Abstract
Patients with end-stage renal disease (ESRD) suffer exceptionally high mortality rates in their first year of chronic hemodialysis. Both vitamin D and fibroblast growth factor (FGF)-23 levels correlate with survival in these patients. Klotho is a protein in the vitamin D/FGF-23 signaling pathway that has been linked with accelerated aging and early mortality in animal models. We therefore hypothesized that genetic variation in the Klotho gene might be associated with survival in subjects with ESRD. We tested the association between 12 single nucleotide polymorphisms (SNPs) in the Klotho gene and mortality in a cohort of ESRD patients during their first year on hemodialysis (n = 1307 white and Asian). We found a significant association between the CC genotype of one tag SNP, rs577912, and increased risk for 1-yr mortality (RR, 1.76; 95% CI, 1.19–2.59; p = 0.003). This effect was even more marked among patients who were not treated with activated vitamin D supplementation (HR, 2.51; 95% CI, 1.18–5.34; p = 0.005). In lymphoblastoid cell lines derived from HapMap subjects, the CC genotype was associated with a 16–21% lower Klotho expression compared with the AA/AC genotype. Our data suggest that a specific Klotho variant (rs577912) is linked to survival in ESRD patients initiating chronic hemodialysis and that therapy with activated vitamin D may modify this risk.
Key words: Klotho, hemodialysis, mortality, vitamin D, fibroblast growth factor-23, HapMap, end-stage renal disease, polymorphism
INTRODUCTION
Approximately 350,000 patients with end-stage renal disease (ESRD) undergo maintenance hemodialysis in the United States.(1) Incident hemodialysis patients suffer from a 1-yr mortality of ∼20%.(1) Whereas much of the high death risk in ESRD may be caused by elevations in traditional risk factors, there is coherent evidence that nontraditional and perhaps genetic risk factors are of particular relevance.(2)
Klotho seems to modulate aging in mice and humans. Mice null for the α-Klotho gene have early appearance of aging-related phenotypes including vascular calcification, osteoporosis, hypogonadism, muscle atrophy, and markedly shortened lifespan.(3,4) Conversely, α-Klotho transgenic mice have increased longevity.(5) Associations with Klotho polymorphisms in humans have been reported for diseases such as osteoporosis, stroke, coronary artery disease, and longevity, and traits such as BMD, high-density lipoprotein (HDL) cholesterol, and blood pressure.(6–11) Rare loss-of-function mutations in the Klotho gene in humans lead to a syndrome including severe vascular calcification with normal renal function.(12) More recently, a gain-of-function mutation in the Klotho gene has been linked with severe hypophosphatemic rickets and hyperparathyroidism in humans.(13)
Klotho encodes a type I cell surface protein with an extracellular domain that can be released as a cleavage product. Klotho participates in complex reciprocal interactions with vitamin D and fibroblast growth factor (FGF)-23. Together with the FGF receptor, Klotho constitutes the functional receptor for FGF-23, a major phosphaturic hormone.(14) α-Klotho–null mice have elevated levels of 1,25 dihydroxyvitamin D [1,25(OH)2D3], because α-Klotho decreases 1-α-hydroxylase activity in the kidney.(15) Many symptoms of α-Klotho–null mice or the similar FGF-null mouse phenotype can be reversed by concurrent ablation of the 1-α-hydroxylase gene, a vitamin D–deficient diet, or most markedly by restriction of dietary phosphate.(15,16) Importantly, administration of 1,25(OH)2D3, a factor correlated with improved survival in ESRD patients, induces elevated expression of α-Klotho in mice.(17) FGF-23 also seems to upregulate Klotho,(18) and FGF-23 levels rise dramatically in the setting of Klotho deficiency, likely because of loss of feedback inhibition. Elevated FGF-23 levels are associated with increased mortality in incident hemodialysis patients,(19) which may implicate low Klotho levels as the intermediary mechanism. This integration with vitamin D and FGF-23 biology, both known factors in mortality on hemodialysis, suggests that Klotho may be part of a causal pathway in survival of ESRD patients.
To determine whether Klotho gene polymorphisms might impact survival in ESRD patients on hemodialysis, we tested SNPs in the Klotho gene for association with improved survival in ESRD patients. We thereafter asked how active vitamin D supplementation and mineral parameters modify the effects of these SNPs. Finally, we showed that the SNP with the strongest association to mortality directly correlates with Klotho mRNA levels in human cell lines.
