Summary
Ca2+ release from the sarcoplasmic reticulum (SR) into the cytosol is a crucial part of excitation-contraction (E-C) coupling. E-C uncoupling, a deficit in Ca2+ release from the SR, is thought to be responsible for at least some of the loss in specific force observed in aging skeletal muscle. E-C uncoupling may be caused by alterations in expression of the voltage-dependent calcium channel α1s (CaV1.1) and β1a (CaVβ1a) subunits, both of which are necessary for E-C coupling to occur. While previous studies have found CaV1.1 expression declines in old rodents, CaVβ1a expression has not been previously examined in aging models. Western blot analysis shows a substantial increase of CaVβ1a expression over the full lifespan of FVB mice. To examine the specific effects of CaVβ1a overexpression, a CaVβ1a -YFP plasmid was electroporated in vivo into young animals. The resulting increase in expression of CaVβ1a corresponded to decline of CaV1.1 over the same time period. YFP fluorescence, used as a measure of CaVβ1a -YFP expression in individual fibers, also showed an inverse relationship with charge movement, measured using the whole-cell patch-clamp technique. Specific force was significantly reduced in young CaVβ1a - YFP electroporated muscle fibers compared to sham-electroporated, age-matched controls. siRNA interference of CaVβ1a in young muscles reduced charge movement, while charge movement in old was restored to young control levels. These studies imply CaVβ1a serves as both a positive and negative regulator CaV1.1 expression, and that endogenous overexpression of CaVβ1a during old age may play a role in the loss of specific force.
Keywords: Sarcopenia, Calcium channel, DHPR, E-C Coupling, Specific Force, Calcium
INTRODUCTION
Depolarization of the sarcolemma leads to muscle fiber contraction and the generation of mechanical force in a process called excitation-contraction (E-C) coupling. Two key proteins involved in E-C coupling are: dihydropyridine receptor (DHPR) and ryanodine receptor (RyR). DHPR serves as a modest L-type Ca2+ channel but is primarily known for its function as a voltage sensor. DHPRs are located within invaginations of the sarcolemma called t-tubules, and are arranged into clusters of four known as tetrads. Each tetrad is positioned directly across from a single RyR, which are embedded within the membrane of the adjacent sarcoplasmic reticulum (SR). DHPRs contain four domains, each composed of six-transmembrane spanning segments. The S4 segment of each domain contains charged amino acid residues, and these residues respond to membrane depolarization by undergoing a conformational shift. This shift results in a proposed physical interaction of DHPR with RyR, causing RyR to open and release intracellular Ca2+ stores from the SR, allowing muscle contraction to occur (for review, see Melzer et al. 1995).
The decline in muscular strength with age, known as sarcopenia, is caused largely by a loss of total muscle mass - but also a disproportionate loss of strength. This loss of specific force (total force/cross sectional area) in old age (Brooks & Faulkner 1988; Gonzalez et al. 2000) is characterized in part by a deficit in Ca2+ release following depolarization (Delbono et al. 1995; Jimenez-Moreno et al. 2008), a phenomenon known as E-C uncoupling. E-C uncoupling is not a result of decreased Ca2+ stores or RyR release function (Jimenez-Moreno et al, 2008), and therefore may be caused by alterations in the functionality and expression of DHPR and its subunits with aging. The primary DHPR subunit in skeletal muscle is CaV1.1, previously known as DHPRα1s (Catterall et al. 2005). CaV1.1 is a large transmembrane protein which contains both the Ca2+ conducting pore and the voltage sensing S4 domain. Four other auxiliary subunits bind CaV1.1 to make up DHPR (for review, see Flucher et al. 2005), with the most widely studied being the cytosolic CaVβ1a subunit. CaVβ1a, a muscle specific member of the CaVβ family of proteins, binds to a region of the I–II intracellular loop of CaV1.1 known as the alpha interaction domain (AID) (Chen et al. 2004). CaVβ1a is classically described by its role in chaperoning CaV1.1 to the plasma membrane and regulating L-type Ca2+ current (Gregg et al. 1996; Strube et al. 1996; Beurg et al. 1997; Neuhuber et al. 1998). Most notably, E-C coupling cannot occur without CaVβ1a (Gregg et al. 1996). CaVβ1a binds to charged residues on RyR (Cheng et al. 2005) and neutralization of these residues reduces E-C coupling, suggesting a direct interaction with RyR. The correct organization of CaV1.1 into tetrads within the t-tubule membrane is also a specific function of the CaVβ1a isoform (Schredelseker et al. 2005).
Although classically known for augmenting the expression and function of CaV1 subfamily of calcium channels, the CaVβ family of subunits may contribute to the down-regulation of CaV1 as well. A family of Ras-related G-proteins (RGKs) mediate the down-regulation of several CaV1 isoforms in a CaVβ dependent manner (Beguin et al. 2001). Additionally, the previously uncharacterized SH3 domain of CaVβ was shown to bind dynamin and mediate endocytosis of CaV1.2 (Gonzalez-Gutierrez et al. 2007).
As previous studies have shown that the CaV1.1 subunit declines in old rodents (Renganathan et al. 1997; Moreno et al. 2006; O'Connell et al. 2008) and this causes an impairment of E-C coupling (Renganathan et al. 1997), we wanted to investigate what effects aging had on CaVβ1a expression, as this subunit is also critical for E-C coupling. We have found that CaVβ1a expression is highly increased in old mice, and that experimental overexpression of CaVβ1a reduces both the expression of CaV1.1 and specific force in dissociated single fibers of young mice. Additionally, siRNA inhibition of CaVβ1a restores charge movement in aged muscle. These findings suggest that overexpression of CaVβ1a with aging contributes to E-C uncoupling by reducing the level of CaV1.1.
RESULTS
CaVβ1a subunit expression increases with age
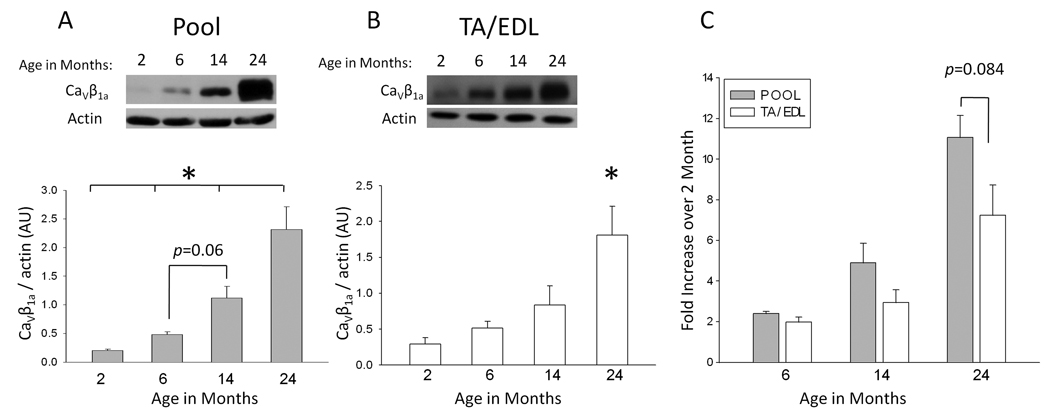
As our laboratory had earlier shown that CaV1.1 expression declines in old animals (Renganathan et al. 1997), we investigated whether CaVβ1a might also change with age. Hindlimb muscles from FVB mice were harvested at 2, 6, 14, and 24 months of age and subjected to a membrane extraction protocol (see Methods) for use in western blot analyses. Gluteus and hamstring muscles (designated “pool”) contain a mixture of type I (slow) and type II (fast) fiber types (Fig. 1A), while tibialis anterior (TA) and extensor digitorum longus (EDL) muscles contain only type II fibers (Manttari and Jarvilehto, 2005; Augusto et al., 2004) (Fig. 1B). Actin was used as a loading control as it appears to remain relatively stable throughout the lifespan of FVB mice. In order to precisely compensate for any variations in loading or possible global alterations in protein expression with aging, the optical density of each CaV1.1 band was divided by that of the corresponding actin band. The resulting values confirm a significant increase of CaVβ1a with age. Relative CaVβ1a expression increased continuously during aging, approximately doubling at each time point. This culminated in a substantial increase between very young and very old age (Fig. 1C) in both muscle groups, though the effect was less pronounced in the TA/EDL muscle groups (~7-fold), than in pool (~10-fold). The mean normalized intensity values (arbitrary units, AU) for each age group (n=4) are as follows: 0.20 ± 0.02 (2 months), 0.49 ± 0.06 (6 months), 1.14 ± 0.25 (14 months), and 2.34 ± 0.49 (24 months) for pool muscles and 0.29 ± 0.09 (2 months), 0.51 ± 0.1 (6 months), 0.84 ± 0.27 (14 months), and 1.81 ± 0.41 (24 months) for TA/EDL muscles.
