Abstract
Non-small cell lung cancers with activating mutations in the epidermal growth factor receptor (EGFR) are highly responsive to EGFR tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib. Such cancers are “addicted” to EGFR, and treatment with a TKI invariably leads to down-regulation of the PI3K-AKT-mTOR and MEK-ERK signaling pathways, resulting in apoptosis. Using a dual PI3K-mTOR inhibitor, NVP-BEZ235, we evaluated whether PI3K-mTOR inhibition alone induced apoptosis in these cancers. In contrast to HER2-amplified breast cancers, we found that PI3K-mTOR inhibition did not promote substantial apoptosis in the EGFR mutant lung cancers. However, blocking both PI3K-mTOR and MEK simultaneously led to apoptosis to similar levels as the EGFR TKIs, suggesting that down-regulation of these pathways may account for much of the apoptosis promoted by EGFR inhibition. In EGFR mutant lung cancers, down-regulation of both intracellular pathways converged on the BH3 family of proteins regulating apoptosis. PI3K inhibition led to down-regulation of Mcl-1, and MEK inhibition led to up-regulation of BIM. In fact, down-regulation of Mcl-1 by siRNA was sufficient to sensitize these cancers to single-agent MEK inhibitors. Surprisingly, an AKT inhibitor did not decrease Mcl-1 levels, and when combined with MEK inhibitors, failed to induce apoptosis. Importantly, we observed that the combination of PI3K-mTOR and MEK inhibitors effectively shrunk tumors in a transgenic and xenograft model of EGFR T790M-L858R cancers. These data indicate simultaneous inhibition of PI3K-mTOR and MEK signaling is an effective strategy for treating EGFR mutant lung cancers, including those with acquired resistance to EGFR TKIs.
Keywords: Mcl-1, MEK, BIM, acquired resistance, AKT
Over the past few years, it has become clear that non-small cell lung cancers (NSCLCs) with activating mutations in epidermal growth factor receptor (EGFR) are particularly sensitive to EGFR tyrosine kinase inhibitors (TKIs), and this has emerged as another example of a successful targeted therapy paradigm [reviewed in (1)]. Similarly, breast cancers with amplification of HER2 are often sensitive to HER2 TKIs (lapatinib) and antibodies (trastuzumab) (2, 3).
Mounting evidence indicates that both the PI3K-AKT-mTOR and the MEK-ERK pathways are strictly regulated by either EGFR or HER2 in cancers that respond to inhibitors of these RTKs (4). For a cancer to respond to an EGFR TKI, treatment must lead to down-regulation of these intracellular signaling pathways. When most cancers, such as KRAS-mutated cancers, are treated with EGFR TKIs, these intracellular pathways are unaffected, and these cancers are thus de novo resistant (5). In contrast, lung cancers with EGFR mutations have PI3K-AKT-mTOR and MEK-ERK under the sole regulation of EGFR, and when treated with an EGFR TKI, these pathways turn off and the cells undergo substantial apoptosis. However, it remains unknown whether down-regulation of the PI3K-AKT-mTOR, MEK-ERK, or both pathways together is sufficient to recapitulate the apoptotic effects induced by the TKI. Indeed, recent data suggests that HER2-amplified cancers are particularly sensitive to single-agent PI3K-mTOR inhibitors and AKT inhibitors (6–8), raising the possibility that EGFR-driven cancers will be similarly sensitive to PI3K pathway inhibitors.
Although EGFR mutant lung cancers often have initial dramatic responses to EGFR TKIs, these cancers invariably become resistant, and usually in less than 12 months (1, 4). About 50% of these cancers escape EGFR TKI treatment through a secondary threonine to methionine substitution at codon 790 (T790M), located in the kinase domain of EGFR, which renders gefitinib and erlotinib ineffective EGFR inhibitors (9–13). These resistance mutations can be overcome by a new generation of irreversible EGFR inhibitors that covalently bind EGFR (14). Approximately another 20–25% escape EGFR TKI treatment through amplification of another RTK, MET (11). When amplified, MET causes resistance by maintaining PI3K-AKT and MEK-ERK signaling despite continued EGFR inhibition (11). MET inhibitors re-sensitize these cancers to EGFR TKIs. Moreover, preliminary evidence suggests that multiple resistance mechanisms occur simultaneously in the same patient (10, 11). Thus, it is unclear whether attempts to overcome individual resistance mechanisms will provide substantial clinical benefit to patients with acquired resistance to EGFR TKIs, and whether there may be an advantage to directly targeting downstream signaling to overcome multiple resistance mechanisms.