MATERIALS AND METHODS
Study population
ArMORR, Accelerated Mortality on Renal Replacement, is a nationally representative prospective cohort study of 10,044 incident hemodialysis patients throughout the United States, which has been previously described in detail.(19,20) Remnant whole blood samples from 2490 random ArMORR subjects were available for DNA extraction and establishment of the ArMORR DNA Repository, which was approved by the Institutional Review Board of the Massachusetts General Hospital. The need for informed consent was waived because of the use of remnant samples and the irreversible removal of all personal identifiers (including all direct or indirect links to personal identifiers) and clinical information from samples before entry into the repository. Remnant blood samples for additional testing of novel biomarkers (e.g., FGF-23) were not available in this population. Information on exposures and clinical outcomes collected in ArMORR(19,20) and adequate quality DNA were available for 1865 total subjects.
DNA extraction
Genomic DNA from 3–4 ml of remnant whole blood stored in PaxGene tubes was extracted following an adapted PreAnalytix protocol using a Qiagen AutoPure extraction robot (Harvard Partners Center for Genetics and Genomics, Cambridge, MA, USA). The DNA quality was assessed with 260/280 OD ratios in all samples.
SNP selection
Fourteen tag SNPs were selected using the Tagger algorithm (http://www.broad.mit.edu/mpg/tagger/server.html). We used multimarker tagging mode to select tag SNPs that would capture common HapMap variants (minor allele frequency [MAF] > 0.05) within the Klotho gene sequence (Chr13: 32488571..32538279) with r 2 > 0.8 (actual r 2 = 0.86–0.99). The 14 SNPs capture 100% of HapMap variation in whites (CEU), 84% Chinese from Beijing and Japanese from Tokyo (CHB+JPT), and 32% in Yoruba from Nigeria (YRI) HapMap SNPs. Given the low tagging efficiency for YRI HapMap SNPs, the differential survival rates in black incident dialysis patients compared with other populations,(19) and to minimize population stratification, we excluded black subjects from this study, leaving 1307 subjects in the final analysis. The tagged SNP RS numbers and sequences are listed in Supplementary Table 1.
SNP genotyping
Genotyping was performed using the Sequenom platform. SNPs used in the study were required to meet the following quality control criteria: (1) overall call rate >94%, (2) discordance rate <3%, and (3) conformation to Hardy-Weinberg equilibrium (HWE) among controls. Genotyped SNPs that failed any of these three criteria were excluded from subsequent analyses. Quality control data for all SNPs are shown in Supplementary Table 2. Twelve tag SNPs passed quality control criteria, with average call rates >99%. These 12 tag SNPs captured 84% of HapMap CEU SNPs (r 2 > 0.8) and 84% of CHB and JPT SNPs (10 of 12 available, r 2 > 0.8), but only 28% (r 2 > 0.8) of YRI SNPs. A subject was excluded if the proportion of missing genotype data exceeded 20%.
Klotho gene expression
We used raw data generated by Stranger et al.(21) and deposited at www.sanger.ac.uk/humgen/genevar, in which 210 HapMap cell lines were profiled for gene expression levels. Genotypes for each subject were obtained from www.hapmap.org. Gene expression levels for Klotho polymorphisms of interest were plotted against genotype for each ancestral group: CEU, JPT, CHB, and YRI. When homozygotes for the minor allele were too infrequent for meaningful statistical analyses, they were pooled with heterozygotes and compared with major allele homozygotes. To pool data from all four ancestral groups into a single group for analysis, Klotho expression levels were normalized for ancestry such that each group's average gene expression level was equal. We used net expression values to calculate percent difference between genotypes and log2 normalized expression values to calculate statistical significances. Linkage disequilibrium (LD) values and diagrams were generated with Haploview.
Statistics
The primary exposure was Klotho variant, and the primary outcome was 1-yr mortality. Patient characteristics according to each variant of interest were compared with the use of Student's t-test, the χ2 test, or Fisher's exact test, as appropriate. The Cochrane-Armitage test was used to test for trend associations. The RR and its 95% CI were also calculated. Additive and dominant models were initially used for the analyses, and when power was limited (e.g., small number of subjects with a specific genotype), dominant models with combined groups were preferred.
We used Kaplan-Meier curves with log-rank tests to examine 1-yr survival after initiation of hemodialysis according to Klotho variant. Patients were censored when they discontinued dialysis as a result of recovery of renal function or kidney transplantation or were lost to follow-up because they transferred their care to a non–Fresenius Medical (the source of all ArMORR subjects) Center.