Fig. 1. Relative CaVβ1a expression across the lifespan of FVB mice.
(A) Representative immunoblot of CaVβ1a (52 kDa) expression in P100 fractions collected from gluteus and hamstring muscles (pool) at 2, 6, 14, and 24 months of age (24 months represents the very old age of this strain). Below is the mean CaVβ1a band intensity for each age group (n=4), normalized to the intensity of the corresponding actin band. Each age group reached a statistically significant difference from all of the others, with the exception of 6 and 14 month age groups. Data represent mean ± SE. *P ≤ 0.01 (one-way ANOVA). (B) The same as A, except representative of TA and EDL muscle groups (n=4). The 24 month age group reached significant difference from all other ages. *P < 0.01(one-way ANOVA). (C) Mean fold increase of pool and TA/EDL samples by age. Values represent normalized sample values from each age group divided by the corresponding 2 month normalized value.
Overexpression of CaVβ1a results in decreased CaV1.1 expression
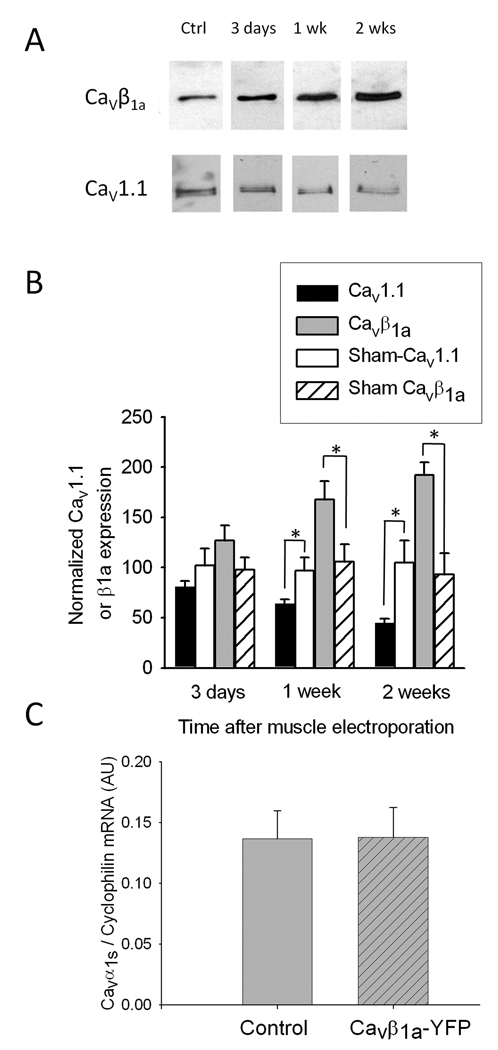
We next sought to determine if the observed increase in CaVβ1a expression was directly involved in the age-related decline in CaV1.1. In order to separate the effects of CaVβ1a overexpression from all other age-related changes, a CaVβ1a-YFP plasmid was electroporated into the TA muscle of young (4–5 month old) FVB mice in vivo. For these experiments we used TA because it is a superficial muscle suitable for electroporation in vivo, and large enough to provide tissue for protein analysis. Western blots of membrane fractions from electroporated muscles at 3 days (n=4), 7 days (n=3), and 2 weeks (n=4) confirm an increase in expression of endogenous CaVβ1a, which continued to rise during the two weeks following in vivo electroporation (Fig 2A). Conversely, CaV1.1 expression levels from the same samples declined steadily over the two week time course. Quantification of CaV1.1 band optical density shows an approximately 50% decrease compared to control at two weeks following CaVβ1a-YFP electroporation (Fig 2B). Sham electroporated muscles (n=4 for each time point) exhibited no significant changes in CaV1.1 or CaVβ1a expression relative to non-electroporated controls. Additionally, quantitative real time RT-PCR was used to assess CaV1.1 transcript levels at 2 weeks after CaVβ1a-YFP electroporation (Fig 2C). No difference was seen in CaVβ1a-YFP electroporated (n=4) vs. controls (n=3), suggesting that CaV1.1 down regulation during CaVβ1a overexpression occurs only at the protein level. These results are significant for two reasons. First, they show use of the CaVβ1a-YFP plasmid to artificially overexpress endogenous CaVβ1a in vivo. Second, CaVβ1a overexpression directly correlates to a decline in CaV1.1 protein levels in young mice, thus providing evidence for CaVβ1a involvement in CaV1.1 down-regulation; namely when present in high levels such as those observed during senescence.
Fig. 2. CaV1.1 and CaVβ1a expression in TA muscles following in vivo electroporation of a CaVβ1a-YFP plasmid.
(A) Representative immunoblots from control muscle (lane 1) and from muscles harvested 3 days (lane 2), 1 week (lane 3), and 2 weeks (lane 4) post-electroporation. (B) CaV1.1 and endogenous CaVβ1a protein expression in muscle following CaVβ1a-YFP electroporation (n=4 for 3 days and 2 weeks, n=3 at 1 week), normalized to control (non-electroporated) muscle. Sham electroporation (n=4 at each time point) produced no significant difference in relative CaV1.1 or CaVβ1a expression. Data represent mean ± SE. *P <0.001 (one-way ANOVA). (C) CaV1.1 mRNA levels measured using qRT-PCR in control (non electroporated, n=3) and CaVβ1a-YFP electroporated (2 weeks, n=4) animals.