Drugs that directly target the PI3K-AKT-mTOR pathway and the MEK-ERK pathway are entering the clinic. Amongst these are the dual PI3K-mTOR inhibitor, NVP-BEZ235, and the MEK-ERK inhibitor, AZD6244, both of which are currently in clinical trials (15, 16). We recently determined that these drugs used in combination were highly effective in mutant KRAS-induced adenocarcinomas in vivo (17). To determine the impact of inhibiting PI3K and MEK signaling in EGFR mutant lung cancers, we compared the effects of NVP-BEZ235, AZD6244, and their combination in models of EGFR addicted cancers, and models of acquired resistance to EGFR TKIs in vitro and in vivo. We find that, unlike HER2-amplified breast cancers, EGFR-addicted lung cancers do not undergo substantial apoptosis to single-agent PI3K-mTOR inhibitors, but require concomitant inhibition of both PI3K-mTOR and MEK. In EGFR-addicted cancers, we observe that inhibition of PI3K down-regulates Mcl-1, and inhibition of MEK up-regulates BIM to induce apoptosis. These studies suggest that single-agent PI3K pathway inhibitors will not be effective for EGFR-driven lung cancers, but that combined PI3K-mTOR and MEK inhibition may be an effective strategy to overcome multiple means of acquired resistance to EGFR TKIs.
Results
HER2-Amplified Breast Cancer Cell Lines Are Sensitive to Single-Agent PI3K-mTOR Inhibition, but EGFR Mutant Lung Cancer Cell Lines Require Combined PI3K-mTOR and MEK Inhibition.
We determined the efficacy of the dual PI3K-mTOR inhibitor (NVP-BEZ235), the MEK inhibitor (AZD6244) and their combination to treat EGFR mutant lung cancers and HER2-amplified breast cancers. To identify the optimal concentrations of each drug to use in these experiments, we assessed a range of doses for effective target inhibition in all of the cell lines evaluated in this study (Fig. S1). We used the lowest drug concentration that maximally inhibited signaling, as indicated by AKT, S6, and ERK phosphorylation. These doses (NVP-BEZ235 0.2 μM and AZD6244 0.2–2 μM) were similar to concentrations used in previous studies by others (7, 16–19).
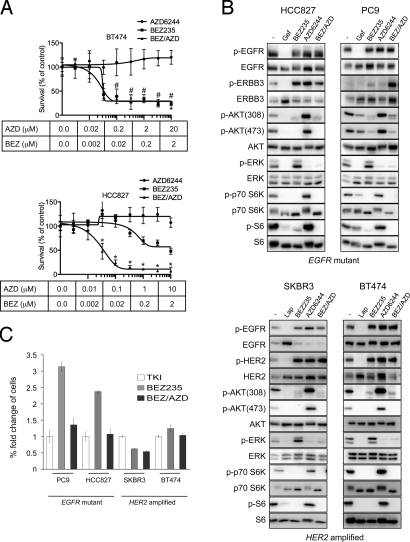
We next compared the efficacy of single-agent NVP-BEZ235, single-agent AZD6244, and their combination (BEZ/AZD), in EGFR mutant (HCC827) and HER2-amplified (BT474) cells. Cells were treated for 72 h and the change in number of viable cells was determined (Fig. 1A). In BT474 cells, single-agent NVP-BEZ235 (0.2 μM) treatment effectively reduced viable cells in agreement with previous reports (7), and this treatment was as potent as BEZ/AZD (0.2 μM NVP-BEZ235/2 μM AZD6244) combination treatment. In contrast, single-agent NVP-BEZ235 (0.2 μM) had little effect on cell viability in HCC827 cells, but the combination BEZ/AZD (0.2 μM NVP-BEZ235/1 μM AZD6244) reduced viability substantially (approximately 5-fold over NVP-BEZ235 (0.2 μM) treatment alone; P < 0.001) (Fig. 1A). In fact, combined BEZ/AZD treatment at the doses that adequately suppress PI3K and MEK signaling (0.2 μM NVP-BEZ235/1 μM AZD6244) decreased cell viability with a similar potency as the EGFR inhibitor, gefitinib (P = ns) (Fig. S2A).
Fig. 1.
PI3K-mTOR inhibition effectively reduces cell viability in HER2-amplified breast cancers, but combined PI3K-mTOR and MEK inhibition is necessary to effectively reduce cell viability in EGFR mutant lung cancer cells. (A) HER2-amplified BT474 cells (top) and EGFR mutant HCC827 cells (lower) and were treated with increasing doses of the PI3K-mTOR inhibitor NVP-BEZ235, the MEK inhibitor AZD6244, or the combination of both (BEZ/AZD), and total cell viability was determined after 72 h by staining cells with the nucleic acid stain, Syto60. Data are presented as the percent of viable cells versus DMSO (-) treated cells. ± S.D. Student's t-test were performed comparing BEZ with BEZ/AZD at indicated concentrations; * indicates a P value <0.001, # indicates P > 0.05 (not significant). (B) Cells were treated with either DMSO (-) or the indicated drug(s) for 6 h (TKIs 1 μM, NVP-BEZ235 0.2 μM for all cell lines, AZD6244 0.2 μM for SKBR3 cells, 1 μM for HCC827 and PC9 cells, and 2 μM for BT474 cells). Protein lysates were immunoblotted and probed with the indicated antibodies. (C) Cells were treated with the indicated drug(s) as in (B) for 16 days, and cell viability was determined by Syto60 assay. The fold difference of viable cells is presented relative to the viable cells treated with tyrosine kinase inhibitor [TKI, gefitinib (gef) for HCC827 and PC9 cells, and lapatinib (lap) for SKBR3 and BT474 cells]. BEZ versus BEZ/AZD (HCC827, P < 0.001; PC9, P < 0.001), and BEZ versus TKI (HCC827, P < 0.001; PC9, P < 0.001).
