Parakeet (Psittacula krameri) haemoglobin has been purified and crystallized under low salt buffered conditions. Preliminary analysis of the crystal that belonged to monoclinic system (C2) is reported.
Keywords: avian haemoglobin, Psittacula krameri
Abstract
Birds often show efficient oxygen management in order to meet the special demands of their metabolism. However, the structural studies of avian haemoglobins (Hbs) are inadequate for complete understanding of the mechanism involved. Towards this end, purification, crystallization and preliminary X-ray diffraction studies have been carried out for parakeet Hb. Parakeet Hb was crystallized as the met form in low-salt buffered conditions after extracting haemoglobin from crude blood by microcentrifugation and purifying the sample by column chromatography. Good-quality crystals were grown from 10% PEG 3350 and a crystal diffracted to about 2.8 Å resolution. Preliminary diffraction data showed that the Hb crystal belonged to the monoclinic system (space group C2), with unit-cell parameters a = 110.68, b = 64.27, c = 56.40 Å, β = 109.35°. Matthews volume analysis indicated that the crystals contained a half-tetramer in the asymmetric unit.
1. Introduction
The strategies adopted by animals to regulate the supply of oxygen to their respective biological demands may be broadly classified into two groups. One is related to the anatomy of the respiratory system and the other to their physiology, including the structural characteristics of the oxygen-transport protein, i.e. haemoglobin. The anatomies of the respiratory systems of mammals and birds differ fundamentally. In birds, part of the respiratory system forms small air sacs which serve as tidal ventilation for the lungs and have no significant exchange across their walls. The respiratory tract forms a large proportion of the body’s total oxygen-storage capacity in birds, whereas in mammals the oxygen in the respiratory tract forms a much smaller proportion of the body’s total oxygen store. Haemoglobin is a well studied biological molecule that is known for its allosteric mechanism. The molecule has two α and β subunits, each of which contains a ferrous haem group to which oxygen binds reversibly. A characteristic structural feature of haemoglobins is the presence of seven or eight helices, called the ‘globin fold’, in each subunit. This protein preferentially exhibits two states, namely the unliganded tense (T) state and the liganded relaxed (R) state, according to the MWC model proposed by Monod, Wyman and Changeux (Monod et al., 1965 ▶). Haemoglobin can also adopt an oxidized Fe3+ state (the met form) in which water replaces oxygen. In addition to the T and R states, another liganded conformation, R2, has been proposed by Silva et al. (1992 ▶). RR2 and R3 conformations have been proposed by Safo & Abraham (2005 ▶). The quaternary structures of the T and R3 conformations show large differences compared with T-state and R2-state Hb. Avian Hbs are known for their high oxygen affinity and show variations arising from amino-acid mutations, especially at positions α119 (G helix) and β55 (D helix) in the switch region between the two subunits. The amino-acid sequence of parakeet Hb shows 82 and 80% identity to those of greylag goose Hb and bar-headed goose Hb, the crystal structures of which have previously been reported (Liang et al., 2001 ▶; Zhang et al., 1996 ▶). Thus, parakeet Hb was investigated with a view to identifying quaternary structural differences and to understanding how birds have adapted to high oxygen affinity as well as surviving hypoxia.
2. Experimental procedures
2.1. Isolation and purification
Parakeet (Psittacula krameri) haemoglobin was prepared and purified as described previously (Neelagandan et al., 2007 ▶). Briefly, fresh blood was obtained from the veins of parakeets, transferred immediately to 0.01% EDTA to avoid clotting and stored at 277 K. Red blood cells were isolated by centrifugation at 12 000 rev min−1. The supernatant was discarded and the pellets were washed three times with 0.9% NaCl and haemolysed by adding two volumes of Millipore water. To remove cell debris and other organelles, 0.2 ml CCl4 was added and the mixture was centrifuged at 5000 rev min−1 for 10 min. The supernatant was immediately transferred onto a gel-filtration (Sephadex G-25) column (2 × 40 cm) equilibrated with 50 mM Tris (pH 7.5). The isolated protein was extensively dialyzed against water for 24 h to remove trace salts. Quantification of the Hb content was carried out according to the method of Bradford (1976 ▶) and the concentration was estimated at around 35 mg ml−1. Electrophoretic and spectroscopic techniques were used to assess its purity. Bovine serum albumin (BSA) was used as a protein marker in 12% SDS–PAGE (Laemmli, 1970 ▶) followed by the silver-staining method.