CaVβ1a -YFP intensity corresponds to reduced charge movement
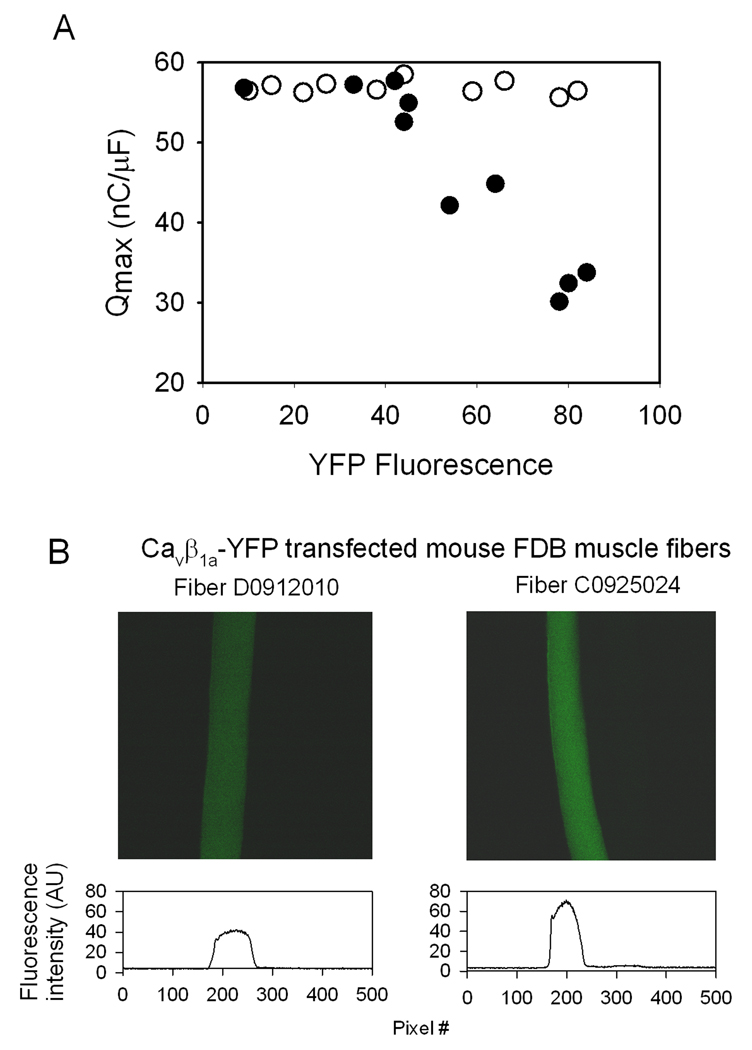
The intensity of YFP fluorescence (F, arbitrary units) varied between individual FDB fibers following in vivo electroporation with the CaVβ1a-YFP plasmid. Thus, the relative level of YFP intensity can be used to represent differences in individual fiber’s CaVβ1a-YFP expression level. Similarly, charge movement (Q) is a measure of single cell CaV1.1 expression at the plasma membrane (Wang et al. 2000; for review, see Rios & Pizarro 1991). Maximal charge movement (Qmax) was plotted against the corresponding fiber’s YFP intensity (Fig 3A). The resulting plot shows an inverse relationship between CaVβ1a-YFP fluorescence level and CaV1.1 membrane expression (r=0.91, n=10), where electroporated fibers with high YFP fluorescence (F > 40 AU) show increasingly impaired Qmax. Fibers with relatively low YFP fluorescence (F ≤ 40 AU) are presumed to exhibit minimal overexpression of CaVβ1a -YFP, and subsequently showed no loss of Qmax. Fibers from muscles electroporated with a GFP plasmid (n=10) showed no difference in Qmax, regardless of relative F intensity. Figure 3B and Figure 4 display an example of two fibers with different YFP intensity, and the resulting difference in Q. Fiber D0912, a fiber with lower CaVβ1a -YFP expression (F=42 AU) exhibited unimpaired maximal charge movement (Qmax =52.6 nC/µF). Conversely, fiber C0925 showed high levels of CaVβ1a -YFP (F=71.3 AU) and substantially reduced maximal charge movement (Qmax =26.6 nC/µF). While the absolute Q-V relationship was reduced in fiber C0925 and others showing high AU (Fig. 4D), the relative Q-V relationship was unchanged (Fig. 4E). Therefore overexpression of CaVβ1a -YFP does not shift the Q-V curve in the voltage axis, reaffirming the notion that the effects of CaVβ1a-YFP are on CaV1.1 membrane expression and not other alterations of the channel’s function.
Fig. 3. CaV1.1 charge movement (Q) and YFP (CaVβ1a -YFP) fluorescence (F) in electroporated FDB fibers.
(A) Qmax-F relationship of single fibers electroporated with CaVβ1a –YFP (●)(n=10) or GFP (○)(n=10). Detection of F above ~40 AU correlates with decreased charge movement (r2=0.91). (B) Top - two representative fibers from A. Fiber D0912 exhibits F=42 AU, Q=52.6 nC/µF. Fiber C0925 exhibits F=71.3 AU, Q=26.6 nC/µF. (Also see Fig. 4 for more detail on these two fibers). Fluorescence acquisition parameters on confocal microscope: 20x objective, zoom = 3.5x, Iris = 3.8mm, Gain = 80, box size = 512×512 pixels (172.6×172.6 µm), pixel dwell time 37.76 µs. Excitation wavelength = 488 nm, Emission = 528 nm. Bottom – Illustration of the fluorescence intensity across the fibers.
Fig. 4. Charge movement from FDB fibers expressing CaVβ1a-YFP.
(A – B) Charge movement recordings from fiber D0912 (A), representing a fiber expressing low levels of CaVβ1a -YFP (indicated by F intensity in Figure 3), and from fiber C0925 (B), representing a fiber expressing high levels of CaVβ1a-YFP (indicated by F intensity in Figure 3). (C) The voltage step protocol. The holding potential was −80 mV. A 2-sec pre-pulse at −30 mV was followed by a 15-ms repolarization to −50 mV, followed by a 25-ms test pulse of varying membrane voltage and repolarization to −50 mV. (D) Absolute Q-V relationship of fibers D0912 (●) and C0925 (○). Note that the fiber which expresses higher levels of CaVβ1a-YFP displays lower charge movement. (E) Relative Q-V relationship of the same fibers in D. Q values were normalized to Qmax for each fiber. Overexpression of CaVβ1a -YFP does not shift the Q-V curve in the voltage axis or modify the steepness of the curve. Data points were fitted to a Boltzmann equation of the form: Q = Qmax/ [1+ exp(V1/2 − Vm)/k], where Qmax is the maximal charge; V1/2 is the charge half-activation potential; Vm is the membrane potential; and k is the steepness of the curve. Qmax, V1/2, and K values were: 56.9 nC/µF, 12.3 mV and 3.37 for fiber D0912, and 30.2 nC/µF, 17.1 mV, and 14.9 for fiber C0925.
CaVβ1a -YFP causes a decline in specific force
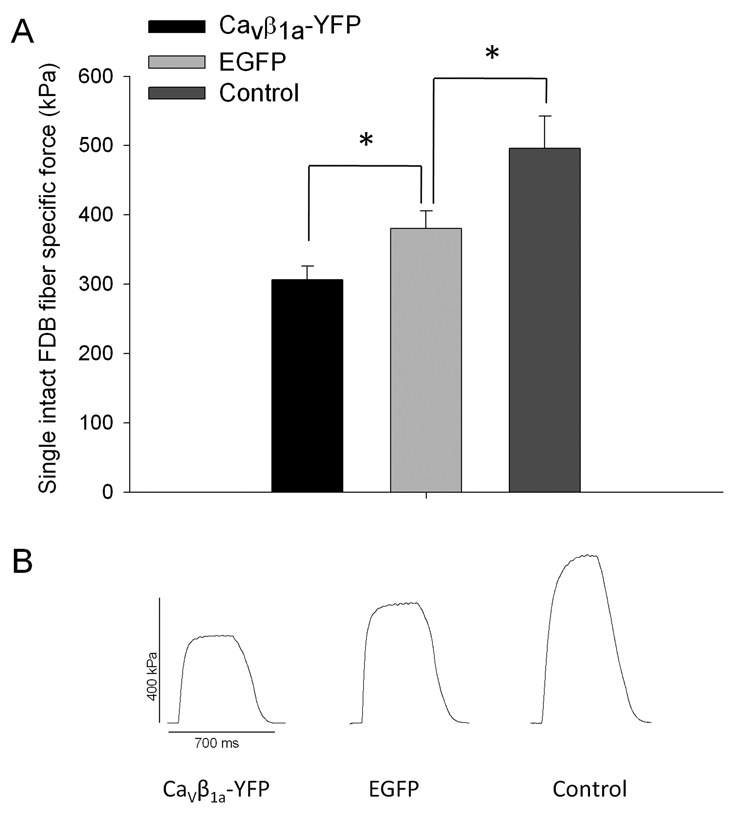
Loss of muscle strength with aging is thought to be partially caused by reduction of CaV1.1, specifically those coupled to RyR in the T-tubule membrane, thereby resulting in an impairment of E-C coupling. As CaVβ1a overexpression lead to a marked reduction CaV1.1, shown by both western blot and charge movement studies, it seemed likely that CaVβ1a overexpression would also result in a loss of specific force. Tetanic specific force (kPa) was measured 14 days after single fibers from FDB muscles were electroporated in vivo with either CaVβ1a-YFP (n=7), GFP (n=7), or non-electroporated (n=3) (Fig 5A). The electroporation process itself causes short-term damage to the muscle fiber in the days immediately following the procedure (Schertzer et al. 2006) and as such the GFP group showed a significant reduction in specific force compared to controls. However, the CaVβ1a-YFP group showed an even greater and statistically significant decline in specific force compared to the YFP electroporated fibers. Figure 5B shows representative tetanic contraction traces from CaVβ1a-YFP, GFP, and control groups. These results support the idea that overexpression of CaVβ1a impairs single fiber specific force by reducing the amount of CaV1.1 expressed at the plasma membrane. No significant difference was found on average time to peak torque (ms), or half relaxation time (ms), between control, GFP, and CaVβ1a-YFP electroporated groups (data not shown), indicating no alterations in calcium buffering activity or rate of cross bridge formation. Twitch specific force (kPA) appeared to decline in CaVβ1a-YFP electroporated fibers compared to GFP and non-electroporated controls, although the reduction did not reach significance (p=0.081).