These survival assays were performed over 72 h, but do not necessarily reflect the efficacies of different treatments over longer periods of time. Thus, we performed long-term survival assays over 16 days to further compare the potencies of NVP-BEZ235, AZD6244, the BEZ/AZD combination, and TKIs in EGFR mutant lung cancers and HER2-amplified breast cancers. As shown in Fig. 1B, the concentrations of NVP-BEZ235 and AZD6244 used suppressed PI3K-AKT and ERK signaling equally to the corresponding TKI. Of note, obvious feedbacks were observed as MEK inhibition led to marked increases in AKT phosphorylation both on Thr-308 and Ser-473 in all four cell lines. In both EGFR mutant cancer cells (PC9 and HCC827), BEZ/AZD treatment for 16 days decreased cell viability to levels similar to those observed by gefitinib treatment (Fig. 1C). NVP-BEZ235 treatment also decreased cell numbers, but substantially less effectively than the TKI or BEZ/AZD combo (P < 0.001). In contrast, in both HER2-amplified cells (SKBR3 and BT474), single-agent NVP-BEZ235 was sufficient to sustain growth inhibition to levels approaching those following lapatinib treatment. Of note, in both EGFR mutant and HER2-amplified cells, both the DMSO and AZD6244 treated cells grew to confluence before the completion of the experiment.
Combination BEZ/AZD Treatment Leads to Cell Death in EGFR-Mutated Cancers.
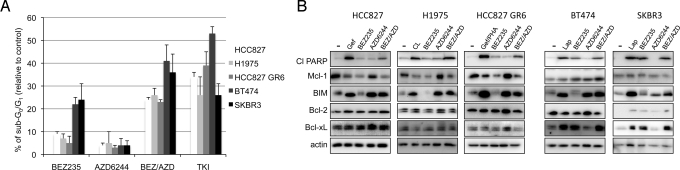
Drug treatments can reduce cell viability by inhibiting growth and/or inducing cell death. Furthermore, it is unclear whether growth inhibition observed in vitro with PI3K pathway inhibitors will accurately predict their activity in vivo. We hypothesized that the greater potency of NVP-BEZ235 in HER2-amplified cancers may reflect a greater capacity to promote cell death, and that the greater potency of the BEZ/AZD combination in the EGFR mutant cancer cell lines may also indicate increased cell death. Thus, we quantified cell death by propidium iodide (PI) staining to determine the percent of cells with subG0/G1 DNA content (20). We compared two HER2-amplified breast cancer cell lines (BT474 and SKBR3) to three different EGFR mutant lung cancer cells lines. We used the EGFR mutant HCC827 cancer cell line, and two models of acquired resistance to EGFR TKIs, H1975 (L858R/T790M), and the MET-amplified HCC827 gefitinib resistant (GR) cells (11). As above, we used the minimum concentrations of NVP-BEZ235 and AZD6244 that effectively inhibited PI3K and MEK signaling in each cell line (Fig. 1B and Fig. S2B). We observed that single-agent NVP-BEZ235 induced markedly more cell death in the HER2-amplified breast cancers than the EGFR mutant lung cancers (Fig. 2A). In the EGFR mutant cancers, PI3K-mTOR inhibition led to an accumulation of cells in G1 after 30 h (Fig. S3), likely accounting for the decreased cell numbers in the long-term assays (Fig. 1C). In addition, we observed that combining the MEK inhibitor with the PI3K-mTOR inhibitor induced marked cell death in the EGFR mutant cancer cells lines, and in fact approached the amount of cell death induced by the effective TKI (Fig. 2A).
Fig. 2.
EGFR mutant cells downregulate Mcl-1 in response to PI3K-mTOR inhibition. (A) EGFR mutant cells (HCC827, H1975, and HCC827 GR6) and HER2-amplified cells (SKBR3 and BT474) were treated with either DMSO control or the indicated drug(s) (TKIs 1 μM, NVP-BEZ235 0.2 μM, AZD6244 0.2 μM for SKBR3 cells, 1 μM for HCC827 and HCC827 GR6 cells, and 2 μM for H1975 and BT474 cells) and 72 h later cells were subjected to flow cytometry to determine subG0/G1 population and the distribution of cycling cells, as described in Materials and Methods. Data are presented as mean ± S.D. of triplicate experiments. (TKI is CL-387,785 (CL) for H1975 cells and gefitinib/PHA-665752 (gef/PHA) for HCC827 GR6 cells). Statistical analyses comparing BEZ versus BEZ/AZD were P < 0.001 for HCC827, H1975, HCC827 GR6, and were not significant (P > 0.01) for BT474 an SKBR3. (B) Cells were treated with either DMSO (-) or drug for 30 h. Cell lysates were prepared and subjected to immunoblotting with the indicated antibodies. The SKBR3 cells were treated for 72 h with the indicated drugs because no substantial PARP cleavage was observed after 30 h with any of the drug treatments.