After ensuring that the proportional hazards assumption was not violated, we adjusted for potential confounders with use of Cox regression analyses. Covariates included in the Cox models have previously been found to be associated with mortality on dialysis(20): age (continuous), sex (binary), race (categorical, white or other), etiology of chronic renal failure (categorical, diabetes, hypertension, glomerulonephritis, other), arteriovenous access (binary, fistula/graft or catheter), baseline systolic blood pressure (BP; continuous, in quantiles, and as a binary covariate divided according to the median levels), body mass index (continuous, in quantiles, and as a binary covariate divided according to the median levels), blood levels of albumin (continuous), white blood cell count (continuous), hemoglobin (continuous), calcium (continuous), phosphorus (continuous), and PTH (continuous, in quantiles, and as a binary covariate divided according to the median levels). The α-Klotho–null mice phenotype seems to be rescued by low levels of 1,25-dihydroxyvitamin D or phosphate deficiency(15,16); therefore, we also examined the relationship between Klotho variants and therapy with activated vitamin D (as a time-dependent variable in Cox models), levels of serum phosphate (continuous and binary, ≤6 versus >6 mg/dl), and whether subjects were treated with a phosphate binder at baseline.
In multivariable analyses, we formally tested the interaction between Klotho variant and covariates (variant × covariate). When significant interaction was detected (p < 0.05) in univariate and multivariable-adjusted analyses (inclusive of models with the variant and the covariate of interest), we examined results from models stratified by the covariate. Hardy-Weinberg equilibrium (HWE) was examined for each variant using 1 df (>3.84) to indicate statistical significance. Those variants that deviated from HWE (p < 0.004 after Bonferroni correction for multiple testing) were excluded from the analyses. We also examined the highly conservative Bonferroni correction to adjust significance values for the number of SNPs successfully genotyped. For gene expression data, normalized (log2) gene expression values were used for statistical analyses. Statistical Analysis System (v 9.1; SAS Institute, Cary, NC, USA) was used for all statistical analyses, and all p values were two-sided.
RESULTS
The general characteristics of the study population are shown in Table 1. The mean age, distribution of race, and other clinical and laboratory characteristics resemble those of larger populations in this same cohort (after exclusion of blacks)(19) and the U.S. dialysis population in general. In this cohort of 1307 incident dialysis subjects from throughout the United States, the 1-yr mortality was 15.5% (n = 202).
Table 1.
Baseline Characteristics (n = 1307)
| Characteristic | Value* |
| Age (yr) | 68 ± 12 |
| Female (%) | 41 |
| Race (%) | |
| White | 89 |
| Asian, Pacific Islander | 1 |
| Native American, Alaskan | 1 |
| Other | 9 |
| BMI (kg/m2) | 27 ± 7 |
| Etiology of ESRD (%) | |
| Diabetes | 48 |
| Hypertension | 30 |
| Glomerulonephritis | 9 |
| Polycystic kidney disease | 2 |
| Other | 11 |
| Vascular access (%) | |
| Catheter | 64 |
| Other | 36 |
| Systolic blood pressure (mmHg) | 141 ± 22 |
| Diastolic blood pressure (mmHg) | 71 ± 12 |
| Albumin (g/dl) | 3.5 ± 0.5 |
| Calcium (mg/dl) | 8.5 ± 0.8 |
| Phosphorus (mg/dl) | 4.6 ± 1.5 |
| Bio-intact PTH (pg/ml)† | 180 (103–304) |
| Hemoglobin (g/dl) | 10.4 ± 1.3 |
| Ferritin (ng/ml)† | 184 (93–379) |
| Creatinine (mg/dl) | 5.6 ± 2.2 |
| White blood cell count (cells/μl) | 8.6 ± 3.1 |
| Platelets (cells/dl) | 226 ± 89 |
* Values are mean ± SD.
† Values are median and interquartile range (25–75%).
Twelve common variants of the Klotho gene were examined in the population according to 1-yr mortality. Table 2 shows the results of the additive models and the level of significance (unadjusted) for each variant among those that died within 1 yr and those that did not. HWE was tested in the controls, and none of the variants deviated from this assumption (p > 0.05 for all variants; Supplementary Table 2).
Table 2.