Fig. 5. Specific force of single intact FDB fibers from young mice electroporated and expressing CaVβ1a-YFP or GFP.
(A) Specific force (kPa) of single intact FDB fibers from CaVβ1a-YFP (n=7), GFP (n=7) and non-electroporated controls (n=3). CaVβ1a-YFP expressing fibers display significantly lower specific force compared to GFP expressing fibers. Data represent mean ± SE. *P<0.05 (one-way ANOVA) (B) Representative tetanic contraction traces from fibers expressing CaVβ1a–YFP, GFP, and control groups.
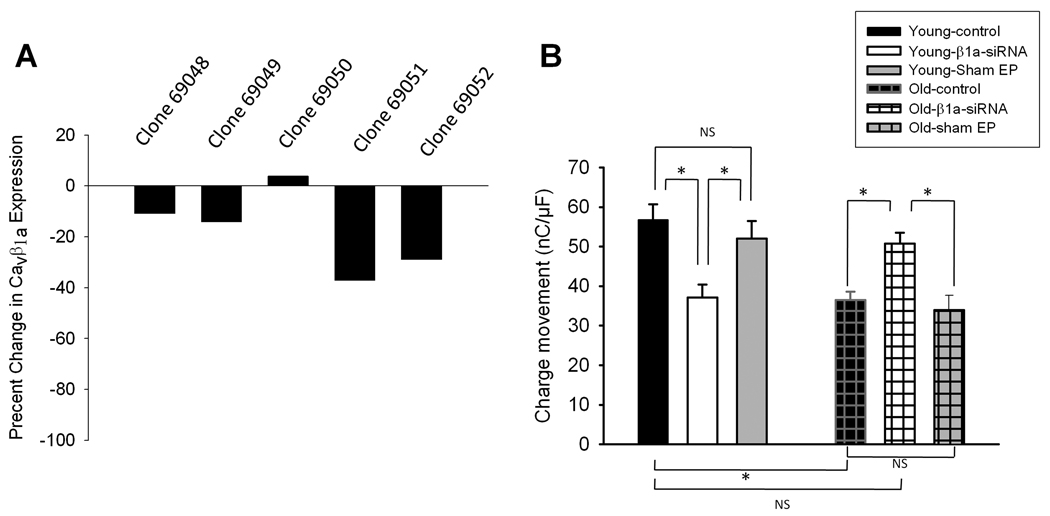
siRNA inhibition of CaVβ1a
If overexpression of CaVβ1a is responsible for muscle impairment with aging, then inhibition of CaVβ1a may provide a way to reverse these effects. We created several siRNA sequences against CaVβ1a (Fig 6A). The effectiveness of these sequences was determined by transfecting each of them into separate C2C12 cell cultures and performing western blot analyses on the harvested cell lysates. Each sample was probed for CaVβ1a, and the optical density of each band was compared to that of a control, untransfected sample. Clones 69051 and 69052 caused the greatest decline in CaVβ1a expression (37% and 28% decline, respectively). Both of these clones were then electroporated in vivo into the FDB muscle of young and old FVB mice (Fig. 6B). In young animals, siRNA against CaVβ1a caused a significant reduction of charge movement in dissociated FDB cells, recorded 7–11 days post electroporation. As demonstrated in Fig 3, electroporation of GFP alone does not result in a loss of charge movement. Old (24 month) mice naturally exhibit reduced charge movement, which was confirmed by our results. Interestingly, siRNA against CaVβ1a restored charge movement of old mice to young control levels. Sham electroporation of old muscles did not result in a further decline in charge movement, suggesting that the deleterious effect of electroporation on force reported above is due primarily to damage to contractile proteins and not due to reduced Cav1.1 expression.
Fig. 6. siRNA restoration of charge movement in vivo.
(A) Effect of five different siRNA sequences on CaVβ1a expression in C2C12 myotubes. Data are presented as percent change in immunoblot band intensity versus control. Clones 69051 and 69052 cause the greatest decline in endogenous CaVβ1a expression (37% and 28% decline, respectively). (B) Charge movement from FDB muscle fibers 7–11 days post-electroporation. FDB muscles were electroporated with 20 µg of siRNA sequence 69051 and 10 µg of siRNA sequence 69052. Fibers were dissociated, patch-clamped, and subjected to the same protocol as in Figure 4. The number of fibers examined for young control, sham GFP-electroporated and CaVβ1a siRNA electroporated was 13, 10, and 17, respectively, and for old control, CaVβ1a siRNA and sham electroporated was 21, 16 and 11, respectively. Data represent mean ± SE. *P < 0.05 (one-way ANOVA).
DISCUSSION
Here we show that CaVβ1a is significantly overexpressed in aging muscle, and present a model by which this phenomenon may contribute to loss of specific force with aging. In young mice, overexpression of CaVβ1a corresponds to decreased CaV1.1 expression at the sarcolemma, as shown by both western blot and charge movement studies. CaVβ1a overexpression also results in a loss maximal specific force, presumably by reducing the number of CaV1.1 channels in the t-tubule membrane coupled to RyRs. Because CaV1.1 subunits are critical for the transduction of sarcolemmal depolarizations into Ca2+ release from the SR, a decreased number of CaV1.1 subunits in the membrane would cause less Ca2+ to be released from intracellular stores, resulting in weakened contractions (Delbono, 2002). To further implicate overexpression of CaVβ1a directly with decreased CaV1.1, we show that using siRNA to partially inhibit CaVβ1a in old muscle restores charge movement to young control levels. Expectedly, inhibition of CaVβ1a in young muscle fibers significantly reduced charge movement. Thus, both the under and overexpression of CaVβ1a results in reduced expression of CaV1.1.