Combined PI3K and MEK Inhibition Leads to Apoptosis in EGFR Mutated Cancers.
The appearance of subG0/G1 DNA following drug treatment reflects cell death, that is, apoptosis, necrosis, and/or autophagy. Gefitinib has been reported to induce cell death via apoptosis in EGFR mutant cells (21–23). Similarly, we observed that BEZ/AZD combination treatment induced apoptosis in EGFR mutant cancers as demonstrated by the detection of the p85 (cleaved) form of poly (ADP-ribose) (PARP) (24) (Fig. 2B) and increased caspase 3 activity (Fig. S4A). To identify the mechanism underlying how combined PI3K and MEK inhibition led to apoptosis in the EGFR mutant lung cancers, we assessed expression levels of Bcl-2 family members, which act in concert in response to apoptotic/survival signaling to modulate mitochondrial integrity (25). In EGFR mutant cancers, we observed both an increase in BIM expression and a reduction in Mcl-1 expression following TKI or BEZ/AZD combination treatment (i.e., apoptosis-inducing regimens). MEK inhibition induced BIM expression, and PI3K-mTOR inhibition reduced Mcl-1 expression (Fig. 2B). Quantitative RT-PCR analyses revealed that the changes in BIM and Mcl-1 protein expression were not due to changes in RNA abundance (Fig. S4B). This is consistent with previous reports that their expression is often regulated by posttranslational modifications (26, 27). In contrast, Mcl-1 levels were not reduced in response to PI3K-mTOR inhibition in the sensitive HER2-amplified breast cancers (Fig. 2B).
We also observed PI3K-dependent down-regulation of Mcl-1 in the A431 cells (EGFR wild-type and gefitinib-sensitive (28)). Indeed, there was marked apoptosis only when both MEK and PI3K were inhibited (Fig. S5 A and B). SUM102 breast cancer cells that are sensitive to EGFR inhibition (29) also demonstrate Mcl-1 down-regulation in response to PI3K inhibition (Fig. S6A). In these cells, gefitinib only had modest effects on AKT phosphorylation and Mcl-1 expression. This may explain why gefitinib induced only a G1 accumulation in these cells, but combined PI3K/MEK promoted marked apoptosis (Fig. S6 B and C). Of note, when we examined a HER2-amplified lung cancer cell line, Calu-3, neither lapatinib nor NVP-BEZ235 down-regulated Mcl-1 (Fig. S6F). Thus, this data together suggests that PI3K regulates Mcl-1 expression in cancers that are reliant on EGFR, whether EGFR is wild type or mutant, and regardless of the tissue of origin. However, HER2-amplified cancers do not regulate Mcl-1 via PI3K. To this point, although expression of EGFR L858R in BT474 and BT20 cells (a breast cancer cell line without HER2 amplification) induced resistance to single-agent NVP-BEZ235 (Fig. S6 D and E), these cells continued to have PI3K-independent regulation of Mcl-1. Inhibition of MEK re-sensitized these cells to NVP-BEZ235 (Fig. S6E). Thus, it seems that PI3K regulation of Mcl-1 occurs during the development of an EGFR-driven cancer.
Several groups recently reported that BIM up-regulation is a critical component of apoptosis in EGFR mutant cancers, and down-regulation of BIM via siRNA protects cells from apoptosis (21, 22, 30, 31). Similarly, we observed that abrogation of BIM induction protected the EGFR mutant cancers from BEZ/AZD-induced apoptosis (Fig. S7 A and B). We observed that both gefitinib and BEZ/AZD led to Bax activation, likely in response to down-regulation of Mcl-1 and up-regulation of BIM (Fig. S7C).
Reducing Mcl-1 Expression Sensitizes EGFR Mutant NSCLCs Cells to MEK Inhibitors.
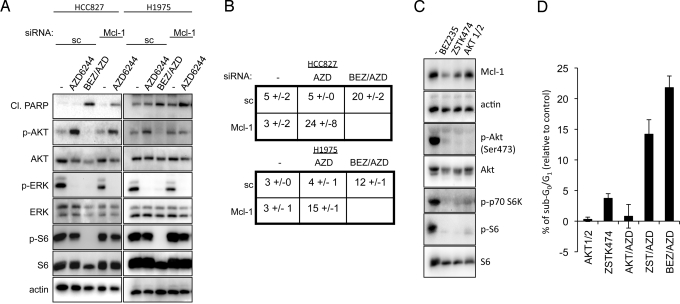
We investigated whether maintenance of Mcl-1 protein expression was a major survival output of the PI3K-AKT-mTOR pathway in EGFR mutant NSCLCs. Remarkably, down-regulation of Mcl-1 via siRNA sensitized the NSCLCs to single-agent MEK inhibition (Fig. 3 A and B and Fig. S8A). The level of Mcl-1 expression in the siRNA experiments (≈75% knockdown) approximated the down-regulation induced by PI3K-mTOR inhibition (Fig. S8B). Moreover, the amount of apoptosis promoted by MEK inhibitors in the Mcl-1 knockdown cells recapitulated the level of cell death induced by combined BEZ/AZD treatment. This suggests that loss of Mcl-1 expression accounts for much of the effect of NVP-BEZ235 in promoting cell death in the combination treatment. These data implicate Mcl-1 and BIM expression as integral survival outputs controlled by the PI3K pathway and MEK-ERK pathway in EGFR mutant lung cancers.