Allele Frequency
| SNP | Allele | Deaths (%) | Non-deaths (%) | Trend p value |
| rs562020 | C/C | 74 (37.8%) | 447 (41.4%) | 0.282 |
| C/T | 93 (47.4%) | 494 (45.8%) | ||
| T/T | 29 (14.8%) | 138 (12.8%) | ||
| rs495392 | G/G | 95 (47.5%) | 555 (52.4%) | 0.421 |
| G/T | 91 (45.5%) | 421 (39.7%) | ||
| T/T | 14 (7.0%) | 84 (7.9%) | ||
| rs385564 | C/C | 21 (10.7%) | 120 (11.0%) | 0.879 |
| C/G | 84 (42.6%) | 446 (41.1%) | ||
| G/G | 92 (46.7%) | 520 (47.9%) | ||
| rs576404 | G/G | 64 (32.8%) | 395 (36.5%) | 0.352 |
| G/T | 98 (50.3%) | 517 (47.8%) | ||
| T/T | 33 (16.9%) | 169 (15.6%) | ||
| rs2283368 | C/C | 4 (2.1%) | 8 (0.7%) | 0.376 |
| T/C | 40 (20.6%) | 219 (20.2%) | ||
| T/T | 150 (77.3%) | 855 (79.0%) | ||
| rs2320762 | G/G | 26 (14.1%) | 119 (11.4%) | 0.364 |
| G/T | 87 (47.0%) | 499 (47.6%) | ||
| T/T | 72 (38.9%) | 430 (41.0%) | ||
| rs9536282 | C/C | 141 (71.9%) | 781 (72.5%) | 0.957 |
| C/T | 52 (26.5%) | 271 (25.2%) | ||
| T/T | 3 (1.5%) | 25 (2.3%) | ||
| rs580332 | A/A | 28 (14.6%) | 155 (14.4%) | 0.485 |
| A/T | 99 (51.6%) | 517 (48.1%) | ||
| T/T | 65 (33.9%) | 402 (37.4%) | ||
| rs577912 | A/A | 3 (1.5%) | 24 (2.3%) | 0.005 |
| C/A | 33 (16.8%) | 273 (26.0%) | ||
| C/C | 161 (81.7%) | 754 (71.7%) | ||
| rs9536254 | G/G | 1 (0.5%) | 9 (0.8%) | 0.647 |
| G/T | 25 (12.4%) | 142 (13.1%) | ||
| T/T | 175 (87.1%) | 934 (86.1%) | ||
| rs567170 | C/C | 59 (30.6%) | 395 (36.6%) | 0.245 |
| C/G | 103 (53.4%) | 512 (47.5%) | ||
| G/G | 31 (16.1%) | 171 (15.9%) | ||
| rs1888057 | C/C | 140 (70.0%) | 685 (63.4%) | 0.063 |
| C/T | 54 (27.0%) | 347 (32.1%) | ||
| T/T | 6 (3.0%) | 49 (4.5%) |
Among the 12 tagging SNPs eligible for testing, only rs577912 achieved a noteworthy level of significance (p = 0.005). The next most significant SNP was rs1888057, which achieved a p value of 0.063. Given the low prevalence of the AA genotype, we examined dominant models. Compared with the AA/AC genotypes, the CC genotype was associated with an increased risk for 1-yr mortality (RR, 1.76; 95% CI, 1.19–2.59; p = 0.003; Bonferroni-adjusted p = 0.036). Table 3 shows the characteristics of the population according to their rs577912 genotype. Baseline serum levels of calcium, phosphate, and PTH were not significantly different between the two groups. In fact, only serum ferritin levels—a measure of iron status and inflammation—were significantly different. The frequency of being treated with activated vitamin D was also not significantly different between the groups (AA/AC, 75% versus CC, 70%, p = 0.103).
Table 3.
Baseline Characteristics According to the rs577912 Genotype
| Characteristic | A/A and A/C* | C/C* | p |
| Age (yr) | 69 ± 12 | 68 ± 12 | 0.245 |
| Female (%) | 39 | 42 | 0.475 |
| Race (%) | 0.221 | ||
| White | 87 | 90 | |
| Asian, Pacific Islander | 2 | 1 | |
| Native American, Alaskan | 0 | 1 | |
| Other | 11 | 8 | |
| BMI (kg/m2) | 27 ± 7 | 27 ± 7 | 0.195 |
| Etiology of ESRD (%) | 0.743 | ||
| Diabetes | 48 | 48 | |
| Hypertension | 30 | 31 | |
| Glomerulonephritis | 9 | 8 | |
| Polycystic kidney disease | 1 | 2 | |
| Other | 12 | 11 | |
| Vascular Access (%) | 0.550 | ||
| Catheter | 65 | 63 | |
| Other | 35 | 37 | |
| Systolic blood pressure (mmHg) | 142 ± 23 | 141 ± 22 | 0.470 |
| Diastolic blood pressure (mmHg) | 70 ± 13 | 70 ± 12 | 0.847 |
| Albumin (g/dl) | 3.5 ± 0.5 | 3.5 ± 0.5 | 0.415 |
| Calcium (mg/dl) | 8.5 ± 0.9 | 8.5 ± 0.8 | 0.982 |
| Phosphorus (mg/dl) | 4.7 ± 1.6 | 4.6 ± 1.5 | 0.341 |
| Bio-intact PTH (pg/ml)† | 207 (114–372) | 201 (117–351) | 0.879 |
| Hemoglobin (g/dl) | 10.4 ± 1.3 | 10.5 ± 1.3 | 0.201 |
| Ferritin (ng/ml)† | 187 (101–387) | 208 (48–421) | 0.040 |
| Creatinine (mg/dl) | 5.7 ± 2.4 | 5.6 ± 2.2 | 0.310 |
| White blood cell count (cells/mcl) | 8.7 ± 3.1 | 8.6 ± 3.1 | 0.688 |
| Platelets (cells/dl) | 230 ± 100 | 226 ± 86 | 0.612 |
* Mean ± SD.