CaVβ1a Overexpression with Aging
While the most striking increase in CaVβ1a expression appears to be between middle aged and very old mice, the rate of CaVβ1a increase is surprisingly uniform, with the normalized expression level roughly doubling at each time point. Relative CaVβ1a level in very young mice is substantially lower than the level seen even at 6 months of age (young adulthood). Thus, increasing CaVβ1a expression may also be a necessary component of muscle development. It is therefore tempting to speculate that the extreme levels of CaVβ1a seen in very old animals may reflect a developmental program hyperfunction, a common attribute of aging (Blagosklonny, 2006). Interestingly, the rate of increase of CaVβ1a expression did not seem to be as high in the TA/EDL muscle group compared to that of the Pool group. TA and EDL muscles are of exclusively type II composition, while there is a mixture of type I and type II fibers found in the gluteus and hamstring muscles used for our pool group. The possible resistance to CaVβ1a overexpression seen in the type II muscle group is perplexing, as type I fibers are thought to be more resistant to age related changes such as denervation (for review, see Larsson, 1995). One possible explanation of this could be due to the increased cell turnover seen in type II fibers, as indicated by increased apoptotic signaling (Phillips and Leeuwenburgh, 2005) and depletion of satellite cells with age (Verdijk et al., 2007) compared to type I fibers, thus somewhat limiting the accumulation of CaVβ1a with age. However, the mouse is not a preferred model to examine fiber type due to the lack of pure type I muscles as seen in the rat, and thus any conclusions on the fiber type-specific rate of CaVβ1a increase are limited. Still, the notion that CaVβ1a is highly overexpressed in both fiber types is strongly supported by these experiments.
The two most likely explanations of CaVβ1a overexpression with age are an increase in its transcription levels, or a failure in its ability to be properly degraded. In regards to the latter, impairment of proteolysis is already known to be a hallmark of aging muscle (for review, see Combaret et al. 2009). Further experiments examining CaVβ1a mRNA levels during aging should shed light on whether transcriptional upregulation plays any role. These explanations are not mutually exclusive and indeed it would seem probable that both contribute to the accumulation of CaVβ1a in old muscle. Although we present a model by which CaVβ1a overexpression causes deleterious effects on muscle function by reducing the number of voltage sensing CaV1.1 subunits in the t-tubule membrane, the relative expression of CaVβ1a in old muscle is so high that it may also interfere with other key processes in a nonspecific manner. CaVβ subunits contain two conserved protein interaction domains: GK and SH3 (Chen et al., 2004) and have recently been shown to interact with several other proteins besides CaV1, such as RGKs (kir/Gem, Rad, Rem), dynamin (for review, see Hidalgo and Neely, 2007), chromobox protein 2/heterochromatin protein 1γ (Hibino et al., 2003), and Akt (Catalucci et al., 2009). It is therefore possible that CaVβ1a may interact with additional proteins in a currently uncharacterized manner.
Potential mechanisms of CaVβ1a involvement in CaV1.1 down-regulation
The notion that CaV1.1 expression declines during old age is supported by several studies (Renganathan et al.1997; Ryan et al. 2000; Moreno et al. 2006; Wang et al. 2007; O’Connell et al. 2008). Importantly, CaV1.1 mRNA does not decrease significantly with age (Zheng et al. 2001), implicating some other mechanism responsible for the decline in its protein level. Here we demonstrate that CaV1.1 expression also declines following induced overexpression of CaVβ1a. Thus, CaVβ1a overexpression, both during natural aging and experimental overexpression in young cells, coincides with a decline in CaV1.1 expression. While we present evidence that CaVβ1a overexpression causes a decline of CaV1.1 at the protein level, the precise mechanism behind this occurrence is not known. CaV1.1 mRNA does not decline in young mice following CaVβ1a overexpression, suggesting CaVβ1a does not regulate CaV1.1 gene expression, in agreement with the aforementioned aging studies on CaV1.1 mRNA. Due to the multiple regulatory functions that CaVβ1a exerts on CaV1.1, both classically and those discovered more recently, there are several hypothetical mechanisms by which overexpression of CaVβ1a could lead to a reduction of CaV1.1. The two most important classical functions of CaVβ1a in skeletal muscle are increasing the trafficking of newly formed CaV1.1 subunit to the t-tubule membrane (Neuhuber et al. 1998; Bichet et al. 2000), and arranging DHPRs into orthogonal arrays, or tetrads (Schredelseker et al. 2005), which are presumed to be necessary for skeletal muscle E-C coupling. Interfering with tetrad formation is one possible way that CaVβ1a overexpression could reduce CaV1.1 insertion into the membrane. Tetrads are critical for the precise alignment of DHPRs with RyR, which is required for proper transduction during E-C coupling. Because CaVβ1a also binds to RyR (Cheng et al. 2005), it seems possible that overexpression of CaVβ1a could interfere with the alignment and coupling of DHPR tetrads to RyR. Also, our group in collaboration with others has found that CaVβ1a interacts with the junctional protein JP-45 (Anderson et al. 2006). Like CaVβ1a, JP-45 is localized to the t-tubule/SR junction (triad) and interacts with CaV1.1 via the AID. In co-immunoprecipitation experiments, treatment with exogenous purified CaVβ1a reduces the ability of JP-45 to pull down CaV1.1, suggesting CaVβ1a interferes with this interaction. As JP-45 KO mice have impaired muscle strength due to reduced levels of CaV1.1 (Delbono et al. 2007), disruption of the JP-45-CaV1.1 interaction associated with excess CaVβ1a is another possible mechanism by which CaVβ1a overexpression reduces the level of CaV1.1 at the membrane. Disruption of the DHPR tetrad – RyR complex, through any of the mechanisms mentioned above, could in turn make CaV1.1 more susceptible to endocytosis and proteolytic degradation.
Much attention has been given recently to the involvement of the RGK family of Ras-related GTP-binding proteins in CaV1 channel function and expression. CaVβ isoforms are necessary for the RGK (kir/Gem) mediated down-regulation of CaV1.2 (Beguin et al. 2001), and CaVβ2a forms a trimeric complex with the RGK Rem and the CaV1 AID (Finlin et al., 2006). In skeletal muscle, overexpression of Rem leads to a decline in the number of CaV1.1 channels in the membrane (Bannister et al. 2008). Thus overexpression of CaVβ1a may contribute to CaV1.1 down-regulation via interaction with RGKs, although the necessity for up-regulation of CaVβ1a itself is unclear. Interestingly, Beguin and colleagues (2006) have recently shown that the RGKs Rad and Rem may sequester CaVβ3 subunits into the nucleus in a calmodulin and 14-3-3 regulated manner. A previous study by Colecraft et al. (2002) also showed the presence of CaVβ1b, CaVβ2a, and CaVβ4 isoforms in the nucleus of cardiomyocytes. In additional to nuclear localization, Hibino et al. (2003) demonstrated that the CaVβ4 splice variant CaVβ4c acts as a transcriptional regulator by binding to and inhibiting the gene silencing ability of the nuclear protein CHCB2/HP1γ. Although we found no influence of CaVβ1a on CaV1.1 mRNA levels, based on these observations it is worth speculating on the possibility of CaVβ1a acting as transcription factor, perhaps even in a self regulating fashion.