Fig. 3.
Loss of Mcl-1 expression sensitizes EGFR mutant cells to single-agent MEK inhibition. (A) EGFR mutant HCC827 cells were transfected with scrambled (sc) or Mcl-1 siRNA followed by treatment with either DMSO (-) or the indicated drugs for 30 h. Protein lysates were assessed with the indicated antibodies. (B) Cells were transfected and drug treated as in (A). Forty-eight hours following drug treatment, cells were assessed for subG0/G1 DNA content. (C) HCC827 cells were treated for 16 h with either DMSO (-) or the PI3K/mTOR dual inhibitor BEZ235 (.2 μM), the PI3K inhibitor, ZSTK474 (1 μM), or the AKT inhibitor, AKT 1/2 (1 μM), and cell lysates were prepared and subjected to immunoblotting with the indicated antibodies. (D) HCC827 cells were treated for 72 h with either DMSO (-) or indicated drugs, and cells were assessed for subG0/G1 DNA content. Mean + S.D. of triplicate experiments is shown. BEZ/AZD versus AKT/AZD, P < 0.001; BEZ/AZD versus ZST/AZD, P > 0.01, not significant.
To better define how NVP-BEZ235 regulates Mcl-1, we compared NVP-BEZ235 to ZSTK474 [a PI3K inhibitor that does not directly inhibit mTOR (32)] and AKT1/2, an allosteric AKT inhibitor (8). The ZSTK474 compound down-regulated Mcl-1, and induced cell death when combined with a MEK inhibitor (Fig. 3 C and D). To our surprise, the AKT inhibitor did not downregulate Mcl-1 despite marked suppression of AKT and mTORC1 activity (Fig. 3C). Accordingly, the AKT inhibitor did not induce substantial cell death when combined with a MEK inhibitor in the HCC827 cells (Fig. 3D). Thus, this data suggests that PI3K regulation of Mcl-1 is not primarily an AKT-dependent process.
BEZ/AZD Combination Therapy Is Effective in EGFR Mutant Xenografts and T790M/L858R (TL) Mice.
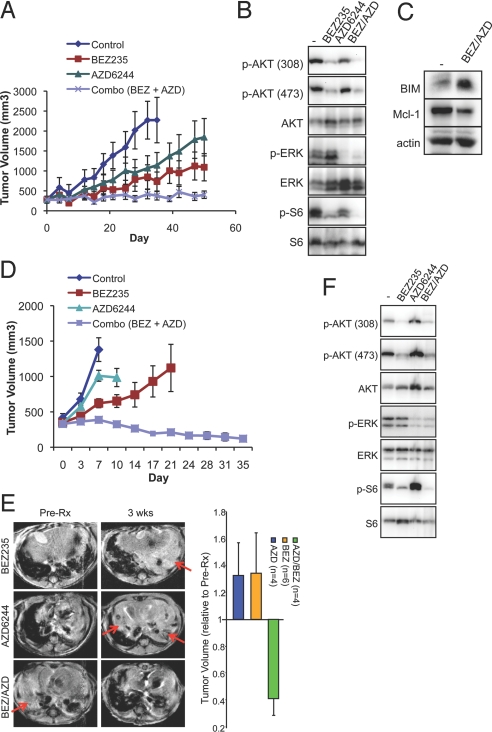
To determine the activity of the BEZ/AZD combination treatment in vivo, we treated two EGFR mutant lung cancer models with the PI3K-mTOR and MEK inhibitors. We first assessed HCC827 xenografts, which have previously been shown to be sensitive to EGFR TKI inhibitors (33). While we did not see substantial responses to either single-agent alone (Fig. 4A), BEZ/AZD combination therapy effectively blocked tumor growth for 50 days (Fig. 4A) (BEZ versus BEZ/AZD, P = 0.014). In vivo, both AKT (Thr-308 and Ser-473) and S6 phosphorylation were reduced by NVP-BEZ235, and ERK phosphorylation was reduced by AZD6244 (Fig. 4B). The combined treatment effectively increased BIM expression and reduced Mcl-1 expression in agreement with the in vitro studies (Fig. 4C). There was no apparent toxicity from the combined treatment. Necropsy examination revealed no evidence of kidney, liver, heart, or lung pathology, and there were no apparent effects on body weight or blood counts. In addition, we treated the gefitinib-resistant H1975 (EGFR L858R/T790M) xenograft tumors and observed marked tumor regression only with BEZ/AZD combination treatment (Fig. 4D) (average tumor is reduced to 36% of pretreatment size, BEZ versus BEZ/AZD, P = 0.002). We next scrutinized genetically engineered mice with inducible expression of an EGFR T790M-L858R transgene upon doxycycline administration (34). This model faithfully recapitulates the T790M mediated mechanism of resistance, the type of acquired resistance to EGFR TKIs that is observed in approximately 50% of patients (9–13, 35). We observed that BEZ/AZD combination therapy, but not either single-agent alone, reduced tumor volume in TL mice (Fig. 4E). This was accompanied by reductions in AKT, S6, and ERK activation (Fig. 4F), and induction of apoptosis as determined by TUNEL staining (Fig. S9 A and B). The dramatic tumor shrinkage induced by BEZ/AZD was even greater than that observed with irreversible EGFR inhibitor, BIBW2992 (36). Of note, we did not identify any effects on tumor vessel numbers following these short-term experiments in the transgenic mouse model, but we did observe that both NVP-BEZ235 and BEZ/AZD diminished the number of vessel endothelial cells after 50 days of treatment in the HCC827 xenograft model consistent with other studies (Fig. S9D) (37, 38). It is possible that the anti-angiogenic activity of NVP-BEZ235 contributes to the therapeutic efficacy of the BEZ/AZD combination.