† Values are median and interquartile range.
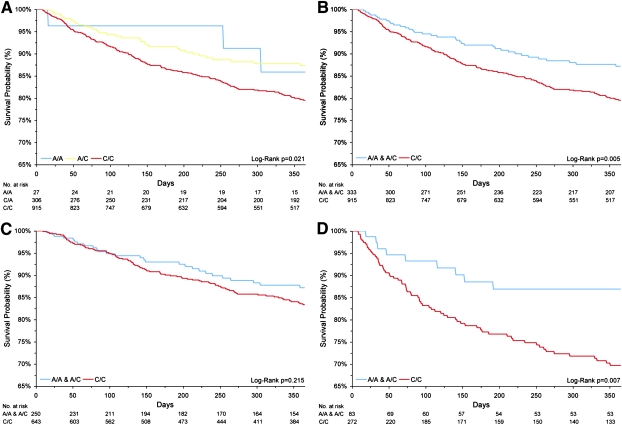
The Kaplan-Meier survival analysis of the entire population using the additive and dominant models are shown in Figs. 1A and 1B., respectively. We performed Cox proportional hazards analysis to determine whether the relationship between rs577912 genotype and mortality was independent of potential confounders (age, sex, race [white versus other], etiology of chronic renal failure, arteriovenous access, baseline systolic BP, body mass index, therapy with activated vitamin D, and baseline blood levels of albumin, white blood cell count, ferritin, hemoglobin, calcium, phosphorus, and PTH). In adjusted analyses, those with the CC genotype showed a significantly increased risk of 1-yr mortality (HR, 1.77; 95% CI, 1.20–2.62; p = 0.004). The other covariates that remained significant in the adjusted analyses included age, systolic blood pressure, and serum ferritin and albumin (all p < 0.05). Alternative modeling strategies to examine the influence of potential nonlinear relationships (e.g., as quantiles or as a binary variable) with body mass index, systolic BP, and PTH levels did not materially change (>10%) the point estimate between the CC genotype and 1-yr mortality.
FIG. 1.
Kaplan-Meier survival analysis based on genotype at rs577912. (A) All subjects, additive model. (B) All subjects, dominant model. (C) Patients receiving 1,25 vitamin D therapy. (D) Patients not receiving 1,25 vitamin D therapy.
We next examined first-order interactions and found that only the interaction term rs577912 variant × therapy with activated vitamin D showed a statistically significant interaction (p = 0.041) in the fully adjusted model with both terms also included. Interaction terms examining the rs577912 variant and baseline categories of serum phosphate, baseline phosphate binder use, and baseline levels of PTH did not reach statistical significance (p = 0.580, p = 0.180, and p = 0.360, respectively). In an effort to further tease apart potential causal pathways, models with and without serum minerals and PTH were examined, but these analyses neither altered the point estimates or the significance of the CC genotype and mortality.
Kaplan-Meier analysis stratified by therapy with activated vitamin D (Figs. 1C and 1D) showed a significant risk of early mortality among untreated subjects with the CC genotype. In the multivariable analysis conducted on all treated subjects, the CC genotype was not significantly associated with increased risk for early mortality (HR, 1.48; 95% CI, 0.93–2.37), whereas among all untreated subjects, the risk among the CC subjects was markedly elevated (HR, 2.51; 95% CI, 1.18–5.34, p = 0.005).