The GK domain of CaVβ subunits binds with high affinity to the AID of CaV1 channels (Chen et al.2004). While this is the consensus binding site of the classically defined CaV1- CaVβ complex, there is evidence that an additional CaVβ subunit can bind to a region on the C-terminus of CaV1 channels (in addition to the high affinity AID) and that this secondary binding modulates the functional properties of CaV1 (Qin et al. 1996; Birnbaumer et al. 1998; Gerster et al. 1999; Canti et al. 2001; García et al. 2005). The question of CaV1 containing multiple CaVβ binding sites is particularly relevant to our present results. Colecraft’s group present model (Dalton et al. 2005) in which CaVβ initially traffics CaV1 to the plasma membrane, then either remains bound to the AID or dissociates, producing two populations of Cav1 with either high (CaVβ associated) or low (CaVβ-less) gating activity. However Colecraft’s model does not exclusively rule out the possibility of one or more additional binding sites on CaV1. The possibility of CaV1 containing multiple CaVβ binding sites of differing affinities offers a logical explanation for the pleiotropic effect of CaVβ1a shown here. If CaVβ1a is present in high concentrations (eg; exogenously applied or due to overexpression with aging), it may then be able to bind the proposed secondary, low affinity binding site with increasing frequency. In additional support of this hypothesis, the SH3 domain of CaVβ2 has recently been shown to interact with dynamin to promote endocytosis of CaV1.2 (Gonzalez-Guiterrez et al,. 2007). Interestingly, this group found that disruption of the AID was necessary for the CaVβ2-dynamin mediated endocytosis, further supporting the possibility that the presence of CaVβ at a secondary binding site results in CaV1 endocytosis. Alternatively, rather than directly mediating channel endocytosis via protein-protein interactions, CaVβ overexpression may alter CaV1 expression indirectly via Ca2+ dependent mechanisms. Garcia et al. (2005) showed that pressure injection of purified CaVβ1a into dissociated muscle fibers produces a rapid increase in both L-type Ca2+ current and intracellular Ca2+ release, without altering charge movement. Endogenous overexpression of CaVβ1a in the short term may produce similar physiological effects. In turn, a chronic increase in intracellular Ca2+ may activate some form of negative feedback, perhaps culminating in CaV1.1 endocytosis and proteolysis. Indeed, Sanchez’s group has also shown that long-term increase of L-type Ca2+ current in skeletal muscle leads to proteolytic down-regulation of CaV1.1, likely via a local Ca2+-dependent protease such as calpain (Carrillo et al. 2004).
Sarcopenia is a major cause of loss of independence in the elderly and presents a substantial public health cost (Janssen et al. 2004). As sarcopenia is caused in part by muscle dysfunction beyond the obvious loss in mass, E-C uncoupling may be a significant contributor to sarcopenia in aging humans. E-C uncoupling appears to be primarily caused by a decline in functional CaV1.1 subunits, which leads to an impairment of the mechanical coupling between neural signals and Ca2+ release necessary for contraction. Here we have shown that another protein, CaVβ1a, with several regulatory influences on CaV1.1, also exhibits significant changes in expression level with aging. Combined with evidence that CaVβ1a overexpression causes CaV1.1 decline in young muscle, our findings present a potentially novel and physiologically significant contributing factor to the loss of skeletal muscle strength with aging.
Experimental Procedures
Animals
Muscles were dissected from FVB (Friend Virus B, our colony) mice between 1.5 and 24 months of age. FVB mice have a maximum lifespan of 25 months and have been used previously as a model of aging skeletal muscle in our laboratory (Renganathan et al. 1998; Payne et al. 2004). Animals were housed at Wake Forest University School of Medicine (WFUSM). Mice were killed by cervical dislocation. Animal handling and procedures were approved by the Animal Care and Use Committee of WFUSM.
Microsome preparation
Isolation of the t-tubule membrane was performed using a modified version of the protocol by (Knudson et al. 1989). Briefly, whole muscles were dissected and pulverized in liquid nitrogen and then homogenized in ice-cold Buffer A (20 mM sodium pyrophosphate, 20 mM sodium phosphate monobasic, 1 mM MgCl2, 0.5 mM EDTA, 303 mM sucrose with complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA) using a handheld tissue tearor. Homogenate was centrifuged at 7,000 g for 20 min at 4°C, and the pellet discarded. Supernatant was filtered through four layers of cheesecloth and centrifuged at 100,000 g for 90 min at 4°C in a Beckman Type Ti.70i rotor. The pellet was rinsed with ice cold PBS and resuspended with a glass homogenizer in fresh Digitonin buffer (1% digitonin (w/v), 185 mM KCl, 1.5 mM CaCl2, 10 mM HEPES pH 7.4 with complete protease inhibitor cocktail). Samples were left on ice for 1h and then vortexed. Protein concentration was measured using bicinchoninic protein assay using BSA digitonin standards.
Antibodies
Primary antibodies used for immunoblots were monoclonal VD21 to CaVβ1a (Developmental Studies Hybridoma Bank, Iowa City, IA, USA), monoclonal IIF7 to CaV1.1; a generous gift from Dr. Kevin P. Campbell of the University of Iowa, and actin (Chemicon International, Temecula, CA, USA). NA931V goat anti-mouse (Amersham Health, Little Chalfont, Buckinghamshire, UK) was used as a secondary antibody.
Western blots
For CaVβ1a subunit, microsomes were mixed with equal volume β-mercaptoethanol buffer and boiled for 5 minutes. For CaV1.1, microsomes were mixed with equal volume double strength Urea buffer and incubated at room temperature for 30 min (Murray & Ohlendieck 1998).SDS-PAGE was conducted using a 4.5 % stacking gel with a 10% resolving gel in a Mini-Protean gel system (BioRad Laboratories, Hemel-Hemptstead, Herts., UK). Gels were transferred to PVDF membranes (Amersham Health, Little Chalfont, Buckinghamshire, UK) overnight at 4°C. Blots were blocked in 5% non-fat dry milk with 0.1% Tween in TBS for CaVβ1a and PBS for all other antibodies. Primary antibody concentrations were as follows: 1:1000 (CaVβ1a), 1:5000 (CaV1.1) and 1:250,000 for actin. Secondary antibodies were used at a 1:5000 dilution. Band intensity was measured using Kodak Gel Doc imaging system.
Quantitative Real Time RT-PCR
Total RNA was isolated using TriReagent according to the manufacturers protocol (Molecular Research Center, Cincinnati, OH), treated with DNAse I (New England Biolabs, Ipswich, MA) and reverse transcribed to cDNA using random hexamers and reverse transcriptase (Promega, Madison, WN) according to manufacturers protocol. Following reverse transcription, cDNA expression was assessed by quantitative real time RT-PCR (Taqman Gene Expression Master Mix kit, Applied Biosystems, Foster City, CA, USA) on a Stratagene MX3000P. Primers and probes for CaV1.1 were purchased from Applied Biosystems.
Muscle Electroporation
Intramuscular plasmid injection and electroporation were performed according to Schertzer et al. (2006) and DiFranco et al. (2006). Briefly, FDB or TA muscles were injected with 30 Al of 0.5 U/Al hyaluronidase and injected 1 hr later with 20 µg CaVβ1a–YFP or 20 µg CaVβ1a siRNA equal volume saline solution. A pair of platinum plate electrodes was placed under the skin on adjacent sides of the muscle. Eight, 150 V, 20-ms square-wave pulses of 1-Hz frequency were generated using a Grass stimulator (Grass S48; W. Warwick, RI, USA) and delivered to the muscle. The polarity was then reversed and a further 8 pulses were delivered to the muscle. For sham electroporations, the same protocol was followed with a saline-only injection.