Fig. 4.
The combination of BEZ/AZD is an effective treatment strategy against in EGFR mutant cancers in vivo. (A) HCC827 xenografts were treated with the indicated drug regimens (as described in SI Methods), and tumor volumes (± S.E.M.) were plotted over time. (B and C) HCC827 xenografts were treated with the indicated drug regimens. Tumors were harvested 1.5 h after treatment on Day 3. Tumor lysates were analyzed by immunoblotting with the indicated antibodies. (D) H1975 xenografts were treated with the indicated drug regimens [as described in Materials and Methods and identical to treatments in (A)], and average tumor volumes (± S.E.M.) were plotted over time. (E) (Left) Representative Magnetic Resonance Images (MRI) of T790M/L858R (TL) mice fed doxycycline to induce tumor formation (Pre-Rx) and treated with the indicated drug regimens for 3 weeks. Note: Red arrows point to tumor. (Right) Tumor volumes of TL mice treated with NVP-BEZ235 (BEZ), AZD6244 (AZD), or AZD/BEZ. The y axis at “1” is the normalized tumor volume at “Day 0” of the respected drug treatments. Tumor volumes of AZD/BEZ versus BEZ alone, P < 0.001, AZD/BEZ versus AZD, P < 0.001). (F) Tumor lysates were prepared from TL mice treated with either vehicle treatment (-) or the indicated drug treatment as in (B). Immunoblotting was performed with the indicated antibodies.
Discussion
In this study, we assessed the relative contributions of the PI3K-AKT-mTOR and MEK-ERK signaling pathways in maintaining the survival of EGFR mutant NSCLCs and HER2-amplified breast cancers. Unlike HER2-amplified breast cancers, EGFR mutant lung cancers did not undergo substantial apoptosis with single-agent PI3K inhibitors. Using several in vitro and in vivo models, we observed that EGFR mutant lung cancers require concomitant PI3K and MEK inhibition to promote apoptosis and shrink cancers. In addition, the combination of NVP-BEZ235 and AZD6244 also effectively induced apoptosis in multiple models of acquired resistance (H1975 cells, HCC827 GR6 cells, and EGFR T790M-L858R transgenic mice) in vitro and in vivo. These data suggest that this combination could be advantageous to patients with TKI-sensitive EGFR mutant NSCLCs as well as those with acquired resistance to EGFR TKI therapy. Since more than one acquired resistance mechanism may simultaneously exist in an individual (11), targeting downstream signaling might provide a useful way to overcome multiple resistance mechanisms.
To understand the mechanism of inhibitor-induced apoptosis in both EGFR mutant and HER2-amplified cancer cells, we analyzed the levels of Bcl-2 family proteins before and after drug treatments. In both cancer types, inhibition of the MEK-ERK pathway resulted in induction of pro-apoptotic BIM protein. Recently, four independent groups observed that BIM was up-regulated in EGFR mutant NSCLCs undergoing apoptosis in response to EGFR TKIs (21, 22, 30, 31). In this study, we found that the levels of the anti-apoptotic Mcl-1 protein were greatly reduced following inhibition of the PI3K-AKT-mTOR pathway in EGFR mutant NSCLCs. In HER2-amplified cancers, there was no decrease in Mcl-1 following PI3K-mTOR inhibitors or any of the drug treatments, further suggesting that EGFR mutant cancers and HER2-amplified cancers use different mechanisms to escape tonic death signaling.
In this study, we also assessed whether the particular oncogene addiction (mutant EGFR vs. wild-type EGFR vs. HER2) or the tissue type (lung vs. breast) determines whether PI3K regulates Mcl-1, and if cell death requires combined PI3K and MEK inhibition. Cancer cell lines from organs other than lung that require wild-type EGFR for growth (A431 epidermoid cancer and SUM102 breast cancer) also had Mcl-1 under the regulation of PI3K, and concomitant PI3K and MEK inhibition was necessary to induce their death (Fig. S6 A–C). This indicates that PI3K-dependent regulation of Mcl-1 expression may be a common feature of most EGFR-driven cancers regardless of tissue of origin, whether EGFR is wild type or mutant. Conversely, a lung cancer cell line with HER2 amplification, Calu-3, did not have Mcl-1 regulation under the control of PI3K (Fig. S6F). In addition, expression of EGFR L858R in BT474 and BT20 breast cancer cell lines conferred resistance to cell death induced by single-agent BEZ235. However, they were sensitive to concomitant targeted inhibition of the PI3K and MEK pathways (Fig. S6 D and E). In total, these results suggest that intrinsic differences between HER2- and EGFR-addicted cancers, rather than their tissues of origin, determine if the PI3K pathway regulates Mcl-1 and the therapeutic potential of these different treatment strategies.