2.2. Crystallization
Parakeet Hb was crystallized under low-salt buffered conditions. PEGs with various molecular weights were used to screen for initial crystallization conditions and PEG 3350 was found to be suitable for obtaining good crystals. Good-quality tetragonal-shaped single crystals of parakeet Hb were obtained by the hanging-drop vapour-diffusion method from drops containing 3 µl protein solution, 2 µl 10%(w/v) PEG 3350 and 2 µl 50 mM sodium phosphate buffer (pH 7.5). The drops were equilibrated against reservoir solution containing 10% PEG 3350 at 291 K.
2.3. Data collection and processing
Hb crystals were mounted in 0.5 mm quartz capillaries at 291 K and X-ray diffraction data were collected on a Rigaku RU-H3R rotating-anode generator using Cu Kα radiation (1.5418 Å) with a MAR 345 image-plate detector system. The crystal-to-detector distance was fixed at 100 mm. A total of 180 frames were collected with a time interval of 420 s for every 1°. The diffraction images were indexed, integrated and scaled using the AUTOMAR program package (Bartels & Klein, 2003 ▶). Molecular-replacement and refinement studies were carried out using the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
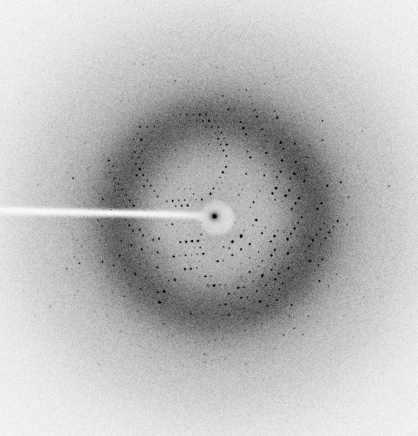
Analysis of parakeet haemoglobin using electrophoretic and spectroscopic techniques indicated that its purity was sufficient for crystallization (Fig. 1 ▶). The purified Hb samples were analyzed by UV–visible spectrophotometry in the visible region (500–600 nm). The peak confirmed that the present parakeet Hb was in the met form. Parakeet Hb was crystallized under low-salt buffered conditions. Crystals suitable for X-ray diffraction were obtained after 2 d (Fig. 2 ▶). The parakeet Hb crystal initially diffracted to 2.8 Å resolution (Fig. 3 ▶), but the diffraction data were processed to 3.0 Å resolution owing to the occurrence of low-resolution spots towards the end of data collection. A summary of the X-ray diffraction data is given in Table 1 ▶. Solvent-content analysis indicated that half a tetramer was present in the asymmetric unit and gave a Matthews coefficient (Matthews, 1968 ▶) of 2.77 Å3 Da−1 and a solvent content of about 57%. Attempts were made to solve the structure by the molecular-replacement method using AMoRe (Navaza, 1994 ▶) as implemented in the CCP4 suite. The amino-acid sequence of parakeet Hb was found to be similar to those of both greylag goose and bar-headed goose Hbs. The coordinates of liganded and unliganded goose Hbs were used as initial search models for molecular replacement. Water molecules were removed from the models to avoid model bias. The resolution range was kept between 15.0 and 4.0 Å and the best solution was obtained using the met form of bar-headed goose Hb (PDB code 1c40; Liu et al., 2001 ▶) as the model, giving an R factor of 0.53%. Refinement was carried out in REFMAC5.0 (Murshudov et al., 1999 ▶) as implemented in the CCP4 suite. 10% of the total reflections were used for R free calculations. The final R factor and R free were 19% and 27%, respectively. Attempts are being made to crystallize Hb in deoxy and carbonmonoxy forms using PEG 3350 and PEG 4000 as precipitants after suitable exposure to dithionite or CO.
Figure 1.