siRNA sequences
C2C12 cells were transfected with five siRNA sequences (Open Biosystems, Huntsville, AL, USA) using FuGENE 6 (Roche Diagnostics, Indianapolis, IN, USA). The source of shRNA has accession # NM_031173. The following five siRNA sequences were used:
Seq. 69048: CCGGCCAGTGGTAATGAAATGACTACTCGAGTAGTCATTTCATTACCACTGGTTTTTG
Seq. 69049: CCGGCCCAGCAAACACATCATCATTCTCGAGAATGATGATGTGTTTGCTGGGTTTTTG
Seq. 69050: CCGGCGAGGGAAGTCTCAATCCAAACTCGAGTTTGGATTGAGACTTCCCTCGTTTTTG
Seq. 69051: CCGGCCTCGGATACAACATCCAACACTCGAGTGTTGGATGTTGTATCCGAGGTTTTTG
Seq. 69052: CCGGGCTCAGGAGAAATCTCAGCTTCTCGAGAAGCTGAGATTTCTCCTGAGCTTTTTG
Charge movement recordings
Enzymatically dissociated flexor digitorum brevis (FDB) fibers were transferred to a small, flow-through Lucite chamber positioned on a microscope stage. Fibers were continuously perfused with the external solution using a push-pull syringe pump (WPI). Only fibers exhibiting a clean surface and no contracture were used for electrophysiological recordings. Muscle fibers were voltage-clamped using an Axopatch-200B amplifier (Molecular Devices, Sunnyvale, CA, USA) in the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981; Wang et al. 1999). Patch pipettes were pulled from borosilicate glass (Boralex, WPI, Sarasota, FL, USA) using a Flaming Brown micropipette puller (P97, Sutter Instrument Co., Novato, CA, USA) and then fire-polished to obtain electrode resistances ranging from 450 to 650 kΩ. In the cell-attached configuration, the seal resistance was in the range of 1–4.5 GΩ, and in the whole-cell configuration, values ranged between 75 and 120 MΩ (Wang et al. 1999). The pipette was filled with the following solution: 140 mM Cs-aspartate, 5 mM Mg-aspartate2, 20 mM Cs2EGTA (ethylene glycol-bis(aminoethyl ether)-N,N,N’N’-tetraacetic acid), and 10 mM HEPES (N-[2-hydroxyethyl]piperazine-N’-[2-ethanesulfonic acid]), and pH was adjusted to 7.4 with CsOH (Adams et al., 1990; Wang et al., 1999). The external solution contained: 150 mM TEA(tetraethylammonium hydroxide)-CH3SO3, 2 mM MgCl2, 2 mM CaCl2, 10 mM Na-HEPES, 0.05 mM BTS(N-benzyl-p-toluenesulfonamide), and 0.001 mM tetrodotoxin (Delbono 1992; Delbono et al. 1997). Solution pH was adjusted to 7.4 with CsOH. All the experiments were conducted at room temperature (21–22°C).
Confocal microscopy
FDB fibers fluorescence was analyzed using a Radiance 2100 confocal microscope (Bio-Rad/Zeiss, Thornwood, NY, USA). YFP fluorescence was detected at 488nm. Confocal microscope fluorescence acquisition parameters were maintained constant across recordings are described above.
Single Intact Muscle Fiber Contraction
At time of sacrifice, FDB muscles were carefully dissected and pinned into a Petri dish lined with Sylgard (Dow Corning, Auburn, MI, USA) in a Ca2+-containing physiological solution (see below). All contraction experiments were carried out at room temperature (21–22°C). Single intact fiber dissection followed procedures previously published (Lannergren & Westerblad 1987; Gonzalez et al. 2000). Following dissection, tendons of single intact fibers were placed in custom-made micro-clips, and these clips were connected to a force transducer and a micropositioner for length control. Fibers were adjusted to optimum length (LO) by using single twitches, elicited by 0.5-ms square wave pulses at 10 V. Once at LO, fibers were stimulated with 350-ms trains of pulses, using frequencies varying from 50 to 100 Hz. The stimulation frequency that elicited maximum force was used for the remainder of the experiment (Payne et al. 2004).
Statistical analysis
All data are presented as means ± SE. Data were analyzed with Student t-test or one-way repeated measures ANOVA, with Tukey’s multiple comparisons test applied post hoc when appropriate. An alpha value of P < 0.05 was considered significant.
ACKNOWLEDGMENTS
The present study was supported by grants to Osvaldo Delbono from the National Institutes of Health/National Institute on Aging (AG13934, AG033385, and AG15820), the Muscular Dystrophy Association (MDA #33149), and the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332). The authors would like to thank Dr. Kurt G. Beam (University of Colorado Health Sciences, Department of Physiology and Biophysics) for providing the CaVβ1a–YFP plasmid, Dr. Franz Hofmann (University of Saarland, Pharmacology and Toxicology) for the initial cDNA sequence of CaVβ1a, and Dr. Hang Shi and Dr. Juan Codina for their expert assistance with the qRT-PCR experiments.
Footnotes
AUTHOR CONTRIBUTIONS
O.D. designed research; J.R.T., Z-M.W., Z.Z., M.L.M., A.M.P. and O.D. performed research; J.R.T., Z-M.W., Z.Z., A.M.P. and O.D. analyzed data, and J.R.T. and O.D. wrote the paper.
REFERENCES
- Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Anderson AA, Altafaj X, Zheng Z, Wang ZM, Delbono O, Ronjat M, Treves S, Zorzato F. The junctional SR protein JP-45 affects the functional expression of the voltage-dependent Ca2+ channel Cav1.1. J Cell Sci. 2006;119:2145–2155. doi: 10.1242/jcs.02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto V, Padovani CR, Campos GER. Skeletal muscle fibertypes in C57BL6J mice. Braz J Morphol Sci. 2004;21:89–94. [Google Scholar]
- Bannister RA, Colecraft HM, Beam KG. Rem Inhibits Skeletal Muscle EC Coupling by Reducing the Number of Functional L-Type Ca2+ Channels. Biophysical Journal. 2008;94:2631–2638. doi: 10.1529/biophysj.107.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Ikeda H, Yamada Y, Seino Y, Hunziker W. Nuclear sequestration of beta-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J Mol Biol. 2006;355:34–46. doi: 10.1016/j.jmb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- Beurg M, Sukhareva M, Strube C, Powers PA, Gregg RG, Coronado R. Recovery of Ca2+ current, charge movements, and Ca2+ transients in myotubes deficient in dihydropyridine receptor beta 1 subunit transfected with beta 1 cDNA. Biophys J. 1997;73:807–818. doi: 10.1016/S0006-3495(97)78113-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. The I–II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel beta subunits. J Bioenerg Biomembr. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canti C, Davies A, Berrow NS, Butcher AJ, Page KM, Dolphin AC. Evidence for two concentration-dependent processes for beta-subunit effects on alpha1B calcium channels. Biophys J. 2001;81:1439–1451. doi: 10.1016/S0006-3495(01)75799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo E, Galindo JM, Garcia MC, Sanchez JA. Regulation of muscle Cav1.1 channels by long-term depolarization involves proteolysis of the alpha1s subunit. J Membr Biol. 2004;199:155–161. doi: 10.1007/s00232-004-0683-x. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- Cheng W, Altafaj X, Ronjat M, Coronado R. Interaction between the dihydropyridine receptor Ca2+ channel beta-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proc Natl Acad Sci U S A. 2005;102:19225–19230. doi: 10.1073/pnas.0504334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca2+ channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Bechet D, Taillandier D, Mosoni L, Attaix D. Skeletal muscle proteolysis in aging. Curr Opin Clin Nutr Metab Care. 2009;12:37–41. doi: 10.1097/MCO.0b013e32831b9c31. [DOI] [PubMed] [Google Scholar]
- Dalton S, Takahashi SX, Miriyala J, Colecraft HM. A single CaV{beta} can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J Physiol. 2005;567:757–769. doi: 10.1113/jphysiol.2005.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O. Calcium current activation and charge movement in denervated mammalian skeletal muscle fibres. J Physiol. 1992;451:187–203. doi: 10.1113/jphysiol.1992.sp019160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O, O'Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995;148:211–222. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- Delbono O, Renganathan M, Messi ML. Regulation of mouse skeletal muscle L-type Ca2+ channel by activation of the insulin-like growth factor-1 receptor. J Neurosci. 1997;17:6918–6928. doi: 10.1523/JNEUROSCI.17-18-06918.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O. Molecular mechanisms and therapeutics of the deficit in specific force in ageing skeletal muscle. Biogerontology. 2002;3:265–270. doi: 10.1023/a:1020189627325. [DOI] [PubMed] [Google Scholar]