Intriguingly, we observed that reduction of Mcl-1 levels with siRNA sensitized the EGFR mutant NSCLCs to single-agent AZD6244. In fact, this recapitulated the apoptotic response invoked by BEZ/AZD combination in both HCC827 and H1975 cells. This result suggests that down-regulation of Mcl-1 is a critical output of PI3K-mTOR inhibition that cooperates with MEK inhibition to promote apoptosis. Thus, we hypothesize that in EGFR mutant NSCLCs, high levels of Mcl-1 may directly sequester Bax and/or Bak to prevent their oligomerization or may bind preexisting BIM, thereby preventing BIM-mediated activation, or “priming,” of Bax/Bak. Indeed, interactions between Mcl-1 with BIM have been reported in the gefitinib-sensitive A431 cells (39). Furthermore, it may be the case that only when BIM levels become excessive, contingent upon both Mcl-1 degradation and BIM up-regulation, that BIM primes Bax/Bak, and commits cells to apoptosis as we observed. This is consistent with the model put forth by Certo et al. (40), and could provide a therapeutic insight into precisely how active PI3K-AKT-mTOR and MEK-ERK signaling keep EGFR mutant cells alive. However, not every single EGFR mutant cancer may share this regulation of BIM and Mcl-1. For example, we did not observe Mcl-1 down-regulation in PC9 cells in response to PI3K-mTOR or EGFR inhibition consistent with previously reported results (30).
Mcl-1 is a pro-survival member of the Bcl-2 family with a short protein half-life (≈3 h). Mcl-1 contains two N-terminal PEST sequences, which appear to contribute to its instability (26). There are many proposed mechanisms that may account for the effect of NVP-BEZ235 on Mcl-1 expression. Phosphorylation of Mcl-1 at Ser-159 (located in one of the PEST domains) by glycogen synthase kinase 3β (GSK3β) promotes Mcl-1 degradation. The mTORC1 pathway, which functions downstream of AKT, can also stabilize Mcl-1, and treatment with the mTOR inhibitor, rapamycin, leads to reduced Mcl-1 levels in several cancer lymphoma and leukemia models (41–43). However, in our studies on EGFR mutant lung cancer cell lines, we found that loss of Mcl-1 cannot be recapitulated by loss of AKT or mTORC1 activity (Fig. 3 B and C). Thus, these experiments predict that a combination of PI3K with MEK inhibitors will be substantially more effective than a combination of AKT and MEK inhibitors for patients with EGFR mutant lung cancers. In light of the recent findings from Garraway and colleagues that describe critical AKT-independent functions in the growth and survival of PIK3CA mutated cancers (44), the observations in this study further support the notion that there may be substantial therapeutic differences between PI3K and AKT inhibitors.
The observations in this study are quite relevant to the treatment of EGFR mutant NSCLCs. Our data suggest that down-regulation of the MEK-ERK and PI3K-AKT-mTOR pathways accounts for much of the apoptotic activity induced by EGFR TKIs. Importantly, our results suggest that patients with EGFR mutant NSCLCs may not be sensitive to single-agent PI3K pathway inhibitors. However, they may be quite sensitive to combined PI3K and MEK inhibition, even if they have acquired resistance to EGFR TKIs. Thus, this combination holds promise as an effective component of therapy to overcome acquired resistance to EGFR TKIs.
Materials and Methods
Please see SI Methods for the description of the cell lines and reagents, survival assays, immunohistochemical, quantitative RT-PCR, Bax activity assay, mouse treatment studies, statistical analyses, and immunoblotting techniques.
Flow Cytometry.
Cells were prepared for cell cycle analysis essentially as previously described (45). Briefly, following treatments, cells were collected, washed twice in PBS, and resuspended in 70% EtOH. After 24 h, cells were analyzed on an LSR flow cytometer (Becton-Dickinson). Routinely, >15,000 acquired events were gated, and cells were quantified for subG0/G1 population, or analyzed for cell cycle populations by Modfit LT V3.0 (Verity Software House).
Short Interfering (si)RNA Experiments.
Scrambled, Bim, or Mcl-1 siRNA were mixed in 0.75 mL Optimem and 10 min later mixed with 0.75 mL Optimem containing 75 μL Hiperfect reagent. Fifteen minutes later the solution was added drop-wise to HCC827 or H1975 cells plated in antibiotic-free media, to give a final siRNA concentration of 50 nM. Following a 24-h transfection period, cells were trypsinized and re-seeded evenly in 6-well plates. The following day cells were treated with either DMSO (-) or the indicated drugs and cells were prepared either 30 h later (immunoblot) or 48 h later (flow cytometry).