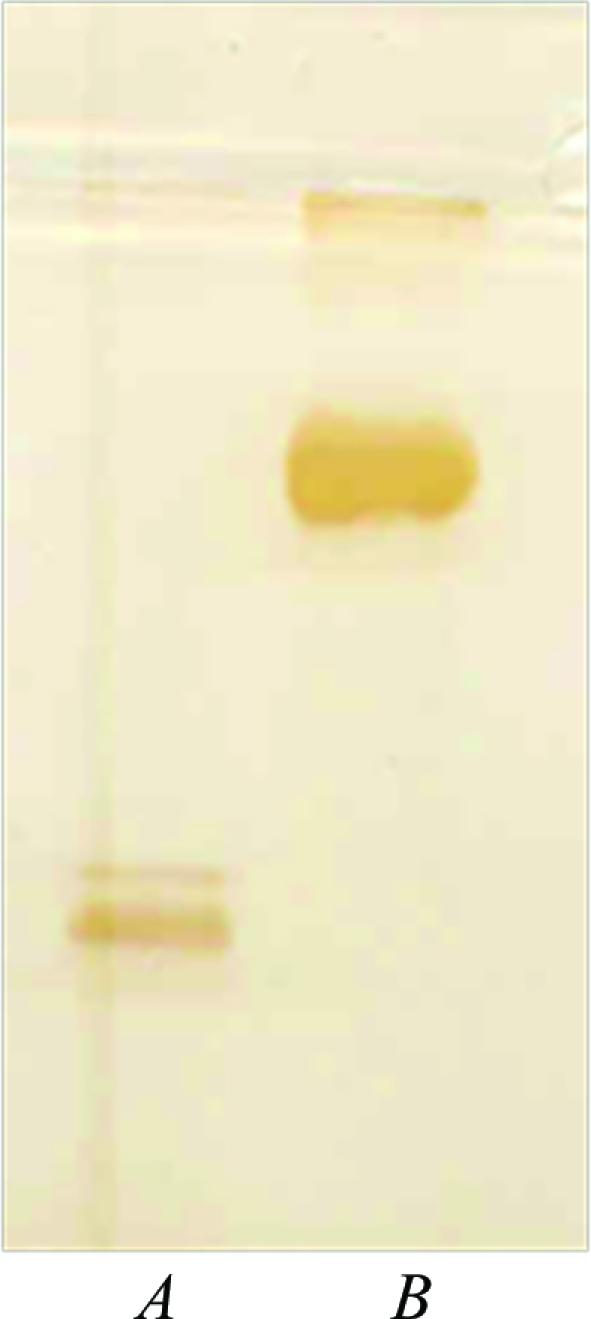
Parakeet Hb (lane A) and bovine serum albumin (lane B) appear as single bands on SDS–PAGE.
Figure 2.
Three-dimensional single crystal of parakeet Hb.
Figure 3.
X-ray diffraction pattern of parakeet Hb.
Table 1. X-ray diffraction data-collection and processing statistics.
| X-ray source | Cu Kα |
| Wavelength (Å) | 1.5418 |
| Oscillation angle (°) | 1 |
| No. of crystals used | 1 |
| Crystal-to-detector distance (mm) | 100 |
| No. of frames | 180 |
| Exposure time (s) | 420 |
| No. of observations | 11523 |
| No. of unique reflections (observed) | 6543 |
| Resolution (Å) | 15.00–3.00 |
| Space group | C2 |
| Unit-cell parameters (Å, °) | a = 110.68, b = 64.27, c = 56.40, β = 109.35 |
| Asymmetric unit | αβ dimer |
| Completeness (%) | 96.0 |
| 〈I/σ(I)〉 | 2.5 |
| Rmerge (%) | 19.52 |
References
- Bartels, K. S. & Klein, C. (2003). The AUTOMAR Manual, v.1.4.3. Norderstedt, Germany: MAR Research GmbH.
- Bradford, M. M. (1976). Anal. Biochem.72, 248–254. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Laemmli, U. K. (1970). Nature (London), 227, 409–413.
- Liang, Y.-H., Liu, X.-Z., Liu, S.-H. & Lu, G.-Y. (2001). Acta Cryst. D57, 1850–1856. [DOI] [PubMed]
- Liu, X.-Z., Li, S.-L., Jing, H., Liang, Y.-H., Hua, Z.-Q. & Lu, G.-Y. (2001). Acta Cryst. D57, 775–783. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Monod, J., Wyman, J. & Changeux, J. P. (1965). J. Mol. Biol.12, 88–118. [DOI] [PubMed]
- Murshudov, G. N., Vagin, A. A., Lebedev, A., Wilson, K. S. & Dodson, E. J. (1999). Acta Cryst. D55, 247–255. [DOI] [PubMed]
- Navaza, J. (1994). Acta Cryst. A50, 157–163.
- Neelagandan, K., Moorthy, P. S., Balasubramanian, M. & Ponnuswamy, M. N. (2007). Acta Cryst. F63, 887–889. [DOI] [PMC free article] [PubMed]
- Safo, M. K. & Abraham, D. J. (2005). Biochemistry, 44, 8347–8359. [DOI] [PubMed]
- Silva, M. M., Rogers, P. H. & Arnone, A. (1992). J. Biol. Chem.267, 17248–17256. [PubMed]
- Zhang, J., Hua, Z., Tame, J. R. H., Lu, G., Zhang, R. & Gu, X. (1996). J. Mol. Biol.255, 484–493. [DOI] [PubMed]