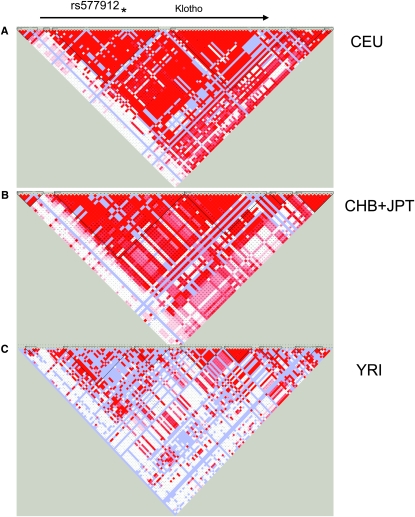
We examined the haplotype structure of the Klotho gene on chromosome 13, both in HapMap populations and in our ARMORR cohort. In the HapMap white, Japanese, and Chinese subjects, there is high LD across the gene region, whereas LD is markedly lower in subjects of African ancestry (Figs. 2A–2C). This difference in LD between populations suggests that rs577912 would serve as an effective tag SNP in white and Asian populations, but may be far less effective in the black population in the United States. Of note, the SNP (rs1888057) with the second best correlation to dialysis mortality (p = 0.06) was the only tag SNP in even modest LD (r 2 = 0.34) with rs577912 (Supplementary Fig. 1).
FIG. 2.
LD maps for α-Klotho in the white (CEU), Asian (CHB+JPT), and African (YRI) HapMap populations. These maps show strong LD in the white and Asian populations but much weaker LD in the African population. Bright red indicates strong LD.
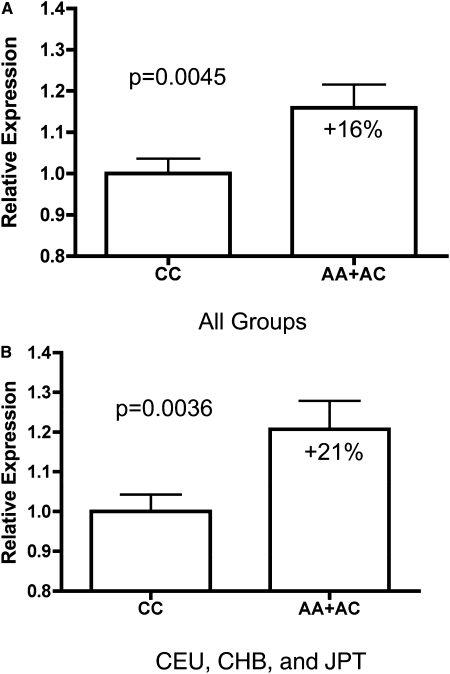
We asked how rs577912, located in intron 1 of the Klotho gene, might affect Klotho function. rs577912 is in strong LD (r 2 > 0.9) with 11 other known SNPs in whites, although neither the location of rs577912 or other SNPs in strong LD with rs577912 suggested obvious functional consequences. We next asked whether rs577912 was associated with Klotho gene expression levels, testing this hypothesis in lymphoblast cell lines from HapMap subjects who have all been genotyped at rs577912. These cell lines included subjects from four different ancestral groups. In each group, cell lines from subjects who had either AA or AC at rs577912 expressed higher levels of Klotho mRNA compared with cell lines from subjects with CC at rs577912. Expression level differences ranged from 7% in Yorubans to 36% in Chinese. Whites with either AA or AC had 27% higher Klotho expression than those with CC at rs577912.
Given that each set of cell lines had limited statistical power to detect differences independently, we pooled data from all four ancestral groups. After pooling all groups, the AA/AC subjects had 16% higher Klotho mRNA expression levels than CC subjects (p = 0.0045; Fig. 3). However, analysis of LD maps showed that, whereas subjects of European or Asian origin had strong LD in the region of the Klotho gene, Yorubans had characteristically weaker LD. Whereas rs577912 efficiently tagged 11 other SNPs in whites (r 2 > 0.9), it tagged only 2 SNPs in those of African ancestry, making it a much weaker proxy for potentially functional SNPs. We analyzed the pooled expression data without including Africans and found a stronger correlation: AA/AC subjects had 21% higher Klotho mRNA expression than CC subjects (p = 0.0036).
FIG. 3.
α-Klotho mRNA expression for different genotypes at rs577912. (A) α-Klotho mRNA expression in 210 HapMap subjects from four populations. (B) α-Klotho mRNA expression, excluding YRI subjects (N = 150). Subjects with the CC genotype at rs577912 have less Klotho mRNA expression than subjects with AA or AC at rs577912.