Supplementary Material
Acknowledgments.
We thank Michael Waring (Ragon Institute, Massachusetts General Hospital) for help with flow cytometry experiments, Alan Eastman (Dartmouth Medical School) for helpful discussions regarding SUM102 cells, and Thomas Gilmore (Department of Biology, Boston University) for the CHAPS. This work was supported by National Institutes of Health Grants K08 CA120060-01 (to J.A.E.), R01CA137008-01 (to J.A.E.), 1R01CA140594 (to J.A.E. and K.-K.W.), K08 AG024004 (to K.-K.W.), R01 CA122794 (to K.-K.W.), and R01 AG2400401 (to K.-K.W.); Lung SPORE Grant P50CA090578 (to J.A.E. and K.-K.W.); Dana Farber/Harvard Cancer Center GI Cancer SPORE Grant P50 CA127003 (to J.A.E.); the V Foundation (to J.A.E.); the Ellison Foundation Scholar (to J.A.E.); American Cancer Society RSG-06-102-1 (to J.A.E.); the Joan Scarangello Foundation to Conquer Lung Cancer (to K.-K.W.); the Cecily and Robert Harris Foundation (to K.-K.W.); and the Flight Attendant Medical Research Institute (to K.-K.W.).
Footnotes
Conflict of interest statement: Sauveur-Michel Maira and Carlos Garcia-Echeverria are employees and stockholders of Novartis.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905056106/DCSupplemental.
References
- 1.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 2.Arteaga CL. Trastuzumab, an appropriate first-line single-agent therapy for HER2-overexpressing metastatic breast cancer. Breast Cancer Res. 2003;5:96–100. doi: 10.1186/bcr574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulhoa-Cintra A, Greenberg L, Geyer CE. The emerging role of lapatinib in HER2-positive breast cancer. Curr Oncol Rep. 2008;10:10–17. doi: 10.1007/s11912-008-0004-0. [DOI] [PubMed] [Google Scholar]
- 4.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18:73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Benvenuti S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 6.Ihle NT, et al. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serra V, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 8.She QB, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:1–11. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bean J, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka T, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12:5764–5769. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- 14.Kwak EL, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh TC, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 16.Maira SM, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 17.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks JL, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68:5524–5528. doi: 10.1158/0008-5472.CAN-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friday BB, et al. BRAF V600E disrupts AZD6244-induced abrogation of negative feedback pathways between extracellular signal-regulated kinase and Raf proteins. Cancer Res. 2008;68:6145–6153. doi: 10.1158/0008-5472.CAN-08-1430. [DOI] [PubMed] [Google Scholar]
- 20.Ormerod MG, Collins MK, Rodriguez-Tarduchy G, Robertson D. Apoptosis in interleukin-3-dependent haemopoietic cells. Quantification by two flow cytometric methods. J Immunol Methods. 1992;153:57–65. doi: 10.1016/0022-1759(92)90305-d. [DOI] [PubMed] [Google Scholar]
- 21.Costa DB, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–1680. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–1690. doi: 10.1371/journal.pmed.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono M, et al. Sensitivity to gefitinib (Iressa, ZD1839) in non-small cell lung cancer cell lines correlates with dependence on the epidermal growth factor (EGF) receptor/extracellular signal-regulated kinase 1/2 and EGF receptor/Akt pathway for proliferation. Mol Cancer Ther. 2004;3:465–472. [PubMed] [Google Scholar]
- 24.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 25.Heath-Engel HM, Shore GC. Regulated targeting of Bax and Bak to intracellular membranes during apoptosis. Cell Death Differ. 2006;13:1277–1280. doi: 10.1038/sj.cdd.4401961. [DOI] [PubMed] [Google Scholar]
- 26.Warr MR, Shore GC. Unique biology of Mcl-1: Therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 27.Luciano F, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 28.Guix M, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoadley KA, et al. EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics. 2007;8:258. doi: 10.1186/1471-2164-8-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng J, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–11875. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaguchi S, et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006;98:545–556. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- 33.Amann J, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–235. [PubMed] [Google Scholar]
- 34.Li D, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Balak MN, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 36.Li D, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan TL, et al. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. Proc Natl Acad Sci USA. 2008;105:9739–9744. doi: 10.1073/pnas.0804123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnell CR, et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: Implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 39.Wilsbacher JL, et al. Insulin-like growth factor-1 receptor and ErbB kinase inhibitor combinations block proliferation and induce apoptosis through cyclin D1 reduction and Bax activation. J Biol Chem. 2008;283:23721–23730. doi: 10.1074/jbc.M708360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Certo M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Vega F, et al. Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res. 2006;66:6589–6597. doi: 10.1158/0008-5472.CAN-05-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei G, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Mills JR, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci USA. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasudevan KM, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faber AC, et al. Inhibition of phosphatidylinositol 3-kinase-mediated glucose metabolism coincides with resveratrol-induced cell cycle arrest in human diffuse large B-cell lymphomas. Biochem Pharmacol. 2006;72:1246–1256. doi: 10.1016/j.bcp.2006.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.