- Delbono O, Xia J, Treves S, Wang ZM, Jimenez-Moreno R, Payne AM, Messi ML, Briguet A, Schaerer F, Nishi M, Takeshima H, Zorzato F. Loss of skeletal muscle strength by ablation of the sarcoplasmic reticulum protein JP45. Proc Natl Acad Sci U S A. 2007;104:20108–20113. doi: 10.1073/pnas.0707389104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco M, Neco P, Capote J, Meera P, Vergara JL. Quantitative evaluation of mammalian skeletal muscle as a heterologous protein expression system. Protein Expr Purif. 2006;47:281–288. doi: 10.1016/j.pep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Finlin BS, Correll RN, Pang C, Crump SM, Satin J, Andres DA. Analysis of the complex between Ca2+ channel beta-subunit and the Rem GTPase. J Biol Chem. 2006;33:23557–23566. doi: 10.1074/jbc.M604867200. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Obermair GJ, Tuluc P, Schredelseker J, Kern G, Grabner M. The role of auxiliary dihydropyridine receptor subunits in muscle. J Muscle Res Cell Motil. 2005;26:1–6. doi: 10.1007/s10974-005-9000-2. [DOI] [PubMed] [Google Scholar]
- Garcí Carrillo E, Galindo JM, Hernálndez A, Copello JA, Fill M, Sánchez JA. Short-Term Regulation of Excitation-Contraction Coupling by the [beta]1a Subunit in Adult Mouse Skeletal Muscle. Biophysical Journal. 2005;89:3976–3984. doi: 10.1529/biophysj.105.067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster U, Neuhuber B, Groschner K, Striessnig J, Flucher BE. Current modulation and membrane targeting of the calcium channel alpha1C subunit are independent functions of the beta subunit. J Physiol. 1999;517(Pt 2):353–368. doi: 10.1111/j.1469-7793.1999.0353t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gutierrez G, Miranda-Laferte E, Neely A, Hidalgo P. The Src homology 3 domain of the beta-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J Biol Chem. 2007;282:2156–2162. doi: 10.1074/jbc.M609071200. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol. 2000;178:175–183. doi: 10.1007/s002320010025. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell JA, Coronado R, Powers PA. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation-contraction coupling. Proc Natl Acad Sci U S A. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Rousset M, Charnet P, Hudspeth AJ, Lesage F. Direct interaction with a nucear protein and regulation of gene silencing by a variant of the Ca 2+ -channel Î2 4 subunit. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:307–312. doi: 10.1073/pnas.0136791100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo P, Neely A. Multiplicity of protein interactions and functions of the voltage-gated calcium channel beta-subunit. Cell Calcium. 2007;42:389–396. doi: 10.1016/j.ceca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Moreno R, Wang ZM, Gerring RC, Delbono O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J. 2008;94:3178–3188. doi: 10.1529/biophysj.107.118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CM, Chaudhari N, Sharp AH, Powell JA, Beam KG, Campbell KP. Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J Biol Chem. 1989;264:1345–1348. [PubMed] [Google Scholar]
- Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H. Action potential fatigue in single skeletal muscle fibres of Xenopus. Acta Physiol Scand. 1987;129:311–318. doi: 10.1111/j.1748-1716.1987.tb08074.x. [DOI] [PubMed] [Google Scholar]
- Mänttäri S, Järvilehto M. Comparative analysis of mouse skeletal muscle fibre type composition and contractile responses to calcium channel blocker. BMC Physiol. 2005;5:4. doi: 10.1186/1472-6793-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Moreno RJ, Messi ML, Zheng Z, Wang ZM, Ye P, D'Ercole JA, Delbono O. Role of sustained overexpression of central nervous system IGF-I in the age-dependent decline of mouse excitation-contraction coupling. J Membr Biol. 2006;212:147–161. doi: 10.1007/s00232-006-0044-z. [DOI] [PubMed] [Google Scholar]
- Murray BE, Ohlendieck K. Complex formation between calsequestrin and the ryanodine receptor in fast- and slow-twitch rabbit skeletal muscle. FEBS Lett. 1998;429:317–322. doi: 10.1016/s0014-5793(98)00621-8. [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Gerster U, Doring F, Glossmann H, Tanabe T, Flucher BE. Association of calcium channel alpha1S and beta1a subunits is required for the targeting of beta1a but not of alpha1S into skeletal muscle triads. Proc Natl Acad Sci U S A. 1998;95:5015–5020. doi: 10.1073/pnas.95.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell K, Gannon J, Doran P, Ohlendieck K. Reduced expression of sarcalumenin and related Ca2+-regulatory proteins in aged rat skeletal muscle. Experimental Gerontology. 2008;43:958–961. doi: 10.1016/j.exger.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Payne AM, Zheng Z, Gonzalez E, Wang ZM, Messi ML, Delbono O. External Ca(2+)-dependent excitation--contraction coupling in a population of ageing mouse skeletal muscle fibres. J Physiol. 2004;560:137–155. doi: 10.1113/jphysiol.2004.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber-specific apoptosis and TNF-α signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;04:2870. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Qin N, Olcese R, Zhou J, Cabello OA, Birnbaumer L, Stefani E. Identification of a second region of the beta-subunit involved in regulation of calcium channel inactivation. Am J Physiol. 1996;271:C1539–C1545. doi: 10.1152/ajpcell.1996.271.5.C1539. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol. 1997;157:247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Delbono O. Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J Biol Chem. 1998;273:28845–28851. doi: 10.1074/jbc.273.44.28845. [DOI] [PubMed] [Google Scholar]
- Rios E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol. Rev. 1991;71:849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Ryan M, Carlson BM, Ohlendieck K. Oligomeric Status of the Dihydropyridine Receptor in Aged Skeletal Muscle. Mol Cell Biol Res Commun. 2000;4:224–229. doi: 10.1006/mcbr.2001.0282. [DOI] [PubMed] [Google Scholar]
- Schertzer JD, Plant DR, Lynch GS. Optimizing Plasmid-Based Gene Transfer for Investigating Skeletal Muscle Structure and Function. Mol Ther. 2006;13:795. doi: 10.1016/j.ymthe.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Schredelseker J, Di Biase V, Obermair GJ, Felder ET, Flucher BE, Franzini-Armstrong C, Grabner M. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube C, Beurg M, Powers PA, Gregg RG, Coronado R. Reduced Ca2+ current, charge movement, and absence of Ca2+ transients in skeletal muscle deficient in dihydropyridine receptor beta 1 subunit. Biophys J. 1996;71:2531–2543. doi: 10.1016/S0006-3495(96)79446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HHCM, van Loon LJC. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292:E151–E157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Messi ML, Delbono O. Patch-clamp recording of charge movement, Ca(2+) current, and Ca(2+) transients in adult skeletal muscle fibers. Biophys J. 1999;77:2709–2716. doi: 10.1016/s0006-3495(99)77104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Messi ML, Delbono O. L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys J. 2000;78:1947–1954. doi: 10.1016/S0006-3495(00)76742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Zheng Z, Messi ML, Delbono O. Muscle fibers from senescent mice retain excitation-contraction coupling properties in culture. In Vitro Cell Dev Biol Anim. 2007;43:222–234. doi: 10.1007/s11626-007-9047-z. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Messi ML, Delbono O. Age-dependent IGF-1 regulation of gene transcription of Ca2+ channels in skeletal muscle. Mech Ageing Dev. 2001;122:373–384. doi: 10.1016/s0047-6374(00)00236-0. [DOI] [PubMed] [Google Scholar]