DISCUSSION
In this study, we identified a common variant in the Klotho gene, rs577912, associated with mortality in chronic hemodialysis patients. A dominant model suggested that the CC genotype conferred an excess risk of death compared with the AA or AC genotypes. This excess risk was most apparent in hemodialysis patients not treated with activated vitamin D, and activated vitamin D supplementation seemed to nullify the risk differential. This specific genotype was also associated with lower Klotho mRNA expression in lymphoblast cell lines from HapMap subjects genotyped at rs577912.
rs577912 is located in an intron, distant from any exons, and does not itself have obvious functional significance. However, rs577912 does tag 11 other SNPs in whites with strong LD (r 2 > 0.9), any one of which could be a functional SNP explaining the effect on hemodialysis mortality. Because none of these potentially functional SNPs changed an amino acid in the Klotho protein, we suspected that a functional SNP might quantitatively affect Klotho gene expression at the mRNA level. We used gene expression profiling data from the HapMap subjects to assess the contribution of rs577912 to Klotho mRNA expression levels. In all four ancestral groups, the risk CC genotype was associated with a tendency toward lower Klotho expression. In sum, cell lines with the AA and AC genotype expressed 16% more Klotho than CC cell lines (p = 0.0045), an effect that was stronger (21% increased expression, p = 0.0036) when blacks were not included in the analysis. Examination of the haplotype structures supports more powerful tagging by rs577912 in white and Asian populations than in African populations, so the improvement after exclusion of Yoruban samples was not surprising. There are currently no cell lines ideally suited to studying Klotho's complex biology, and the effect of genotype at rs577912 on Klotho gene expression might be substantially stronger in vivo.
We also predict that rs577912 would show a better correlation with hemodialysis mortality in whites (and Asians) than in blacks. Whereas rs577912 tags 11 HapMap SNPs at r 2 > 0.9 in whites, it only tags 2 SNPs at r 2 > 0.9 in Africans, meaning that a functional SNP may be successfully tagged by rs577912 in whites but not in those with significant African ancestry. This observation is typical of LD across the genome, where LD blocks are longer in whites, with less heterogeneity in each block, than Africans.
Although the Klotho gene is linked with the biology of calcium, phosphorus, and PTH metabolism,(22) we did not observe significant effect modification or differences in levels between our specific Klotho variant and these measures. The reasons for this include the possibility that human serum levels may not be reflective of cellular metabolism, or we could not control for all potential confounders (e.g., diet, dialysis dose, binder use) that also affect these levels. It also suggests that other mechanisms linked with Klotho that have been recently uncovered including interactions with insulin/IGF signaling, TRPV5 calcium channel function, oxidative stress, and Wnt signaling, which may play a vital role in mortality.(5,23–25) In this light, it was interesting to note that only serum ferritin, a measure of inflammation,(26) was significantly different according to the rs577912 genotype. A recent study has shown that activation of peroxisome proliferator-activated receptor-γ (PPARγ) with thiazolidinediones induces Klotho gene expression,(27) which has raised the possibility that Klotho may be involved in the PPARγ-mediated anti-inflammatory responses.(28) Furthermore, given the high mortality burden in this patient population because of vascular disease, it is tempting to speculate that Klotho is promoting beneficial effects on vascular homeostasis unrelated to blood mineral levels. Our evidence for interaction between Klotho and vitamin D, along with evidence that vitamin D has direct effects on vascular health, makes a direct vascular effect of Klotho another intriguing possibility.
α-Klotho–null mice have elevated levels of FGF-23, suggesting that Klotho is downstream of FGF-23 and there is a failure of feedback inhibition in the α-Klotho–null mouse. For this reason, the correlation between high FGF-23 levels and mortality in hemodialysis patients as we recently reported(19) may be caused by high FGF-23 levels acting as a surrogate marker for low Klotho levels. We did not have remnant blood samples in this repository to measure FGF-23 levels; however, this study included a subset of subjects from the same population among whom we found a strong relationship between serum FGF-23 levels and mortality.
α-Klotho–null mice have significantly elevated activity of the 1-α-hydroxylase enzyme and subsequently 1,25 dihydroxyvitamin D levels, suggesting that vitamin D is also upstream of Klotho and regulates Klotho levels, whereas Klotho inhibits activated vitamin D production through a classic feedback loop. We therefore tested for an interaction with activated vitamin D therapy, first expecting it would increase risk for early mortality among those with the CC genotype (lower Klotho expression). Importantly, the frequency of being treated with activated vitamin D therapy was similar in the AA/AC versus CC groups, supporting the finding that this variant does not lead to dramatic changes in serum levels of PTH or minerals (primary reasons to treat or withhold therapy with activated vitamin D therapy(29)). In these analyses, among those treated with activated vitamin D therapy, the CC genotype was not associated with increased risk of early mortality. This suggested that activated vitamin D therapy might well overwhelm the ≅20% difference in Klotho levels because of variation at rs577912. Indeed, 1,25 dihydroxyvitamin D administration increases renal Klotho expression in mice.(17) Whereas interesting to speculate that upregulation of renal Klotho by vitamin D may be an important mechanism underlying the mortality benefit of vitamin D administration to hemodialysis patients, Klotho expression in extrarenal tissues is low, and we did not have measures of residual renal function in subjects for this study. Future studies may test this hypothesis in other populations, including those with chronic kidney disease before initiation of dialysis.
