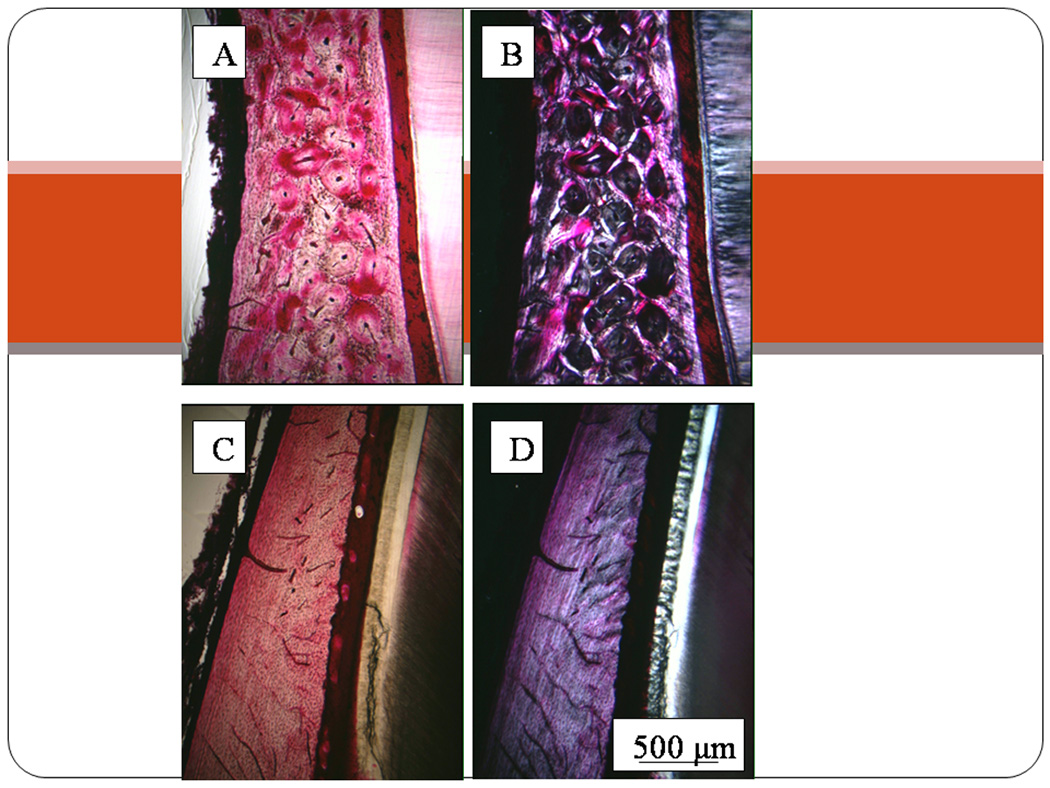
Photomicrographs of alveolar bone in a two year old beagle dog (panels A and B) and a one year old rabbit (panels C and D). The difference in osteon density, illustrated using brightfield microscopy of basic fuchsin stained sections (A&C), clearly illustrates the lower rate of secondary osteonal remodeling in the rabbit mandible. This can also be appreciated using polarized light (B and D), which shows the rabbit mandible alveolar process is unremodeled primary bone even in this mature animal. If bisphosphonate-related osteonecrosis of the jaw (BRONJ) is found more often in regions of bone that have high basal remodeling rates, then rabbits are not likely to be a very good animal model for the pathogenesis of BRONJ.
It seems that the dog, because of its ability to remodel in ways that are similar to humans, may represent the best potential animal model for BRONJ. Demonstration of large areas of necrotic bone in a subpopulation of dogs treated with BPs suggests the possibility that true ONJ could develop under conditions of dental trauma or infection in these animals.
Conclusions
There is no animal model currently exists that mimics BRONJ. Treatment of dogs for 1–3 years with oral BPs, or for 6 months with i.v. zoledronate, is associated with the development of areas of tissue necrosis and dead osteocytes in the mandible and the rib, both sites of high bone turnover rates. It is not clear whether tissue necrosis is related to the toxic effects of BPS at high dose, skeletal accumulation of BPs that are given for prolonged periods of time, or whether the necrosis is simply the result of the natural process of cell death , and that the areas are retained because suppressed remodeling prevents their replacement. These are not questions that are likely to be answered from human clinical studies. The development of an animal model that mimics the most important aspects of BRONJ is vital to our understanding of the pathogenesis of this serious condition.
Acknowledgements
This work was supported by NIH Grants R01 AR51555, R01 AR047838, and T32 AR007581, and research grants from the National Osteoporosis Foundation, The Alliance for Better Bone Health (Procter and Gamble Pharmaceuticals and sanofi-aventis), Eli Lilly and Co. and Amgen. Merck and Co. kindly provided the alendronate. This investigation utilized an animal facility constructed with support from Research Facilities Improvement Program Grant Number C06RR10601 from the NIH National Center for Research Resources.
References
- 1.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra B, Fantasia J, Nissel-Horowitz S, Vinarsky S, Sheth M, Ruggiero S. Osteonecrosis of the maxilla: an unusual complication of prolonged bisphosphonate therapy. A case report. Proc Am Soc Clin Oncol. 2003;22 (abstr 3194) [Google Scholar]
- 4.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 6.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Allen MR, Follet H, Khurana M, Sato M, Burr DB. Antiremodeling agents influence osteoblast activity differently in modeling and remodeling sites of canine rib. Calcif Tiss Int. 2006;79:255–261. doi: 10.1007/s00223-006-0031-5. [DOI] [PubMed] [Google Scholar]
- 8.Allen MR, Burr DB. Mineralization, microdamage, and matrix: How bisphosphonates influence material properties of bone. BoneKEy-Osteovision. 2007;4:49–60. [Google Scholar]
- 9.Allen MR, Burr DB. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 10.Allen MR, Burr DB. Changes in vertebral strength-density and energy absorption-density relationships following bisphosphonate treatment in beagle dogs. Osteoporos Int. 2008;19:95–99. doi: 10.1007/s00198-007-0451-8. [DOI] [PubMed] [Google Scholar]
- 11.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–337. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 12.Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg. 2008;66:987–994. doi: 10.1016/j.joms.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost HM. In vivo osteocyte death. J Bone Joint Surg. 1960;42A:138–143. [PubMed] [Google Scholar]
- 14.Burr DB, Stafford T. Validity of the bulk staining technique to separate artifactual from in vivo bone microdamage. Clin Orthop Rel Res. 1990;260:305–308. [PubMed] [Google Scholar]
- 15.Burr DB, Hooser M. Alterations to the en bloc basic fuchsin staining protocol for the demonstration of microdamage produced in vivo. Bone. 1995;17:431–433. doi: 10.1016/s8756-3282(95)00241-3. [DOI] [PubMed] [Google Scholar]
- 16.Enlow DH. Functions of the Haversian system. Am J Anat. 1962;11:269–282. doi: 10.1002/aja.1001100305. [DOI] [PubMed] [Google Scholar]
- 17.Idris AI, Rojas J, Greig IR, van’t Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tiss Int. 2008;82:191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 18.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Follet H, Li J, Phipps RJ, Hui S, Condon K, Burr DB. Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone. 2007;40:1172–1177. doi: 10.1016/j.bone.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 20.Iwata K, Li J, Follet H, Phipps RJ, Burr DB. Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone. 2006;39:1053–1058. doi: 10.1016/j.bone.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Tobias JH, Chow JWM, Chambers TJ. 3-Amino-1-hydrozypropylidine-1-bisphosphonate (AHPrBP) suppresses not only the induction of new, but also the persistence of existing bone-forming surfaces in rat cancellous bone. Bone. 1993;14:619–623. doi: 10.1016/8756-3282(93)90083-m. [DOI] [PubMed] [Google Scholar]
- 22.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, et al. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone. 2006;38:617–627. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Allen MR, Iwata K, Sato M, Burr DB. Raloxifene enhances vertebral mechanical properties independent of bone density. Bone. 2006;39:1130–1135. doi: 10.1016/j.bone.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Tricker ND, Dixon RB, Garretto LP. Cortical bone turnover and mineral apposition in dentate dog mandible. In: Garetto LP, Turner CH, Duncan RL, Burr DB, editors. Bridging the gap between dental and orthopaedic implants. Indianapolis: Indiana University School of Dentistry; 2002. pp. 226–228. [Google Scholar]
- 25.Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec. 2006;288A:1243–1249. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 27.Meta IF, Fernandez SA, Gulati P, Huja SS. Alveolar process anabolic activity in C3H/HeJ and C57BL/6J inbred mice. J Periodontol. 2008;vol. 79 doi: 10.1902/jop.2008.070610. 1255-162. [DOI] [PubMed] [Google Scholar]
- 28.Gotcher JE, Jee WSS. The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium. J Periodont Res. 1981;16:441–455. doi: 10.1111/j.1600-0765.1981.tb00995.x. [DOI] [PubMed] [Google Scholar]