Several studies have reported various phenotypes that correlate with a Klotho haplotype called KL-VS, which contains two coding polymorphisms in perfect LD. The two individual polymorphisms seem to have opposite effects on Klotho levels, neutralizing one another to some degree. In our study, SNP rs9536282 tags these coding polymorphisms but did not show any relationship to ESRD hemodialysis mortality (p = 0.956).
Unlike genetic studies in diseases such as type 2 diabetes or coronary artery disease, where many cohorts are available for replicating results, carefully phenotyped cohorts of hemodialysis patients with archived genetic material are rare. Whereas our p values for genetic association between rs577912 and mortality are significant even after correction for multiple hypotheses testing, genuine concern remains that positive results may be caused by chance or undetected bias (either in our epidemiologic or genetic approaches). For this reason, we performed in vitro gene expression data based on genotype. Because the same SNP was associated with both increased mortality and decreased Klotho expression levels (the expected Klotho–mortality relationship based on mouse and human data), we believe that the chance of a false-positive association is low. Furthermore, this human study supports the elegant in vivo and in vitro studies linking Klotho alterations and accelerated mortality.(3,5) Nevertheless, we must acknowledge that this study will need to be confirmed by subsequent replication as additional cohorts become available. In addition, we would hope to find Klotho variants that are informative in black patients, a task that will likely require a larger number of patients and denser marker sets.
ACKNOWLEDGMENTS
D.F. is supported by DK076868 (NIH). R.T. is supported by DK71674, DK076116, and HL093954, all by the National Institutes of Health.
Footnotes
Dr. Thadhani has received research support from Abbott and speaking honoraria from Abbott and Genzyme. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.U.S. Renal Data System. Bethesda, MD, USA: National Institute of Diabetes and Digestive and Kidney Diseases; 2008. Annual Data Report: Atlas of Chronic Kidney Disease and End-stage Renal Disease in the United States. [Google Scholar]
- 2.Zoccali C, Testa A, Spoto B, Tripepi G, Mallamaci F. Mendelian randomization: A new approach to studying epidemiology in ESRD. Am J Kidney Dis. 2006;47:332–341. doi: 10.1053/j.ajkd.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 4.Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 5.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arking DE, Krebsova A, Macek M, Sr, Macek M, Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, Dietz HC. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 9.Kawano K, Ogata N, Chiano M, Molloy H, Kleyn P, Spector TD, Uchida M, Hosoi T, Suzuki T, Orimo H, Inoue S, Nabeshima Y, Nakamura K, Kuro-o M, Kawaguchi H. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17:1744–1751. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- 10.Ogata N, Matsumura Y, Shiraki M, Kawano K, Koshizuka Y, Hosoi T, Nakamura K, Kuro OM, Kawaguchi H. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone. 2002;31:37–42. doi: 10.1016/s8756-3282(02)00786-x. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of the androgen receptor and klotho genes with bone mineral density in Japanese women. J Mol Med. 2005;83:50–57. doi: 10.1007/s00109-004-0578-4. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7:318–319. [PubMed] [Google Scholar]
- 13.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 15.Torres PU, Prie D, Molina-Bletry V, Beck L, Silve C, Friedlander G. Klotho: An antiaging protein involved in mineral and vitamin D metabolism. Kidney Int. 2007;71:730–737. doi: 10.1038/sj.ki.5002163. [DOI] [PubMed] [Google Scholar]
- 16.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: Lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 18.Strewler GJ. Untangling klotho's role in calcium homeostasis. Cell Metab. 2007;6:93–95. doi: 10.1016/j.cmet.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 21.Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, Ingle CE, Dunning M, Flicek P, Koller D, Montgomery S, Tavare S, Deloukas P, Dermitzakis ET. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuro-o M. Endocrine FGFs and Klothos: Emerging concepts. Trends Endocrinol Metab. 2008;19:239–245. doi: 10.1016/j.tem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 24.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105:9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant. 2004;19:141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N. Klotho is a target gene of PPAR-gamma. Kidney Int. 2008;74:732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 28.Ruan X, Zheng F, Guan Y. PPARs and the kidney in metabolic syndrome. Am J Physiol Renal Physiol. 2008;294:F1032–F1047. doi: 10.1152/ajprenal.00152.2007. [DOI] [PubMed] [Google Scholar]
- 29.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]